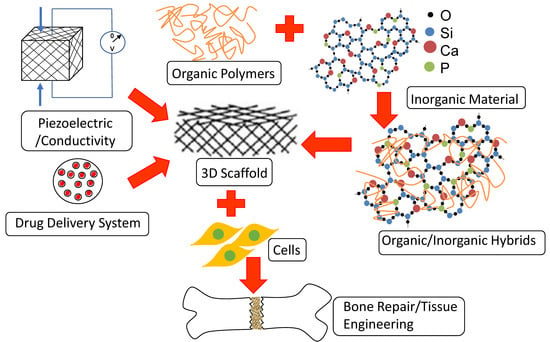
Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment
Abstract
1. Introduction
2. Bone Tissue Engineering Scaffolds Are More than Mere Structural Templates
3. Bone Tissue Engineering Scaffolds Encompass the Chemistry of Inorganic and Organic Polymers
3.1. Biodegradable Inorganic Materials: Crystalline Calcium Phosphates and Non-crystalline Bioactive Glasses
3.1.1. Crystalline Calcium Phosphates
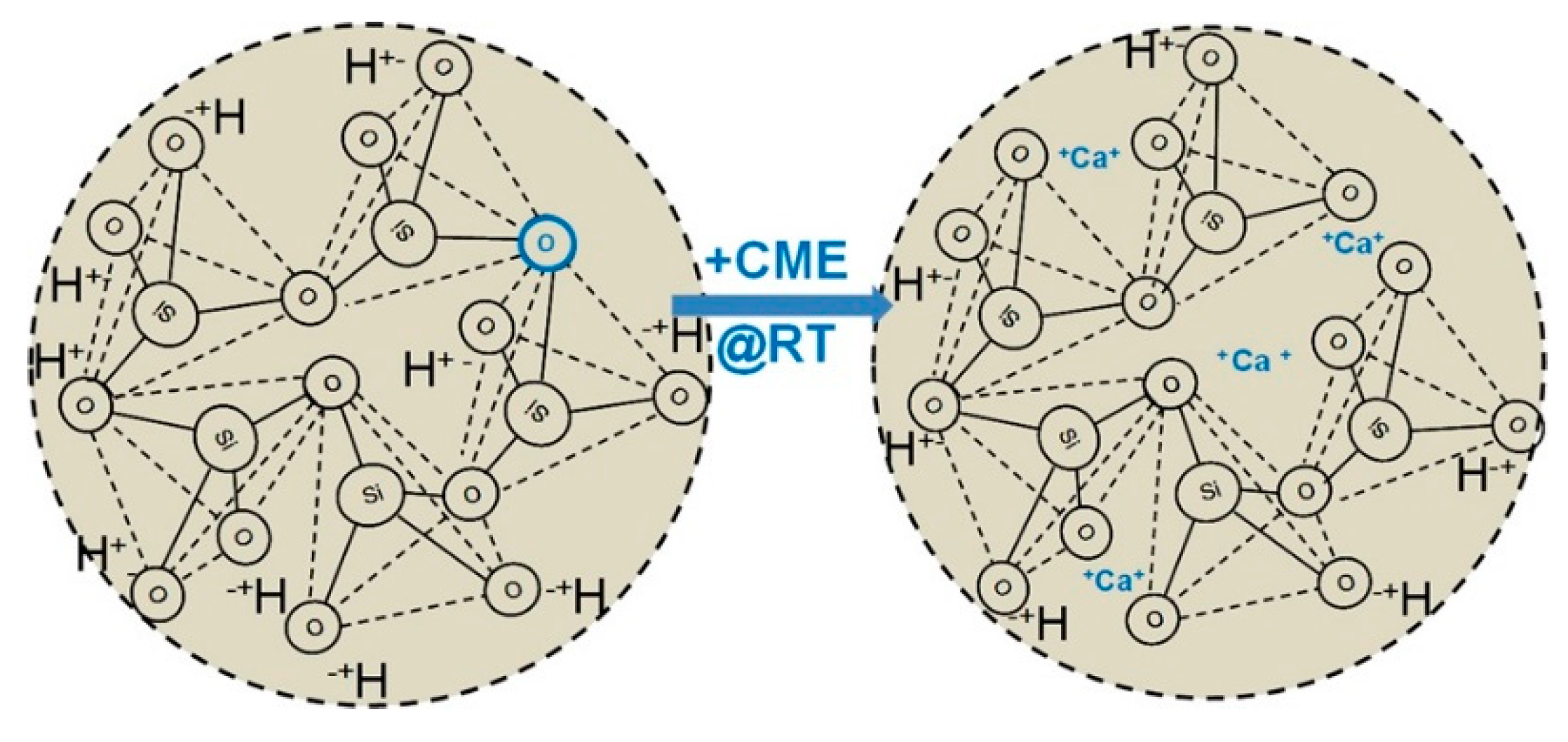
3.1.2. Non-Crystalline Bioactive Glasses
3.2. Biocompatible and Degradable Organic Polymers
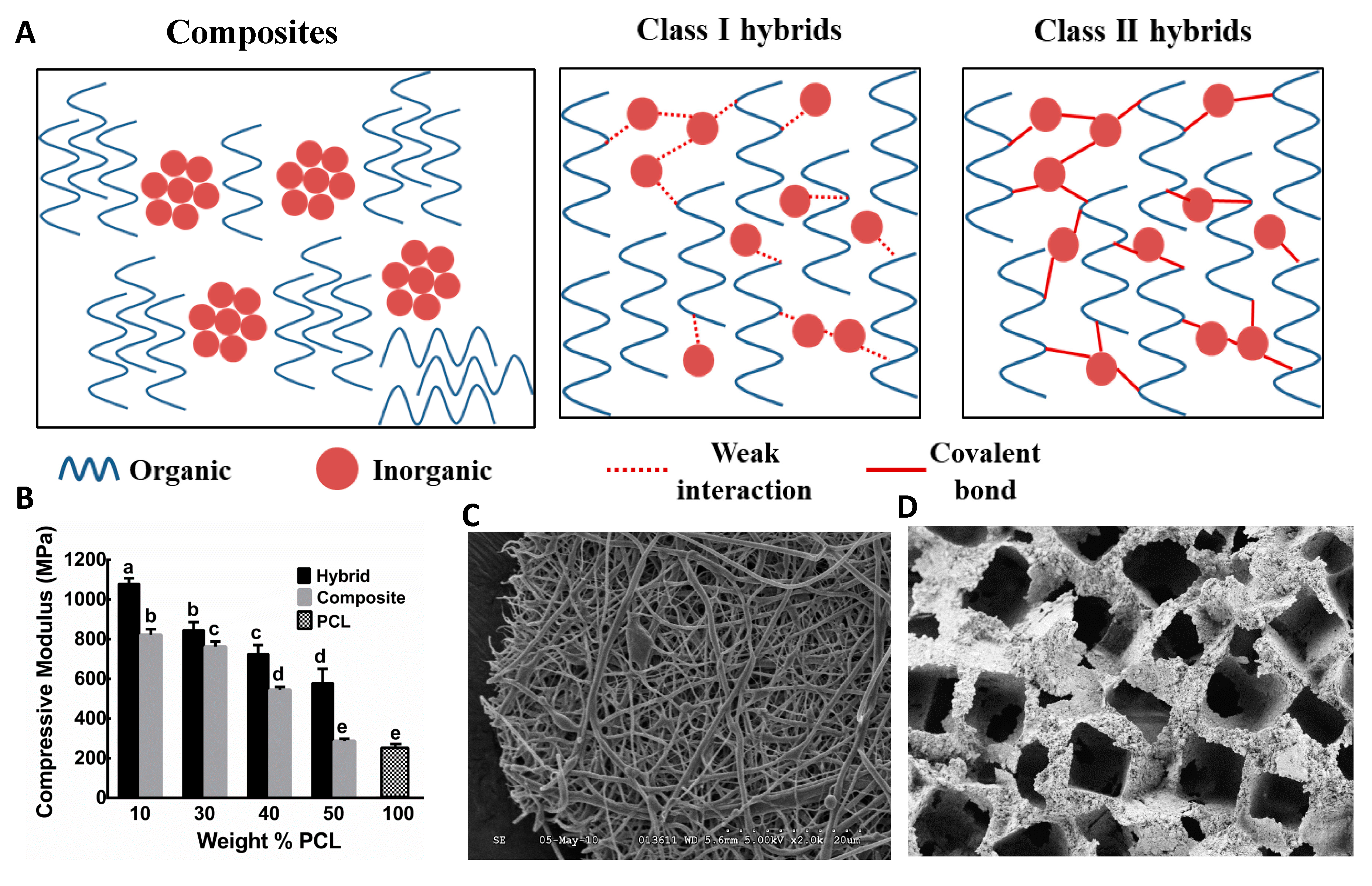
3.3. Composite Bone Biomaterials from Inorganic and Organic Polymers
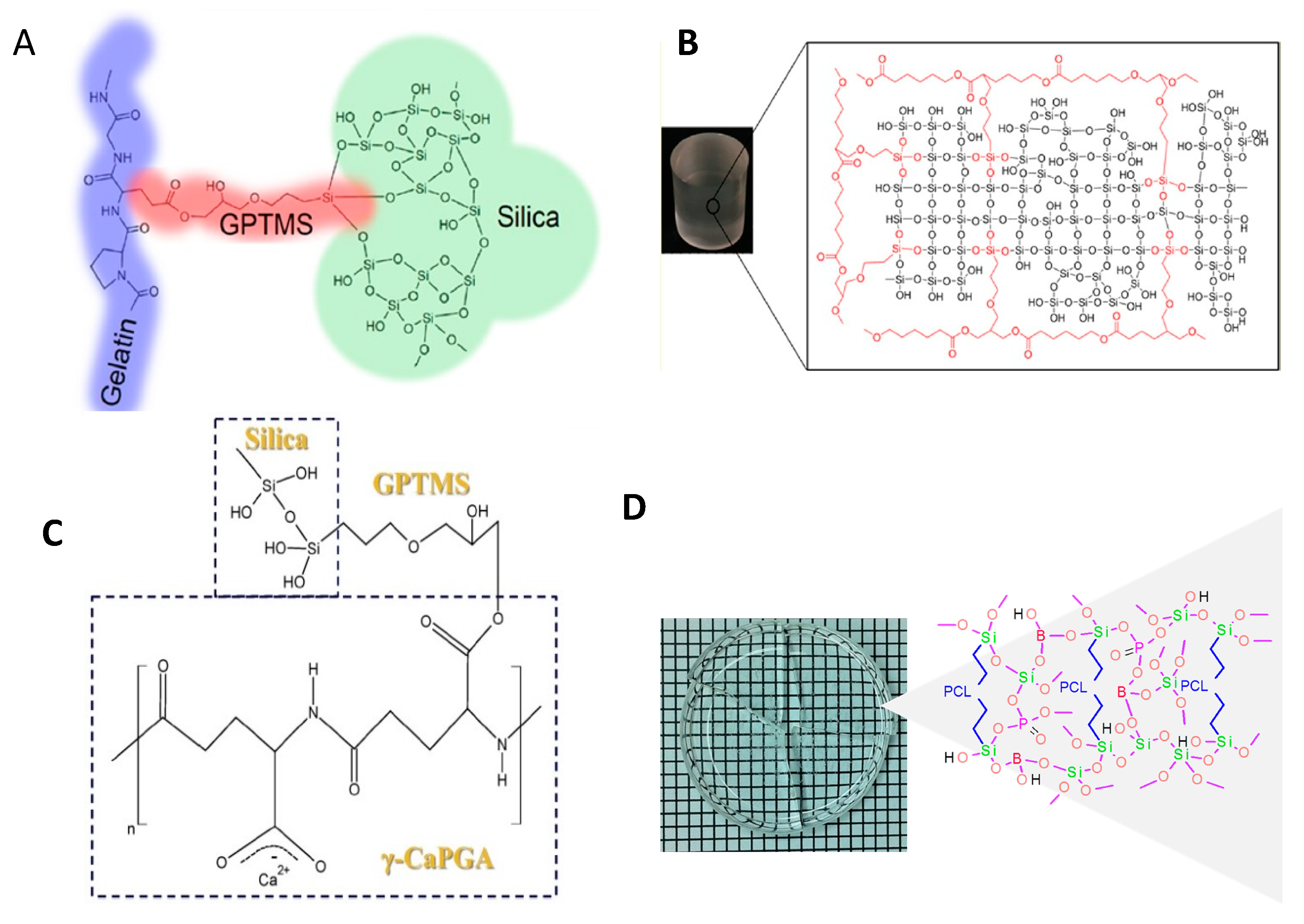
3.4. Hybrid Bone Biomaterials from Inorganic and Organic Polymers
3.4.1. O/I Class I Hybrid Biomaterials
3.4.2. O/I Class II Hybrid Biomaterials
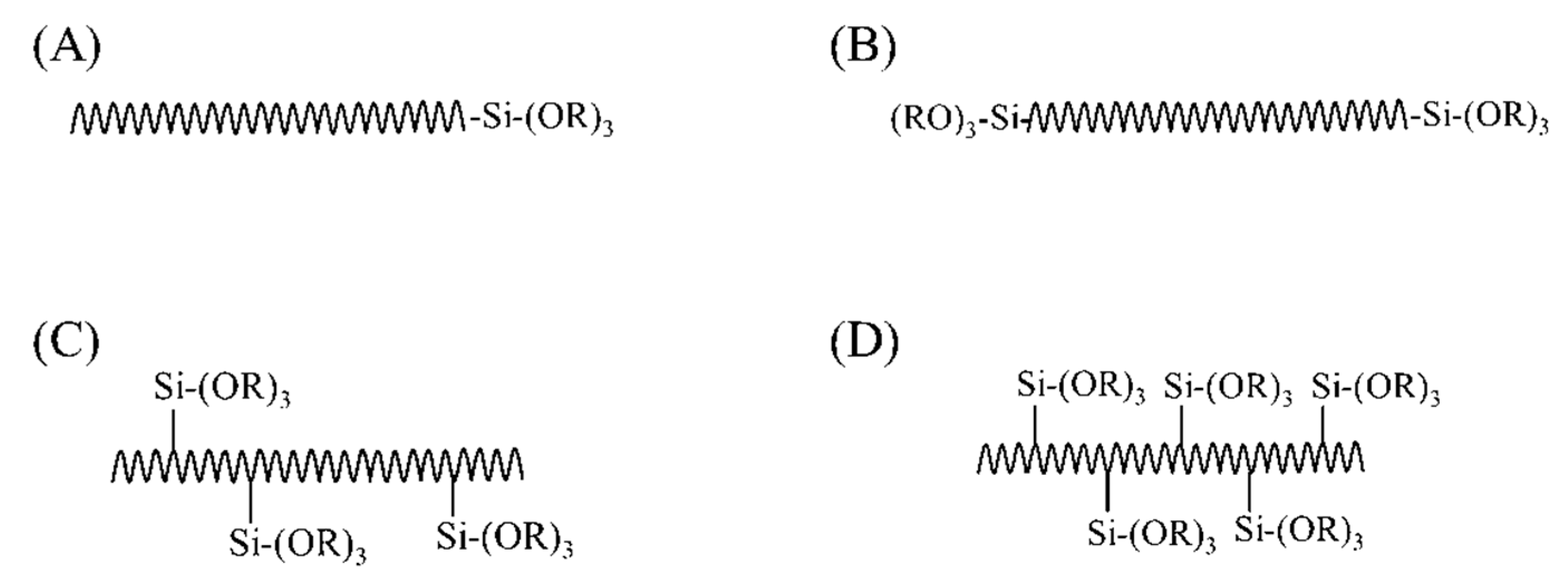
3.4.3. Challenges Associated with the Synthesis of Class II Hybrids
Synthesis Route
Incorporation of Necessary Components
4. New Materials Are Emerging for Bone Tissue Engineering Scaffolds
4.1. Mesoporous Materials
4.2. Piezoelectric Materials
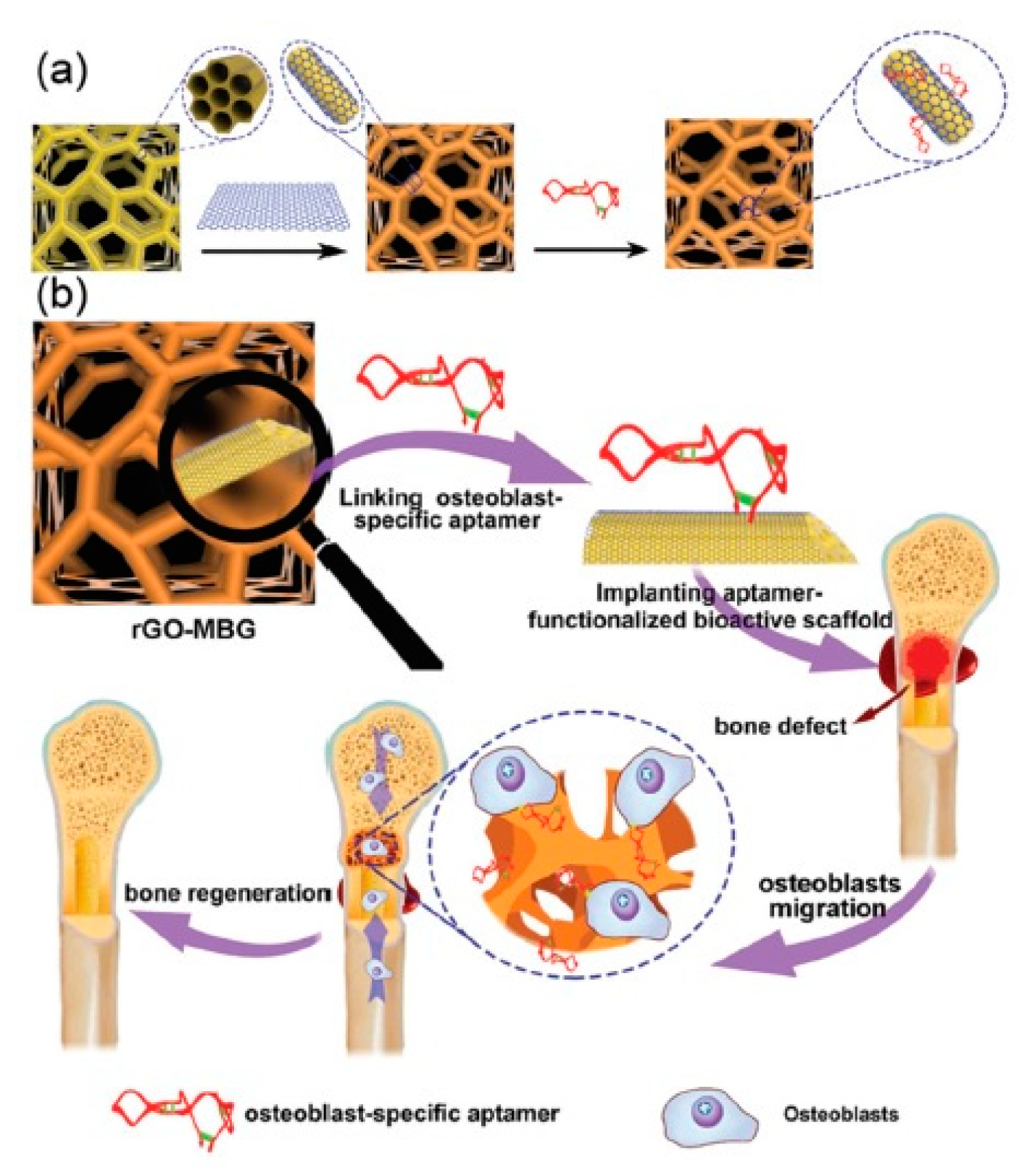
4.3. Conductive Materials
5. Successful Bone Repair and Regeneration Requires Appropriate Primary and Stem Cell Sources
6. Future Directions
Funding
Conflicts of Interest
References
- Riddle, R.C.; Clemens, T.L. Bone Cell Bioenergetics and Skeletal Energy Homeostasis. Physiol. Rev. 2017, 97, 667–698. [Google Scholar] [CrossRef]
- Kane, R.; Ma, P.X. Mimicking the nanostructure of bone matrix to regenerate bone. Mater. Today 2013, 16, 418–423. [Google Scholar] [CrossRef]
- Pivonka, P. Multiscale Mechanobiology of Bone Remodeling and Adaptation; Springer International Publishing: Basel, Switzerland, 2018. [Google Scholar]
- Abdel Meguid, E.; Ke, Y.; Ji, J.; El-Hashash, A.H.K. Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J. Cell. Physiol. 2018, 233, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Schuit, S.C.E.; Van der Klift, M.; Weel, A.E.A.M.; De Laet, C.E.D.H.; Burger, H.; Seeman, E.; Hofman, A.; Uitterlinden, A.G.; Van Leeuwen, J.P.T.M.; Pols, H.A.P. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone 2004, 34, 195–202. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Brown, T.D. Joint injury, repair, and remodeling: Roles in post-traumatic osteoarthritis. Clin. Orthop. Relat. Res. 2004, 423, 7–16. [Google Scholar] [CrossRef]
- Rauch, F.; Glorieux, F.H. Osteogenesis imperfecta. Lancet 2004, 363, 1377–1385. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Pape, H.; Giannoudis, P.; Krettek, C. The timing of fracture treatment in polytrauma patients: Relevance of damage control orthopedic surgery. Am. J. Surg. 2002, 183, 622–629. [Google Scholar] [CrossRef]
- Phukan, R.; Herzog, T.; Boland, P.J.; Healey, J.; Rose, P.; Sim, F.H.; Yazsemski, M.; Hess, K.; Osler, P.; DeLaney, T.F.; et al. How Does the Level of Sacral Resection for Primary Malignant Bone Tumors Affect Physical and Mental Health, Pain, Mobility, Incontinence, and Sexual Function? Clin. Orthop. Relat. Res. 2016, 474, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.H.; Gibbons, C.L.; Athanasou, N.A. The pathology of bone allograft. J. Bone Jt. Surg. Br. 1999, 81, 333–335. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.T.; Scott, D.D. A review of bone substitutes. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Tovar, N.; Jimbo, R.; Gangolli, R.; Perez, L.; Manne, L.; Yoo, D.; Lorenzoni, F.; Witek, L.; Coelho, P.G. Evaluation of bone response to various anorganic bovine bone xenografts: An experimental calvaria defect study. Int. J. Oral Maxillofac. Surg. 2014, 43, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Biomaterials. Science 1980, 208, 826–831. [Google Scholar] [CrossRef]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Melchels, F.P.W.; Barradas, A.M.C.; van Blitterswijk, C.A.; de Boer, J.; Feijen, J.; Grijpma, D.W. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010, 6, 4208–4217. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Martin, I.; Vunjak-Novakovic, G. Frontiers in tissue engineering. In vitro modulation of chondrogenesis. Clin. Orthop. Relat. Res. 1999, 367, S46–S58. [Google Scholar] [CrossRef]
- Ishaug, S.L.; Crane, G.M.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997, 36, 17–28. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Wopenka, B.; Valsami-Jones, E. Bone and Tooth Mineralization: Why Apatite? Elements 2008, 4, 97–104. [Google Scholar] [CrossRef]
- Allo, B.A.; Rizkalla, A.S.; Mequanint, K. Synthesis and Electrospinning of ε-Polycaprolactone-Bioactive Glass Hybrid Biomaterials via a Sol−Gel Process. Langmuir 2010, 26, 18340–18348. [Google Scholar] [CrossRef]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Salinas, A.J.; Esbrit, P.; Vallet-Regí, M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2012, 1, 40–51. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; LeGeros, J.P. Dense Hydroxyapatite: An Introduction to Bioceramics; World Scientific: Singapore, 1993; pp. 139–180. [Google Scholar]
- Jevtić, M.; Mitrić, M.; Škapin, S.; Jančar, B.; Ignjatović, N.; Uskoković, D. Crystal Structure of Hydroxyapatite Nanorods Synthesized by Sonochemical Homogeneous Precipitation. Cryst. Growth Des. 2008, 8, 2217–2222. [Google Scholar] [CrossRef]
- Pang, Y.X.; Bao, X. Influence of temperature, ripening time and calcination on the morphology and crystallinity of hydroxyapatite nanoparticles. J. Eur. Ceram. Soc. 2003, 10, 1697–1704. [Google Scholar] [CrossRef]
- Wang, H.; Lee, J.-K.; Moursi, A.; Lannutti, J.J. Ca/P ratio effects on the degradation of hydroxyapatite in vitro. J. Biomed. Mater. Res. Part A 2003, 67, 599–608. [Google Scholar] [CrossRef]
- Dorozhkin, S. Calcium Orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Hench, L.L.; Best, S.M. Ceramics, Glasses, and Glass-Ceramics: Basic Principles. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 128–151. [Google Scholar]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef]
- Bigi, A.; Fini, M.; Bracci, B.; Boanini, E.; Torricelli, P.; Giavaresi, G.; Aldini, N.N.; Facchini, A.; Sbaiz, F.; Giardino, R. The response of bone to nanocrystalline hydroxyapatite-coated Ti13Nb11Zr alloy in an animal model. Biomaterials 2008, 29, 1730–1736. [Google Scholar] [CrossRef]
