High-Precision Monitoring of Average Molecular Weight of Polyethylene Wax from Waste High-Density Polyethylene
Abstract
1. Introduction
2. Test Procedure
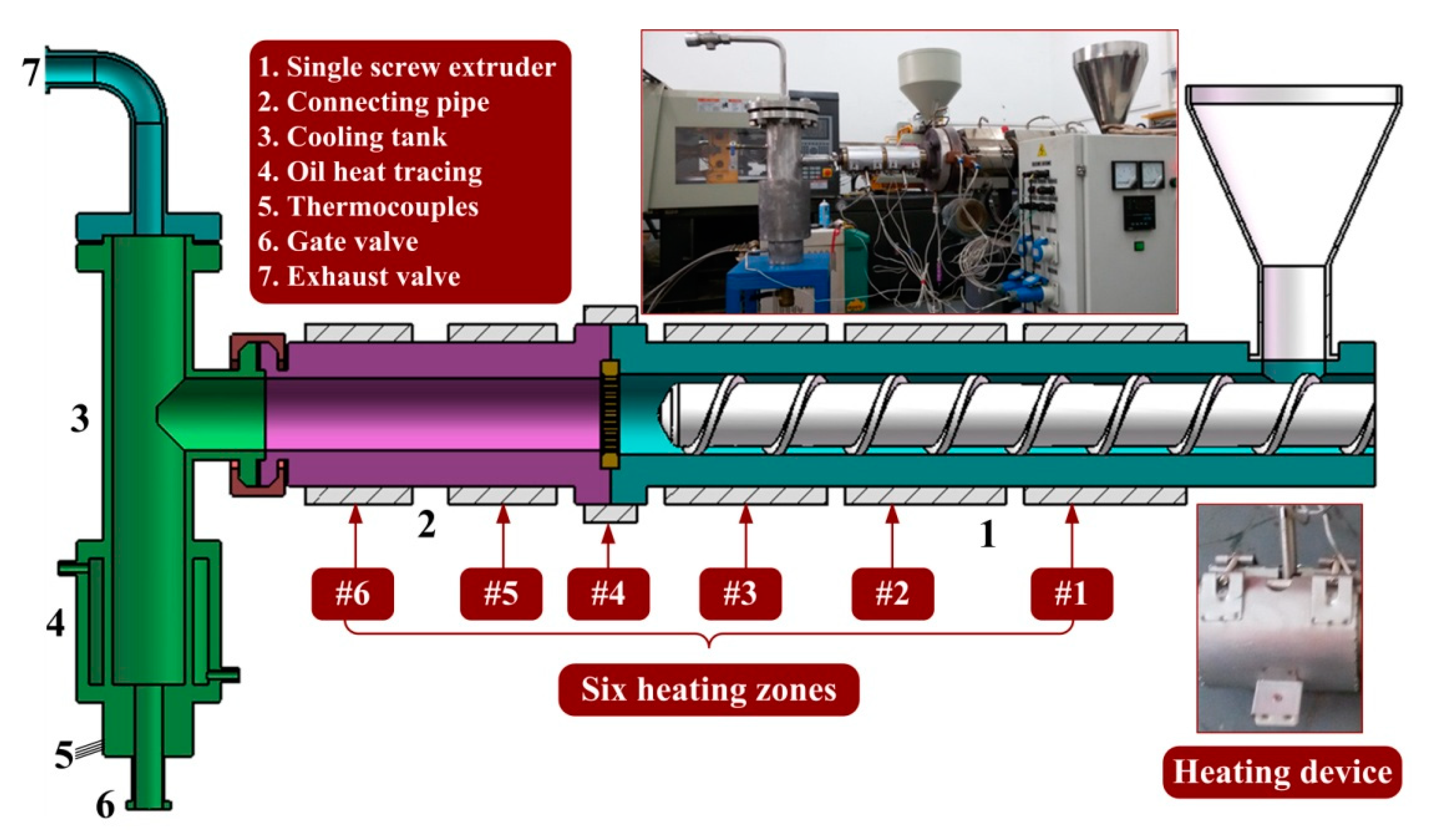
2.1. Test Platform
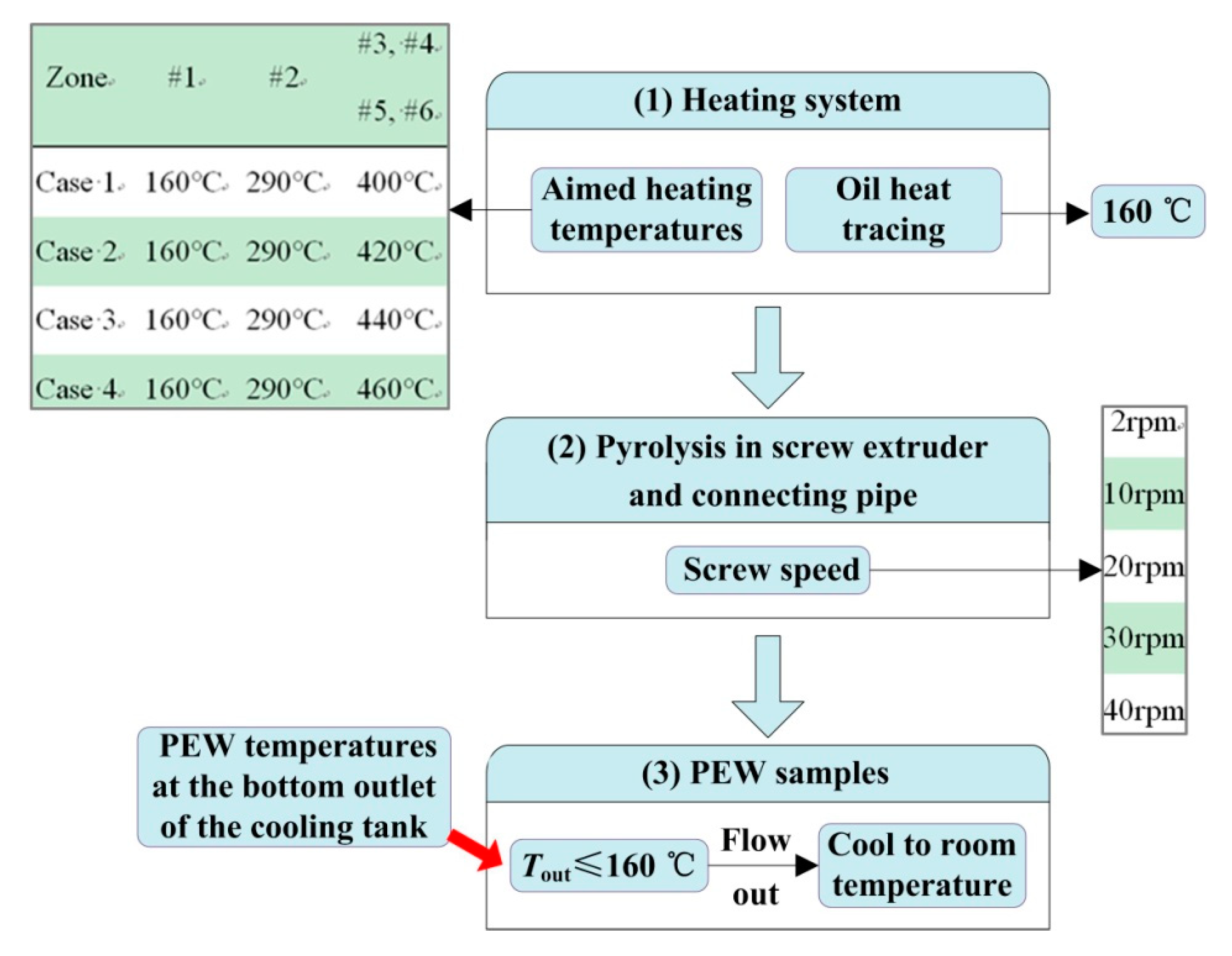
2.2. Preparation of Polyethylene Wax
2.3. Characterization of Polyethylene Wax
3. Theoretical Analysis of Pyrolysis Procedure
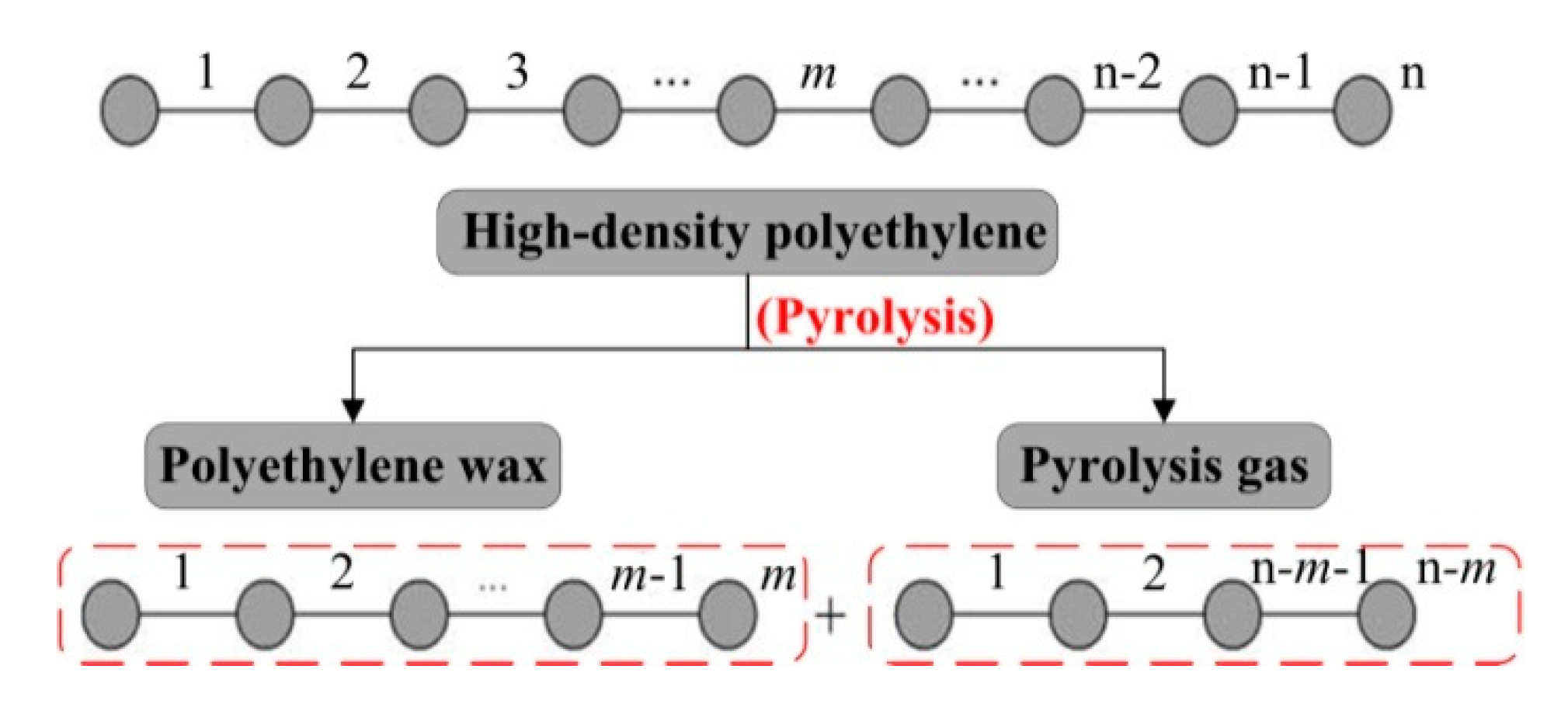
3.1. Pyrolysis Model
3.2. Theoretical Calculation of Average Carbon-Atom Number of PEW
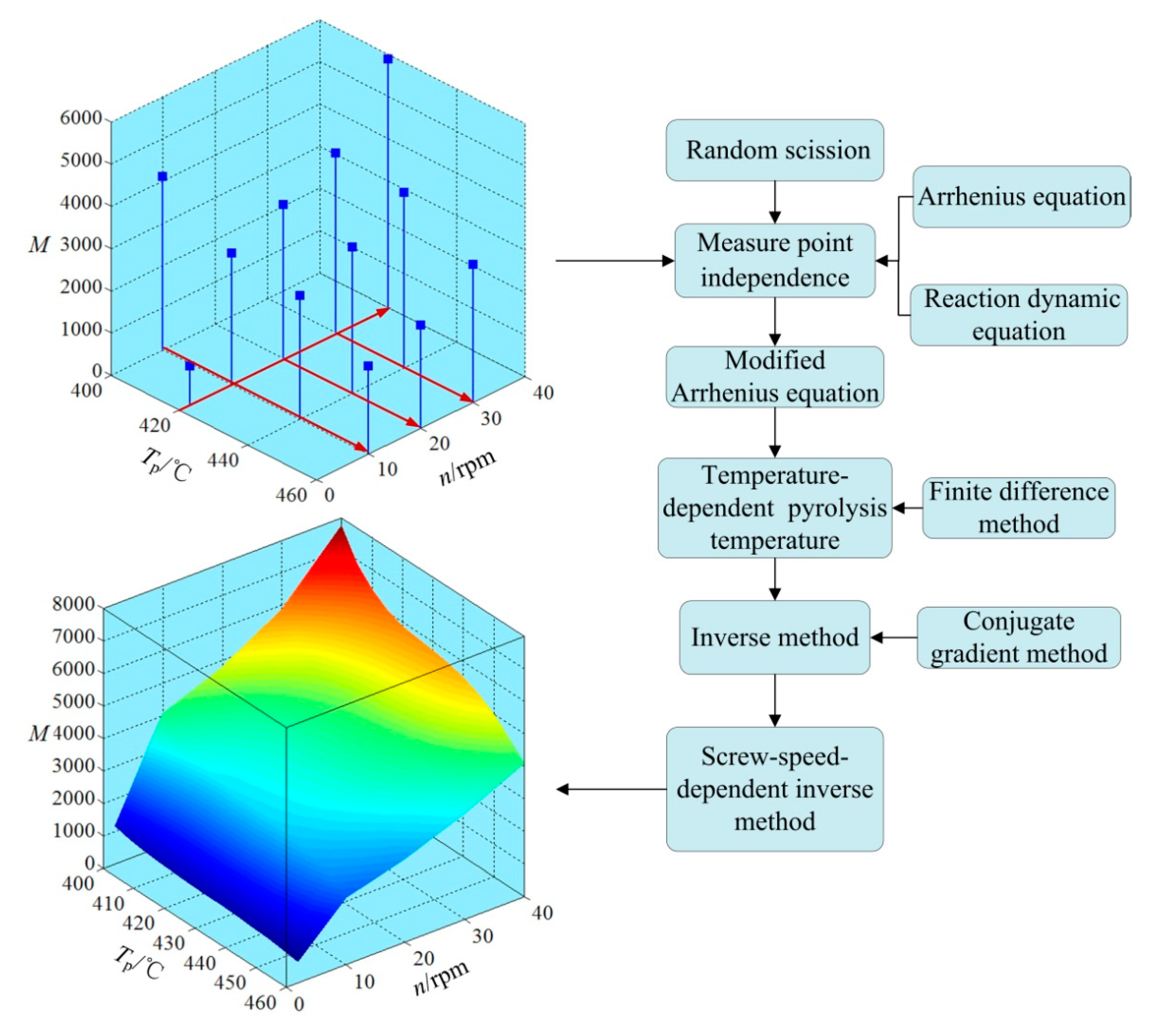
4. Monitoring Procedure for Average Molecular Weight of Polyethylene Wax
4.1. Arrhenius Equation
4.2. Measure-Point-Independence Verification
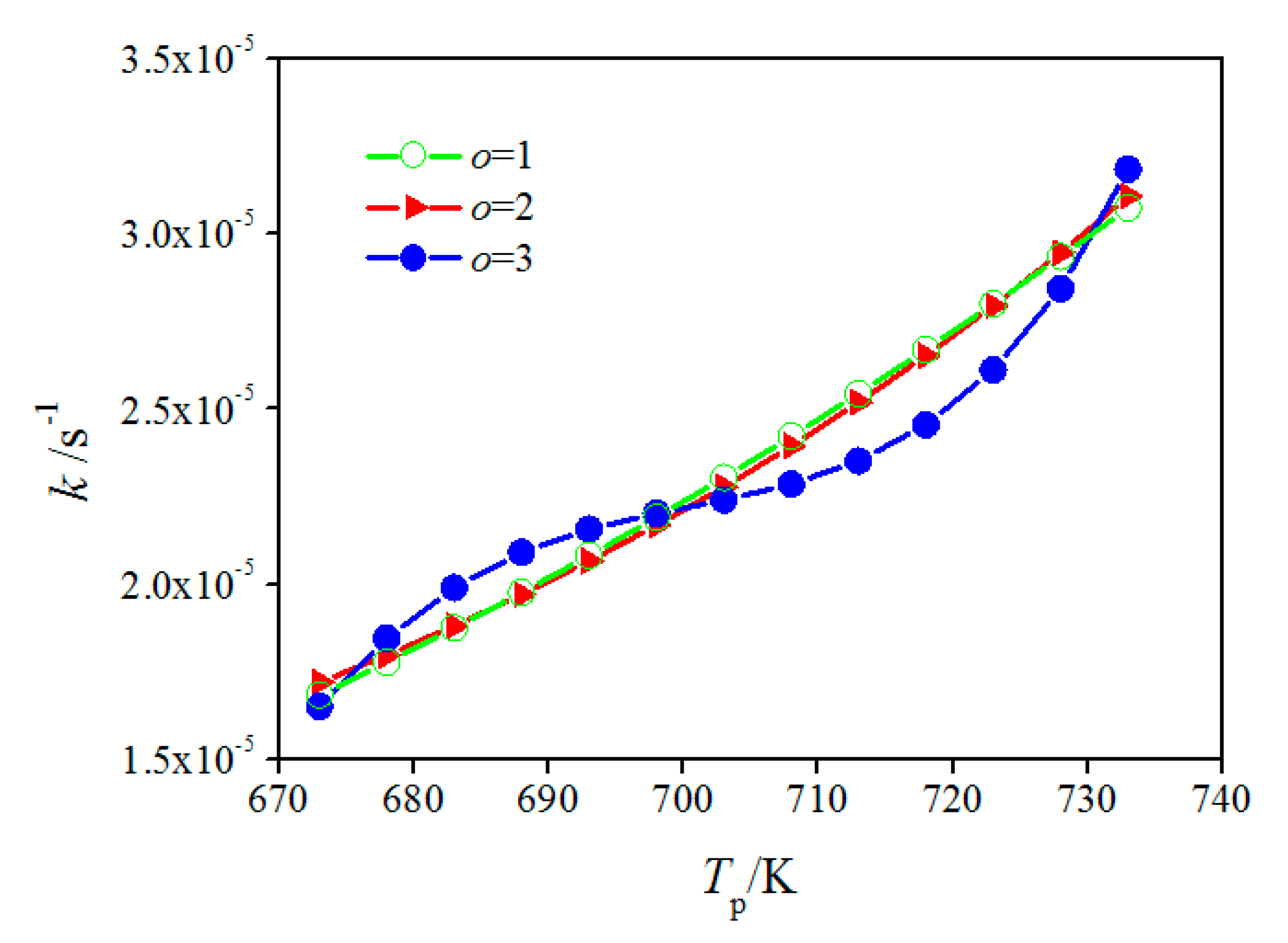
4.3. Modified Arrhenius Equation
4.4. Time-Dependent Pyrolysis Temperature
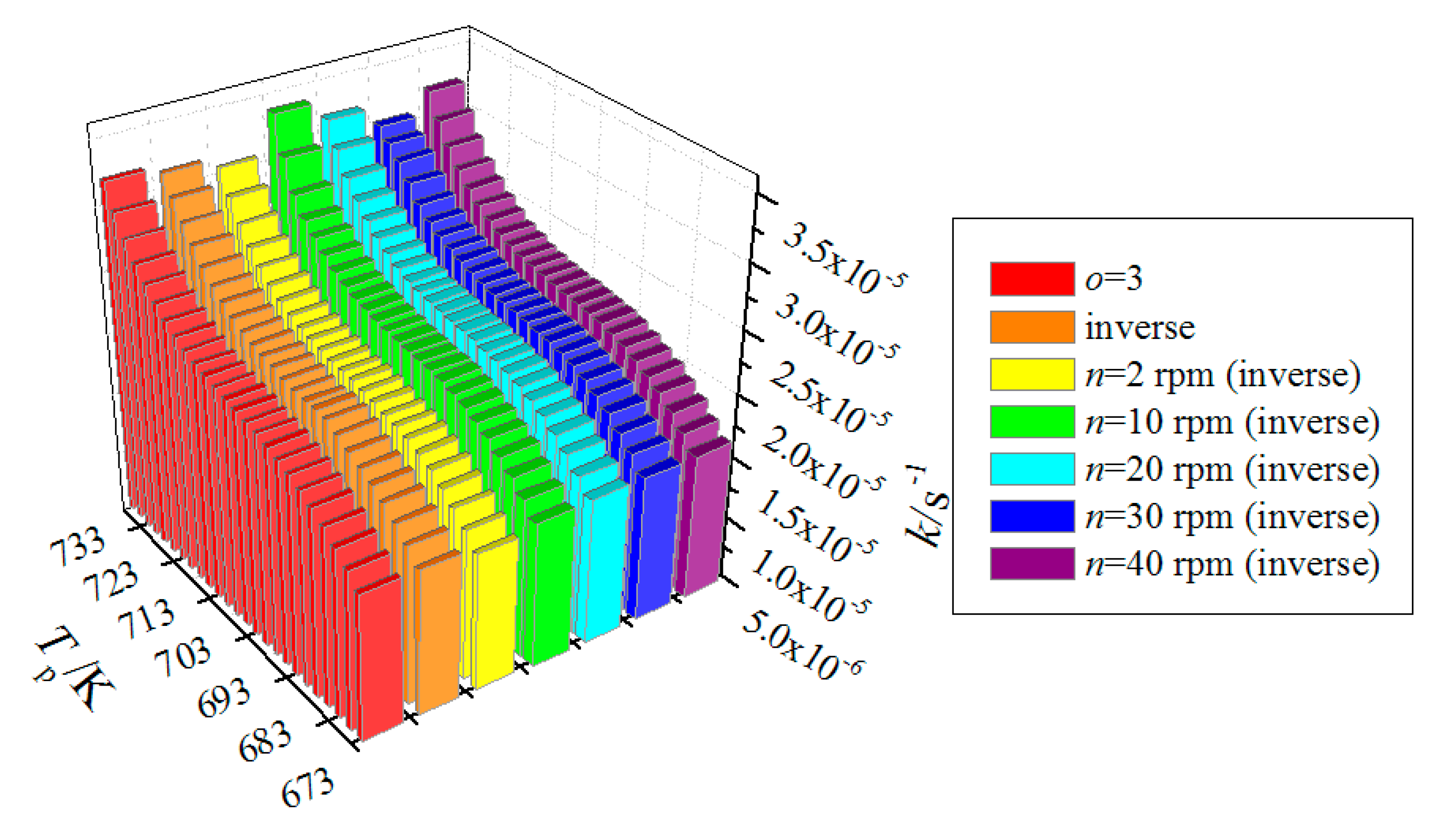
4.5. Inverse Method
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A1: | the factor before exponent, s−1 |
| A2: | the amplitude of temperature fluctuation, K |
| b: | the number of not pyrolyzed nodes |
| d: | search direction |
| E: | activation energy, kJ/mol |
| J: | objective function |
| k: | reaction rate constant |
| l: | the period of temperature fluctuation, s |
| m: | carbon-atom number |
