3.1. Cyclic Moisture Sorption
For the description of moisture absorption curves of epoxies and epoxy-based composites, the classical Fick’s model is usually applied [
4,
5,
7,
14,
15,
16]. In the case of 3D mode, the moisture content
w(
t) in the specimen is expressed as
where
;
w0 and
w∞ are the initial and equilibrium moisture contents; and
a,
b, and
l are respectively the thickness, width, and length of the specimen.
D identifies the diffusion coefficient of the material.
Basically, in Equation (1), there are two independent parameters: the diffusion coefficient
D and the equilibrium moisture content
w∞. The equilibrium moisture content is the maximal achieved moisture content over the sorption test, while the time-varying moisture content (in %) is obtained by using the following expression:
where
m(
t) is the time-varying mass of the specimen at time
t, and
m0 is the mass of the specimen in the initial state (
t = 0). Then, the diffusion coefficient is calculated from the initial slope of the curve
w vs.
[
17]:
Usually, Fick’s model provides a satisfactory description of the experimental data for the moisture absorption of epoxies and epoxy-based composites at room temperature [
4,
7,
14,
15,
16]. However, as a matter of fact, at elevated temperature and humidity, non-Fickian diffusion starts to dominate since the polymer network may be significantly “disturbed”, and large adjustments to segmental conformations are required to reach equilibrium with long relaxation times [
4].
One of non-Fickian models which may improve the description of the experimental data at high relative humidity is the model with a time-variable diffusivity [
15,
16,
18]. According to this model, due to different physical processes (e.g., the plasticization, ageing, post-cure etc.), the diffusion coefficient decreases with time:
where
D0 is the diffusion coefficient at the initial instant of time, but
γ is the coefficient describing the rate of change for it. Then, according to this model the expression for determining the moisture content in the specimen for the three-dimensional case takes the form [
16,
18]
where
γ = 1/
τ, and
τ is the characteristic time of relaxation. Thus, the model contains three parameters—the diffusion coefficient at the initial instant of time
D0, the equilibrium moisture content
w∞, and the coefficient
γ describing the rate of change in the diffusion coefficient.
According to [
6,
7,
9,
19] 1D Fick’s model provided a satisfactory description of experimental data for moisture absorption by the epoxy filled with MWCNTs. In the current study the purpose was to evaluate if the Fick’s model correlates well with the experimental data also for the case of cyclic moisture absorption: moisture absorption, desorption, and resorption. It is worth noting that preliminary evaluation by using 1D, 2D, and 3D models (Equations (1) and (5)) has shown that the moisture sorption curves evaluated by three-dimensional models were the closest to the experimental data. Therefore, hereinafter they were used in the description of moisture kinetics for the epoxy filled with N and SA during moisture absorption, desorption, and resorption.
