Electrochemical Properties and Structure Evolution of Starch-Based Carbon Nanomaterials as Li-Ion Anodes with Regard to Thermal Treatment
Abstract
1. Introduction
2. Materials and Methods
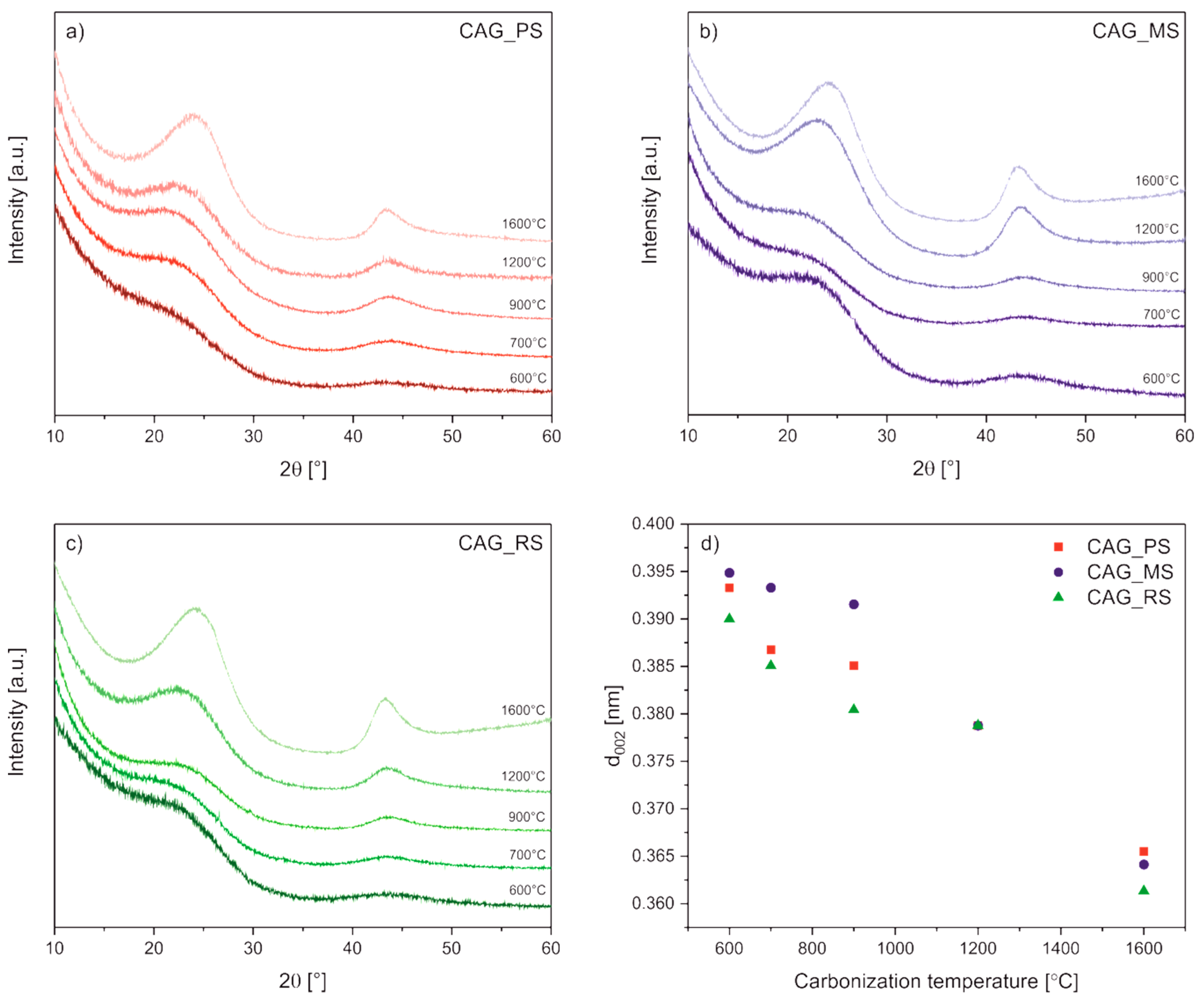
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.; He, M.; Walter, M.; Krumeich, F.; Kravchyk, K.V.; Kovalenko, M.V. Monodisperse CoSn2 and FeSn2 nanocrystals as high-performance anode materials for lithium-ion batteries. Nanoscale 2018, 10, 6827–6831. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, L.; Lou, X. Nanostructured Conversion-type Anode Materials for Advanced Lithium-Ion Batteries. Chem 2018, 4, 972–996. [Google Scholar] [CrossRef]
- Hu, L.; Luo, L.; Tang, L.; Lin, C.; Li, R.; Chen, Y. Ti2Nb2xO4+5x anode materials for lithium-ion batteries: A comprehensive review. J. Mater. Chem. A 2018, 6, 9799–9815. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Hou, S.; Lu, T.; Yao, Y.; Chua, D.H.C.; Pan, L. Metal-organic frameworks derived yolk-shell ZnO/NiO microspheres as high-performance anode materials for lithium-ion batteries. Chem. Eng. J. 2018, 335, 579–589. [Google Scholar] [CrossRef]
- Varzi, A.; Mattarozzi, L.; Cattarin, S.; Guerriero, P.; Passerini, S. 3D Porous Cu–Zn Alloys as Alternative Anode Materials for Li-Ion Batteries with Superior Low T Performance. Adv. Energy Mater. 2018, 8, 1701706. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Pillot, C. The Rechargeable Battery Market and Main Trends 2016-2025. In Proceedings of the 33rd Annual International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 20 March 2017; Available online: http://cii-resource.com/cet/FBC-TUT8/Presentations/Pillot_Christophe.pdf (accessed on 25 July 2019).