| M: | the number-average molecular weight of polyethylene wax |
| : | the average carbon-atom number of moleculars of polyethylene wax |
| n: | screw speed |
| n: | the maximum carbon-atom number of moleculars of polyethylene wax |
| : | the number of moleculars of polyethylene wax with m carbon atoms |
| N: | measure point (a test under a specific process condition) |
| o: | the equation order of initial or modified Arrhenius equation |
| p: | measure point number |
| r: | pyrolysis ratio |
| R: | gas constant (8.314 J/(mol·K)) |
| T: | temperature, K |
| t: | time |
| v: | the order of reaction dynamic equation |
| Z: | the total number of input measure points |
| Greek Symbols | |
| : | search step size |
| : | the increment of pyrolysis ratio |
| : | temperature fluctuation, K |
| : | a small positive number |
| : | computational domain |
| Subscripts | |
| cal: | calculated |
| m: | melting point |
| mea: | measured |
| p: | pyrolysis |
| v: | evaporation |
| Superscripts | |
| i, j: | iteration index |
References
- Verespej, M. HDPE recycling remains stagnant at 29.9%. Waste Recycl. News 2013, 18, 19. [Google Scholar]
- Okan, M.; Aydin, H.M.; Barsbay, M. Current approaches to waste polymer utilization and minimization: A review. J. Chem. Technol. Biotechnol. 2019, 94, 8–21. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F.J.; Arandes, J.M.; Bilbao, J. Fuel production by cracking of polyolefins pyrolysis waxes under fluid catalytic cracking (FCC) operating conditions. Waste Manag. 2019, 93, 162–172. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.A.; Stacey, N.T. Process targeting: An energy based comparison of waste plastic processing technologies. Energy 2019, 170, 273–283. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Ben, H.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A. Pyrolytic Behavior of Major Biomass Components in Waste Biomass. Polymers 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wu, N.; Zheng, A.; Wang, X.; Feng, X. Effect of Glycerol Pretreatment on Levoglucosan Production from Corncobs by Fast Pyrolysis. Polymers 2017, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Dai, L.; Roger, R.; Wang, X. Preparation of fuel from co-pyrolysis of waste vegetable oil and waste polyethylene plastics. Trans. Chin. Soc. Agric. Mach. 2016, 47. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, D.; Lei, H.; Villota, E.; Ruan, R. Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl. Energy 2019, 251, 113337. [Google Scholar] [CrossRef]