- Borsari, V.; Fini, M.; Giavaresi, G.; Tschon, M.; Chiesa, R.; Chiusoli, L.; Salito, A.; Rimondini, L.; Giardino, R. Comparative in vivo evaluation of porous and dense duplex titanium and hydroxyapatite coating with high roughnesses in different implantation environments. J. Biomed. Mater. Res. Part A 2009, 89, 550–560. [Google Scholar] [CrossRef]
- Scaglione, S.; Ilengo, C.; Fato, M.; Quarto, R. Hydroxyapatite-Coated Polycaprolacton Wide Mesh as a Model of Open Structure for Bone Regeneration. Tissue Eng. Part A 2008, 15, 155–163. [Google Scholar] [CrossRef]
- Lickorish, D.; Guan, L.; Davies, J.E. A three-phase, fully resorbable, polyester/calcium phosphate scaffold for bone tissue engineering: Evolution of scaffold design. Biomaterials 2007, 28, 1495–1502. [Google Scholar] [CrossRef]
- Jongwattanapisan, P.; Charoenphandhu, N.; Krishnamra, N.; Thongbunchoo, J.; Tang, I.-M.; Hoonsawat, R.; Smith, S.M.; Pon-On, W. In vitro study of the SBF and osteoblast-like cells on hydroxyapatite/chitosan–silica nanocomposite. Mater. Sci. Eng. C 2011, 31, 290–299. [Google Scholar] [CrossRef]
- Spanos, N.; Misirlis, D.Y.; Kanellopoulou, D.G.; Koutsoukos, P.G. Seeded growth of hydroxyapatite in simulated body fluid. J. Mater. Sci. 2006, 41, 1805–1812. [Google Scholar] [CrossRef]
- Wilke, A.; Orth, J.; Lomb, M.; Fuhrmann, R.; Kienapfel, H.; Griss, P.; Franke, R.P. Biocompatibility analysis of different biomaterials in human bone marrow cell cultures. J. Biomed. Mater. Res. 1998, 40, 301–306. [Google Scholar] [CrossRef]
- Bluteau, G.; Pilet, P.; Bourges, X.; Bilban, M.; Spaethe, R.; Daculsi, G.; Guicheux, J. The modulation of gene expression in osteoblasts by thrombin coated on biphasic calcium phosphate ceramic. Biomaterials 2006, 27, 2934–2943. [Google Scholar] [CrossRef]
- Sun, L.; Wu, L.; Bao, C.; Fu, C.; Wang, X.; Yao, J.; Zhang, X.; van Blitterswijk, C.A. Gene expressions of Collagen type I, ALP and BMP-4 in osteo-inductive BCP implants show similar pattern to that of natural healing bones. Mater. Sci. Eng. C 2009, 6, 1829–1834. [Google Scholar] [CrossRef]
- Rochet, N.; Loubat, A.; Laugier, J.-P.; Hofman, P.; Bouler, J.M.; Daculsi, G.; Carle, G.F.; Rossi, B. Modification of gene expression induced in human osteogenic and osteosarcoma cells by culture on a biphasic calcium phosphate bone substitute. Bone 2003, 32, 602–610. [Google Scholar] [CrossRef]
- Geesink, R.G.T. Osteoconductive Coatings for Total Joint Arthroplasty. Clin. Orthop. Relat. Res. (1976–2007) 2002, 395, 53–65. [Google Scholar] [CrossRef]
- Barrere, F.; Van Der Valk, C.M.; Dalmeijer, R.A.J.; Meijer, G.; Van Blitterswijk, C.A.; De Groot, K.; Layrolle, P. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. J. Biomed. Mater. Res. Part A 2003, 66, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; van der Valk, C.M.; van Blitterswijk, C.A.; de Groot, K.; Meijer, G. Influence of octacalcium phosphate coating on osteoinductive properties of biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Oonishi, H.; Hench, L.L.; Wilson, J.; Sugihara, F.; Tsuji, E.; Kushitani, S.; Iwaki, H. Comparative bone growth behavior in granules of bioceramic materials of various sizes. J. Biomed. Mater. Res. 1999, 44, 31–43. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. Part A 2014, 102, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Brink, M. The influence of alkali and alkaline earths on the working range for bioactive glasses. J. Biomed. Mater. Res. 1997, 36, 109–117. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Sonny Bal, B.; Brown, R.F.; Day, D.E. Mechanical and in vitro performance of 13–93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008, 4, 1854–1864. [Google Scholar] [CrossRef]
- Brovarone, C.V.; Verné, E.; Appendino, P. Macroporous bioactive glass-ceramic scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2006, 17, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Lewis, M.; Olsen, I.; Knowles, J.C. Phosphate glasses for tissue engineering: Part 1. Processing and characterisation of a ternary-based P2O5–CaO–Na2O glass system. Biomaterials 2004, 25, 491–499. [Google Scholar] [CrossRef]
- Uo, M.; Mizuno, M.; Kuboki, Y.; Makishima, A.; Watari, F. Properties and cytotoxicity of water soluble Na2O–CaO–P2O5 glasses. Biomaterials 1998, 19, 2277–2284. [Google Scholar] [CrossRef]
- Bunker, B.C.; Arnold, G.W.; Wilder, J.A. Phosphate glass dissolution in aqueous solutions. J. Non-Cryst. Solids 1984, 64, 291–316. [Google Scholar] [CrossRef]
- Gao, H.; Tan, T.; Wang, D. Dissolution mechanism and release kinetics of phosphate controlled release glasses in aqueous medium. J. Control. Release 2004, 96, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Mizoguchi, T.; Ito, M.; Bitar, M.; Salih, V.; Knowles, J.C. In vitro bioactivity and gene expression by cells cultured on titanium dioxide doped phosphate-based glasses. Biomaterials 2007, 28, 19–2967. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Pickup, D.M.; Carroll, D.L.; Hope, C.K.; Pratten, J.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Effect of Silver Content on the Structure and Antibacterial Activity of Silver-Doped Phosphate-Based Glasses. Antimicrob. Agents Chemother. 2007, 51, 4453–4461. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Fu, H.; Liu, X. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J. Biomed. Mater. Res. Part A 2010, 95, 164–171. [Google Scholar] [CrossRef]
- Han, X.; Day, D.E. Reaction of sodium calcium borate glasses to form hydroxyapatite. J. Mater. Sci. Mater. Med. 2007, 18, 1837–1847. [Google Scholar] [CrossRef]
- Huang, W.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006, 17, 583–596. [Google Scholar] [CrossRef]
- Yao, A.; Wang, D.; Huang, W.; Fu, Q.; Rahaman, M.N.; Day, D.E. In Vitro Bioactive Characteristics of Borate-Based Glasses with Controllable Degradation Behavior. J. Am. Ceram. Soc. 2007, 90, 303–306. [Google Scholar] [CrossRef]
- Xu, S.; Yang, X.; Chen, X.; Shao, H.; He, Y.; Zhang, L.; Yang, G.; Gou, Z. Effect of borosilicate glass on the mechanical and biodegradation properties of 45S5-derived bioactive glass-ceramics. J. Non-Cryst. Solids 2014, 405, 91–99. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.; Zhang, X.; Zhang, Y.; Zhang, C.; Liu, X.; Rahaman, M.N.; Huang, W.; Pan, H. Biodegradable borosilicate bioactive glass scaffolds with a trabecular microstructure for bone repair. Mater. Sci. Eng. C 2014, 36, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bioactive borophosphosilicate-polycaprolactone hybrid biomaterials via a non-aqueous sol gel process. RSC Adv. 2016, 6, 92824–92832. [Google Scholar] [CrossRef]