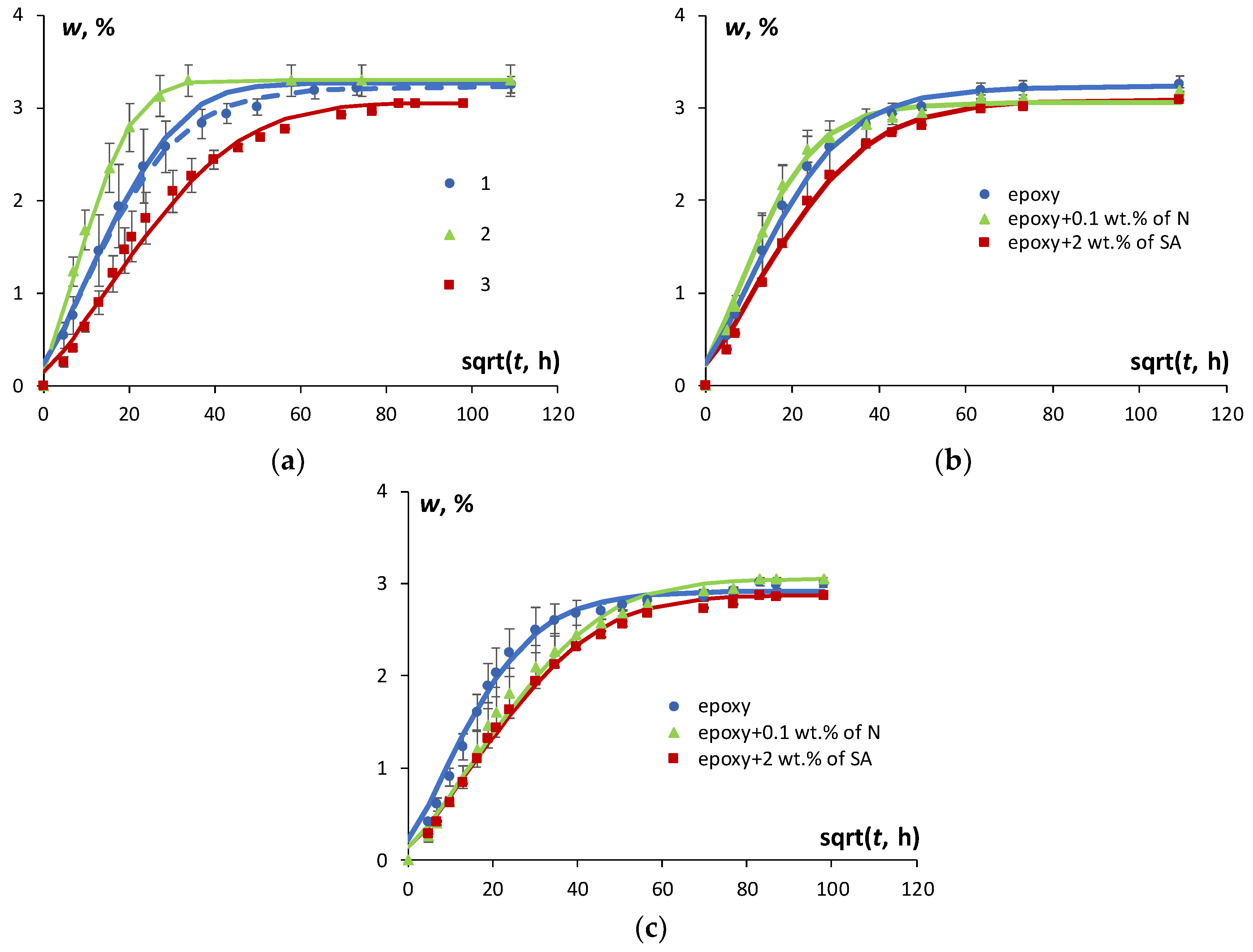
The moisture absorption, desorption, and resorption results for the epoxy resin in an atmosphere with 91% RH are presented in
Figure 2a. To improve the description of experimental data for moisture absorption both Fick’s model and the model with time-variable diffusion coefficient were applied. Obviously, the application of the model with time-variable diffusion coefficient provided better description of the experimental data than the Fick′s model and were used for further analysis of the sorption characteristics during the absorption. It is seen that all sorption processes were characterized by similar trends with a considerably higher sorption rate in the early stages and a progressively slower water uptake as the equilibrium plateau was approached. Similar results were obtained for the epoxy filled with both N and SA.
Figure 2b and c reveal the effects of N and SA nanotubes on moisture absorption and resorption kinetics. Obviously, as shown in
Figure 2a the sorption process in the epoxy (as well as in the NC) was not fully reversible. The rate of moisture diffusion was the highest for desorption, the lowest for resorption, and at intermediate level for the absorption process. Similar results were discussed in [
4] for desorption and resorption, which were shown to occur faster than absorption. Moreover, the difference among predictions computed by Fick′s model and the experimental data vanished comparing moisture absorption and resorption.
The fact that resorption occurred faster than absorption and desorption could be explained by the increase in the free volume of a polymer matrix that occurs due to the swelling effect [
4,
20]. Basically, the free volume in a polymer may be considered as a difference between the measured and occupied volumes of the polymer and its content in a polymer may change over a long period of time due to physical ageing, thus affecting the overall properties of the polymer. Nevertheless, almost the same or a slight increase in the equilibrium moisture content was observed for all sorption processes, due to the hygrothermal ageing of the epoxy [
4]. According to
Figure 2a, desorption was characterized by the highest rate of diffusion and the highest equilibrium moisture content in comparison with absorption and resorption, both for the epoxy and the NC with SA and N, which is in line with the swelling effect discussed previously. However, the moisture absorption–desorption cycle might induce irreversible structural changes and subsequently might change the amount of free volume available for water molecules to diffuse through the material. Therefore, the resorption process may occur differently than absorption with a different diffusion coefficient and equilibrium moisture content.