- Wallis, M.D.; Bhatia, S.K. Thermal degradation of high density polyethylene in a reactive extruder. Polym. Degrad. Stab. 2007, 92, 1721–1729. [Google Scholar] [CrossRef]
- Scheirs, J.; Kaminsky, W.; Scheirs, J.; Kaminsky, W. Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and other Fuels; John Wiley and Sons Ltd.: West Sussex, UK, 2006; p. 816. [Google Scholar]
- Madorsky, S.L.; Straus, S.; Thompson, D.; Williamson, L. Pyrolysis of polyisobutene (vistanex), polyisoprene, polybutadiene, GR-S, and polyethylene in a high vacuum. J. Polym. Sci. 1949, 4, 639–664. [Google Scholar] [CrossRef]
- Jellinek, H.H.G. Thermal degradation of polystyrene and polyethylene. Part III. J. Polym. Sci. 1949, 4, 13–36. [Google Scholar] [CrossRef]
- Wall, L.A.; Madorsky, S.L.; Brown, D.W.; Straus, S.; Simha, R. The Depolymerization of Polymethylene and Polyethylene. J. Am. Chem. Soc. 1954, 76, 3430–3437. [Google Scholar] [CrossRef]
- Poutsma, M.L. Reexamination of the Pyrolysis of Polyethylene: Data Needs, Free-Radical Mechanistic Considerations, and Thermochemical Kinetic Simulation of Initial Product-Forming Pathways. Macromolecules 2003, 36, 8931–8957. