Sintering Reaction and Pyrolysis Process Analysis of Al/Ta/PTFE
Abstract
1. Introduction
2. Experiment Section
2.1. Raw Material and Sample Preparation
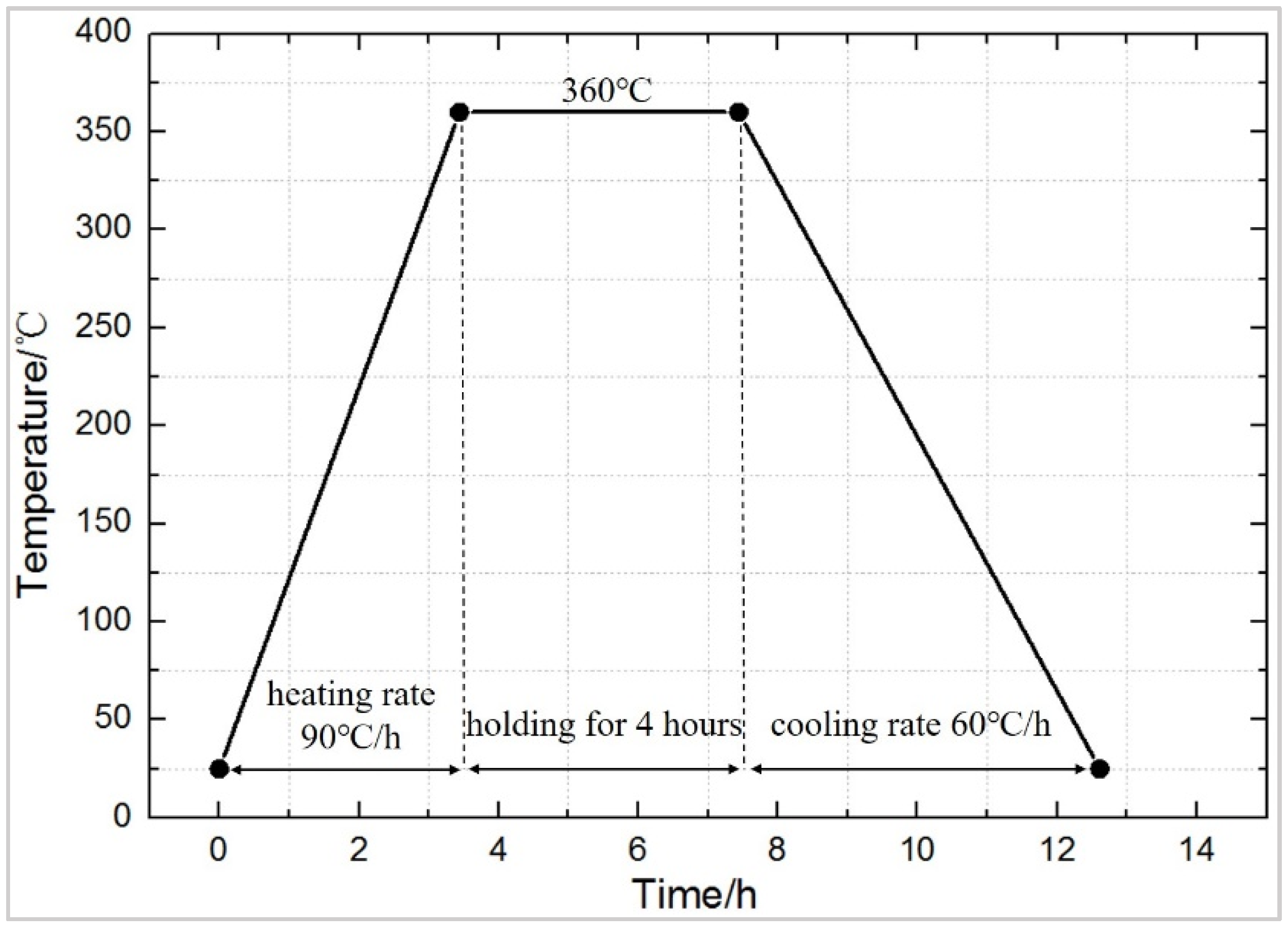
2.2. Experimental Contents
3. Results and Discussion
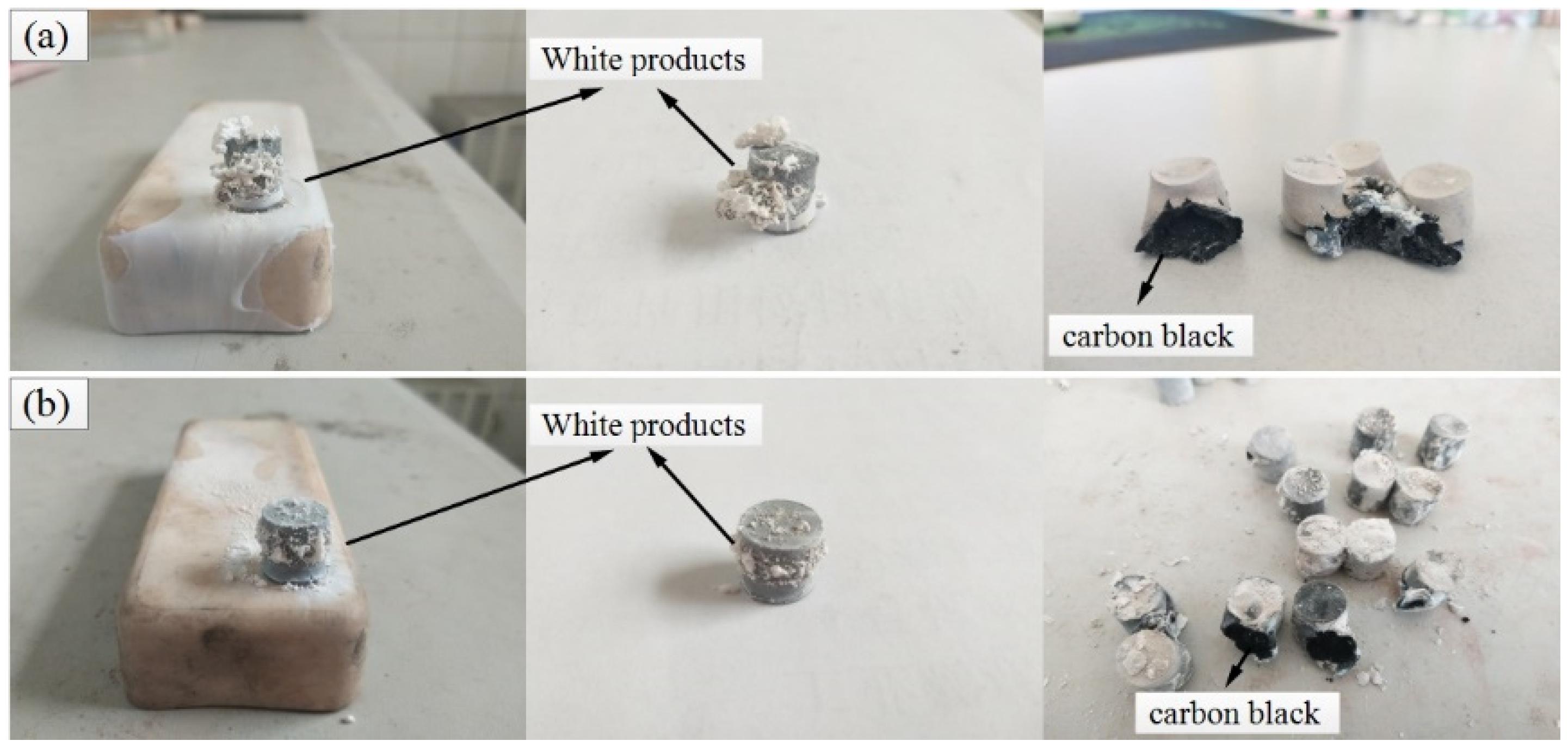
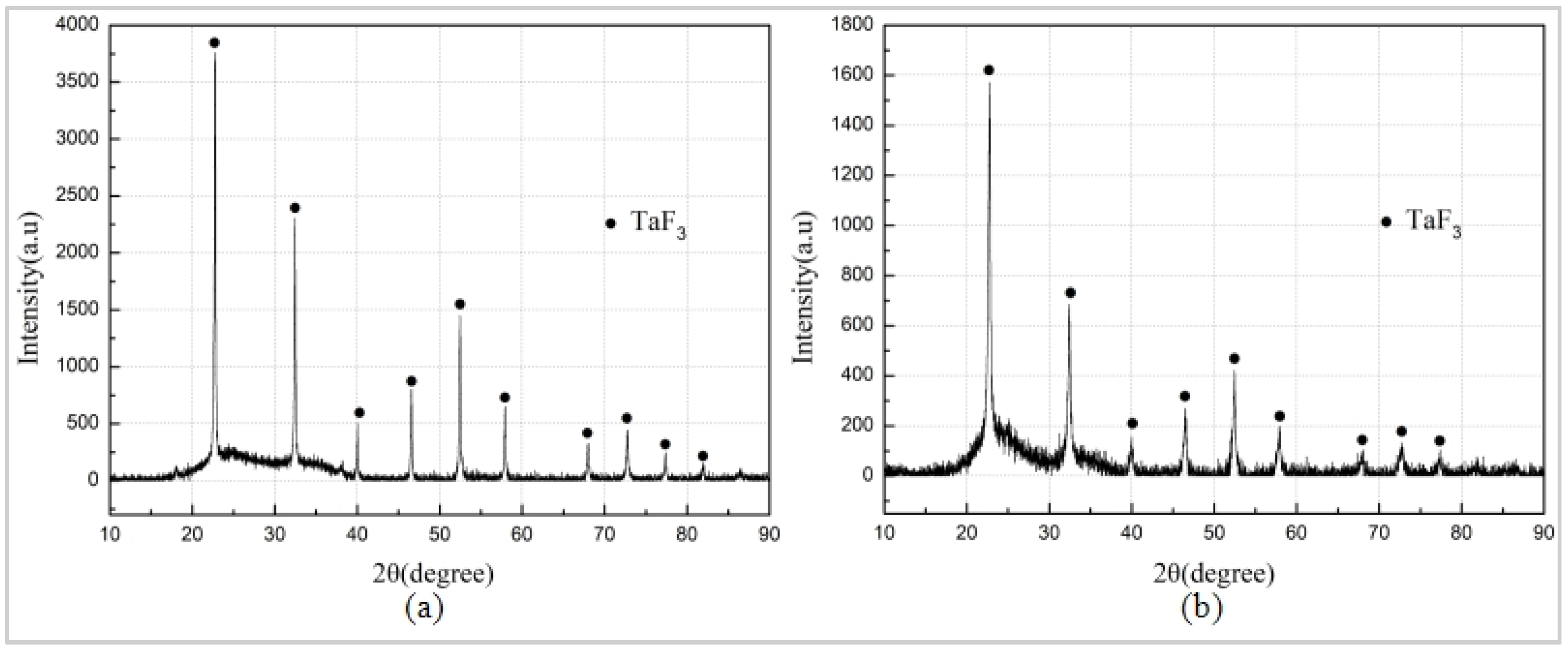