The effect of N and SA loading on the kinetics of moisture absorption and resorption of the epoxy is shown in
Figure 2b,c, respectively. Obviously, a slight reduction of the equilibrium moisture content both for absorption and resorption processes was obtained for the NCs. The sorption isotherms given in
Figure 3 reveal a slight reduction of equilibrium moisture content for the case of the NC compared to the epoxy. The maximal reduction of the equilibrium moisture content in comparison with the epoxy resin was observed in atmosphere with 91% RH: for the epoxy filled with 0.1 wt % of N it was −0.11%, while for the epoxy filled with 2 wt % of SA it was −0.21%, respectively.
The diffusion coefficient which was calculated by Equation (3) in all atmospheres was 7.3 ± 0.2 × 10
3 mm
2/h for the epoxy and 6.6 ± 0.2 × 10
3 mm
2/h for the epoxy filled with N and SA MWCNTs. This value was used as
D0, the diffusion coefficient at the initial instant of time, for the model with time-variable diffusion coefficient. The coefficient of proportionality
γ for the epoxy and all NCs during moisture absorption was almost the same: 0.0004 ± 0.0001 and 0.0003 ± 0.0001, accordingly. In general, due to the presence of three parameters, the model with a time-variable diffusivity was relatively flexible and provided better description of the sorption curves in comparison with Fick′s model. For sure, such a small reduction in the sorption characteristics for the NC can be explained by the high quality, mechanical properties, and resistance to moisture absorption of the epoxy, which is widely applied for aeronautical elements in liquid infusion processes. Similar results and observations were obtained and discussed for Araldite LY556 (Sigma-Aldrich, St. Louis, MO, USA) filled with different types of graphene nanoplatelets [
21].
Generally, the reduction in sorption characteristics of the polymers could be explained by assuming a reduction in the free volume within the polymer due to the addition of different types of nanoparticles, particularly exfoliated platelet-shape nanoparticles [
7,
22]. This might occur mostly due to enhanced physical ageing, differences in cure kinetics, and the segmental mobility of polymer chains upon the addition of the nanofillers.
It is possible to perform indirect evaluation of the changes in the free volume in polymers before and after the absorption–desorption cycle, by calculating the swelling strain, computed as relative change in the length of the test specimens, as follows:
where
l(
t) is the time-varying length of the wet specimen at time
t, and
l0 is the length of the specimen in the initial (dry) state (
t = 0).
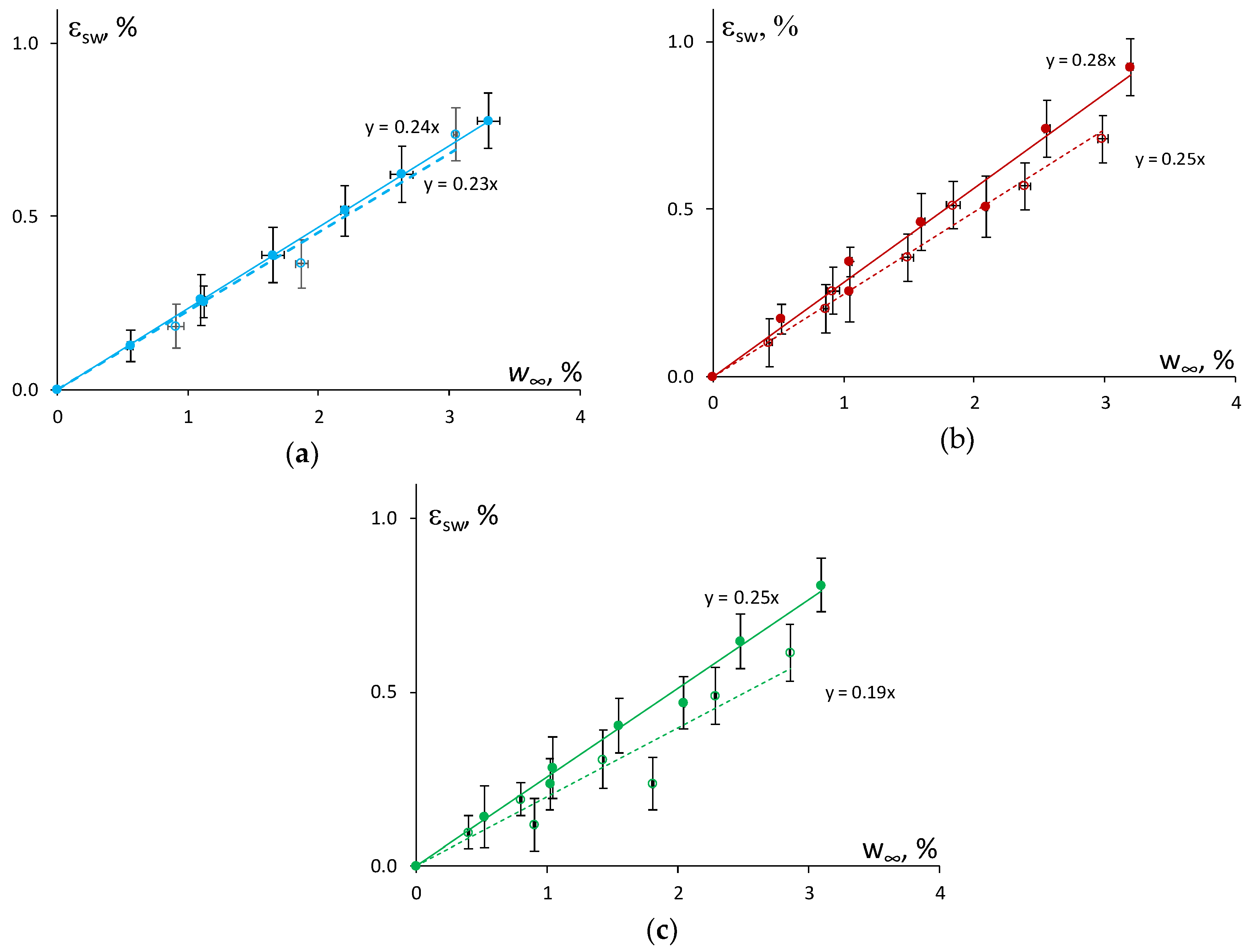
The swelling strain as a function of the moisture content in atmospheres with different relative humidity (47%, 73%, and 91%) is shown in