- Uysal, T.; Ustdal, A.; Sonmez, M.F.; Ozturk, F. Stimulation of Bone Formation by Dietary Boron in an Orthopedically Expanded Suture in Rabbits. Angle Orthod. 2009, 79, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.W.; Liang, W.; Liang, W.; Reilly, G.C.; Day, D.E.; Rahaman, M.N.; Mao, J.J. Borate Glass Supports the In Vitro Osteogenic Differentiation of Human Mesenchymal Stem Cells. Mech. Adv. Mater. Struct. 2005, 12, 239–246. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Miola, M.; Balagna, C.; Verné, E. 3D-glass–ceramic scaffolds with antibacterial properties for bone grafting. Chem. Eng. J. 2008, 137, 129–136. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Bonewald, L.F.; Kuroki, K.; Brown, R.F. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. II. In vitro and in vivo biological evaluation. J. Biomed. Mater. Res. A 2010, 95, 172–179. [Google Scholar] [CrossRef]
- Jia, W.-T.; Zhang, X.; Luo, S.-H.; Liu, X.; Huang, W.-H.; Rahaman, M.N.; Day, D.E.; Zhang, C.-Q.; Xie, Z.-P.; Wang, J.-Q. Novel borate glass/chitosan composite as a delivery vehicle for teicoplanin in the treatment of chronic osteomyelitis. Acta Biomater. 2010, 6, 812–819. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Z.; Zhang, C.; Pan, H.; Rahaman, M.N.; Zhang, X.; Fu, Q.; Huang, W. Bioactive borate glass scaffolds: In vitro and in vivo evaluation for use as a drug delivery system in the treatment of bone infection. J. Mater. Sci. Mater. Med. 2010, 21, 575–582. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, W.T.; Gu, Y.F.; Xiao, W.; Liu, X.; Wang, D.; Zhang, C.; Huang, W.; Rahaman, M.N.; Day, D.E.; et al. Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials 2010, 31, 5865–5874. [Google Scholar] [CrossRef]
- Jung, S.B.; Day, D.E.; Brown, R.F.; Bonewald, L. Potential toxicity of bioactive borate glasses in-vitro and in-vivo. In Proceedings of the Advances in Bioceramics and Porous Ceramics V—A Collection of Papers Presented at the 36th International Conference on Advanced Ceramics and Composites (ICACC 2012), Daytona Beach, FL, USA, 22–27 January 2012; pp. 65–74. [Google Scholar]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. CHAPTER 3—Hydrolysis and Condensation II: Silicates. In Sol-Gel Science; San Diego Academic Press: San Diego, CA, USA, 1990; pp. 96–233. [Google Scholar]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel–derived 58S bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Chen, X.; Wang, Y.; Zhao, N.; Du, C.; Fang, L. Surface nanoscale patterning of bioactive glass to support cellular growth and differentiation. J. Biomed. Mater. Res. Part A 2010, 94, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Shrivats, A.R.; McDermott, M.C.; Hollinger, J.O. Bone tissue engineering: State of the union. Drug Discov. Today 2014, 19, 781–786. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and Biodegradable Nanocomposites and Hybrid Biomaterials for Bone Regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef]
- Martin, C.; Winet, H.; Bao, J.Y. Acidity near eroding polylactide-polyglycolide in vitro and in vivo in rabbit tibial bone chambers. Biomaterials 1996, 17, 2373–2380. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Dziadek, M.; Menaszek, E.; Zagrajczuk, B.; Pawlik, J.; Cholewa-Kowalska, K. New generation poly(ε-caprolactone)/gel-derived bioactive glass composites for bone tissue engineering: Part I. Material properties. Mater. Sci. Eng. C 2015, 56, 9–21. [Google Scholar] [CrossRef]
- Lu, H.H.; El-Amin, S.F.; Scott, K.D.; Laurencin, C.T. Three-dimensional, bioactive, biodegradable, polymer–bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. Part A 2003, 64, 465–474. [Google Scholar] [CrossRef]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Porous poly(α-hydroxyacid)/Bioglass® composite scaffolds for bone tissue engineering. I: Preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, T.; Niiranen, H.; Kellomäki, M.; Törmälä, P. Self-reinforced composites of bioabsorbable polymer and bioactive glass with different bioactive glass contents. Part I: Initial mechanical properties and bioactivity. Acta Biomater. 2005, 1, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Maquet, V.; Jérôme, R.; Boccaccini, A.R.; Nazhat, S.N. Mechanical properties of highly porous PDLLA/Bioglass® composite foams as scaffolds for bone tissue engineering. Acta Biomater. 2005, 1, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Boccaccini, A. Poly(D,L-lactic acid) coated 45S5 Bioglass®-based scaffolds: Processing and characterization. J. Biomed. Mater. Res. Part A 2006, 77, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Bretcanu, O.; Chen, Q.; Misra, S.K.; Boccaccini, A.R.; Roy, I.; Verne, E.; Brovarone, C.V. Biodegradable polymer coated 45S5 Bioglassderived glass-ceramic scaffolds for bone tissue engineering. Eur. J. Glass Sci. Technol. Part A 2007, 48, 227–234. [Google Scholar]
- Kang, Z.; Zhang, X.; Chen, Y.; Akram, M.Y.; Nie, J.; Zhu, X. Preparation of polymer/calcium phosphate porous composite as bone tissue scaffolds. Mater. Sci. Eng. C 2017, 70, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhong, S.; Ma, B.; Shuler, F.D.; Lim, C.T. Controlled biomineralization of electrospun poly(ε-caprolactone) fibers to enhance their mechanical properties. Acta Biomater. 2013, 9, 5698–5707. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, X.; Xie, C.; Zhuang, H.; Zhou, S.; Weng, J. Hydroxyapatite nucleation and growth mechanism on electrospun fibers functionalized with different chemical groups and their combinations. Biomaterials 2010, 31, 4620–4629. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yeh, Y.; Lipner, J.; Xie, J.; Sung, H.; Thomopoulos, S.; Xia, Y. Enhancing the Stiffness of Electrospun Nanofiber Scaffolds with a Controlled Surface Coating and Mineralization. Langmuir 2011, 27, 9088–9093. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; Mooney, D.J. Bioinspired Growth of Crystalline Carbonate Apatite on Biodegradable Polymer Substrata. J. Am. Chem. Soc. 2002, 124, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, J.; Lipner, J.; Yuan, X.; Thomopoulos, S.; Xia, Y. Nanofiber Scaffolds with Gradations in Mineral Content for Mimicking the Tendon-to-Bone Insertion Site. Nano Lett. 2009, 9, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, J. pH-compensation effect of bioactive inorganic fillers on the degradation of PLGA. Compos. Sci. Technol. 2005, 65, 2226–2232. [Google Scholar] [CrossRef]