3.1. Sintering Reaction of Ta/PTFE and Al/ Ta/PTFE and Analysis of the Sintering Products
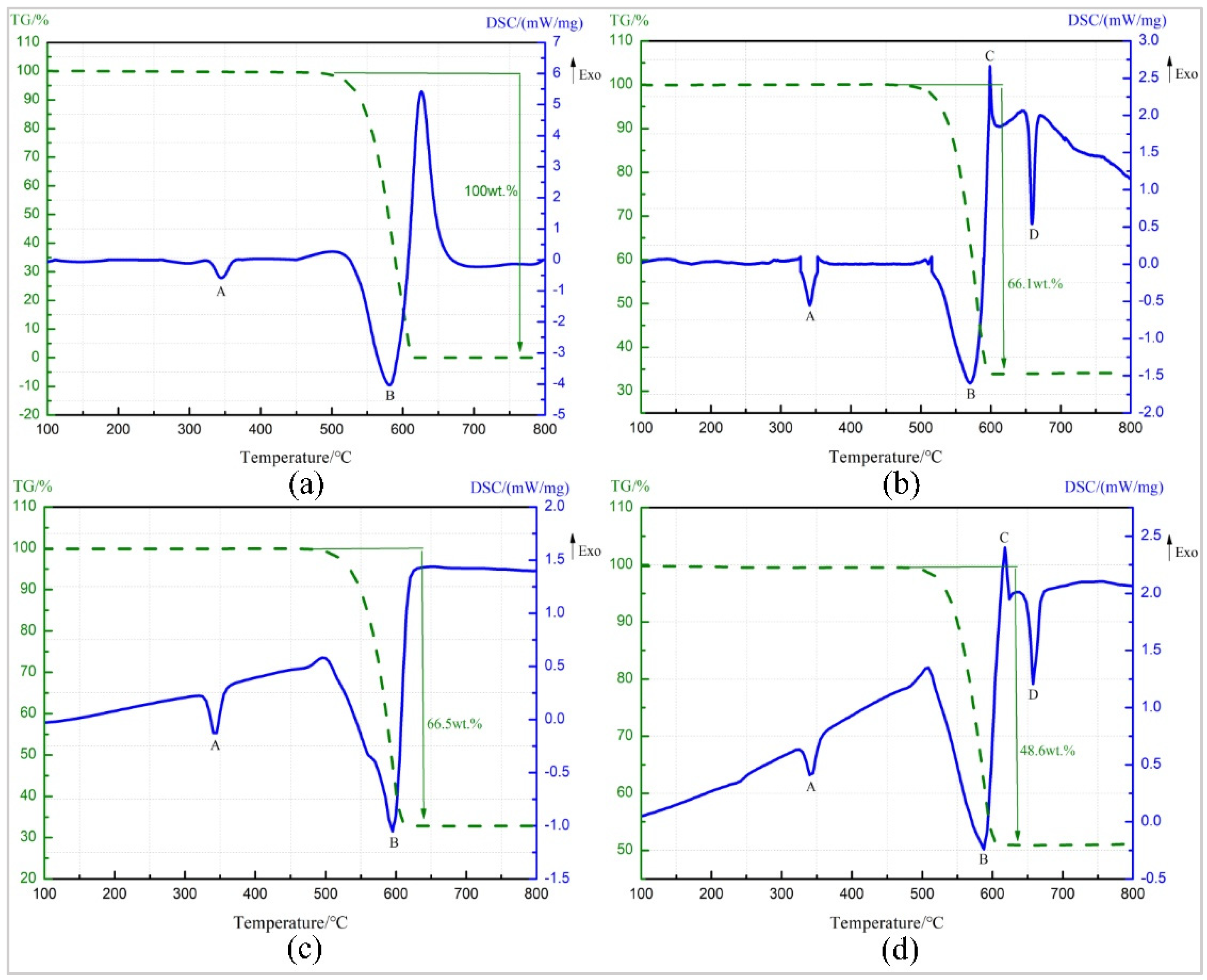
3.2. TG-DSC and XRD Phase Analysis of Four Groups of Reactive Materials
3.3. Analysis of Reaction Mechanism of Al/Ta/PTFE System
4. Conclusions
- Ta and PTFE chemically react when heated to 360 °C to form a soft and fluffy white material TaF3 and carbon black. TaF3 can overflow the surface of the specimen, causing cracking of the compacted cylindrical specimen, and scatter on the placement plate of specimens. This is presumably because the density of the substance is lower than that of the pressed specimens and the melting point is less than 360 °C.
- The results of the XRD phase detection show that there is TaF3 in the residue of TG-DSC specimen at 350 °C and 360 °C, indicating that Ta and PTFE have reacted at 340–350 °C. However, no obvious reaction exothermic peak is found on TG-DSC curves of Ta/PTFE and Al/Ta/PTFE. It is possibly because the reaction generates little energy and coincides with the melting process of PTFE, and the liberated heat is absorbed for PTFE to melt.
