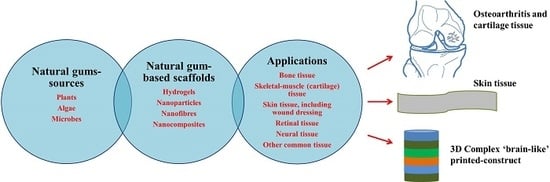
Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review
Abstract
1. Introduction
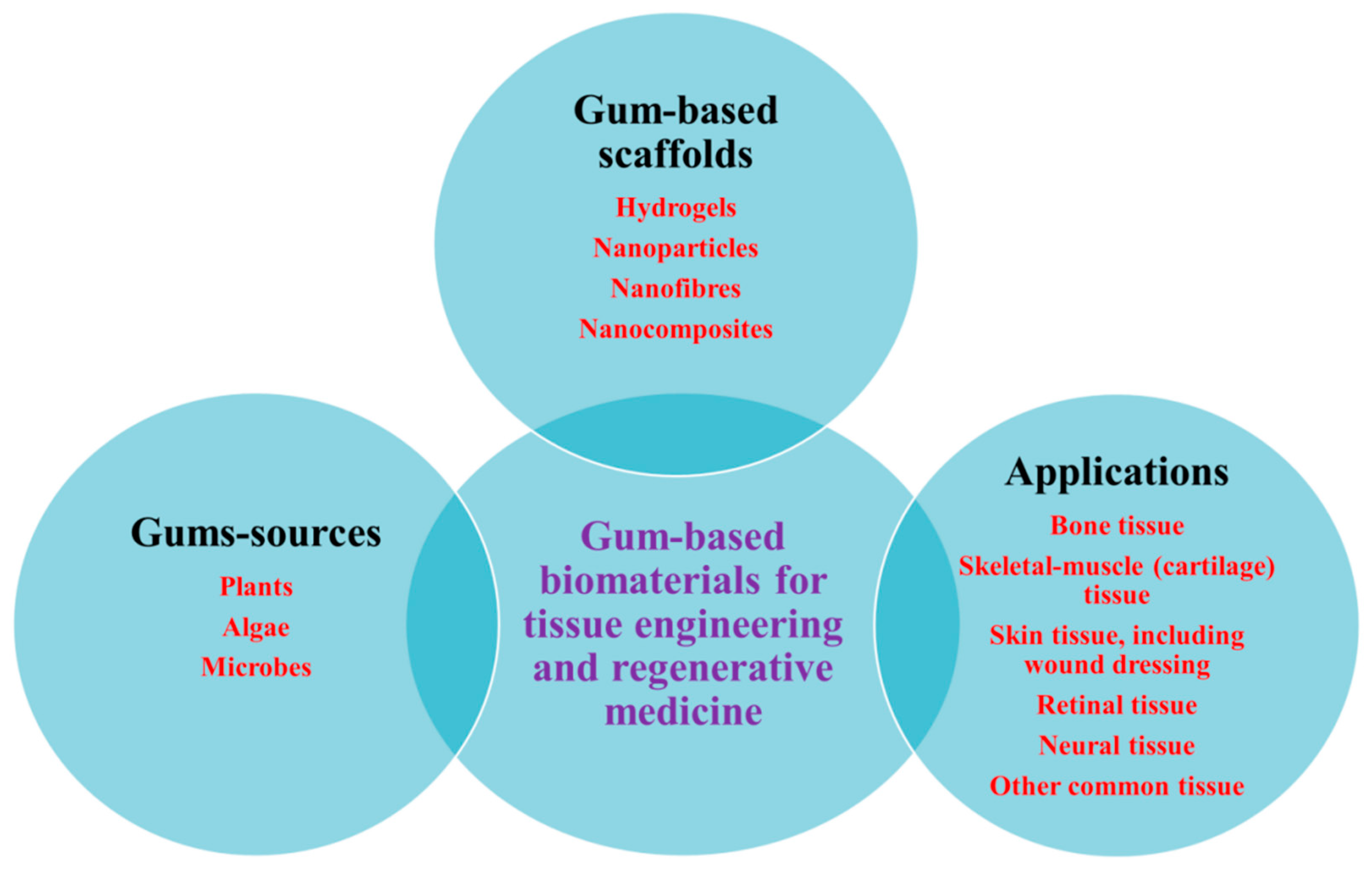
2. Natural Gums as Renewable Bio-Based Materials
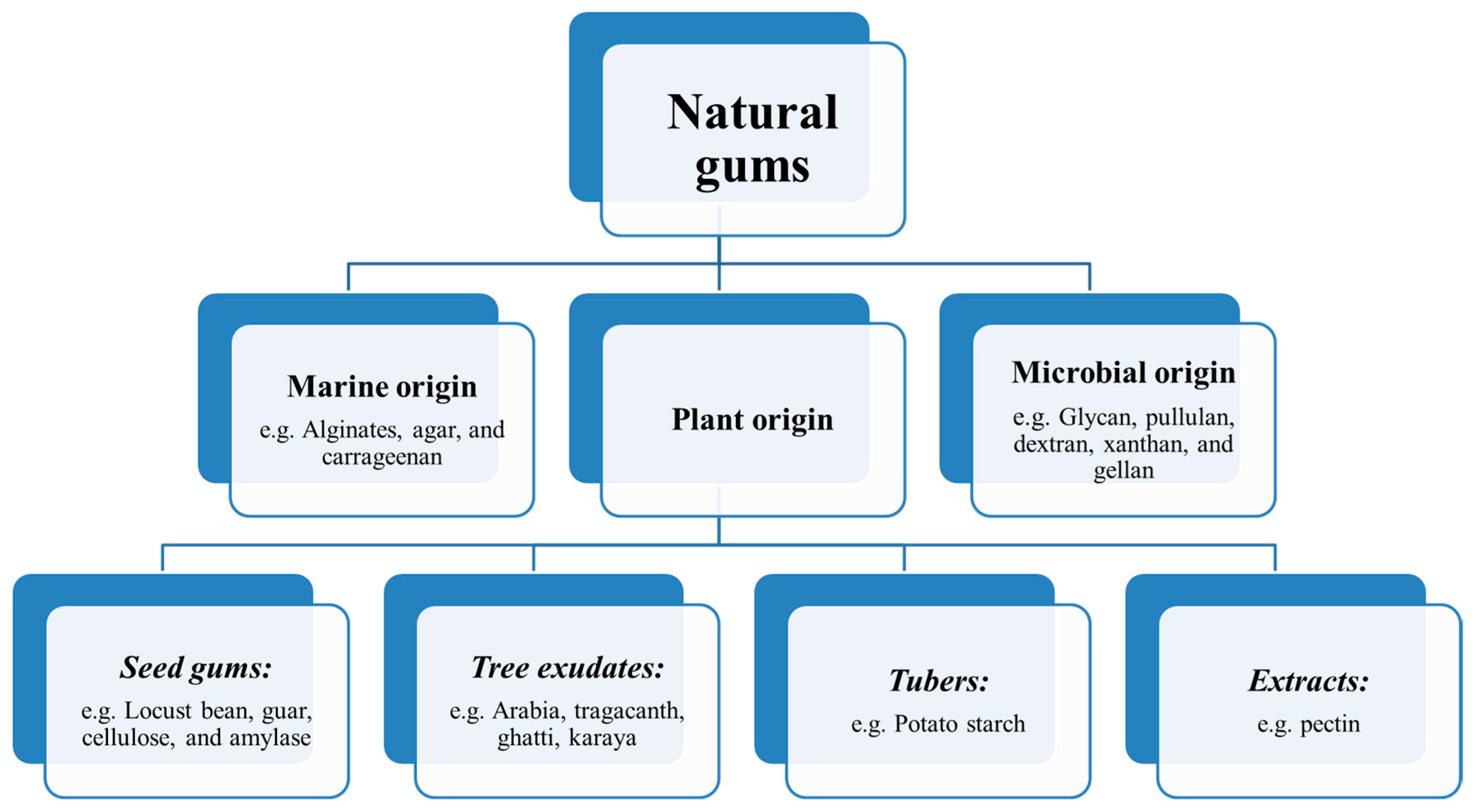
3. Origin of Natural Gums
4. Physico-Chemical Properties of Natural Gums
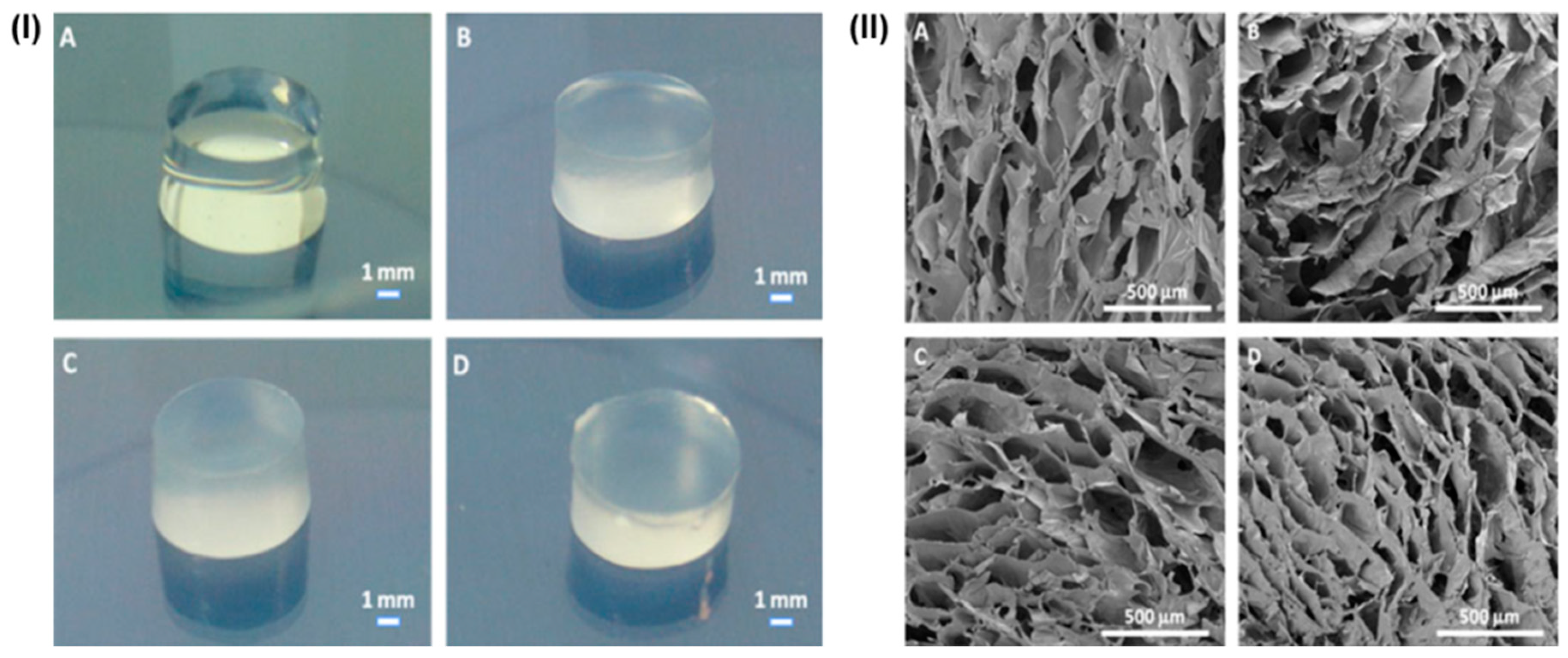
5. Various Forms of Scaffolds Based on Gums
5.1. Gum-Based Nanocomposites
5.2. Gums-Based Nanofibrous Forms
6. Natural Gums as Biocompatible Scaffolds for 3D Cell Culture and Tissue Engineering
6.1. Bone Tissue Engineering
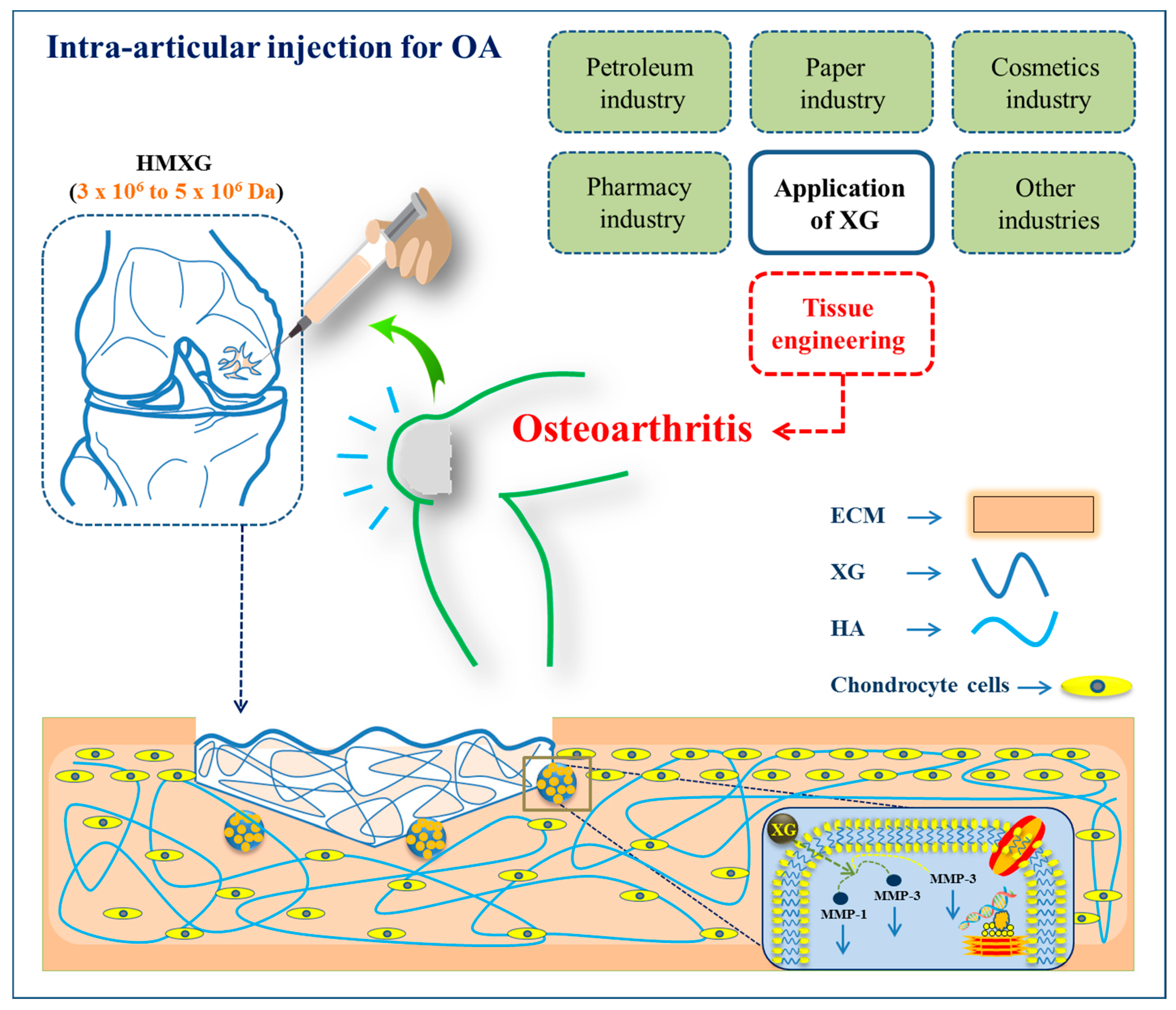
6.2. Osteoarthritis and Cartilage Tissue Engineering
6.3. Skin Tissue Engineering
Wound Dressing
6.4. Retinal Tissue Engineering
6.5. Neural Tissue Engineering
6.6. Other Tissue Engineering
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mokhtarzadeh, A.; Alibakhshi, A.; Hejazi, M.; Omidi, Y.; Dolatabadi, J.E.N. Bacterial-derived biopolymers: Advanced natural nanomaterials for drug delivery and tissue engineering. TrAC Trends Anal. Chem. 2016, 82, 367–384. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [PubMed]
- Renaud, M.; Belgacem, M.N.; Rinaudo, M. Rheological behaviour of polysaccharide aqueous solutions. Polymer 2005, 46, 12348–12358. [Google Scholar] [CrossRef]
- Yaseen, E.; Herald, T.; Aramouni, F.; Alavi, S. Rheological properties of selected gum solutions. Food Res. Int. 2005, 38, 111–119. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef]
- Bhardwaj, T.R.; Kanwar, M.; Lal, R.; Gupta, A. Natural gums and modified natural gums as sustained-release carriers. Drug Dev. Ind. Pharm. 2000, 26, 1025–1038. [Google Scholar] [CrossRef]
- Maciel, J.S.; Azevedo, S.; Correia, C.R.; Costa, A.M.; Costa, R.R.; Magalhães, F.A.; de Sousa Monteiro, A.A.; Costa, J.F.G.; de Paula, R.C.M.; Feitosa, J.P. Oxidized Cashew Gum Scaffolds for Tissue Engineering. Macromol. Mater. Eng. 2019, 1800574. [Google Scholar] [CrossRef]
- Milcovich, G.; Lettieri, S.; Antunes, F.E.; Medronho, B.; Fonseca, A.C.; Coelho, J.F.; Marizza, P.; Perrone, F.; Farra, R.; Dapas, B. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 2017, 249, 163–180. [Google Scholar] [CrossRef]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Patel, M.; Bastioli, C.; Marini, L.; Würdinger, E. Life-Cycle Assessment of Bio-Based Polymers and Natural Fibres; Chapter in the encyclopaedia “Biopolymers”; Wiley-VCH: Weinheim, Germany, 2003; Volume 10. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.D.; Pawar, H.A. Recently investigated natural gums and mucilages as pharmaceutical excipients: An overview. J. Pharm. 2014, 2014, 204849. [Google Scholar] [CrossRef] [PubMed]
- Hamman, H.; Steenekamp, J.; Hamman, J. Use of natural gums and mucilages as pharmaceutical excipients. Curr. Pharm. Des. 2015, 21, 4775–4797. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.H.; Yarce, C.J.; Moreno, R.A.; Prieto, V.; Recalde, J. Natural gum-type biopolymers as potential modified nonpolar drug release systems. Carbohydr. Polym. 2018, 189, 31–38. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Shah, P.A.; Shrivastav, P.S. Guar gum: Versatile natural polymer for drug delivery applications. Eur. Polym. J. 2018, 112, 722–735. [Google Scholar] [CrossRef]
- Hasan, A.M.; Abdel-Raouf, M.E. Applications of guar gum and its derivatives in petroleum industry: A review. Egypt. J. Petrol. 2018, 27, 1042–1050. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Ala’a, H.; Naushad, M.; Ghfar, A.A.; Mola, G.T.; Stadler, F.J. Guar gum and its composites as potential materials for diverse applications: A review. Carbohydr. Polym. 2018, 199, 534–545. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Gani, A.; Bhat, N.A.; Masoodi, F.A. Effect of gamma irradiation on physicochemical, structural and rheological properties of plant exudate gums. Innov. Food Sci. Emerg. Technol. 2017, 44, 74–82. [Google Scholar] [CrossRef]
- Halake, K.; Kim, H.J.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.; Kim, Y.J.; Kim, S.; Ahn, S.; An, S.Y. Recently developed applications for natural hydrophilic polymers. J. Ind. Eng. Chem. 2016, 40, 16–22. [Google Scholar] [CrossRef]
- Ivanova, E.; Bazaka, K. Crawford Natural polymer biomaterials: Advanced applications. In New Functional Biomaterials for Medicine and Healthcare; Woodhead Publishing: New Delhi, India, 2014; pp. 32–70. [Google Scholar]
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira Junior, O.B.; Ribeiro, S.J. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef]
- Yadav, G.; Sharma, N.; Bansal, M.; Thakur, N. Application of natural polysaccharide for delivery of biopharmaceuticals. Int. J. Pharm. Life Sci. 2013, 42756–42765. [Google Scholar]
- Kiani, A.; Shahbazi, M.; Asempour, H. Hydrogel membranes based on gum tragacanth with tunable structure and properties. I. Preparation method using Taguchi experimental design. J. Appl. Polym. Sci. 2012, 124, 99–108. [Google Scholar] [CrossRef]
- Maiti, S.; Ranjit, S.; Sa, B. Polysaccharide-based graft copolymers in controlled drug delivery. Int. J. PharmTech Res. 2010, 2, 1350–1358. [Google Scholar]
- Nadimi, A.E.; Ebrahimipour, S.Y.; Afshar, E.G.; Falahati-Pour, S.K.; Ahmadi, Z.; Mohammadinejad, R.; Mohamadi, M. Nano-scale drug delivery systems for antiarrhythmic agents. Eur. J. Med. Chem. 2018, 157, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mohamad, R.; Rahim, R.A.; Mohammadinejad, R.; Ariff, A.B. Hydrogel beads bio-nanocomposite based on Kappa-Carrageenan and green synthesized silver nanoparticles for biomedical applications. Int. J. Biol. Macromol. 2017, 104, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Mauck, R.L.; Tuan, R.S. Electrospun nanofibrous scaffolds: Production, characterization, and applications for tissue engineering and drug delivery. J. Biomed. Nanotechnol. 2005, 1, 259–275. [Google Scholar] [CrossRef]
- Buckley, C.; O’Kelly, K. Regular scaffold fabrication techniques for investigations in tissue engineering. Top. Bio Mech. Eng. 2004, 147–166. [Google Scholar]
- Shabani, I.; Haddadi-Asl, V.; Soleimani, M.; Seyedjafari, E.; Babaeijandaghi, F.; Ahmadbeigi, N. Enhanced infiltration and biomineralization of stem cells on collagen-grafted three-dimensional nanofibers. Tissue Eng. A 2011, 17, 1209–1218. [Google Scholar] [CrossRef]
- Fayazzadeh, E.; Rahimpour, S.; Ahmadi, S.M.; Farzampour, S.; Anvari, M.S.; Boroumand, M.A.; Ahmadi, S.H.J.A.M.I. Acceleration of skin wound healing with tragacanth (Astragalus) preparation: An experimental pilot study in rats. Acta Med. Iran. 2014, 52, 3–8. [Google Scholar]
- Khan, I.A.; Abourashed, E.A. Leung’s Encyclopedia of Common Natural Ingredients: Used in Food, Drugs and Cosmetics; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Couet, F.; Rajan, N.; Mantovani, D. Macromolecular biomaterials for scaffold-based vascular tissue engineering. Macromol. Biosci. 2007, 7, 701–718. [Google Scholar] [CrossRef]
- Shiroodi, S.G.; Mohammadifar, M.A.; Gorji, E.G.; Ezzatpanah, H.; Zohouri, N.J.J.o.D.R. Influence of gum tragacanth on the physicochemical and rheological properties of kashk. J. Dairy Res. 2012, 79, 93–101. [Google Scholar] [CrossRef]
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Balaghi, S.; Mohammadifar, M.A.; Zargaraan, A.; Gavlighi, H.A.; Mohammadi, M.J.F.H. Compositional analysis and rheological characterization of gum tragacanth exudates from six species of Iranian Astragalus. Food Hydrocoll. 2011, 25, 1775–1784. [Google Scholar] [CrossRef]
- Gavlighi, H.A.; Meyer, A.S.; Zaidel, D.N.; Mohammadifar, M.A.; Mikkelsen, J.D. Stabilization of emulsions by gum tragacanth (Astragalus spp.) correlates to the galacturonic acid content and methoxylation degree of the gum. Food Hydrocoll. 2013, 31, 5–14. [Google Scholar] [CrossRef]