- Naseri, S.; Boccaccini, A.R.; Nazhat, S.N. Chapter 10: Bioactive Glass Particulate-incorporated Polymer Composites. In Bioactive Glasses: Fundamentals, Technology and Applications; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 236–256. [Google Scholar]
- Blaker, J.J.; Bismarck, A.; Boccaccini, A.R.; Young, A.M.; Nazhat, S.N. Premature degradation of poly(α-hydroxyesters) during thermal processing of Bioglass®-containing composites. Acta Biomater. 2010, 6, 756–762. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; Mohn, D.; Stark, W.J.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 2011, 32, 8915–8926. [Google Scholar] [CrossRef]
- Dou, Y.; Wu, C.; Chang, J. Preparation, mechanical property and cytocompatibility of poly(l-lactic acid)/calcium silicate nanocomposites with controllable distribution of calcium silicate nanowires. Acta Biomater. 2012, 8, 4139–4150. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Hong, Z.; Liu, A.; Chen, L.; Chen, X.; Jing, X. Preparation of bioactive glass ceramic nanoparticles by combination of sol–gel and coprecipitation method. J. Non-Cryst. Solids 2009, 355, 368–372. [Google Scholar] [CrossRef]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of scaffolds of poly(l-lactic acid) containing bioactive glass ceramic nanoparticles. Acta Biomater. 2008, 4, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of novel bioactive glass ceramic nanoparticles. J. Biomed. Mater. Res. Part A 2009, 88, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-E.; Knowles, J.C.; Kim, H.-W.; Kim, H. Production and Potential of Bioactive Glass Nanofibers as a Next-Generation Biomaterial. Adv. Funct. Mater. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lee, H.-H.; Chun, G.-S. Bioactivity and osteoblast responses of novel biomedical nanocomposites of bioactive glass nanofiber filled poly(lactic acid). J. Biomed. Mater. Res. Part A 2008, 85, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Song, J.-H.; Kim, H.-E. Bioactive glass nanofiber–collagen nanocomposite as a novel bone regeneration matrix. J. Biomed. Mater. Res. Part A 2006, 79, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Yu, H.-S.; Jang, J.-H.; Kim, H.-W. Bioactivity improvement of poly(ε-caprolactone) membrane with the addition of nanofibrous bioactive glass. Acta Biomater. 2008, 4, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Lee, E.; Shin, D.; Kim, H.; Kim, H.; Koh, Y.; Jang, J. In vitro/in vivo biocompatibility and mechanical properties of bioactive glass nanofiber and poly(ε-caprolactone) composite materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 213–220. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Sheikh, F.A.; Lim, J.K. Air jet spinning of hydroxyapatite/poly(lactic acid) hybrid nanocomposite membrane mats for bone tissue engineering. Colloids Surf. B Biointerfaces 2013, 102, 635–643. [Google Scholar] [CrossRef]
- Mi, R.; Liu, Y.; Chen, X.; Shao, Z. Structure and properties of various hybrids fabricated by silk nanofibrils and nanohydroxyapatite. Nanoscale 2016, 8, 20096–20102. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.; Sousa, S.R.; van Blitterswijk, C.A.; Moroni, L.; Monteiro, F.J. A biocomposite of collagen nanofibers and nanohydroxyapatite for bone regeneration. Biofabrication 2014, 6, 035015. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.A.; Yue, S.; Hanna, J.V.; Lee, P.D.; Newport, R.J.; Smith, M.E.; Jones, J.R. Characterizing the hierarchical structures of bioactive sol–gel silicate glass and hybrid scaffolds for bone regeneration. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1422–1443. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.M. Hybrid Nanocomposite Materials?between inorganic glasses and organic polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Grosso, D.; Ribot, F.; Boissiere, C.; Sanchez, C. Molecular and supramolecular dynamics of hybrid organic–inorganic interfaces for the rational construction of advanced hybrid nanomaterials. Chem. Soc. Rev. 2011, 40, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. New trends in bioactive scaffolds: The importance of nanostructure. J. Eur. Ceram. Soc. 2009, 29, 1275–1281. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Martín, A.I.; Salinas, A.J.; Vallet-Regí, M. Bioactive and degradable organic–inorganic hybrids. J. Eur. Ceram. Soc. 2005, 25, 3533–3538. [Google Scholar] [CrossRef]
- Pereira, M.M.; Jones, J.R.; Hench, L.L. Bioactive glass and hybrid scaffolds prepared by sol–gel method for bone tissue engineering. Adv. Appl. Ceram. 2005, 104, 35–42. [Google Scholar] [CrossRef]
- Pereira, M.M.; Jones, J.R.; Orefice, R.L.; Hench, L.L. Preparation of bioactive glass-polyvinyl alcohol hybrid foams by the sol-gel method. J. Mater. Sci. Mater. Med. 2005, 16, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Shin, K.-H.; Noh, D.-Y.; Jo, I.-H.; Koh, Y.-H.; Choi, W.-Y.; Kim, H.-E. Nanofibrous gelatin–silica hybrid scaffolds mimicking the native extracellular matrix (ECM) using thermally induced phase separation. J. Mater. Chem. 2012, 22, 14133. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Zhan, H.; Yan, F. Synthesis of bioactive poly(ethylene glycol)/SiO2-CaO-P2O5 hybrids for bone regeneration. Mater. Sci. Eng. C 2012, 32, 707–711. [Google Scholar] [CrossRef]
- Allo, B.A.; Rizkalla, A.S.; Mequanint, K. Hydroxyapatite Formation on Sol–Gel Derived Poly(ε-Caprolactone)/Bioactive Glass Hybrid Biomaterials. ACS Appl. Mater. Interfaces 2012, 4, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Lin, S.; Mequanint, K.; Rizkalla, A.S. Role of Bioactive 3D Hybrid Fibrous Scaffolds on Mechanical Behavior and Spatiotemporal Osteoblast Gene Expression. ACS Appl. Mater. Interfaces 2013, 5, 7574–7583. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.; Lee, E.; Jo, J.; Jeon, J.; Kim, M.; Kim, H.; Koh, Y. Fibrous membrane of nano-hybrid poly-L-lactic acid/silica xerogel for guided bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Teng, S.; Jang, T.; Wang, P.; Yook, S.; Kim, H.; Koh, Y. Nanostructured poly(ε-caprolactone)–silica xerogel fibrous membrane for guided bone regeneration. Acta Biomater. 2010, 6, 3557–3565. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Müller, T.; Schubert, D.W.; Boccaccini, A.R.; Yao, Q.; Roether, J.A. Electrospun Polyhydroxybutyrate/Poly(ε-caprolactone)/58S Sol–Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17098–17108. [Google Scholar] [CrossRef]