Figure 4 for the epoxy and the epoxy filled with 0.1 wt % of N and 2 wt % of SA. Obviously, by increasing the relative humidity of the atmosphere, the swelling strain increased as well. However, the relationship between the swelling strain and equilibrium moisture content for the absorption and resorption processes was slightly different for the epoxy and the NC.
It can be hypothesized that a higher reduction in the rate (slope) of swelling of the NC in comparison with the epoxy could be related to the increased contribution of “bound” water and a subsequent decrease in the free volume after a cycle of moisture absorption–desorption. Similar ideas were formulated, and results obtained in [
7] where the neat epoxy and its NC with the content of 0.3–1 wt % of MWCNTs were characterized by nearly the same swelling coefficient
which was equal to 0.24. Herein, the estimation of the swelling coefficient as shown in
Figure 3, gave different results and allowed the effect of the nanofiller content to be revealed. The epoxy resin had a β value of 0.24 ± 0.02 for absorption and 0.23 ± 0.02 for resorption. While the addition of 0.1 wt % of N resulted in the reduction of the swelling coefficient β from 0.28 ± 0.02 to 0.25 ± 0.05, and the addition of 2.0 wt % of SA resulted in a reduction in β from 0.25 ± 0.02 to 0.19 ± 0.02. Thus, the epoxy filled with 2.0 wt % of SA showed the greatest decrease of the swelling coefficient if compared with the epoxy and the epoxy filled with 0.1 wt % of N
Moreover, the diffusion coefficient and equilibrium moisture content in a polymer NC is a function, to a large extent, of the interplay between the free volume content and the tortuosity factor of the nanoparticles [
22]. The tortuosity of the nanoparticles is dependent on many factors, such as the morphology of the dispersed nanoparticles, their arrangement and orientation in the diffusion direction, and their volume fraction [
23]. The result of this interplay (increase or decrease of D) upon the addition of nanoparticles determines which of the two factors would become predominant.
To better understand the differences in diffusion phenomena in the epoxy, filled with different aspect ratio MWCNTs, Nielsen model could be applied considering parallel periodic arrangement of straight filler particles. According to this model the tortuosity (
τ) of any filler particles could be calculated by the following equation [
22,
24]:
where
is the aspect ratio (length
l vs. thickness
h) of the nanoparticles, and
v is the filler volume content which can be estimated by using equation [
25]:
where
c is the filler weight fraction, and
and
are the density of the filler and the polymer matrix, respectively. To compare current results of theoretical tortuosity of MWCNTs in the epoxy resin with the literature data, two additional nanocomposites were chosen: epoxy resin, respectively, filled with NC3152 (Nanocyl, Sambreville, Belgium) having aspect ratio of 100 [
6] and C150P (Bayer, Leverkusen, Germany) having aspect ratio of 77 [
7]. The density of the epoxies and the MWCNTs were taken from the material datasheets [
11,
12,
26,
27,
28,
29], and instead the filler content was kept the same as in [
6,
7].