- Gavlighi, H.A.; Meyer, A.S.; Mikkelsen, J.D. Enhanced enzymatic cellulose degradation by cellobiohydrolases via product removal. Biotechnol. Lett. 2013, 35, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, V. Influence of polymer network parameters of tragacanth gum-based pH responsive hydrogels on drug delivery. Carbohydr. Polym. 2014, 101, 928–940. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.; Tecante, A. Dynamic viscoelastic behavior of gellan-ι-carrageenan and gellan-xanthan gels. Food Hydrocoll. 1999, 13, 59–64. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from Xanthomonas campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Douglas, T.E.; Schietse, J.; Zima, A.; Gorodzha, S.; Parakhonskiy, B.V.; KhaleNkow, D.; Shkarin, R.; Ivanova, A.; Baumbach, T.; Weinhardt, V. Novel self-gelling injectable hydrogel/alpha-tricalcium phosphate composites for bone regeneration: Physiochemical and microcomputer tomographical characterization. J. Biomed. Mater. Res. A 2018, 106, 822–828. [Google Scholar] [CrossRef]
- Popa, N.; Novac, O.; Profire, L.; Lupusoru, C.E.; Popa, M.I. Hydrogels based on chitosan–xanthan for controlled release of theophylline. J. Mater. Sci. Mater. Med. 2010, 21, 1241–1248. [Google Scholar] [CrossRef]
- Izawa, H.; Nishino, S.; Maeda, H.; Morita, K.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Kadokawa, J.-I. Mineralization of hydroxyapatite upon a unique xanthan gum hydrogel by an alternate soaking process. Carbohydr. Polym. 2014, 102, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Mao, J.J. Engineering dextran-based scaffolds for drug delivery and tissue repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for biomedical and pharmaceutical applications: Recent advances and overview of alginate electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Saltz, A.; Kandalam, U. Mesenchymal stem cells and alginate microcarriers for craniofacial bone tissue engineering: A review. J. Biomed. Mater. Res. A 2016, 104, 1276–1284. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- You, J.-O.; Park, S.-B.; Park, H.-Y.; Haam, S.; Chung, C.-H.; Kim, W.-S. Preparation of regular sized Ca-alginate microspheres using membrane emulsification method. J. Microencapsul. 2001, 18, 521–532. [Google Scholar]
- De Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.; Deshpande, M. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Grifoll-Romero, L.; Pascual, S.; Aragunde, H.; Biarnés, X.; Planas, A. Chitin deacetylases: Structures, specificities, and biotech applications. Polymers 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.-H.; Ryu, J.-H. Hyaluronic acid-coated nanomedicine for targeted cancer therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U. Hyaluronan: Its nature, distribution, functions and turnover. J. Int. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.-A.; Kong, J.-H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Q.; Xu, J.; Xu, X.; Tian, F.; Yeung, K.W.; Bian, L. Self-assembled injectable nanocomposite hydrogels stabilized by bisphosphonate-magnesium (Mg2+) coordination regulates the differentiation of encapsulated stem cells via dual crosslinking. Adv. Funct. Mater. 2017, 27, 1701642. [Google Scholar] [CrossRef]
- Kumar, A.; Matari, I.A.I.; Choi, H.; Kim, A.; Suk, Y.J.; Kim, J.Y.; Han, S.S. Development of halloysite nanotube/carboxylated-cellulose nanocrystal-reinforced and ionically-crosslinked polysaccharide hydrogels. Mater. Sci. Eng. C 2019, 104, 109983. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Mohandas, A.; Hwang, N.S.; Jayakumar, R. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2019, 122, 320–328. [Google Scholar] [CrossRef]
- Torres, M.; Hallmark, B.; Wilson, D.I. Effect of concentration on shear and extensional rheology of guar gum solutions. Food Hydrocoll. 2014, 40, 85–95. [Google Scholar] [CrossRef]
- Sittikijyothin, W.; Torres, D.; Gonçalves, M. Modelling the rheological behaviour of galactomannan aqueous solutions. Carbohydr. Polym. 2005, 59, 339–350. [Google Scholar] [CrossRef]
- Ragothaman, M.; Palanisamy, T.; Kalirajan, C. Collagen–poly (dialdehyde) guar gum based porous 3D scaffolds immobilized with growth factor for tissue engineering applications. Carbohydr. Polym. 2014, 114, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, M.; Chourasia, M.K.; Jain, N.K.; Jain, A.; Soni, V.; Gupta, Y.; Jain, S.K. Cross-linked guar gum microspheres: A viable approach for improved delivery of anticancer drugs for the treatment of colorectal cancer. AAPS PharmSciTech 2006, 7, E143. [Google Scholar] [CrossRef] [PubMed]
- Soumya, R.S.; Vineetha, V.P.; Reshma, P.L.; Raghu, K.G. Preparation and characterization of selenium incorporated guar gum nanoparticle and its interaction with H9c2 cells. PLoS ONE 2013, 8, e74411. [Google Scholar] [CrossRef] [PubMed]
- Stout, A.W. Larch Arabogalactan, Industrial Gums; Academic Press: Cambridge, MA, USA, 1959; pp. 307–310. [Google Scholar]
- Goldstein, A.; Alter, E.; Seaman, J.; Whistler, R.; BeMiller, J. Industrial Gums; Academic Press: Cambridge, MA, USA, 1973; p. 303. [Google Scholar]
- Launay, B.; Cuvelier, G.; Martinez-Reyes, S. Viscosity of locust bean, guar and xanthan gum solutions in the Newtonian domain: A critical examination of the log (ηsp) o-log C [η] o master curves. Carbohydr. Polym. 1997, 34, 385–395. [Google Scholar] [CrossRef]
- Deuel, H.; Neukomm, H. Some properties of locust bean gum. In Natural Plant Hydrocolloids; ACS Publications: Washington, DC, USA, 1954; pp. 51–61. [Google Scholar]
- Mathur, N. Industrial Galactomannan Polysaccharides; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ofori-Kwakye, K.; Asantewaa, Y.; Kipo, S.L. Physicochemical and binding properties of cashew tree gum in metronidazole tablet formulations. Int. J. Pharm. Pharm. Sci. 2010, 2, 105–109. [Google Scholar]
- Jiang, J.; Zhu, L.; Zhang, W.; Sun, R. Characterization of galactomannan gum from fenugreek (Trigonella foenum-graecum) seeds and its rheological properties. Int. J. Polym. Mater. 2007, 56, 1145–1154. [Google Scholar] [CrossRef]
- Mathur, V.; Mathur, N. Fenugreek and other lesser known legume galactomannan-polysaccharides: Scope for developments. J. Sci. Ind. Res. 2005, 64, 475–481. [Google Scholar]
- Manchanda, R.; Arora, S.; Manchanda, R. Tamarind seed polysaccharide and its modifications-versatile pharmaceutical excipients—A review. Int. J. Pharm. Technol. Res. 2014, 6, 412–420. [Google Scholar]
- Nayak, A.K.; Pal, D.; Santra, K. Screening of polysaccharides from tamarind, fenugreek and jackfruit seeds as pharmaceutical excipients. Int. J. Biol. Macromol. 2015, 79, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.W. Polysaccharide Gums from Agricultural Products: Processing, Structures and Functionality; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Abbastabar, B.; Azizi, M.H.; Adnani, A.; Abbasi, S. Determining and modeling rheological characteristics of quince seed gum. Food Hydrocoll. 2015, 43, 259–264. [Google Scholar] [CrossRef]
- Sudhakar, Y.; Selvakumar, M.; Bhat, D.K. Biopolymer Electrolytes: Fundamentals and Applications in Energy Storage; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Jefferies, M.; And, G.P.; Phillips, G.O. Viscosity of aqueous solutions of gum ghatti. J. Sci. Food Agric. 1977, 28, 173–179. [Google Scholar] [CrossRef]
- Ogwal, E.; Wu, X.; Hu, W. Analysis of enzymatic degradation of polymer additives. Asian J. Appl. Sci. 2011, 4, 526–534. [Google Scholar] [CrossRef][Green Version]
- Chaudhari, N.B.; Patil, V.R. Isolation and evaluation of Cassia fistula seed gum as film coating material. Int. J. PharmTech Res. 2011, 3, 478–1481. [Google Scholar]