- It is speculated that the reaction mechanism of the Ta/PTFE system is that PTFE decomposes first, and then the product reacts with the highly oxidizable metal Ta to generate TaF3 and carbon black. TG-DSC test of PTFE and Al/PTFE shows that the decomposition temperature of PTFE starts at about 500 °C, which does not agree with the scope of the reaction temperature. It is speculated that PTFE does decompose before 500 °C, which could not be detected effectively, because the decomposition is weak, or the introduction of the metal Ta could affect decomposition of PTFE. Therefore, Ta reacts with PTFE during the sintering process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daniel, B.N.; Richard, M.T.; Benjamin, N.A. Reactive Material Enhanced Projectiles and Related Methods. U.S. Patent 20,080,035,007, 14 February 2008. [Google Scholar]
- Mock, W.; Holt, W.H. Impact Initiation of Rods of Pressed Polytetrafluoroethylene (PTFE) and Aluminum Powders. AIP Conf. Proc. 2006, 845, 1097–1100. [Google Scholar]
- Wang, H.; Zheng, Y.; Yu, Q.; Liu, Z.; Yu, W. Impact-induced Initiation and Energy Release Behavior of Reactive Materials. J. Appl. Phys. 2011, 110, 074904. [Google Scholar]
- Willis, M.J.; Jason, T.D. Effect of Aluminum Particle Size on the Impact Initiation of Pressed PTFE/Al Composite Rods. AIP Conf. Proc. 2007, 955, 971–974. [Google Scholar]
- Leslie, R.B.; Brian, B. Oil Well Perforators. U.S. Patent 20,070,056,462, 15 March 2007. [Google Scholar]
- Ye, W.J.; Wang, T.; Yu, Y.H. Research Progress of Fluoropolymer-Matrix Energetic Reactive Materials. Aerosp. Mater. Technol. 2012, 42, 19–23. [Google Scholar]
- Xu, S.L. Study on the Mechanical Performance of Polytetrafluorethylene/Al Energetic Reactive Materials; National University of Defence Technology: Changsha, China, 2010. [Google Scholar]
- Nielson, D.B.; Rochelle, D.P. Reactive Material Compositions, Shot Shells Including Reactive Materials, and a Method of Producing Same. U.S. Patent 797,742,0B2, 12 July 2011. [Google Scholar]
- Nielson, D.B.; Richard, L.T. High Strength Reactive Materials. U.S. Patent 20,030,096,897, 22 May 2003. [Google Scholar]
- Nielson, D.B.; Richard, L.T.; Gary, K.L. High Strength Reactive Materials. U.S. Patent 6,593,410, 15 July 2003. [Google Scholar]
- Li, L.Q. Research on Detonation Properties of Metal-Fluoride Reactive Materials; North University of China: Taiyuan, China, 2015. [Google Scholar]
- Koch, E.C. Metal-Fluorocarbon-Pyrolants IV: Thermochemical and Combustion Behaviour of Magnesium/Teflon/Viton (MTV). Propellants Explos. Pyrotech. 2002, 27, 340–351. [Google Scholar] [CrossRef]
- Xu, S.; Yang, S.; Zhang, W. The Mechanical Behaviors of Polytetrafluorethylene/Al/W Energetic Composites. J. Phys. Condens. Matter 2009, 21, 285401. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Tu, J.; Zhao, L.J. Influence of Particle Size Grading on Strength of Al/W/PTFE Composite. Ordnance Mater. Sci. Eng. 2014, 37, 17–21. [Google Scholar]
- Wu, J.X.; Wang, H.X.; Fang, X.; Li, Y.; Mao, Y.; Yang, L.; Yin, Q.; Wu, S.; Yao, M.; Song, J. Investigation on the Thermal Behavior, Mechanical Properties and Reaction Characteristics of Al-PTFE Composities Enhanced by Ni Particle. Materials 2018, 11, 1741. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Fang, X.; Li, Y.C.; Feng, B.; Wang, H.; Du, K. The Mechanical and Reaction Behavior of PTFE/Al/Fe2O3 under Impact and Quasi-static Compression. Adv. Mater. Sci. Eng. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Li, Y.-C. Characterization of Thermal Reaction of Aluminum/Copper (II) oxide/Poly (tetrafluoroethene) Nanocomposite by Thermogravimetric Analysis, Differential Scanning Calorimetry, Mass Spectrometry and X-ray Diffraction. Thermochim. Acta 2015, 621, 68–73. [Google Scholar] [CrossRef]