- Mondal, D.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Mechanically-competent and cytocompatible polycaprolactone-borophosphosilicate hybrid biomaterials. J. Mech. Behav. Biomed. Mater. 2017, 75, 180–189. [Google Scholar] [CrossRef]
- Connell, L.S.; Romer, F.; Suarez, M.; Valliant, E.M.; Zhang, Z.; Lee, P.; Smith, M.E.; Hanna, J.V.; Jones, J. Chemical characterisation and fabrication of chitosan–silica hybrid scaffolds with 3-glycidoxypropyl trimethoxysilane. J. Mater. Chem. B 2014, 2, 668–680. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Su, Y.-H.; Lai, J.-Y. In situ crosslinking of chitosan and formation of chitosan–silica hybrid membranes with using γ-glycidoxypropyltrimethoxysilane as a crosslinking agent. Polymer 2004, 45, 6831–6837. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Costa, M.A.; Fernandes, M.H. Physical, chemical and in vitro biological profile of chitosan hybrid membrane as a function of organosiloxane concentration. Acta Biomater. 2009, 5, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Romer, F.; McPhail, D.S.; Hanna, J.V.; Wang, D.; Connell, L.; Walter, C.; Saiz, E.; Yue, S.; Lee, P.D.; Jones, J.R. Highly flexible silica/chitosan hybrid scaffolds with oriented pores for tissue regeneration. J. Mater. Chem. B 2015, 3, 7560–7576. [Google Scholar]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Fernandes, M.H. In vitro cytocompatibility of MG63 cells on chitosan-organosiloxane hybrid membranes. Biomaterials 2005, 26, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Shirosaki, Y.; Okayama, T.; Tsuru, K.; Hayakawa, S.; Osaka, A. Synthesis and cytocompatibility of porous chitosan–silicate hybrids for tissue engineering scaffold application. Chem. Eng. J. 2008, 137, 122–128. [Google Scholar] [CrossRef]
- Toskas, G.; Cherif, C.; Hund, R.; Laourine, E.; Mahltig, B.; Fahmi, A.; Heinemann, C.; Hanke, T. Chitosan(PEO)/silica hybrid nanofibers as a potential biomaterial for bone regeneration. Carbohydr. Polym. 2013, 94, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.P.; Gangadharan, D.; Mohan, N.; Sumathi, B.; Nair, P.D. Hybrid scaffold bearing polymer-siloxane Schiff base linkage for bone tissue engineering. Mater. Sci. Eng. C 2015, 52, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.-H.; Choi, J.-Y.; Kim, H.-M. Preparation of a bioactive and degradable poly(ε-caprolactone)/silica hybrid through a sol–gel method. Biomaterials 2002, 23, 4915–4921. [Google Scholar] [CrossRef]
- Tian, D.; Dubois, P.; Grandfils, C.; Jerome, R.; Viville, P.; Lazzaroni, R.; Bredas, J.-L.; Leprince, P. A Novel Biodegradable and Biocompatible Ceramer Prepared by the Sol−Gel Process. Chem. Mater. 1997, 9, 871–874. [Google Scholar] [CrossRef]
- Rhee, S.-H. Bone-like apatite-forming ability and mechanical properties of poly(ε-caprolactone)/silica hybrid as a function of poly(ε-caprolactone) content. Biomaterials 2004, 25, 1167–1175. [Google Scholar] [CrossRef]
- Rhee, S.-H.; Lee, Y.-K.; Lim, B.-S.; Yoo, J.J.; Kim, H.J. Evaluation of a Novel Poly(ε-caprolactone)−Organosiloxane Hybrid Material for the Potential Application as a Bioactive and Degradable Bone Substitute. Biomacromolecules 2004, 5, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.-H.; Lee, S.J. Effect of acidic degradation products of poly(lactic-co-glycolic)acid on the apatite-forming ability of poly(lactic-co-glycolic)acid-siloxane nanohybrid material. J. Biomed. Mater. Res. Part A 2007, 83, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Kascholke, C.; Hendrikx, S.; Flath, T.; Kuzmenka, D.; Dorfler, H.; Schumann, D.; Gressenbuch, M.; Schulze, F.P.; Schulz-Siegmund, M.; Hacker, M.C. Biodegradable and adjustable sol-gel glass based hybrid scaffolds from multi-armed oligomeric building blocks. Acta Biomater. 2017, 63, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, S.; Kascholke, C.; Flath, T.; Schumann, D.; Gressenbuch, M.; Schulze, F.P.; Hacker, M.C.; Schulz-Siegmund, M. Indirect rapid prototyping of sol-gel hybrid glass scaffolds for bone regeneration—Effects of organic crosslinker valence, content and molecular weight on mechanical properties. Acta Biomater. 2016, 35, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Mahony, O.; Yue, S.; Turdean-Ionescu, C.; Hanna, J.V.; Smith, M.E.; Lee, P.D.; Jones, J.R. Silica–gelatin hybrids for tissue regeneration: Inter-relationships between the process variables. J. Sol-Gel Sci. Technol. 2014, 69, 288–298. [Google Scholar] [CrossRef]
- Sang, T.; Li, S.; Ting, H.-K.; Stevens, M.M.; Becer, C.R.; Jones, J.R. Hybrids of Silica/Poly(caprolactone coglycidoxypropyl trimethoxysilane) as Biomaterials. Chem. Mater. 2018, 30, 3743–3751. [Google Scholar] [CrossRef]
- Wang, D.; Nakamura, J.; Poologasundarampillai, G.; Kasuga, T.; Jones, J.R.; McPhail, D.S. ToF-SIMS evaluation of calcium-containing silica/γ-PGA hybrid systems for bone regeneration. Appl. Surf. Sci. 2014, 309, 231–239. [Google Scholar] [CrossRef]
- Mahony, O.; Tsigkou, O.; Ionescu, C.; Minelli, C.; Ling, L.; Hanly, R.; Smith, M.E.; Stevens, M.M.; Jones, J.R. Silica-Gelatin Hybrids with Tailorable Degradation and Mechanical Properties for Tissue Regeneration. Adv. Funct. Mater. 2010, 20, 3835–3845. [Google Scholar] [CrossRef]
- Lei, B.; Wang, L.; Chen, X.; Chae, S.-K. Biomimetic and molecular level-based silicate bioactive glass–gelatin hybrid implants for loading-bearing bone fixation and repair. J. Mater. Chem. B 2013, 1, 5153. [Google Scholar] [CrossRef]
- Gao, C.; Gao, Q.; Li, Y.; Rahaman, M.N.; Teramoto, A.; Abe, K. In vitro evaluation of electrospun gelatin-bioactive glass hybrid scaffolds for bone regeneration. J. Appl. Polym. Sci. 2013, 127, 2588–2599. [Google Scholar] [CrossRef]
- Lin, S.; Ionescu, C.; Pike, K.J.; Smith, M.E.; Jones, J.R. Nanostructure evolution and calcium distribution in sol–gel derived bioactive glass. J. Mater. Chem. 2009, 19, 1276–1282. [Google Scholar] [CrossRef]