- Kaur, V.; Bera, M.B.; Panesar, P.S.; Kumar, H.; Kennedy, J. Welan gum: Microbial production, characterization, and applications. Int. J. Biol. Macromol. 2014, 65, 454–461. [Google Scholar] [CrossRef]
- Tong, K.; Xiao, G.; Cheng, W.; Chen, J.; Sun, P. Large amplitude oscillatory shear behavior and gelation procedure of high and low acyl gellan gum in aqueous solution. Carbohydr. Polym. 2018, 199, 397–405. [Google Scholar] [CrossRef]
- Fink, J. Petroleum Engineer’s Guide to oil Field Chemicals and Fluids; Gulf Professional Publishing: Houston, TX, USA, 2015. [Google Scholar]
- Morris, E.R. Ordered conformation of xanthan in solutions and “weak gels”: Single helix, double helix–or both? Food Hydrocoll. 2019, 86, 18–25. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Pal, D.; Nayak, A.K. Novel tamarind seed polysaccharide-alginate mucoadhesive microspheres for oral gliclazide delivery: In vitro–in vivo evaluation. Drug Deliv. 2012, 19, 123–131. [Google Scholar] [CrossRef]
- Gerard, T. Tamarind Gum in Hand Book of Water Soluble Gums and Resins; Davidson, R.L., Ed.; McGraw-Hill Book Co: New York, NY, USA, 1980; Volume 12, pp. 1–23. [Google Scholar]
- Nishinari, K.; Yamatoya, K.; Shirakawa, M.; Phillips, G.O. Handbook of Hydrocolloids; Williams, P.A., Ed.; Woodhead Publishing Limited/CRC Press: Cambridge, MA, USA, 2000; pp. 247–267. [Google Scholar]
- Balasubramanian, S.; Bezawada, S.R.; Raghavachari, D. Green, selective, seedless and one-pot synthesis of triangular au nanoplates of controlled size using bael gum and mechanistic study. ACS Sustain. Chem. Eng. 2016, 4, 3830–3839. [Google Scholar] [CrossRef]
- Wang, J.; Nain, A.S. Suspended micro/nanofiber hierarchical biological scaffolds fabricated using non-electrospinning STEP technique. Langmuir 2014, 30, 13641–13649. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Shanti, R.M.; Tuan, R.S. Electrospinning technology for nanofibrous scaffolds in tissue engineering. Nanotechnol. Life Sci. 2007, 9. [Google Scholar] [CrossRef]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–46. [Google Scholar]
- Oktay, B.; Kayaman-Apohan, N.; Erdem-Kuruca, S. Fabrication of nanofiber mats from electrospinning of functionalized polymers. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012011. [Google Scholar] [CrossRef]
- Sajeev, U.; Anand, K.A.; Menon, D.; Nair, S. Control of nanostructures in PVA, PVA/chitosan blends and PCL through electrospinning. Bull. Mater. Sci. 2008, 31, 343–351. [Google Scholar] [CrossRef]
- Pirzada, T.; Farias, B.V.D.; Chu, H.M.A.; Khan, S.A. Fabrication of Guar-Only Electrospun Nanofibers by Exploiting a High-and Low-Molecular Weight Blend. ACS Omega 2019, 4, 10767–10774. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Tabata, Y. Biomaterial technology for tissue engineering applications. J. R. Soc. Interface 2009, 6, S311–S324. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient removal of arsenic using zinc oxide nanocrystal-decorated regenerated microfibrillated cellulose scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Geng, L.; Peng, X.; Zhan, C.; Naderi, A.; Sharma, P.R.; Mao, Y.; Hsiao, B.S. Structure characterization of cellulose nanofiber hydrogel as functions of concentration and ionic strength. Cellulose 2017, 24, 5417–5429. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Functional nanoparticles obtained from cellulose: Engineering the shape and size of 6-carboxycellulose. Chem. Commun. 2013, 49, 8818–8820. [Google Scholar] [CrossRef]
- Shantha, K.; Harding, D. Synthesis and evaluation of sucrose-containing polymeric hydrogels for oral drug delivery. J. Appl. Polym. Sci. 2002, 84, 2597–2604. [Google Scholar] [CrossRef]
- Vilela, C.A.; Correia, C.; da Silva Morais, A.; Santos, T.C.; Gertrudes, A.C.; Moreira, E.S.; Frias, A.M.; Learmonth, D.A.; Oliveira, P.; Oliveira, J.M. In vitro and in vivo performance of methacrylated gellan gum hydrogel formulations for cartilage repair. J. Biomed. Mater. Res. A 2018, 106, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: A review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Xu, J.; Feng, Q.; Lin, S.; Yuan, W.; Li, R.; Li, J.; Wei, K.; Chen, X.; Zhang, K.; Yang, Y.; et al. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials 2019, 210, 51–61. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, J.; Zhang, K.; Yao, H.; Zheng, N.; Zheng, L.; Wang, J.; Wei, K.; Xiao, X.; Qin, L.; et al. Dynamic and Cell-Infiltratable Hydrogels as Injectable Carrier of Therapeutic Cells and Drugs for Treating Challenging Bone Defects. ACS Cent. Sci. 2019, 5, 440–450. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Qin, X.; Wa, Q. 3D printing of concentrated alginate/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering. Mater. Des. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Urruela-Barrios, R.; Ramírez-Cedillo, E.; Díaz de León, A.; Alvarez, A.J.; Ortega-Lara, W. Alginate/Gelatin Hydrogels Reinforced with TiO2 and β-TCP Fabricated by Microextrusion-based Printing for Tissue Regeneration. Polymers 2019, 11, 457. [Google Scholar] [CrossRef]
- Loste, J.; Lopez-Cuesta, J.-M.; Billon, L.; Garay, H.; Save, M. Transparent polymer nanocomposites: An overview on their synthesis and advanced properties. Prog. Polym. Sci. 2018, 89, 133–158. [Google Scholar] [CrossRef]
- Devi, M.I.; Nallamuthu, N.; Rajini, N.; Kumar, T.S.M.; Siengchin, S.; Rajulu, A.V.; Ayrilmis, N. Biodegradable poly (propylene) carbonate using in-situ generated CuNPs coated Tamarindus indica filler for biomedical applications. Mater. Today Commun. 2019, 19, 106–113. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Dadashzadeh, A.; Moghassemi, S.; Ashrafizadeh, M.; Dehshahri, A.; Pardakhty, A.; Sassan, H.A.; Sohrevardi, S.M.; Mandegary, A. Shedding light on gene therapy: Carbon dots for the minimally invasive image-guided delivery of plasmids and noncoding RNAs. J. Adv. Res. 2019, 18, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Huang, Y.; Shim, Y.Y.; Ma, X.; Reaney, M.J.T.; Wang, Y. Novel Flaxseed Gum Nanocomposites are Slow Release Iron Supplements. J. Agric. Food Chem. 2018, 66, 5167–5177. [Google Scholar] [CrossRef]
- Hira, I.; Kumar, A.; Kumari, R.; Saini, A.K.; Saini, R.V. Pectin-guar gum-zinc oxide nanocomposite enhances human lymphocytes cytotoxicity towards lung and breast carcinomas. Mater. Sci. Eng. C 2018, 90, 494–503. [Google Scholar] [CrossRef]
- Dutta, K.; Das, B.; Orasugh, J.T.; Mondal, D.; Adhikari, A.; Rana, D.; Banerjee, R.; Mishra, R.; Kar, S.; Chattopadhyay, D. Bio-derived cellulose nanofibril reinforced poly (N-isopropylacrylamide)-g-guar gum nanocomposite: An avant-garde biomaterial as a transdermal membrane. Polymer 2018, 135, 85–102. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Gentile, P.; Ferreira, A.M.; Cometa, S.; De Giglio, E. Insight into halloysite nanotubes-loaded gellan gum hydrogels for soft tissue engineering applications. Carbohydr. Polym. 2017, 163, 280–291. [Google Scholar] [CrossRef]
- Sridhar, R.; Lakshminarayanan, R.; Madhaiyan, K.; Barathi, V.A.; Lim, K.H.C.; Ramakrishna, S. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: Applications in tissue regeneration, drug delivery and pharmaceuticals. Chem. Soc. Rev. 2015, 44, 790–814. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.J.J.o.I.T. Characteristics of aloe vera incorporated poly (ε-caprolactone)/gum tragacanth nanofibers as dressings for wound care. J. Ind. Text. 2018, 47, 1464–1477. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Ajalloueian, F.; Zeng, G.; Mendes, A.C.; Chronakis, I.S. Electrospun xanthan gum-chitosan nanofibers as delivery carrier of hydrophobic bioactives. Mater. Lett. 2018, 228, 322–326. [Google Scholar] [CrossRef]