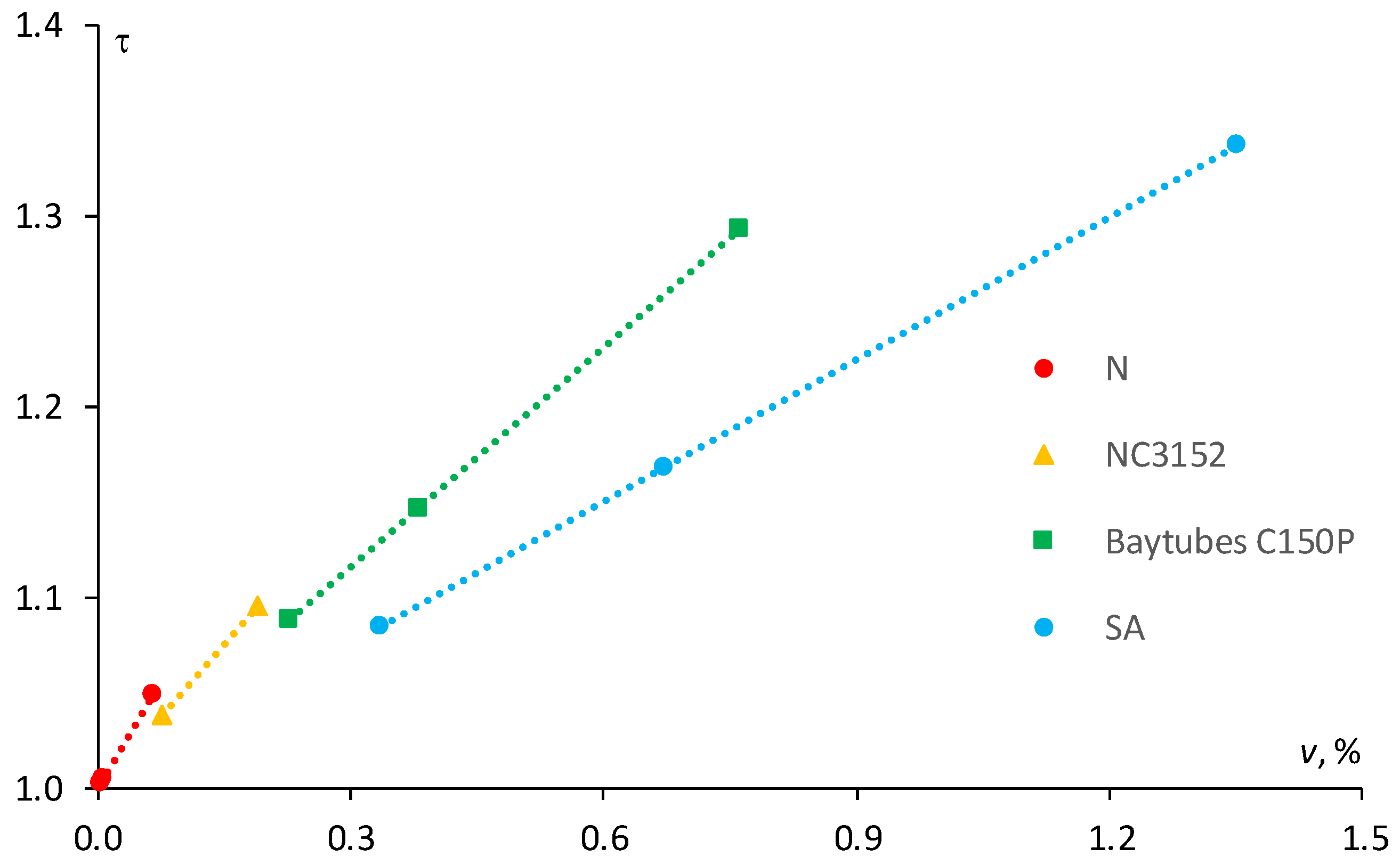
The results of the calculation of the tortuosity factor according to Equation (5) for the epoxies filled with MWCNTs having different aspect ratios are reported in
Figure 5. Obviously, N and NC3152, which have higher aspect ratio, are characterized by higher coefficient of proportionality compared to SA and C150P. Indeed, if it would be possible to increase the NA and NC3152 filler content, regardless of the viscosity increase for the final NC, the tortuosity factor would be much higher at the same filler content. However, due to high viscosity of the NC filled with the MWCNTs of high aspect ratio at a filler content of more than 0.1–0.25 wt %, this can be hardly achieved. As shown in
Figure 4 for the epoxy resin filled with N (α ≈ 158) at filler content ranging from 0.003 to 0.065 vol % (i.e., 0.005–0.1 wt %) the tortuosity factor resulted within the range 1.0–1.05, whereas for the same epoxy resin filled with SA (α = 50) at a filler content within the range 0.34–1.35 vol % (i.e., 0.5–2 wt %), it would be much higher—1.08–1.34.