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sumi, K. Thermal decomposition products of polymethylene. J. Polym. Sci. Part B Polym. Lett. 1968, 6, 357–361. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sumi, K. Thermal decomposition products of polyethylene. J. Polym. Sci. Part A-1 Polym. Chem. 1968, 6, 415–424. [Google Scholar] [CrossRef]
- Seeger, M.; Cantow, H.-J. Thermische spaltungsmechanismen in homo-und copolymeren aus α-olefinen, 1. Grundlegende mechanismen und pyrolyse von linearem polyäthylen. Die Makromolekulare Chemie 1975, 176, 1411–1425. [Google Scholar] [CrossRef]
- Kiran, E.; Gillham, J.K. Pyrolysis-molecular weight chromatography: A new on-line system for analysis of polymers. II. Thermal decomposition of polyolefins: Polyethylene, polypropylene, polyisobutylene. J. Appl. Polym. Sci. 1976, 20, 2045–2068. [Google Scholar] [CrossRef]
- Gascoin, N.; Navarro-Rodriguez, A.; Gillard, P.; Mangeot, A. Kinetic modelling of high density polyethylene pyrolysis: Part 1. Comparison of existing models. Polym. Degrad. Stab. 2012, 97, 1466–1474. [Google Scholar] [CrossRef][Green Version]
- Ranzi, E.; Dente, M.; Faravelli, T.; Bozzano, G.; Fabini, S.; Nava, R.; Cozzani, V.; Tognotti, L. Kinetic modeling of polyethylene and polypropylene thermal degradation. J. Anal. Appl. Pyrolysis 1997, 40, 305–319. [Google Scholar] [CrossRef]