- Deveswaran, R.; Abraham, S.; Bharath, S.; Basavaraj, B.; Furtado, S.; Madhavan, V. Design and characterization of diclofenac sodium tablets containing tamarind seed polysaccharide as release retardant. Int. J. PharmTech Res. 2009, 1, 191–195. [Google Scholar]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J. Synthesis and characterization of hydrogel films of carboxymethyl tamarind gum using citric acid. Int. J. Biol. Macromol. 2017, 105, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Pal, D. Development of pH-sensitive tamarind seed polysaccharide–alginate composite beads for controlled diclofenac sodium delivery using response surface methodology. Int. J. Biol. Macromol. 2011, 49, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Sangnim, T.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P.; Sittikijyothin, W.; Wannachaiyasit, S.; Huanbutta, K. Design and characterization of clindamycin-loaded nanofiber patches composed of polyvinyl alcohol and tamarind seed gum and fabricated by electrohydrodynamic atomization. Asian J. Pharm. Sci. 2018, 13, 450–458. [Google Scholar] [CrossRef]
- Rahimdokht, M.; Pajootan, E.; Ranjbar-Mohammadi, M.J.P.I. Titania/gum tragacanth nanohydrogel for methylene blue dye removal from textile wastewater using response surface methodology. Polym. Int. 2019, 68, 134–140. [Google Scholar] [CrossRef]
- Kurt, A.; Cengiz, A.; Kahyaoglu, T. The effect of gum tragacanth on the rheological properties of salep based ice cream mix. Carbohydr. Polym. 2016, 143, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M. Production of cotton fabrics with durable antibacterial property by using gum tragacanth and silver. Int. J. Biol. Macromol. 2018, 109, 476–482. [Google Scholar] [CrossRef]
- Badakhshanian, E.; Hemmati, K.; Ghaemy, M. Enhancement of mechanical properties of nanohydrogels based on natural gum with functionalized multiwall carbon nanotube: Study of swelling and drug release. Polymer 2016, 90, 282–289. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M.; Rad, M.M. Tragacanth gum as a natural polymeric wall for producing antimicrobial nanocapsules loaded with plant extract. Int. J. Biol. Macromol. 2015, 81, 514–520. [Google Scholar] [CrossRef]
- Hemmati, K.; Ghaemy, M. Synthesis of new thermo/pH sensitive drug delivery systems based on tragacanth gum polysaccharide. Int. J. Biol. Macromol. 2016, 87, 415–425. [Google Scholar] [CrossRef]
- Hemmati, K.; Masoumi, A.; Ghaemy, M. pH responsive tragacanth gum and poly (methyl methacrylate-co-maleic anhydride)-g-poly (caprolactone) conetwork microgel for in vitro quercetin release. Polymer 2015, 59, 49–56. [Google Scholar] [CrossRef]
- Hemmati, K.; Masoumi, A.; Ghaemy, M. Synthesis and characterization of pH-responsive nanohydrogels as biocompatible drug carriers based on chemically modified tragacanth gum polysaccharide. RSC Adv. 2015, 5, 85310–85318. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Hemmati, K.; Ghaemy, M. Synthesis of nanohydrogels based on tragacanth gum biopolymer and investigation of swelling and drug delivery. Int. J. Biol. Macromol. 2016, 82, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Bahrami, S.H.; Joghataei, M. Fabrication of novel nanofiber scaffolds from gum tragacanth/poly (vinyl alcohol) for wound dressing application: In vitro evaluation and antibacterial properties. Mater. Sci. Eng. C 2013, 33, 4935–4943. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly (ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Zarekhalili, Z.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Fabrication and characterization of PVA/Gum tragacanth/PCL hybrid nanofibrous scaffolds for skin substitutes. Int. J. Biol. Macromol. 2017, 94, 679–690. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Chouaibi, M.; Donsì, F.; Ferrari, G.; Hamdi, S. Chemical composition and functional properties of gum exudates from the trunk of the almond tree (Prunus dulcis). Food Sci. Technol. Int. 2012, 18, 241–250. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A.; Tavanai, H. Fractionation and some physicochemical properties of almond gum (Amygdalus communis L.) exudates. Food Hydrocoll. 2016, 60, 461–469. [Google Scholar] [CrossRef]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Sessa, M.; Chouaibi, M.; Ferrari, G.; Donsì, F.; Hamdi, S. Assessment of emulsifying ability of almond gum in comparison with gum arabic using response surface methodology. Food Hydrocoll. 2014, 37, 49–59. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Helbert, C.B.; Kallel, F.; Driss, D.; Kacem, I.; Ghorbel, R.; Chaabouni, S.E. Purification, structural data and biological properties of polysaccharide from Prunus amygdalus gum. Int. J. Food Sci. Technol. 2015, 50, 578–584. [Google Scholar] [CrossRef]
- Rezaei, A.; Tavanai, H.; Nasirpour, A. Fabrication of electrospun almond gum/PVA nanofibers as a thermostable delivery system for vanillin. Int. J. Biol. Macromol. 2016, 91, 536–543. [Google Scholar] [CrossRef]
- Le, X.T.; Turgeon, S.L. Rheological and structural study of electrostatic cross-linked xanthan gum hydrogels induced by β-lactoglobulin. Soft Matter 2013, 9, 3063–3073. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Wang, F.; Wen, Y.; Bai, T. The composite hydrogels of polyvinyl alcohol–gellan gum-Ca2+ with improved network structure and mechanical property. Mater. Sci. Eng. C 2016, 69, 268–275. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.; Shakiba, M.; Wang, B.; Gomes, M.E.; Neves, N.M.; Reis, R.L.; Khademhosseini, A. Microfabricated photocrosslinkable polyelectrolyte-complex of chitosan and methacrylated gellan gum. J. Mater. Chem. 2012, 22, 17262–17271. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polymer Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J. Locust bean gum: A versatile biopolymer. Carbohydr. Polym. 2013, 94, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hadavi, M.; Hasannia, S.; Faghihi, S.; Mashayekhi, F.; Zadeh, H.; Mostofi, S. Novel calcified gum Arabic porous nano-composite scaffold for bone tissue regeneration. Biochem. Biophys. Res. Ccommun. 2017, 488, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Manda, M.G.; da Silva, L.P.; Cerqueira, M.T.; Pereira, D.R.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan gum-hydroxyapatite composite spongy-like hydrogels for bone tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Wlodarczyk, M.; Pamula, E.; Declercq, H.; de Mulder, E.L.; Bucko, M.M.; Balcaen, L.; Vanhaecke, F.; Cornelissen, R.; Dubruel, P. Enzymatic mineralization of gellan gum hydrogel for bone tissue-engineering applications and its enhancement by polydopamine. J. Tissue Eng. Regen. Med. 2014, 8, 906–918. [Google Scholar] [CrossRef]
- Douglas, T.E.; Krawczyk, G.; Pamula, E.; Declercq, H.A.; Schaubroeck, D.; Bucko, M.M.; Balcaen, L.; Van Der Voort, P.; Bliznuk, V.; van den Vreken, N.M. Generation of composites for bone tissue-engineering applications consisting of gellan gum hydrogels mineralized with calcium and magnesium phosphate phases by enzymatic means. J. Tissue Eng. Regen. Med. 2016, 10, 938–954. [Google Scholar] [CrossRef]
- Douglas, T.E.; Piwowarczyk, W.; Pamula, E.; Liskova, J.; Schaubroeck, D.; Leeuwenburgh, S.C.; Brackman, G.; Balcaen, L.; Detsch, R.; Declercq, H. Injectable self-gelling composites for bone tissue engineering based on gellan gum hydrogel enriched with different bioglasses. Biomed. Mater. 2014, 9, 045014. [Google Scholar] [CrossRef]