Finally, the comparison of N and SA nanotube types shows that for SA filler with an aspect ratio three times smaller than N-type nanotube, at maximum content percentage (i.e., 2 wt %) is characterized by an approximately 27% higher tortuosity factor due to the increase in filler content by 21 times. Evidently, the effects on the sorption characteristics such as diffusion coefficient and equilibrium moisture content are the highest for the epoxy resin filled with 2.0 wt % of SA (maximum filler content).
3.2. Thermomechanical Properties
Dynamic mechanical analysis (DMA) is widely used to explore the viscoelastic properties of polymers that occur as a response to the application of oscillating forces and make conclusions about mobility of polymer chains and the deterioration of polymers’ thermomechanical properties due to hygrothermal ageing effects [
6,
7].
To evaluate the reversibility of the moisture absorption effect on the dynamic mechanical properties (storage modulus
E’ and loss factor tan
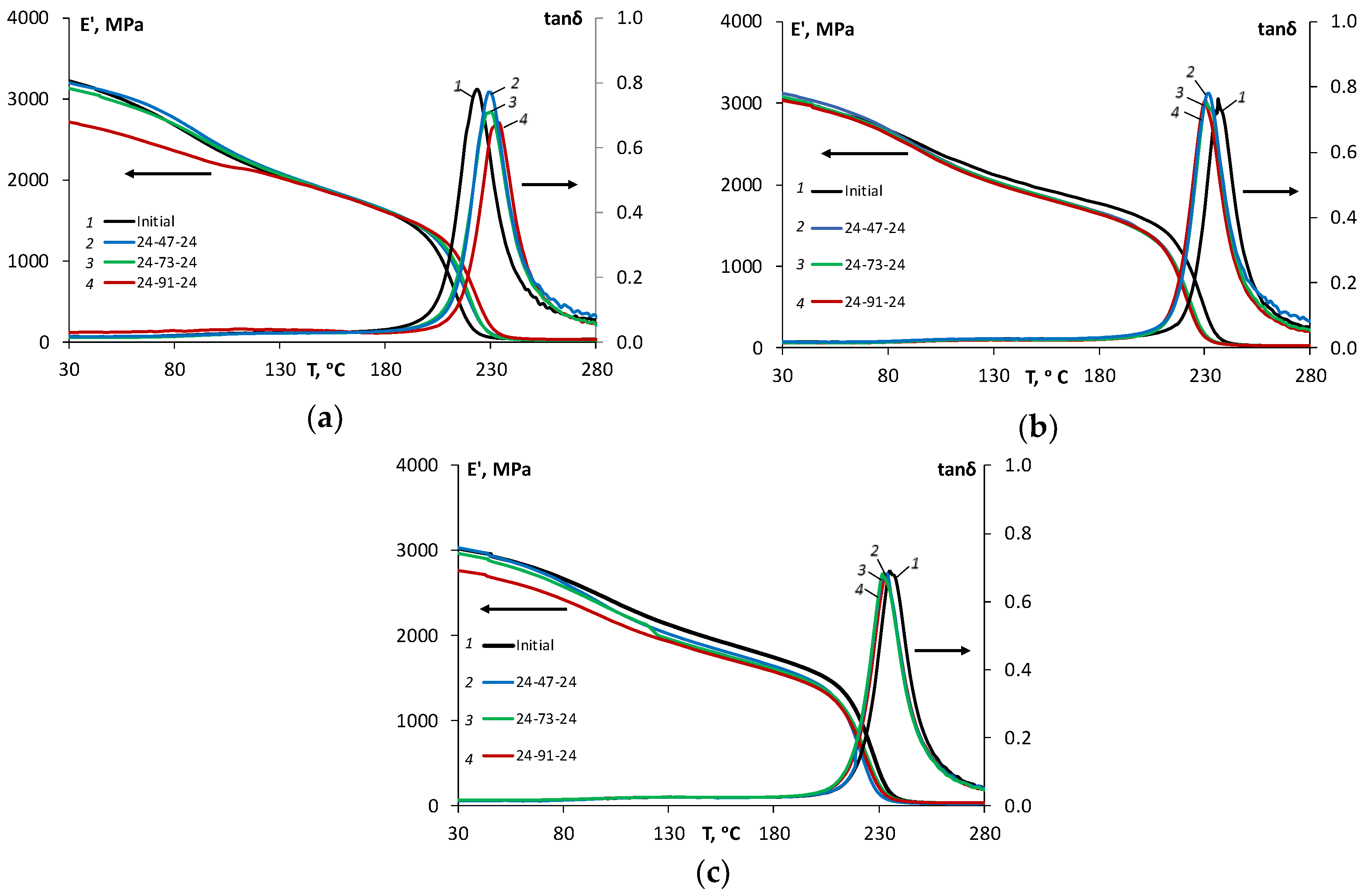
δ) of the epoxy and epoxy filled with N and SA, these characteristics were analyzed in the initial state and after the moisture absorption–desorption cycle in atmospheres with relative humidity levels of 47%, 73%, and 91%. The representative DMA curves for unaged and environmentally aged specimens after a cycle of moisture absorption–desorption in atmospheres with different relative humidity values are shown in
Figure 6, respectively, for the epoxy (a) and the epoxy filled with 0.1 wt % of N (b) and 2 wt % of SA (c), respectively.
It is worth to notice from
Figure 6, that the cycle of moisture absorption–desorption in different atmospheres caused irreversible changes to the thermomechanical properties of all materials, and these changes varied for the epoxy and the NCs. As shown in