- Faravelli, T.; Bozzano, G.; Scassa, C.; Perego, M.; Fabini, S.; Ranzi, E.; Dente, M. Gas product distribution from polyethylene pyrolysis. J. Anal. Appl. Pyrolysis 1999, 52, 87–103. [Google Scholar] [CrossRef]
- Marongiu, A.; Faravelli, T.; Ranzi, E. Detailed kinetic modeling of the thermal degradation of vinyl polymers. J. Anal. Appl. Pyrolysis 2007, 78, 343–362. [Google Scholar] [CrossRef]
- Levine, S.E.; Broadbelt, L.J. Detailed mechanistic modeling of high-density polyethylene pyrolysis: Low molecular weight product evolution. Polym. Degrad. Stab. 2009, 94, 810–822. [Google Scholar] [CrossRef]
- Roudi, A.M.; Chelliapan, S.; Wan, H.M.W.M.; Kamyab, H. Prediction and Optimization of the Fenton Process for the Treatment of Landfill Leachate Using an Artificial Neural Network. Water 2018, 10, 595. [Google Scholar] [CrossRef]
- Roudi, A.; Chelliapan, S.; Kamyab, H.; Md Din, M.F.; Krishnan, S. Removal of COD from landfill leachate by Predication and Evaluation of Multiple Linear Regression (MLR) Model and Fenton process. Egypt. J. Chem. 2019, 62, 1607–1618. [Google Scholar] [CrossRef]
- Wallis, M.; Bhatia, S.K. Kinetic study of the thermal degradation of high density polyethylene. Polym. Degrad. Stab. 2006, 91, 1476–1483. [Google Scholar] [CrossRef]