- Dieudonné, X.; Fayon, F.; Jallot, E.; Lao, J.; Montouillout, V. Bioactive glass–gelatin hybrids: Building scaffolds with enhanced calcium incorporation and controlled porosity for bone regeneration. J. Mater. Chem. B 2016, 4, 2486–2497. [Google Scholar]
- Greenhalgh, R.D.; Ambler, W.S.; Quinn, S.J.; Medeiros, E.S.; Anderson, M.; Gore, B.; Menner, A.; Bismarck, A.; Li, X.; Tirelli, N. Hybrid sol–gel inorganic/gelatin porous fibres via solution blow spinning. J. Mater. Sci. 2017, 52, 9066–9081. [Google Scholar] [CrossRef]
- Gao, C.; Rahaman, M.N.; Gao, Q.; Teramoto, A.; Abe, K. Robotic deposition and in vitro characterization of 3D gelatin−bioactive glass hybrid scaffolds for biomedical applications. J. Biomed. Mater. Res. Part A 2013, 101, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Poologasundarampillai, G.; Ionescu, C.; Tsigkou, O.; Murugesan, M.; Hill, R.G.; Stevens, M.M.; Hanna, J.V.; Smith, M.E.; Jones, J.R. Synthesis of bioactive class II poly(γ-glutamic acid)/silica hybrids for bone regeneration. J. Mater. Chem. 2010, 20, 8952–8961. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Tsigkou, O.; Valliant, E.; Lee, P.D.; Yu, B.; Yue, S.; Hamilton, R.W.; Stevens, M.M.; Kasuga, T.; Jones, J. Bioactive silica–poly(γ-glutamic acid) hybrids for bone regeneration: Effect of covalent coupling on dissolution and mechanical properties and fabrication of porous scaffolds. Soft Matter 2012, 8, 4822. [Google Scholar] [CrossRef]
- Gao, C.; Ito, S.; Obata, A.; Mizuno, T.; Jones, J.R.; Kasuga, T. Fabrication and in vitro characterization of electrospun poly (γ-glutamic acid)-silica hybrid scaffolds for bone regeneration. Polymer 2016, 91, 106–117. [Google Scholar] [CrossRef]
- Obata, A.; Ito, S.; Iwanaga, N.; Mizuno, T.; Jones, J.R.; Kasuga, T. Poly(γ-glutamic acid)–silica hybrids with fibrous structure: Effect of cation and silica concentration on molecular structure, degradation rate and tensile properties. RSC Adv. 2014, 4, 52491–52499. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Yu, B.; Tsigkou, O.; Wang, D.; Romer, F.; Bhakhri, V.; Giuliani, F.; Stevens, M.M.; McPhail, D.S.; Smith, M.E. Poly(γ-glutamic acid)/Silica Hybrids with Calcium Incorporated in the Silica Network by Use of a Calcium Alkoxide Precursor. Chemistry 2014, 20, 8149–8160. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wu, D.; Li, A.; Shen, H.; Wang, C.; Martin, R.A.; Qiu, D. Bioactive organic/inorganic hybrids with improved mechanical performance. J. Mater. Chem. B 2015, 3, 1379–1390. [Google Scholar]
- Russo, L.; Gabrielli, L.; Valliant, E.M.; Nicotra, F.; Jimenez-Barbero, J.; Cipolla, L.; Jones, J.R. Novel silica/bis(3-aminopropyl) polyethylene glycol inorganic/organic hybrids by sol–gel chemistry. Mater. Chem. Phys. 2013, 140, 168–175. [Google Scholar] [CrossRef]
- Gabrielli, L.; Russo, L.; Poveda, A.; Jones, J.R.; Nicotra, F.; Jimenez-Barbero, J.; Cipolla, L. Epoxide Opening versus Silica Condensation during Sol-Gel Hybrid Biomaterial Synthesis. Chemistry 2013, 19, 7856–7864. [Google Scholar] [CrossRef] [PubMed]
- Vueva, Y.; Connell, L.S.; Chayanun, S.; Wang, D.; McPhail, D.S.; Romer, F.; Hanna, J.V.; Jones, J.R. Silica/alginate hybrid biomaterials and assessment of their covalent coupling. Appl. Mater. Today 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Kickelbick, G. The search of a homogeneously dispersed material—The art of handling the organic polymer/metal oxide interface. J. Sol-Gel Sci. Technol. 2008, 46, 281–290. [Google Scholar] [CrossRef]
- Yabuta, T.; Bescher, E.P.; Mackenzie, J.D.; Tsuru, K.; Hayakawa, S.; Osaka, A. Synthesis of PDMS-Based Porous Materials for Biomedical Applications. J. Sol-Gel Sci. Technol. 2003, 26, 1219–1222. [Google Scholar] [CrossRef]
- Chen, Q.; Kamitakahara, M.; Miyata, N.; Kokubo, T.; Nakamura, T. Preparation of Bioactive PDMS-Modified CaO–SiO2–TiO2 Hybrids by the Sol-Gel Method. J. Sol-Gel Sci. Technol. 2000, 19, 101–105. [Google Scholar] [CrossRef]
- Salinas, A.J.; Merino, J.M.; Babonneau, F.; Gil, F.J.; Vallet-Regí, M.; Vallet-Regí, M.; Salinas, A.; Vallet-Regi, M. Microstructure and macroscopic properties of bioactive CaO–SiO2–PDMS hybrids. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and mechanical properties of PDMS-modified CaO-SiO2-TiO2 hybrids prepared by sol-gel process. J. Biomed. Mater. Res. 2000, 51, 605–611. [Google Scholar] [CrossRef]
- Chen, Q.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and Mechanical Properties of Poly(dimethylsiloxane)-Modified Calcia-Silica Hybrids with Added Titania. J. Am. Ceram. Soc. 2003, 86, 806–810. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, D.; Tang, L.; Hutchins, M.K. Synthesis, characterization, and properties of new polystyrene-SiO2 hybrid sol-gel materials. J. Mater. Res. 1993, 8, 1143–1152. [Google Scholar] [CrossRef]
- Costa, R.O.R.; Vasconcelos, W.L. Structural modification of poly(2-hydroxyethyl methacrylate)–silica hybrids utilizing 3-methacryloxypropyltrimethoxysilane. J. Non-Cryst. Solids 2002, 304, 84–91. [Google Scholar] [CrossRef]
- John, Ł.; Bałtrukiewicz, M.; Sobota, P.; Brykner, R.; Cwynar-Zając, Ł.; Dzięgiel, P. Non-cytotoxic organic–inorganic hybrid bioscaffolds: An efficient bedding for rapid growth of bone-like apatite and cell proliferation. Mater. Sci. Eng. C 2012, 32, 1849–1858. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Miyazaki, T.; Tanihara, M. Development of bioactive organic–inorganic hybrid for bone substitutes. Mater. Sci. Eng. C 2002, 22, 27–34. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ohtsuki, C.; Tanihara, M. Synthesis of Bioactive Organic-Inorganic Nanohybrid for Bone Repair through Sol-Gel Processing. J. Nanosci. Nanotechnol. 2003, 3, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Uchino, T.; Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T.; Hayakawa, S.; Osaka, A. Synthesis of bioactive HEMA-MPS-CaCl2 hybrid gels: Effects of catalysts in the sol-gel processing on mechanical properties and in vitro hydroxyapatite formation in a simulated body fluid. J. Biomater. Appl. 2009, 23, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.G.; Lin, F.J. Organic–inorganic composite materials from acrylonitrile–butadiene–styrene copolymers (ABS) and silica through an in situ sol-gel process. J. Appl. Polym. Sci. 2000, 75, 275–283. [Google Scholar] [CrossRef]