- Gantar, A.; da Silva, L.P.; Oliveira, J.M.; Marques, A.P.; Correlo, V.M.; Novak, S.; Reis, R.L. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Mater. Sci. Eng. C 2014, 43, 27–36. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.; Oliveira, J.T.; Sousa, R.; Mano, J.; Reis, R. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Mo, J.; Lyu, Y.; Tang, X.; Shen, X. Effects of guar gum on adhesion properties of soybean protein isolate onto porcine bones. Int. J. Adhes. Adhes. 2017, 75, 124–131. [Google Scholar] [CrossRef]
- Haeri, S.M.J.; Sadeghi, Y.; Salehi, M.; Farahani, R.M.; Mohsen, N. Osteogenic differentiation of human adipose-derived mesenchymal stem cells on gum tragacanth hydrogel. Biologicals 2016, 44, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Kwon, S.E.; Lee, Y.N.; Han, S.S. Xanthan gum/bioactive silica glass hybrid scaffolds reinforced with cellulose nanocrystals: Morphological, mechanical and in vitro cytocompatibility study. Mater. Lett. 2017, 193, 274–278. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym. Test. 2017, 63, 214–225. [Google Scholar] [CrossRef]
- Mirza, S.; Zia, I.; Jolly, R.; Kazmi, S.; Owais, M.; Shakir, M. Synergistic combination of natural bioadhesive bael fruit gum and chitosan/nano-hydroxyapatite: A ternary bioactive nanohybrid for bone tissue engineering. Int. J. Biol. Macromol. 2018, 119, 215–224. [Google Scholar] [CrossRef]
- Mei, L.; Shen, B.; Xue, J.; Liu, S.; Ma, A.; Liu, F.; Shao, H.; Chen, J.; Chen, Q.; Liu, F. Adipose tissue–derived stem cells in combination with xanthan gum attenuate osteoarthritis progression in an experimental rat model. Biochem. Biophys. Res. Commun. 2017, 494, 285–291. [Google Scholar] [CrossRef]
- Han, G.-Y.; Mei, X.-F.; Ling, P.-X.; Guo, Y.-W.; Zhu, X.-Q.; Shao, H.-R.; Liu, F.; Zhang, T.-M. Xanthan gum inhibits cartilage degradation by down-regulating matrix metalloproteinase-1 and-3 expressions in experimental osteoarthritis. J. Bioact. Compat. Polym. 2014, 29, 180–189. [Google Scholar] [CrossRef]
- Chen, Q.; Mei, X.; Han, G.; Ling, P.; Guo, B.; Guo, Y.; Shao, H.; Wang, G.; Cui, Z.; Bai, Y. Xanthan gum protects rabbit articular chondrocytes against sodium nitroprusside-induced apoptosis in vitro. Carbohydr. Polym. 2015, 131, 363–369. [Google Scholar] [CrossRef]
- Han, G.; Wang, G.; Zhu, X.; Shao, H.; Liu, F.; Yang, P.; Ying, Y.; Wang, F.; Ling, P. Preparation of xanthan gum injection and its protective effect on articular cartilage in the development of osteoarthritis. Carbohydr. Polym. 2012, 87, 1837–1842. [Google Scholar] [CrossRef]
- Shao, H.; Jin, Y.; Han, G.; Jiang, P.; Zhu, X.; Liu, F.; Song, Z.; Li, M.; Ling, P. Viscosupplementation of synovial fluid with xanthan gum for treatment of osteoarthritis and its clearance kinetics in the rabbit knee joint. Biorheology 2014, 51, 305–314. [Google Scholar] [CrossRef]
- Han, G.; Chen, Q.; Liu, F.; Cui, Z.; Shao, H.; Liu, F.; Ma, A.; Liao, J.; Guo, B.; Guo, Y. Low molecular weight xanthan gum for treating osteoarthritis. Carbohydr. Polym. 2017, 164, 386–395. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, S.J.; Wang, H.-Y.; Tang, S.C.; Yang, S.-W. Evaluation of the ability of xanthan gum/gellan gum/hyaluronan hydrogel membranes to prevent the adhesion of postrepaired tendons. Carbohydr. Polym. 2014, 114, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Martins, L.; Picciochi, R.; Malafaya, P.; Sousa, R.; Neves, N.; Mano, J.; Reis, R.L. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 93, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, J.; Fan, H.; Zhang, X. An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation. Carbohydr. Polym. 2012, 88, 46–53. [Google Scholar] [CrossRef]
- Perugini, V.; Guildford, A.L.; Silva-Correia, J.; Oliveira, J.M.; Meikle, S.T.; Reis, R.L.; Santin, M. Anti-angiogenic potential of VEGF blocker dendron loaded on to gellan gum hydrogels for tissue engineering applications. J. Tissue Eng. Regen. Med. 2018, 12, e669–e678. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Dreesen, I.; Kobayashi, S.; Vitalone, A.; Casadei, M.A. Injectable and photocross-linkable gels based on gellan gum methacrylate: A new tool for biomedical application. Int. J. Biol. Macromol. 2015, 72, 1335–1342. [Google Scholar] [CrossRef]
- Lee, H.; Fisher, S.; Kallos, M.S.; Hunter, C.J. Optimizing gelling parameters of gellan gum for fibrocartilage tissue engineering. J. Biomed. Mater. Res. B 2011, 98, 238–245. [Google Scholar] [CrossRef]
- Shin, E.Y.; Park, J.H.; Shin, M.E.; Song, J.E.; Carlomagno, C.; Khang, G. Evaluation of Chondrogenic Differentiation Ability of Bone Marrow Mesenchymal Stem Cells in Silk Fibroin/Gellan Gum Hydrogels Using miR-30. Macromol. Res. 2019, 27, 369–376. [Google Scholar] [CrossRef]
- Pereira, D.; Canadas, R.; Silva-Correia, J.; da Silva Morais, A.; Oliveira, M.; Dias, I.; Mano, J.; Marques, A.; Reis, R.L.; Oliveira, J. Injectable gellan-gum/hydroxyapatite-based bilayered hydrogel composites for osteochondral tissue regeneration. Appl. Mater. Today 2018, 12, 309–321. [Google Scholar] [CrossRef]
- Tsai, R.-Y.; Kuo, T.-Y.; Hung, S.-C.; Lin, C.-M.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Use of gum arabic to improve the fabrication of chitosan–gelatin-based nanofibers for tissue engineering. Carbohydr. Polym. 2015, 115, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C 2018, 93, 356–366. [Google Scholar]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar]
- Singh, B.; Sharma, S.; Dhiman, A. Acacia gum polysaccharide based hydrogel wound dressings: Synthesis, characterization, drug delivery and biomedical properties. Carbohydr. Polym. 2017, 165, 294–303. [Google Scholar] [CrossRef]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef]
- Bouaziz, F.; Romdhane, M.B.; Helbert, C.B.; Buon, L.; Bhiri, F.; Bardaa, S.; Driss, D.; Koubaa, M.; Fakhfakh, A.; Sahnoun, Z. Healing efficiency of oligosaccharides generated from almond gum (Prunus amygdalus) on dermal wounds of adult rats. J. Tissue Viability 2014, 23, 98–108. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Li, X.; Zhu, H.; Xu, Z.; Liu, L.; Ma, J.; Zhang, M. A Bioinspired Alginate-Gum Arabic Hydrogel with Micro-/Nanoscale Structures for Controlled Drug Release in Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 22160–22175. [Google Scholar] [CrossRef]
- Moreira, B.R.; Batista, K.A.; Castro, E.G.; Lima, E.M.; Fernandes, K.F. A bioactive film based on cashew gum polysaccharide for wound dressing applications. Carbohydr. Polym. 2015, 122, 69–76. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin loaded gellan gum–chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef]
- Cerqueira, M.T.; da Silva, L.P.; Santos, T.C.; Pirraco, R.P.; Correlo, V.M.; Marques, A.P.; Reis, R.L. Human skin cell fractions fail to self-organize within a gellan gum/hyaluronic acid matrix but positively influence early wound healing. Tissue Eng. Part. A 2014, 20, 1369–1378. [Google Scholar] [CrossRef]
- Cerqueira, M.T.; da Silva, L.P.; Santos, T.C.; Pirraco, R.P.; Correlo, V.M.; Reis, R.L.; Marques, A.P. Gellan gum-hyaluronic acid spongy-like hydrogels and cells from adipose tissue synergize promoting neoskin vascularization. ACS Appl. Mater. Interfaces 2014, 6, 19668–19679. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-W.; Chen, H.-J.; Tsao, S.-W. Preparation, characterization and biological properties of Gellan gum films with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide cross-linker. Carbohydr. Polym. 2010, 82, 920–926. [Google Scholar] [CrossRef]
- Horii, Y.; Uchiyama, K.; Toyokawa, Y.; Hotta, Y.; Tanaka, M.; Yasukawa, Z.; Tokunaga, M.; Okubo, T.; Mizushima, K.; Higashimura, Y. Partially hydrolyzed guar gum enhances colonic epithelial wound healing via activation of RhoA and ERK1/2. Food Funct. 2016, 7, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Jana, P.; Mitra, T.; Selvaraj, T.K.R.; Gnanamani, A.; Kundu, P. Preparation of guar gum scaffold film grafted with ethylenediamine and fish scale collagen, cross-linked with ceftazidime for wound healing application. Carbohydr. Polym. 2016, 153, 573–581. [Google Scholar] [CrossRef]