- Xing, B.; Zhang, C.; Cao, Y.; Huang, G.; Liu, Q.; Zhang, C.; Chen, Z.; Yi, G.; Chen, L.; Yu, J. Preparation of synthetic graphite from bituminous coal as anode materials for high performance lithium-ion batteries. Fuel Process. Technol. 2018, 172, 162–171. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.; Liu, H.; Fan, H.; Wang, G. Naturally nitrogen doped porous carbon derived from waste shrimp shells for high-performance lithium ion batteries and supercapacitors. Mesoporous Mesoporous Mater. 2017, 246, 72–80. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef]
- Świętosławski, M.; Bakierska, M.; Pacek, J.; Chudzik, K.; Lis, M.; Marszałowicz, W.; Knura, R.; Molenda, M. Integrated and Sustainable Solutions for Li-ion Energy Storage Systems. Adv. Inorg. Chem. 2018, 72, 287–321. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithium ion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Z.; Yin, S.; Guo, Z.; Wang, S.; Feng, C. Biomass carbon micro/nano-structures derived from ramie fibers and corncobs as anode materials for lithium-ion and sodium-ion batteries. Appl. Surf. Sci. 2016, 379, 73–82. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.; Shi, L.; Dong, Y.; Ma, J.; Chen, X.; Song, H. Nitrogen-doped biomass-based ultra-thin carbon nanosheets with interconnected framework for High-Performance Lithium-Ion Batteries. Appl. Surf. Sci. 2018, 437, 136–143. [Google Scholar] [CrossRef]
- Ye, G.; Zhu, X.; Chen, S.; Li, D.; Yin, Y.; Lu, Y.; Komarneni, S.; Yang, D. Nanoscale engineering of nitrogen-doped carbon nanofiber aerogels for enhanced lithium ion storage. J. Mater. Chem. A 2017, 5, 8247–8254. [Google Scholar] [CrossRef]
- Zheng, F.; Liu, D.; Xia, G.; Yang, Y.; Liu, T.; Wu, M.; Chen, Q. Biomass waste inspired nitrogen-doped porous carbon materials as high-performance anode for lithium-ion batteries. J. Alloys Compd. 2017, 693, 1197–1204. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X.S. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K.; Tian, N.; Qin, A.; Liao, L.; Du, R.; Wei, C. Biomass carbon derived from sisal fiber as anode material for lithium-ion batteries. Mater. Lett. 2015, 142, 193–196. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Feng, N.; Qiao, L.; Li, X.; He, D. A new carbonaceous material derived from biomass source peels as an improved anode for lithium ion batteries. J. Anal. Appl. Pyrol. 2013, 100, 181–185. [Google Scholar] [CrossRef]
- Bakierska, M.; Chojnacka, A.; Świętosławski, M.; Natkański, P.; Gajewska, M.; Rutkowska, M.; Molenda, M. Multifunctional Carbon Aerogels Derived by Sol-Gel Process of Natural Polysaccharides of Different Botanical Origin. Materials 2017, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Bakierska, M.; Molenda, M.; Majda, D.; Dziembaj, R. Functional Starch Based Carbon Aerogels for Energy Applications. Procedia Eng. 2014, 98, 14–19. [Google Scholar] [CrossRef]
- Bakierska, M.; Lis, M.; Pacek, J.; Świętosławski, M.; Gajewska, M.; Tąta, A.; Proniewicz, E.; Molenda, M. Bio-derived carbon nanostructures for high-performance lithium-ion batteries. Carbon 2019, 145, 426–432. [Google Scholar] [CrossRef]
- Lis, M.; Chudzik, K.; Bakierska, M.; Świętosławski, M.; Gajewska, M.; Rutkowska, M.; Molenda, M. Aqueous Binder for Nanostructured Carbon Anode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A5354–A5361. [Google Scholar] [CrossRef]
- BeMiller, J.; Whistler, R. Starch-Chemistry and Technology, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Herrero-Martınez, J.M.; Schoenmakers, P.J.; Kok, W.T. Determination of the amylose–amylopectin ratio of starches by iodine-affinity capillary electrophoresis. J. Chromatogr. A 2004, 1053, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Górka, J.; Vix-Guterl, C.; Ghimbeu, C.M. Recent Progress in Design of Biomass-Derived Hard Carbons for Sodium Ion Batteries. J. Carbon Res. 2016, 2, 24. [Google Scholar] [CrossRef]