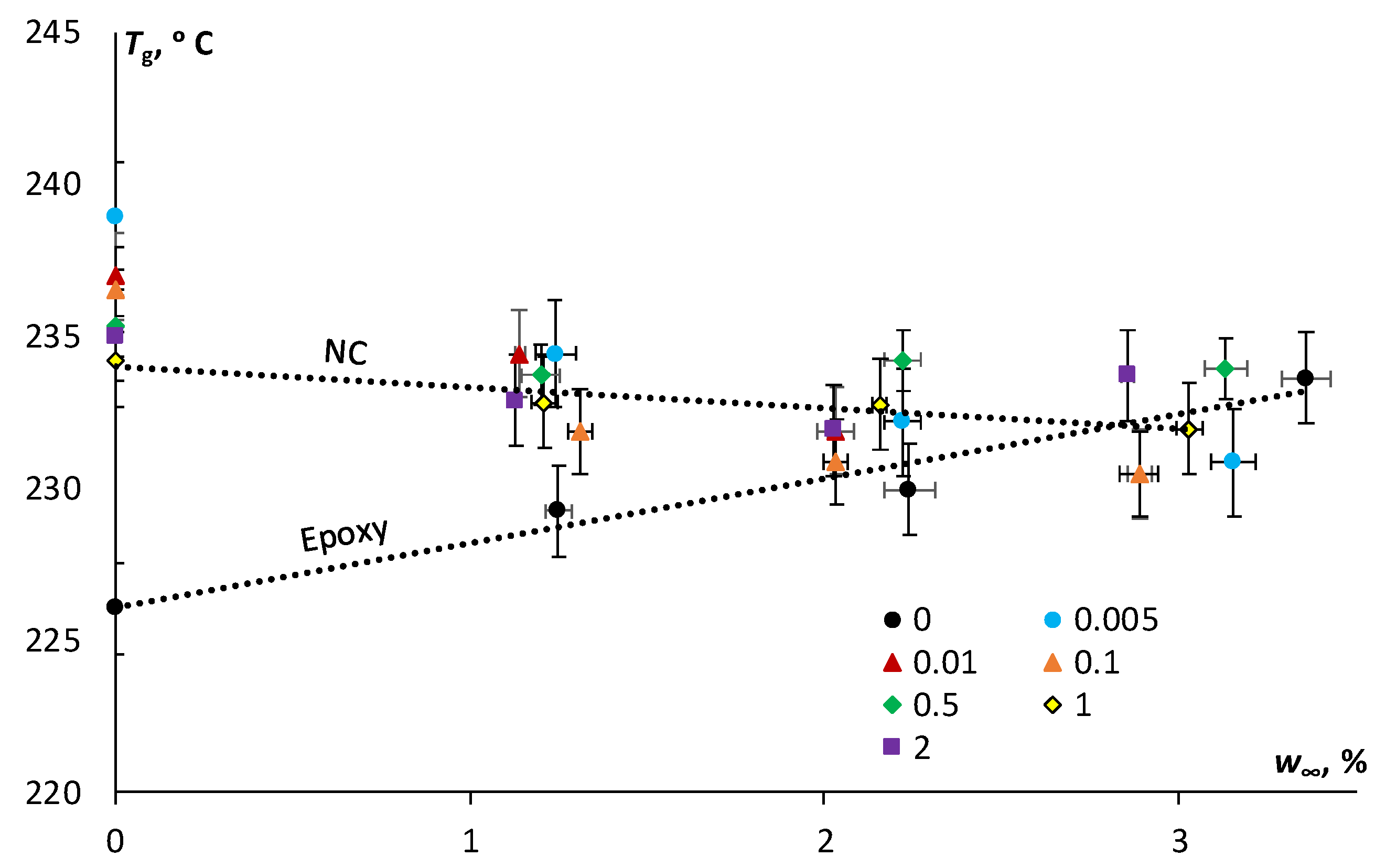
Figure 7, for the epoxy, the glass transition temperature increased from 226 °C (in the initial state) to 234 °C (after moisture cycle 24-91-24); for the epoxy filled with 0.1 wt % of N, it decreased from 236 °C (in the initial state) to 230 °C (after moisture cycle 24-91-24); and for the epoxy filled with 2 wt % of SA, it also decreased from 235 °C (in the initial state) to 233 °C (after moisture cycle 24-91-24).
Rather contrary results for DMA curves and
Tg were published in the literature. Almost total reversibility of the plastization was obtained by water after desorption, causing to return to the same glass transition temperature as that of epoxy polymer under the initial conditions. This was explained by the reformation of secondary bounds [
30,
31]. Though, in the case of non-complete curing of polymers, especially at high temperatures, post-curing phenomena can occur during water absorption, leading to no change or even an increase in
Tg, proving that the post-curing process dominates over plastization [
5,
6], especially for cold-cured polymers [
20,
32].
Herein, according to
Figure 7 the glass transition temperature of the epoxy increased with the higher desorbed moisture content after the absorption–desorption cycle likely due to possible post-cure and decreasing of the free volume, as previously discussed. Similar results of
Tg increasing after moisture absorption were attributed to the post-curing phenomenon during physical ageing for a partially cured system [
5]. The minimal effect of the addition of both N and SA on the glass transition temperature is fully in line with available literature and it can be attributed to several contrasting factors such as the existence of interphases/interfaces on the surface of the nanoparticles, agglomerates, changes in crystallinity, and the crosslinking density of the epoxy resin [