- Singh, B.; Varshney, L.; Francis, S. Designing tragacanth gum based sterile hydrogel by radiation method for use in drug delivery and wound dressing applications. Int. J. Biol. Macromol. 2016, 88, 586–602. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M.; Rad, M.M. Encapsulation of Aloe Vera extract into natural Tragacanth Gum as a novel green wound healing product. Int. J. Biol. Macromol. 2016, 93, 344–349. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Rao, K.S.V.K.; Haider, A.; Han, S.S. Biodegradable Tragacanth Gum Based Silver Nanocomposite Hydrogels and Their Antibacterial Evaluation. J. Polym. Environ. 2018, 26, 778–788. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Han, S.S. Development of antibacterial paper coated with sodium hyaluronate stabilized curcumin-Ag nanohybrid and chitosan via polyelectrolyte complexation for medical applications. Mater. Res. Express 2017, 4, 115401. [Google Scholar]
- Rao, K.M.; Kumar, A.; Haider, A.; Han, S.S. Polysaccharides based antibacterial polyelectrolyte hydrogels with silver nanoparticles. Mater. Lett. 2016, 184, 189–192. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, J.; Seo, Y.; Jo, Y.; Son, J.; Choi, J. Engineered chitosan–xanthan gum biopolymers effectively adhere to cells and readily release incorporated antiseptic molecules in a sustained manner. J. Ind. Eng. Chem. 2017, 46, 68–79. [Google Scholar] [CrossRef]
- Singh, R.; Cuzzani, O.; Binette, F.; Sternberg, H.; West, M.D.; Nasonkin, I.O. Pluripotent stem cells for retinal tissue engineering: Current status and future prospects. Stem Cell Rev. Rep. 2018, 14, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering retinal pigment epithelial cells regeneration for transplantation in regenerative medicine using PEG/Gellan gum hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Leach, J.B. Neural tissue engineering: Strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef] [PubMed]
- Albani, D.; Gloria, A.; Giordano, C.; Rodilossi, S.; Russo, T.; D’Amora, U.; Tunesi, M.; Cigada, A.; Ambrosio, L.; Forloni, G. Hydrogel-based nanocomposites and mesenchymal stem cells: A promising synergistic strategy for neurodegenerative disorders therapy. Sci World J. 2013, 2013, 270260. [Google Scholar] [CrossRef]
- Tunesi, M.; Raimondi, I.; Russo, T.; Colombo, L.; Micotti, E.; Brandi, E.; Cappelletti, P.; Cigada, A.; Negro, A.; Ambrosio, L.; et al. Hydrogel-based delivery of Tat-fused protein Hsp70 protects dopaminergic cells in vitro and in a mouse model of Parkinson’s disease. NPG Asia Mater. 2019, 11, 28. [Google Scholar] [CrossRef]
- Russo, T.; Tunesi, M.; Giordano, C.; Gloria, A.; Ambrosio, L. Hydrogels for central nervous system therapeutic strategies. Proc. Inst. Mech. Eng. Part. H J. Eng. Med. 2015, 229, 905–916. [Google Scholar] [CrossRef]
- Koivisto, J.T.; Joki, T.; Parraga, J.E.; Pääkkönen, R.; Ylä-Outinen, L.; Salonen, L.; Jönkkäri, I.; Peltola, M.; Ihalainen, T.O.; Narkilahti, S. Bioamine-crosslinked gellan gum hydrogel for neural tissue engineering. Biomed. Mater. 2017, 12, 025014. [Google Scholar] [CrossRef]
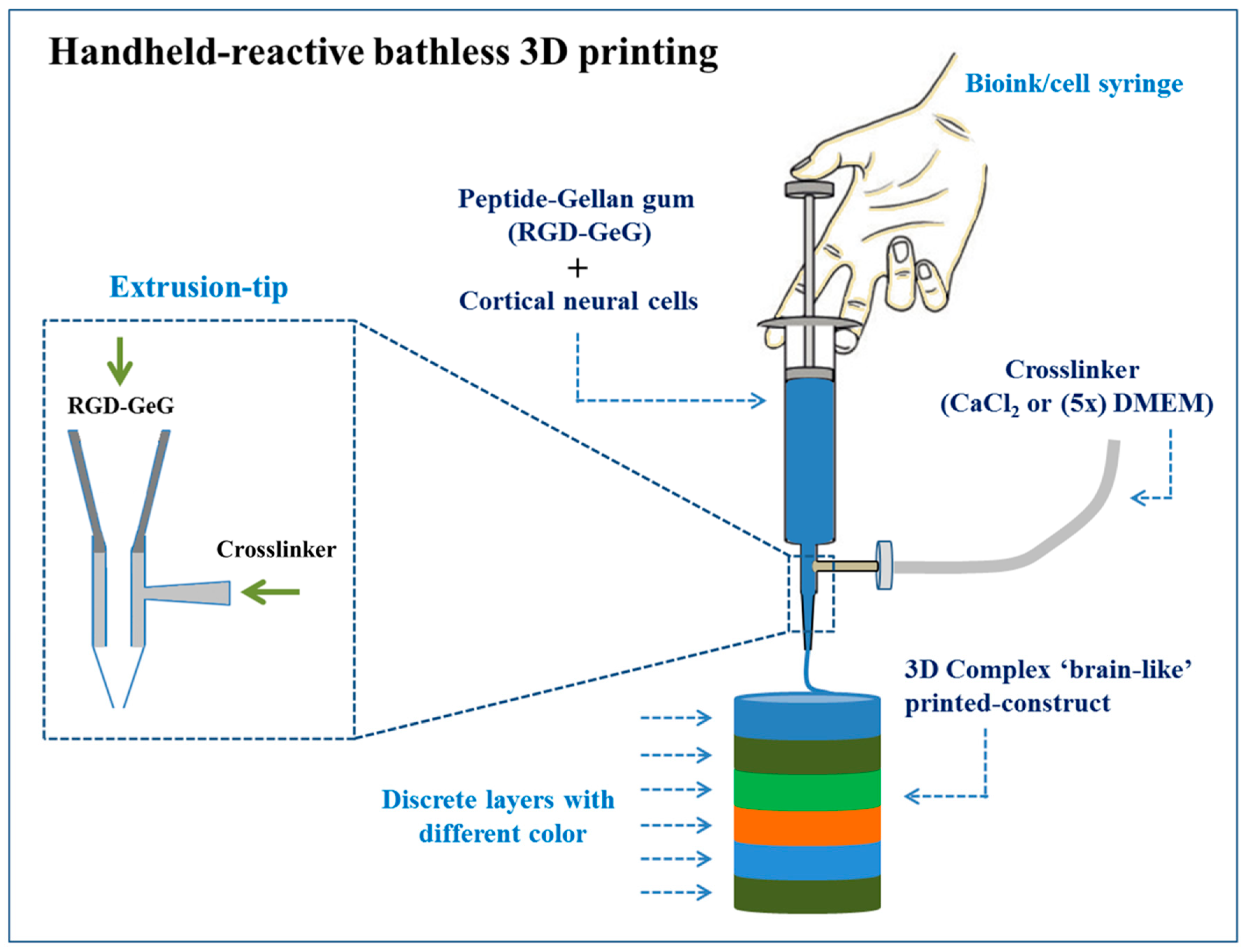
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R., III; Stewart, E.M.; in het Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Gum tragacanth/poly (l-lactic acid) nanofibrous scaffolds for application in regeneration of peripheral nerve damage. Carbohydr. Polym. 2016, 140, 104–112. [Google Scholar] [CrossRef]
- Canadas, R.F.; Ren, T.; Tocchio, A.; Marques, A.P.; Oliveira, J.M.; Reis, R.L.; Demirci, U. Tunable anisotropic networks for 3-D oriented neural tissue models. Biomaterials 2018, 181, 402–414. [Google Scholar] [CrossRef]
- Kang, D.; Cai, Z.; Jin, Q.; Zhang, H. Bio-inspired composite films with integrative properties based on the self-assembly of gellan gum–graphene oxide crosslinked nanohybrid building blocks. Carbon 2015, 91, 445–457. [Google Scholar] [CrossRef]
- da Silva, L.P.; Cerqueira, M.T.; Sousa, R.A.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Engineering cell-adhesive gellan gum spongy-like hydrogels for regenerative medicine purposes. Acta Biomater. 2014, 10, 4787–4797. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.V.; Srisuk, P.; da Silva, L.P.; Marques, A.P.; Reis, R.L.; Correlo, V.M. Synthesis and characterization of electroactive gellan gum spongy-like hydrogels for skeletal muscle tissue engineering applications. Tissue Eng. A 2017, 23, 968–979. [Google Scholar] [CrossRef]
- Yu, I.; Kaonis, S.; Chen, R. A study on degradation behavior of 3D printed gellan gum scaffolds. Procedia CIRP 2017, 65, 78–83. [Google Scholar] [CrossRef]
- Aadil, K.R.; Nathani, A.; Sharma, C.S.; Lenka, N.; Gupta, P. Investigation of poly (vinyl) alcohol-gellan gum based nanofiber as scaffolds for tissue engineering applications. J. Drug Deliv. Sci. Technol. 2019, 54, 101276. [Google Scholar] [CrossRef]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Y.; Ding, P.; Zhang, T.; Zan, Y.; Ni, T.; Lin, R.; Lui, M.; Pei, R. Bone Marrow-Derived Mesenchymal Stem Cells Encapsulated in Functionalized Gellan Gum/Collagen Hydrogel for Effective Vascularization. ACS Appl. Bio Mater. 2018, 1, 1408–1415. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Han, S.S. Polysaccharide based hydrogels reinforced with halloysite nanotubes via polyelectrolyte complexation. Mater. Lett. 2018, 213, 231–235. [Google Scholar]
- Rao, K.M.; Kumar, A.; Han, S.S. Polysaccharide-based magnetically responsive polyelectrolyte hydrogels for tissue engineering applications. J. Mater. Sci. Technol. 2018, 34, 1371–1377. [Google Scholar] [CrossRef]
- Kumar, A.; Zo, S.M.; Kim, J.H.; Kim, S.C.; Han, S.S. Enhanced physical, mechanical, and cytocompatibility behavior of polyelectrolyte complex hydrogels by reinforcing halloysite nanotubes and graphene oxide. Compos. Sci. Technol. 2019, 175, 35–45. [Google Scholar] [CrossRef]
- Tiwari, A.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Biodegradable hydrogels based on novel photopolymerizable guar gum–methacrylate macromonomers for in situ fabrication of tissue engineering scaffolds. Acta Biomater. 2009, 5, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, N.M.; Paiva, B.; Camassola, M.; Rosenthal-Kim, E.Q.; Garcia, K.C.; dos Santos, F.P.; Soares, R.M. Gelatin and galactomannan-based scaffolds: Characterization and potential for tissue engineering applications. Carbohydr. Polym. 2015, 133, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Maharana, V.; Gaur, D.; Nayak, S.K.; Singh, V.K.; Chakraborty, S.; Banerjee, I.; Ray, S.S.; Anis, A.; Pal, K. Reinforcing the inner phase of the filled hydrogels with CNTs alters drug release properties and human keratinocyte morphology: A study on the gelatin-tamarind gum filled hydrogels. J. Mech. Behav. Biomed. Mater. 2017, 75, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, A.R.; Grenha, A.; da Costa, A.M.R.; Belo, J.A. Locust bean gum as an alternative polymeric coating for embryonic stem cell culture. Mater. Sci. Eng. C 2014, 40, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng. C 2016, 58, 521–531. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
| Gum Type | Monosaccharid Composition | Main Chain | Molecular Weight (kDa) | References |
|---|---|---|---|---|
| Larch gum | Arabinose, galactose | Arabinogalactan | 100–120 | [69] |
| Guar gum | Mannose, galactose | Galactomannan | 220–250 | [70,71] |
| Locust bean gum | Mannose, galactose | Galactomannan | 310 | [71,72] |
| Tara gum | Mannose, galactose | Galactomannan | 500 | [73] |