- Chung, J.J.; Li, S.; Stevens, M.M.; Georgiou, T.K.; Jones, J.R. Tailoring Mechanical Properties of Sol–Gel Hybrids for Bone Regeneration through Polymer Structure. Chem. Mater. 2016, 28, 6127–6135. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, D.; Brennan, D.J.; Rivera, D.N.; Zhuang, Q.; DiNardo, N.J.; Qiu, K. Atomic Force Microscopy Study of Organic−Inorganic Hybrid Materials. Chem. Mater. 1998, 10, 769–772. [Google Scholar] [CrossRef]
- Ravarian, R.; Zhong, X.; Barbeck, M.; Ghanaati, S.; Kirkpatrick, C.J.; Murphy, C.M.; Schindeler, A.; Chrzanowski, W.; Dehghani, F. Nanoscale chemical interaction enhances the physical properties of bioglass composites. ACS Nano 2013, 7, 8469–8483. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Rhee, S.-H. The mechanical properties and bioactivity of poly(methyl methacrylate)/SiO2-CaO nanocomposite. Biomaterials 2009, 30, 3444–3449. [Google Scholar] [CrossRef]
- Rhee, S.H.; Hwang, M.H.; Si, H.J.; Choi, J.Y. Biological activities of osteoblasts on poly(methyl methacrylate)/silica hybrid containing calcium salt. Biomaterials 2003, 24, 901–906. [Google Scholar] [CrossRef]
- Ravarian, R.; Wei, H.; Rawal, A.; Hook, J.; Chrzanowski, W.; Dehghani, F. Molecular interactions in coupled PMMA–bioglass hybrid networks. J. Mater. Chem. B 2013, 1, 1835–1845. [Google Scholar] [CrossRef]
- Chung, J.J.; Fujita, Y.; Li, S.; Stevens, M.M.; Kasuga, T.; Georgiou, T.K.; Jones, J.R. Biodegradable inorganic-organic hybrids of methacrylate star polymers for bone regeneration. Acta Biomater. 2017, 54, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Valliant, E.M.; Jones, J.R. Softening bioactive glass for bone regeneration: Sol–gel hybrid materials. Soft Matter 2011, 7, 5083–5095. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/Inorganic Hybrid Network Materials by the Sol−Gel Approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. New developments in the nonaqueous and/or non-hydrolytic sol–gel synthesis of inorganic nanoparticles. Electrochim. Acta 2010, 55, 7717–7725. [Google Scholar] [CrossRef]
- Hay, J.N.; Raval, H.M. Synthesis of Organic−Inorganic Hybrids via the Non-hydrolytic Sol−Gel Process. Chem. Mater. 2001, 13, 3396–3403. [Google Scholar] [CrossRef]
- Vioux, A. Nonhydrolytic Sol−Gel Routes to Oxides. Chem. Mater. 1997, 9, 2292–2299. [Google Scholar] [CrossRef]
- Karmakar, B.; De, G.; Kundu, D.; Ganguli, D. Silica microspheres from the system tetraethyl orthosilicate-acetic acid-water. J. Non-Cryst. Solids 1991, 135, 29–36. [Google Scholar] [CrossRef]
- Sharp, K.G. A two-component, non-aqueous route to silica gel. J. Sol-Gel Sci. Technol. 1994, 2, 35–41. [Google Scholar] [CrossRef]
- Skipper, L.J.; Sowrey, F.E.; Pickup, D.M.; Drake, K.O.; Smith, M.E.; Saravanapavan, P.; Hench, L.L.; Newport, R.J. The structure of a bioactive calcia–silica sol–gel glass. J. Mater. Chem. 2005, 15, 2369–2374. [Google Scholar] [CrossRef]
- Yu, B.; Turdean-Ionescu, C.A.; Martin, R.A.; Newport, R.J.; Hanna, J.V.; Smith, M.E.; Jones, J.R. Effect of Calcium Source on Structure and Properties of Sol–Gel Derived Bioactive Glasses. Langmuir 2012, 28, 17465–17476. [Google Scholar] [CrossRef] [PubMed]
- Poologasundarampillai, G.; Yu, B.; Jones, J.R.; Kasuga, T. Electrospun silica/PLLA hybrid materials for skeletal regeneration. Soft Matter 2011, 7, 10241. [Google Scholar] [CrossRef]
- Rámila, A.; Balas, F.; Vallet-Regí, M. Synthesis Routes for Bioactive Sol−Gel Glasses: Alkoxides versus Nitrates. Chem. Mater. 2002, 14, 542–548. [Google Scholar] [CrossRef]
- Dieudonné, X.; Montouillout, V.; Jallot, É.; Fayon, F.; Lao, J. Bioactive glass hybrids: A simple route towards the gelatin–SiO2–CaO system. Chem. Commun. 2014, 50, 8701. [Google Scholar] [CrossRef] [PubMed]
- Valliant, E.M.; Romer, F.; Wang, D.; McPhail, D.S.; Smith, M.E.; Hanna, J.V.; Jones, J.R. Bioactivity in silica/poly(γ-glutamic acid) sol–gel hybrids through calcium chelation. Acta Biomater. 2013, 9, 7662–7671. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Lin, S.; Rizkalla, A.S.; Mequanint, K. Porous and biodegradable polycaprolactone-borophosphosilicate hybrid scaffolds for osteoblast infiltration and stem cell differentiation. J. Mech. Behav. Biomed. Mater. 2019, 92, 162–171. [Google Scholar] [CrossRef]
- Lepry, W.C.; Nazhat, S.N. Highly Bioactive Sol-Gel-Derived Borate Glasses. Chem. Mater. 2015, 27, 4821–4831. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. CHAPTER 2—Hydrolysis and Condensation I: Nonsilicates. In Sol-Gel Science; San Diego Academic Press: San Diego, CA, USA, 1990; pp. 20–95. [Google Scholar]
- Isaac, J.; Nohra, J.; Lao, J.; Jallot, E.; Nedelec, J.-M.; Berdal, A.; Sautier, J.-M. Effects of strontium-doped bioactive glass on the differentiation of cultured osteogenic cells. Eur. Cell Mater. 2011, 21, 130–143. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.-H.; Kim, H.-W. Osteoinductive Fibrous Scaffolds of Biopolymer/Mesoporous Bioactive Glass Nanocarriers with Excellent Bioactivity and Long-Term Delivery of Osteogenic Drug. ACS Appl. Mater. Interfaces 2015, 7, 1140–1152. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, H.-W. Mesoporous bioactive nanocarriers in electrospun biopolymer fibrous scaffolds designed for sequential drug delivery. RSC Adv. 2014, 4, 4444–4452. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Zhu, Y.; Zheng, R.; Appleyard, R.; Howard, A.; Zreiqat, H. The effect of mesoporous bioactive glass on the physiochemical, biological and drug-release properties of poly(dl-lactide-co-glycolide) films. Biomaterials 2009, 30, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, L.; He, Q.; Zhao, J.; Limin, G.; Shi, J. Mesoporous bioactive glass-coated poly( l -lactic acid) scaffolds: A sustained antibiotic drug release system for bone repairing. J. Mater. Chem. 2011, 21, 1064–1072. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Zhu, Y.; Friis, T.; Xiao, Y. Structure–property relationships of silk-modified mesoporous bioglass scaffolds. Biomaterials 2010, 31, 3429–3438. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Tandon, B.; Blaker, J.J.; Cartmell, S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018, 73, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Lanceros-Méndez, S.; Lanceros-Méndez, S. Polymer-Based Magnetoelectric Materials. Adv. Funct. Mater. 2013, 23, 3371–3385. [Google Scholar] [CrossRef]