- Howe, J.Y.; Rawn, C.J.; Jones, L.E.; Ow, H. Improved crystallographic data for graphite. Powder Diffr. 2003, 18, 150–154. [Google Scholar] [CrossRef]
- Winter, M.; Moeller, K.C.; Besenhard, J.O. Carbonaceous and Graphitic Anodes. In Lithium Batteries Science and Technology; Nazri, G.A., Pistoia, G., Eds.; Springer: New York, NY, USA, 2003; pp. 144–194. [Google Scholar]
- Gong, J.; Wu, H.; Yang, Q. Structural and electrochemical properties of disordered carbon prepared by the pyrolysis of poly(p-phenylene) below 1000 °C for the anode of a lithium-ion battery. Carbon 1999, 37, 1409–1416. [Google Scholar] [CrossRef]
- Gao, G.; Cheong, L.Z.; Wang, D.; Shen, C. Pyrolytic Carbon derived from spent coffee grounds as anode for sodium-ion batteries. Carbon Resour. Convers. 2018, 1, 104–108. [Google Scholar] [CrossRef]
- Dahn, J.R.; Xing, W.; Gao, Y. The “falling cards model” for the structure of microporous carbons. Carbon 1997, 35, 825–830. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Su, S.; Wang, J. Evaluation of the porous structure development of chars from pyrolysis of rice straw: Effects of pyrolysis temperature and heating rate. J. Anal. Appl. Pyrol. 2012, 98, 177–183. [Google Scholar] [CrossRef]




| CAG_PS | SBET [m2/g] | SEXT [m2/g] | VMIC[cm3/g] | VTOT (p/p0=0.99) [cm3/g] | SEXT/SBET [-] | VMIC/VTOT [-] |
| 600 | 371 | 19 | 0.179 | 0.213 | 0.05 | 0.83 |
| 700 | 302 | 73 | 0.116 | 0.187 | 0.24 | 0.62 |
| 900 | 569 | 93 | 0.187 | 0.258 | 0.16 | 0.72 |
| 1200 | 287 | 31 | 0.104 | 0.127 | 0.11 | 0.81 |
| 1600 | 29 | 30 | 0.001 | 0.098 | ----- | ----- |
| CAG_RS | SBET [m2/g] | SEXT [m2/g] | VMIC[cm3/g] | VTOT (p/p0=0.99) [cm3/g] | SEXT/SBET [-] | VMIC/VTOT [-] |
| 600 | 221 | 10 | 0.107 | 0.118 | 0.04 | 0.91 |
| 700 | 520 | 143 | 0.149 | 0.277 | 0.27 | 0.53 |
| 900 | 1743 | 475 | 0.503 | 0.929 | 0.27 | 0.54 |
| 1200 | 342 | 60 | 0.112 | 0.284 | 0.17 | 0.39 |
| 1600 | 51 | 46 | 0.002 | 0.147 | ----- | ----- |
| CAG_MS | SBET [m2/g] | SEXT [m2/g] | VMIC[cm3/g] | VTOT (p/p0=0.99) [cm3/g] | SEXT/SBET [-] | VMIC/VTOT [-] |
| 600 | 169 | 6 | 0.083 | 0.092 | 0.03 | 0.90 |
| 700 | 549 | 167 | 0.152 | 0.310 | 0.30 | 0.49 |
| 900 | 657 | 136 | 0.205 | 0.327 | 0.21 | 0.63 |
| 1200 | 178 | 20 | 0.062 | 0.077 | 0.11 | 0.81 |
| 1600 | 31 | 29 | 0.001 | 0.079 | ----- | ----- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubicka, M.; Bakierska, M.; Chudzik, K.; Rutkowska, M.; Pacek, J.; Molenda, M. Electrochemical Properties and Structure Evolution of Starch-Based Carbon Nanomaterials as Li-Ion Anodes with Regard to Thermal Treatment. Polymers 2019, 11, 1527. https://doi.org/10.3390/polym11091527
Kubicka M, Bakierska M, Chudzik K, Rutkowska M, Pacek J, Molenda M. Electrochemical Properties and Structure Evolution of Starch-Based Carbon Nanomaterials as Li-Ion Anodes with Regard to Thermal Treatment. Polymers. 2019; 11(9):1527. https://doi.org/10.3390/polym11091527
Chicago/Turabian StyleKubicka, Marcelina, Monika Bakierska, Krystian Chudzik, Małgorzata Rutkowska, Joanna Pacek, and Marcin Molenda. 2019. "Electrochemical Properties and Structure Evolution of Starch-Based Carbon Nanomaterials as Li-Ion Anodes with Regard to Thermal Treatment" Polymers 11, no. 9: 1527. https://doi.org/10.3390/polym11091527
APA StyleKubicka, M., Bakierska, M., Chudzik, K., Rutkowska, M., Pacek, J., & Molenda, M. (2019). Electrochemical Properties and Structure Evolution of Starch-Based Carbon Nanomaterials as Li-Ion Anodes with Regard to Thermal Treatment. Polymers, 11(9), 1527. https://doi.org/10.3390/polym11091527