| Cashew gum | Mannose, galactose, glucoronic acid | Galactan | 180 | [73,74] |
| Fenugreek seed gum | Mannose, galactose | Mannan | 30 | [75,76] |
| Tamarind gum | Glucose, galactose, xylose | Glucan | 52.4 | [77,78] |
| Flaxseed gum | Glucose, xylose, galactose, rhamnose | Xylan | 285 | [79] |
| Quince seed gum | Galactose, arabinose, xylose | Galactan | 150 | [69,80] |
| Gum arabic | Galactose, arabinose, rhamnose, glucoronic acid, 4-O-methylglucoronic acid | Galactan | 250–600 | [70,81] |
| Gum karaya | D-galactose, L-rhamnose, D-galacturonic acid | Galactan | 9500 | [72] |
| Gum tragacanth | D-galactose, L-fucose, D-xylose, L-arabinose, L-rhamnose | Galactan | 840 | [73] |
| Gum ghatti | L-arabinose, D-galactose, D-mannose, D-xylose, D-glucuronic acid | Galactan | 12,000 | [73,82] |
| Corn fiber gum | D-xylose, L-arabinose, galactose, glucose, D-glucuronic acid | Xylan | 278–394 | [76] |
| Sesbania gum | Mannose, galactose | Galactomannan | 241.5–357 | [76,83] |
| Cassia tora gum | Mannose, galactose | Galactomannan | 200–300 | [84] |
| Guar gum | Mannose, galactose | Galactomannan | 100–200 | [12] |
| Welan gum | L-mannose, L-rhamnose, D-glucose, D-glucuronic acid | 100 | [85] | |
| Gellan gum | D-glucoronic acid, D-glucose, L-rhamnose | 500–2000 | [81,86,87] | |
| Xanthan Gum | D-mannose, D-glucose, Pyruvate | D-glucose | 2000 to 20,000 | [81,88,89] |
| Tamarind seed gum | D-xylopyranose, D-galactopyranosyl, D-xylpyranose, glucose | D-glucan | 115–2500 | [90,91,92] |
| Bael gum | D-galactose, L-arabinose, L-rhamnose, and D-galacturonic | Xyloglucan | [93] | |
| Carrageenan | D-galactose | 100–1000 | [81] |
| Constituting Materials | Engineering of Tissue Type | Cell Type | Remarks | References | |
|---|---|---|---|---|---|
| Gum Type | Other Materials and Biomolecules | ||||
| AcG | Crosslinked polyacrylic acid polymer (carbopol), N-vinylpyrollidone (NVP), Moxifloxacin, Glutaraldehyde (GA) | Skin wound tissue | Inflammatory cells | Non-haemolytic, antioxidant, and mucoadhesive in nature | [187] |
| AcG | SA, ZnONPs, and Glutaraldehyde (crosslinker) | Skin wound tissue | Peripheral blood mononuclear cells (PBMCs) and Sheep fibroblast cells | Significant reduction in toxicity to cells, while maintaining antibacterial and healing effect. Low doses of ZnONPs are beneficial and may reduce undesirable side effects | [188] |
| AlG | Commercial cream formulation and/or Oligosaccharide (OAG) | Dermal wound healing | Host epithelial cells (skin keratinocytes and fibroblasts) | OAG alone or supplemented to cream formulation exhibits acceleration of wound healing, by promoting neo-blood vessels and collagen | [189] |
| ArG | HAp urea-formaldehyde (crosslinker) | Bone tissue | C2C12 cells | Scaffolds with 40%–50% of HAp showed highest mechanical properties and supported enhanced biomineralization | [156] |
| ArG | CS, gelatin, PVA, glutaraldehyde (crosslinker) | Skin tissue | KP-hMSCs | Enhanced mechanical properties and cytocompatibility | [184] |
| ArG | PCL and Zein | Skin tissue | L929 fibroblast cells | Enhanced mechanical and good antibacterial properties with favorable cell viability | [185] |
| ArG | PCL, Zein, C. officinalis | Skin tissue | L929 fibroblast cells | Desirable mechanical properties, gradual and controlled release of C. officinalis, and better antibacterial and cell viability than PCL/Zein/ArG scaffolds | [186] |
| ArG | Alg and Recombinant human MG53 protein (rhMG53) | Dermal wound healing | Provided micro-/nanoscale structure, adhesion characteristics, and tunable properties for quick and sustained delivery of rhMG53 | [190] | |
| CG | PVA and trypsin | Wound healing | Human PDL fibroblast cell | No cytotoxicity was observed for cells and became bioactive by the immobilization of trypsin | [191] |
| CGG | Whitlockite (Ca18Mg2(HPO4)2(PO4)12) NPs and dimethyloxallylglycine (an angiogenic drug) | Bone tissue | human umbilical vein endothelial cells | Enhanced in vitro osteogenesis and angiogenesis | [63] |
| GaTG | Gelatin | Wound and tissue engineering | Rat mesenchymal stem cells (rMSCs) | Enhanced mechanical properties and good cell adhesion with no cytotoxicity | [225] |
| GeG | ALP, PDA | Bone tissue | MC3T3-E1 cells (osteoblastic cells) | Enhanced ALP-mediated enzymatic mineralization of GeG by the PDA functionalization | [158] |
| GeG | ALP | Bone tissue | MC3T3-E1 cells and RAW 264.7 monocytic cells | Enhanced osteoblast cell adhesion nd proliferation on hydrogels with Mg-loaded mineral (i.e., mineralized in B–E media) | [159] |
| GeG | CS, PEG, and APN | Wound healing | Enhanced biocompatibility, entrapment and sustained release of drug, moist nature and antioxidant property | [192] | |
| GeG | HAp | Bone tissue | hASCs | Enhanced mechanical properties, sustained degradation, and cell adhesion and proliferation | [157] |
| GeG | HAp | Osteochondral tissue | Mouse lung L929 fibroblast cells | Provided temporary load while neotissue formation, good in vivo integration with surrounding tissues and supporting formation of cartilage and bone-like tissue | [183] |
| GeG | SF and MicroRNAs | Articulate cartilage tissue | BMSCs | Effective and suitable for cell growth and nutrients perfusion; BMSCs-loaded hydrogel transfected with miR-30a promote chondrogenesis of BMSCs with up-regulation of cartilage specific gene | [182] |
| GeGMA | GelMA | Cartilage tissue | NIH3T3 fibroblast cells | High mechanical strength and cytocompatibility | [175] |
| GeG | Cartilage tissue | Human nasal chondrocyte cells | High cell entrapment with homogenous distribution, good viscoelastic properties and cytocompatibility | [176] | |
| Oxidized-GeG | CMCS | Cartilage tissue | Chondrocyte cells | Enhanced gelation temperature, mechanical properties, and cell viability | [178] |
| iGeG-MA | FF-Gen3K(WHLPFKC)16 | Enhanced anti-angiogenesis potential in vitro and in vivo | [179] | ||
| GeG-MA | PEG-DMA, sulindac, and vitamin B12 | Cartilage tissue | Human fibroblast cells (WI-38 cells) | Better mechanical properties and in vivo cytocompatibility, tunable release of small molecule, whereas no significant difference with large molecules | [180] |
| GeG | Musculoskeletal tissues/fibrocartilage tissue | Low acyl-GeG (2% w/v) was found most suitable for cell encapsulation with appropriate mechanics, gelling temperature, and degradation properties | [181] | ||
| GeG | GO | Good fracture strength and strain, tensile modulus, and biocompatibility | [214] | ||
| GeG | Wound dressing and cartilage tissue | Scaffolds with high surface area to mass ratio and high degradation, improvement in mechanical properties after degradation in SBF | [217] | ||
| GeG | PVA | Not specified | Embryonic stem cells (ESCs) | Good stability in aqueous medium and good cell attachment and growth | [218] |
| GeG | GelMA, PCL, alginate | Not specified | BMSCs | Highly complex structures were achieved; fabrication and sacrificing process did not affect cell viability | [219] |
| GeGMA | GelMA | NIH3T3 fibroblast cells | 3D constructs with tunable microporosity capable of directing cellular responses at millimeter scale (e.g., anisotropic outgrowth) | [213] | |
| GeGMA | Collagen | Vasculogenic differentiation | Bone marrow-derived mesenchymal stem cells (BMSCs) | Effectively promoted BMSCs to differentiate into endothelial cells | [220] |
| GeGMA | Tissue engineering (not specified) | NIH-3T3 fibroblast cells | Highly tunable degradation and mechanical properties as well as high cell viability | [185] | |
| GeG | Peptides | Soft tissue engineering (not specified) | Human adipose stem cells (hASCs), dermal microvascular endothelial cells (hDMECs) and keratinocytes (hKC) from human adult skin and human osteoblast-like cells SaOs-2 | Enhanced mechanical properties and flexibility, cell-adhesiveness of spongy-like hydrogels due to pre-incubation with cell-adhesive protein | [215] |