- Guex, A.G.; Puetzer, J.L.; Armgarth, A.; Littmann, E.; Stavrinidou, E.; Giannelis, E.P.; Malliaras, G.G.; Stevens, M.M. Highly porous scaffolds of PEDOT: PSS for bone tissue engineering. Acta Biomater. 2017, 62, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Dai, J.; Wang, J.; Tan, Y.; Yang, X.; Yang, S.; Yuan, Q.; Zhang, Y. A 3D graphene coated bioglass scaffold for bone defect therapy based on the molecular targeting approach. J. Mater. Chem. B 2017, 5, 6794–6800. [Google Scholar] [CrossRef]
- Cheng, X.; Wan, Q.; Pei, X. Graphene Family Materials in Bone Tissue Regeneration: Perspectives and Challenges. Nanoscale Res. Lett. 2018, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Alegret, N.; Dominguez-Alfaro, A.; Mecerreyes, D. 3D Scaffolds Based on Conductive Polymers for Biomedical Applications. Biomacromolecules 2019, 20, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Fini, M.; Di Primio, R.; Mattioli-Belmonte, M. Biofabrication and bone tissue regeneration: Cell source, approaches, and challenges. Front. Bioeng. Biotechnol. 2017, 5. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.S.; Corradetti, B.; Chan, P.; Van Eps, J.L.; Janecek, T.; Rameshwar, P.; Weiner, B.K.; Tasciotti, E. Enhanced osteogenic potential of mesenchymal stem cells from cortical bone: A comparative analysis. Stem Cell Res. Ther. 2015, 6, 203. [Google Scholar]
- Corradetti, B.; Taraballi, F.; Powell, S.; Sung, D.; Minardi, S.; Ferrari, M.; Weiner, B.K.; Tasciotti, E. Osteoprogenitor cells from bone marrow and cortical bone: Understanding how the environment affects their fate. Stem Cells Dev. 2015, 24, 1112–1123. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Colnot, C. Cell sources for bone tissue engineering: Insights from basic science. Tissue Eng. Part B Rev. 2011, 17, 449–457. [Google Scholar] [CrossRef]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef]
- Russell, K.C.; Phinney, D.G.; Lacey, M.R.; Barrilleaux, B.L.; Meyertholen, K.E.; O’Connor, K.C. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 2010, 28, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J. The mesenchymal stromal cells dilemma—Does a negative phase iii trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 2013, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, M.; Grayson, W.L.; Wan, L.Q.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr. Stem Cell Res. Ther. 2008, 3, 254–264. [Google Scholar]
- El Tamer, M.; Reis, R. Progenitor and stem cells for bone and cartilage regeneration. J. Tissue Eng. Regener. Med. 2009, 3, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Szpalski, C.; Barbaro, M.; Sagebin, F.; Warren, S.M. Bone tissue engineering: Current strategies and techniques—Part ii: Cell types. Tissue Eng. Part B Rev. 2012, 18, 258–269. [Google Scholar] [CrossRef]
- Zanetti, A.S.; Sabliov, C.; Gimble, J.M.; Hayes, D.J. Human adipose-derived stem cells and three-dimensional scaffold constructs: A review of the biomaterials and models currently used for bone regeneration. J. Biomed. Mater. Res. B 2013, 101b, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Lafosse, A.; Dufeys, C.; Beauloye, C.; Horman, S.; Dufrane, D. Impact of hyperglycemia and low oxygen tension on adipose-derived stem cells compared with dermal fibroblasts and keratinocytes: Importance for wound healing in type 2 diabetes. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Ohnishi, H.; Oda, Y.; Aoki, T.; Tadokoro, M.; Katsube, Y.; Ohgushi, H.; Hattori, K.; Yuba, S. A comparative study of induced pluripotent stem cells generated from frozen, stocked bone marrow- and adipose tissue-derived mesenchymal stem cells. J. Tissue Eng. Regener. Med. 2012, 6, 261–271. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.Y.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Wissing, S.; Munoz-Lopez, M.; Macia, A.; Yang, Z.Y.; Montano, M.; Collins, W.; Garcia-Perez, J.L.; Moran, J.V.; Greene, W.C. Reprogramming somatic cells into ips cells activates line-1 retroelement mobility. Hum. Mol. Genet. 2012, 21, 208–218. [Google Scholar] [CrossRef]
- English, K.; Wood, K.J. Immunogenicity of embryonic stem cell-derived progenitors after transplantation. Curr. Opin. Organ Transplant. 2011, 16, 90–95. [Google Scholar] [CrossRef]
- Rana, D.; Kumar, S.; Webster, T.J.; Ramalingam, M. Impact of induced pluripotent stem cells in bone repair and regeneration. Curr. Osteoporos. Rep. 2019, 17, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kimura, T.; Swami, S.; Wu, J.Y. Pluripotent stem cells as a source of osteoblasts for bone tissue regeneration. Biomaterials 2019, 196, 31–45. [Google Scholar] [CrossRef]
- Wu, Q.Q.; Yang, B.; Hu, K.; Cao, C.; Man, Y.; Wang, P. Deriving osteogenic cells from induced pluripotent stem cells for bone tissue engineering. Tissue Eng. Part B Rev. 2017, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Sotome, S.; Torigoe, I.; Maehara, H.; Sugata, Y.; Yamada, T.; Shinomiya, K.; Okawa, A. Isolation of osteogenic progenitor cells from trabecular bone for bone tissue engineering. Tissue Eng. Part A 2009, 16, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Hsu, Y.; Cheng, Y.; Li, C.; Ruaan, R.; Chien, C.; Chung, C.; Tsao, C. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C 2014, 37, 28–36. [Google Scholar] [CrossRef]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics 2013, 34, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Thrivikraman, G.; Lee, P.S.; Hess, R.; Haenchen, V.; Basu, B.; Scharnweber, D. Interplay of Substrate Conductivity, Cellular Microenvironment, and Pulsatile Electrical Stimulation toward Osteogenesis of Human Mesenchymal Stem Cells in Vitro. ACS Appl. Mater. Interfaces 2015, 7, 23015–23028. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslankoohi, N.; Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers 2019, 11, 1437. https://doi.org/10.3390/polym11091437
Aslankoohi N, Mondal D, Rizkalla AS, Mequanint K. Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers. 2019; 11(9):1437. https://doi.org/10.3390/polym11091437
Chicago/Turabian StyleAslankoohi, Neda, Dibakar Mondal, Amin S. Rizkalla, and Kibret Mequanint. 2019. "Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment" Polymers 11, no. 9: 1437. https://doi.org/10.3390/polym11091437
APA StyleAslankoohi, N., Mondal, D., Rizkalla, A. S., & Mequanint, K. (2019). Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers, 11(9), 1437. https://doi.org/10.3390/polym11091437