- Westerhout, R.W.J.; Waanders, J.; Kuipers, J.A.M.; van Swaaij, W.P.M. Kinetics of the Low-Temperature Pyrolysis of Polyethene, Polypropene, and Polystyrene Modeling, Experimental Determination, and Comparison with Literature Models and Data. Ind. Eng. Chem. Res. 1997, 36, 1955–1964. [Google Scholar] [CrossRef]
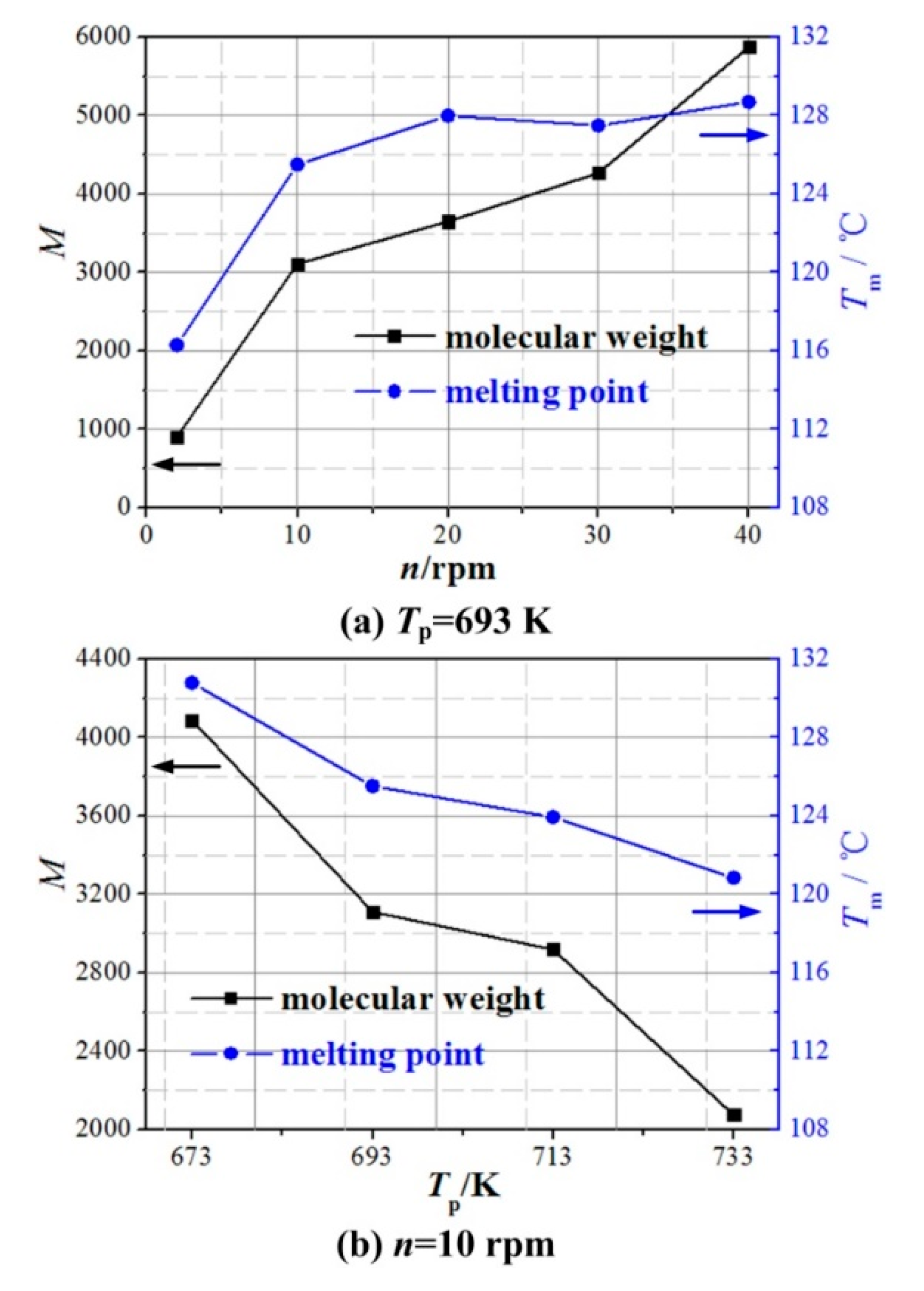
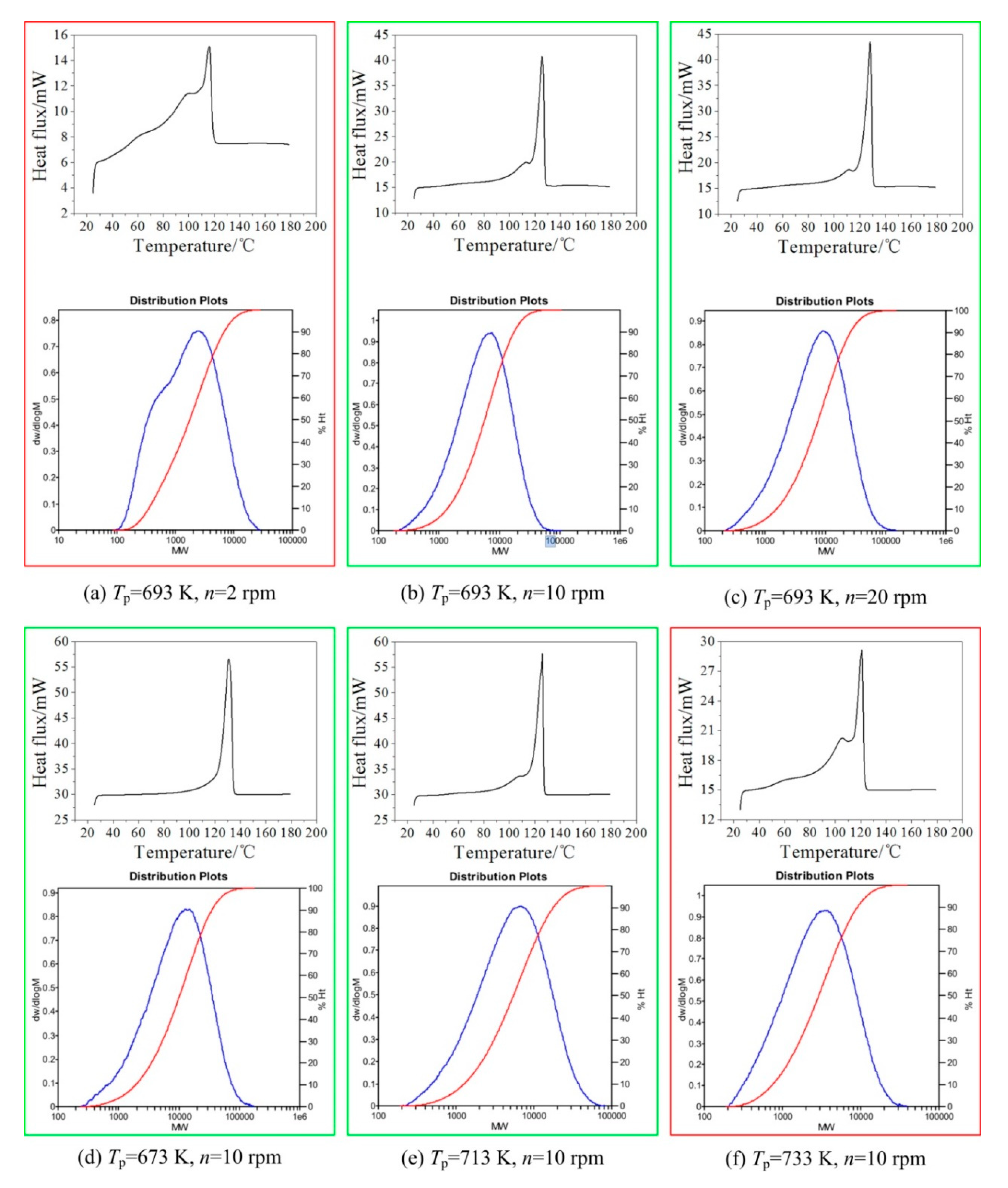


| Screw Speed (rpm) | 2 | 10 | 20 | 30 | 40 |
| Pyrolysis Time (s) | 842 | 213 | 179 | 146 | 110 |
| Pyrolysis Temperature (K) | 673 | 693 | 713 | 733 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screw Speed (rpm) | 10 | 2 | 10 | 20 | 30 | 40 | 10 | 20 | 30 | 10 | 20 | 30 |
| NAMW of PEW | 4088 | 908 | 3111 | 3649 | 4268 | 5876 | 2919 | 3458 | 4151 | 2075 | 2445 | 3274 |
| Measure point | N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | N10 | N11 | N12 |
| p = 4 | ✓ | ✓ | ✓ | ✓ | ||||||||
| p = 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| p = 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Equation Order | Initial or Modified Arrhenius Equation | N4 (%) | N5 (%) | N7 (%) | N11 (%) | Mean (%) |
|---|---|---|---|---|---|---|
| o = 1 | 5.8 | 10.4 | 8.0 | 8.3 | 8.1 | |