7,
33].
Moreover, it can be noticed that for the epoxy resin, the height of tanδ curves decreased after the moisture absorption–desorption cycle, which can be attributed to lower degree of mobility of polymer chains caused by microstructural reorganization during hygrothermal ageing and the subsequent decrease of the free volume in the epoxy resin. This result correlates well with the reduced diffusion coefficient and equilibrium moisture for moisture resorption in comparison with moisture absorption for all materials studied. For the NC, this effect was not so pronounced, apparently due to the possible reduction of free volume (in comparison with the epoxy resin) in the initial state caused by enhanced physical ageing. Similar observation and ideas regarding a difference in cure kinetics of the polymers and NC, as well as the reduced segmental mobility of the polymer chains were formulated in [
7,
22,
23,
34].
The effect of moisture absorption–desorption on the storage modulus can be observed as an overall slight decrease in both glassy and rubbery regions for all materials. It can be seen from
Figure 6 that the higher the relative humidity for the sorption process was, the lower is the storage modulus in the desorbed state was for all materials. The maximal decrease in the storage modulus was observed after desorption in the atmosphere with a relative humidity of 91%. For the epoxy resin, at 30 and 280 °C, the storage modulus decreased by 15% and 13%, but for the epoxy resin filled with 0.1 wt % N, it decreased by 3% and 9%, and for the epoxy filled with 2 wt % SA, it decreased by 9% and 10%, respectively. These results correlate well with the reduced sorption characteristics and considerations about the reduced free volume for the NC.