| GeGMA | PBS | Intervertebral discs (IVDs) regeneration | Rat lung fibroblast L929 cells | Enhanced mechanical, degradation, and water uptake properties with good cytocompatibility | [162] |
| GeG | BG | Bone tissue | Rat mesenchymal stem cells (rMSCs) | The incorporation of BG promoted mineralizability and antibacterial properties and differentiation of rMSCs depending on BG-type | [160] |
| GeG | BG | Bone tissue | Human adipose-derived stem cells (ADMSCs) | Good apatite-forming ability, improved mechanical properties, and cell viability | [161] |
| GeG | Glycerol and HNTs | Dermal tissue (soft tissue) | Human dermal fibroblast (NHDF-Neo) cells | Tuneable mechanical properties (compressive modulus: 20–75 kPa) and high metabolic activities of cells on 25% HNT loaded-GeG/Gly hydrogels | [120] |
| GeG | HA and cellular mediators (adipose tissue cells) | Skin tissue | Human microvascular endothelial cells (hAMECs) | Fast wound closure and re-epithelialization, a distinct dermal matrix remodeling, and improved neovascularization was observed | [194] |
| GeG | HA, Ca2+ | Wound tissue | Epidermal and dermal cellular fractions (Keratinocytes, fibroblasts, endothelial cells) | Accelerated rate of wound closure and re-epithelialization, including tissue neovascularization | [193] |
| GeG | EDC | Wound healing | Fibroblast (L929) cells | High reduction in wound size (%) and collagen content | [195] |
| GeG | Neural tissue | Primary cortical neural cells | Successful printing of complex, layered, and viable 3D cell structures (i.e., brain-like structures) | [211] | |
| GeG | Bioamines (SPD, SPM) and peptide (RGD) | Neural tissue engineering | Human pluripotent stem cell-derived neuronal cells (hPSCs) | Properties mimicking naïve rabbit brain tissue under relevant physiological stress and strain; cell type-specific behavior after functionalization with laminin | [210] |
| GuG | GMA | Common tissue | Human endothelial cell line (EA.hy926) | Excellent endothelial cell viability | [224] |
| GeG | PEG | ARPE-19 cell | Promotion of retinal regeneration compared to only GeG and 3 wt.% PEG-GeG could be applied as an alternative for retinal regeneration | [205] | |
| PHGuG | Wound tissue | Young adult mouse colonic (YAMC) epithelial cells | Promotion of colonic epithelial cell wound healing through RhoA activation that occurs downstream of ERK1/2 activation | [196] | |
| GuG | SPI | Bone | Significant improvement of bond strength of SPI adhesives onto porcine bones | [163] | |
| CMGuG | Ethylenediamine, fish collagen, and Ceftazidime drug | Wound healing | NIH3T3 fibroblast cells | Enhanced biocompatibility and antibacterial properties; release of 90–95% Ceftazidime from film after 96 h of incubation at physiological pH | [197] |
| TaG | Gelatin, CNTs, and salicylic acid | Wound, tissue, and drug delivery | Human keratinocyte (HaCaT) cells | Enhanced mechanical stability, diffusion-mediated drug release, and cytocompatibility | [226] |
| GT | Bone tissue | Adipose-derived mesenchymal stem cells (ADMSCs) | Supporting and the acceleration of adhesion, proliferation, and osteogenic differentiation of stem cells | [164] | |
| GT | PVA, glutaraldehyde (crosslinker) | Wound healing | human fibroblast AGO cells | Good antibacterial properties against Gram-negative bacteria and cell adhesion and proliferation | [137] |
| GT | PCL, Cur | Wound healing | Mesenchyme stem cells (MSCs) | Enhanced mechanical properties, sustained release of Cur up to 20 days, and cell adhesion and proliferation for PCL-GT-Cur3%; and significantly fast wound closure with well-formed granulation tissue | [138,139] |
| GT | Good antibacterial and mechanical properties with suitable biocompatibility and hydrophilic nature | [140] | |||
| GT | PVA, SA, and Moxifloxacin drug | Wound dressing | Good biocompatibility with impermeability to microbes and the release of drug via non-Fickian mechanism; best fitting in the Hixson–Crowell model | [198] | |
| GT | Aloe vera extract, Al3+ as crosslinker | Wound healing | Human fibroblast cells | Excellent wound healing behavior with significant migration rate of fibroblast cells | [199] |
| GT | Acrylamide, Terminalia chebula (TC), AgNPs | Wound healing | Good antibacterial properties against both B. subtilis and E. coli bacteria | [200] | |
| GT | PLLA | Nerve tissue engineering | Nerve cell (PC12) | Enhanced mechanical properties, cell viability, neurite outgrowth and better cellular phenotype | [212] |
| XG | Osteoarthritis | ADMSCs | ADMSCs with XG reduced pain associated with osteoarthritis | [168] | |
| XG | Articular cartilage | Chondrocyte cells | XG significantly reversed SNP-reduced cell proliferation and prevented cell early apoptosis rate in a dose-dependent manner | [170] | |
| XG | HAp | Bone tissue | Change in microstructure of gel by mineralization process and enhanced mechanical properties | [44] | |
| XG | BG, CNCs, Borax | Bone tissue | MC3T3-E1 osteoblast cells | Enhanced mechanical properties and cytocompatibility | [165] |
| XG | SA, HNTs, and CNCs | Bone tissue | MC3T3-E1 osteoblast cells | Enhanced rheological and mechanical properties as well as cytocompatibility | [166] |
| MWXG | Articular cartilage | Prepared injection of high transparency with low protein and free of endotoxin; significantly protects joint cartilage | [171] | ||
| LWXG | Articular cartilage | Rabbit articular chondrocytes | Promoted cell proliferation as well as decreased chondrocyte apoptosis through down-regulation of the protein levels of caspases-3 and bax, and up-regulation of the protein level of bcl-2 in cartilage (in vitro and in vivo) | [173] | |
| XG | GeG/HA | Skeletal muscle tissue (tendon) | Decreased tendon adhesion without reducing tendon strength, rapid swelling, slow degradation, and rapid and close blanketing onto tendon tissue | [174] | |
| XG | CS and Chlorhexidine (CHX) | Wound healing | Human dermal fibroblast cells | Good viscoplastic behavior, cytocompatibility, non-Fickian diffusion mechanism of CHX release in vitro and selective antibacterial behavior against P. gingivalis | [203] |
| XG | CS and HNTs | Not specified | MC3T3-E1 osteoblast cells | Excellent mechanical properties with good cell viability (in vitro) | [221] |
| XG | CS, Fe3O4 MNPs, GDL | Multiple tissues | NIH3T3 fibroblast cell | Enhanced rheological and mechanical properties as well as cytocompatibility | [222] |
| LBG | Tissue engineering (not specified) | Mouse embryonic stem cell (ESCs) | Coating of LBG promoted mouse ESCs growth in an undifferentiated state | [227] | |
| BFG | HAp | Bone tissue | Osteoblast MG-63 cells | Enhancement in mechanical properties, protein adsorption, antibacterial behavior, cell viability and osteogenic differentiation | [167] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. https://doi.org/10.3390/polym12010176
Mohammadinejad R, Kumar A, Ranjbar-Mohammadi M, Ashrafizadeh M, Han SS, Khang G, Roveimiab Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers. 2020; 12(1):176. https://doi.org/10.3390/polym12010176
Chicago/Turabian StyleMohammadinejad, Reza, Anuj Kumar, Marziyeh Ranjbar-Mohammadi, Milad Ashrafizadeh, Sung Soo Han, Gilson Khang, and Ziba Roveimiab. 2020. "Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review" Polymers 12, no. 1: 176. https://doi.org/10.3390/polym12010176
APA StyleMohammadinejad, R., Kumar, A., Ranjbar-Mohammadi, M., Ashrafizadeh, M., Han, S. S., Khang, G., & Roveimiab, Z. (2020). Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers, 12(1), 176. https://doi.org/10.3390/polym12010176