| o = 2 | 6.6 | 11.3 | 7.1 | 7.2 | 8.0 | |
| o = 3 | 2.2 | 6.7 | 0.8 | 4.7 | 3.6 |
| Relative Error (%) | N4 | N5 | N7 | N11 | Mean |
|---|---|---|---|---|---|
| modified Arrhenius equation (o = 3) | 2.2 | 6.7 | 0.8 | 4.7 | 3.6 |
| Inverse method | 0.2 | 4.6 | 0.9 | 6.7 | 3.1 |
| Screw-speed-dependent inverse method | 0.6 | 4.8 | 0.2 | 4.5 | 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Lan, X.; Xue, P. High-Precision Monitoring of Average Molecular Weight of Polyethylene Wax from Waste High-Density Polyethylene. Polymers 2020, 12, 188. https://doi.org/10.3390/polym12010188
Guo Z, Lan X, Xue P. High-Precision Monitoring of Average Molecular Weight of Polyethylene Wax from Waste High-Density Polyethylene. Polymers. 2020; 12(1):188. https://doi.org/10.3390/polym12010188
Chicago/Turabian StyleGuo, Zhouchao, Xia Lan, and Ping Xue. 2020. "High-Precision Monitoring of Average Molecular Weight of Polyethylene Wax from Waste High-Density Polyethylene" Polymers 12, no. 1: 188. https://doi.org/10.3390/polym12010188
APA StyleGuo, Z., Lan, X., & Xue, P. (2020). High-Precision Monitoring of Average Molecular Weight of Polyethylene Wax from Waste High-Density Polyethylene. Polymers, 12(1), 188. https://doi.org/10.3390/polym12010188