- Yu, Z.S.; Fang, X.; Gao, Z.R.; Wang, H.X.; Huang, J.Y.; Yao, M.; Li, Y.C. Mechanical and Reaction Properties of Al/TiH2/PTFE under Quasi-Static Compression. Adv. Eng. Mater. 2018, 20, 1800019. [Google Scholar] [CrossRef]
- Marinelli, G.; Martina, F.; Ganguly, S.; Williams, S. Microstructure, hardness and mechanical properties of two different unalloyed tantalum wires deposited via wire + arc additive manufacture. Int. J. Refract. Met. Hard Mater. 2019, 83, 104974. [Google Scholar] [CrossRef]
- Ding, D. Preparation and Biological Properties of Tantalum Based Coatings on Bone Implant; University of Chinese Academy of Sciences: Shanghai, China, 2018. [Google Scholar]
- Peng, J.X.; Li, Y.L.; Li, D.H. An Experimental Study on the Dynamic Constitutive Relation of Tantalum. Explos. Shock Waves 2003, 23, 183–187. [Google Scholar]
- Qian, Z.M. Fluorine Resin Properties and Processing Applications (Continuation 10). Chem. Prod. Technol. 2006, 13, 7–13. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics—80th edition 1999–2000; CRC Press: Boca Raton, FL, USA, 1999; Volume 15, p. 504. [Google Scholar]
- Zhang, J.; Huang, J.Y.; Fang, X.; Li, Y.C.; Yu, Z.; Gao, Z.; Wu, S.; Yang, L.; Wu, J.; Kui, J. Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite. Materials 2018, 11, 2541. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.T.; Pantoya, M.L. Effect of Al particle size on the thermal degradation of Al/Teflon mixtures. Combust. Sci. Technol. 2007, 179, 1467–1480. [Google Scholar] [CrossRef]
- Dolgoborodov, A.Y.; Makhov, M.N.; Kolbanev, I.V.; Streletskii, A.N.; Fortov, V.E. Detonation in an aluminum-Teflon mixture. JETP Lett. 2005, 81, 311–314. [Google Scholar] [CrossRef]
- Li, X.Y. Preparation and Reactivity of Fluoro-Nanothermite; Nanjing University of Science & Technology: Nanjing, China, 2016. [Google Scholar]
- Simon, C.; Kaminsky, W. Chemical recycling of polytetrafluoroethylene by pyrolysis. Polym. Degrad. Stab. 1998, 62, 1–7. [Google Scholar] [CrossRef]
- Arai, N. Transient ablation of Teflon in intense radiative and convective environments. AIAA J. 1979, 17, 634–640. [Google Scholar] [CrossRef]

| Reactive Material | Composition (wt.%) | ||
|---|---|---|---|
| Al | Ta | PTFE | |
| PTFE | - | - | 100 |
| Al/PTFE | 26.5 | - | 73.5 |
| Ta/PTFE | - | 48.55 | 51.45 |
| Al/Ta/PTFE | 18.55 | 30 | 51.45 |
| Materials | Starting Temperature/°C | Peak Temperature/°C | Termination Temperature/°C | Melting Enthalpy/J·g−1 |
|---|---|---|---|---|
| PTFE | 325.2 | 344.45 | 363.1 | 78.56 |
| Al/PTFE | 329.8 | 341.4 | 350.6 | 52.54 |
| Ta/PTFE | 331.0 | 343.0 | 353.5 | 29.57 |
| Al/Ta/PTFE | 330.5 | 342.4 | 353.1 | 40.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Huang, J.; Li, Y.; Liu, Q.; Yu, Z.; Wu, J.; Gao, Z.; Wu, S.; Kui, J.; Song, J. Sintering Reaction and Pyrolysis Process Analysis of Al/Ta/PTFE. Polymers 2019, 11, 1469. https://doi.org/10.3390/polym11091469
Zhang J, Huang J, Li Y, Liu Q, Yu Z, Wu J, Gao Z, Wu S, Kui J, Song J. Sintering Reaction and Pyrolysis Process Analysis of Al/Ta/PTFE. Polymers. 2019; 11(9):1469. https://doi.org/10.3390/polym11091469
Chicago/Turabian StyleZhang, Jun, Junyi Huang, Yuchun Li, Qiang Liu, Zhongshen Yu, Jiaxiang Wu, Zhenru Gao, Shuangzhang Wu, Jiaying Kui, and Jiaxing Song. 2019. "Sintering Reaction and Pyrolysis Process Analysis of Al/Ta/PTFE" Polymers 11, no. 9: 1469. https://doi.org/10.3390/polym11091469
APA StyleZhang, J., Huang, J., Li, Y., Liu, Q., Yu, Z., Wu, J., Gao, Z., Wu, S., Kui, J., & Song, J. (2019). Sintering Reaction and Pyrolysis Process Analysis of Al/Ta/PTFE. Polymers, 11(9), 1469. https://doi.org/10.3390/polym11091469






