Hydrophobilization of Furan-Containing Polyurethanes via Diels–Alder Reaction with Fatty Maleimides
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Chemicals
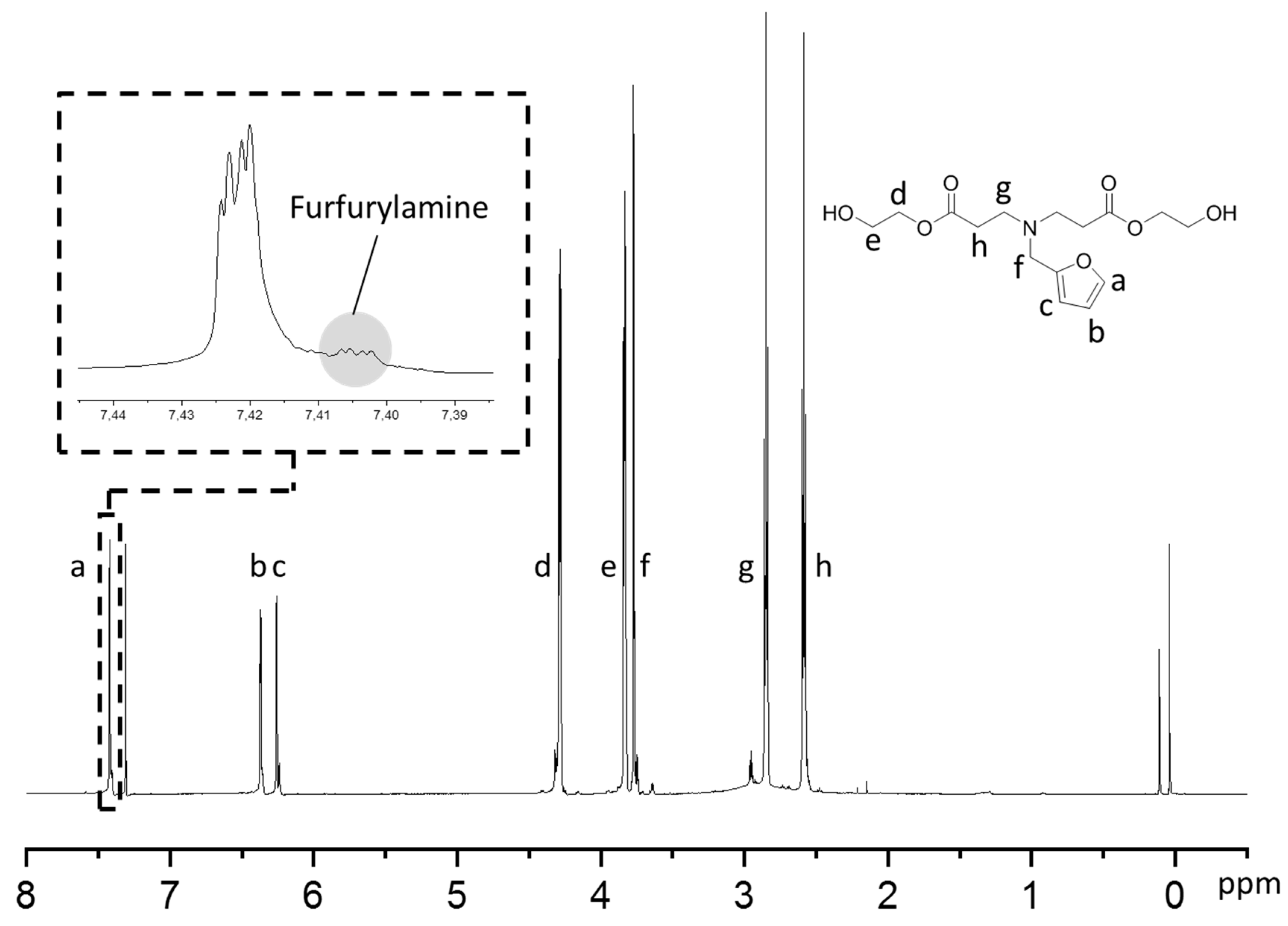
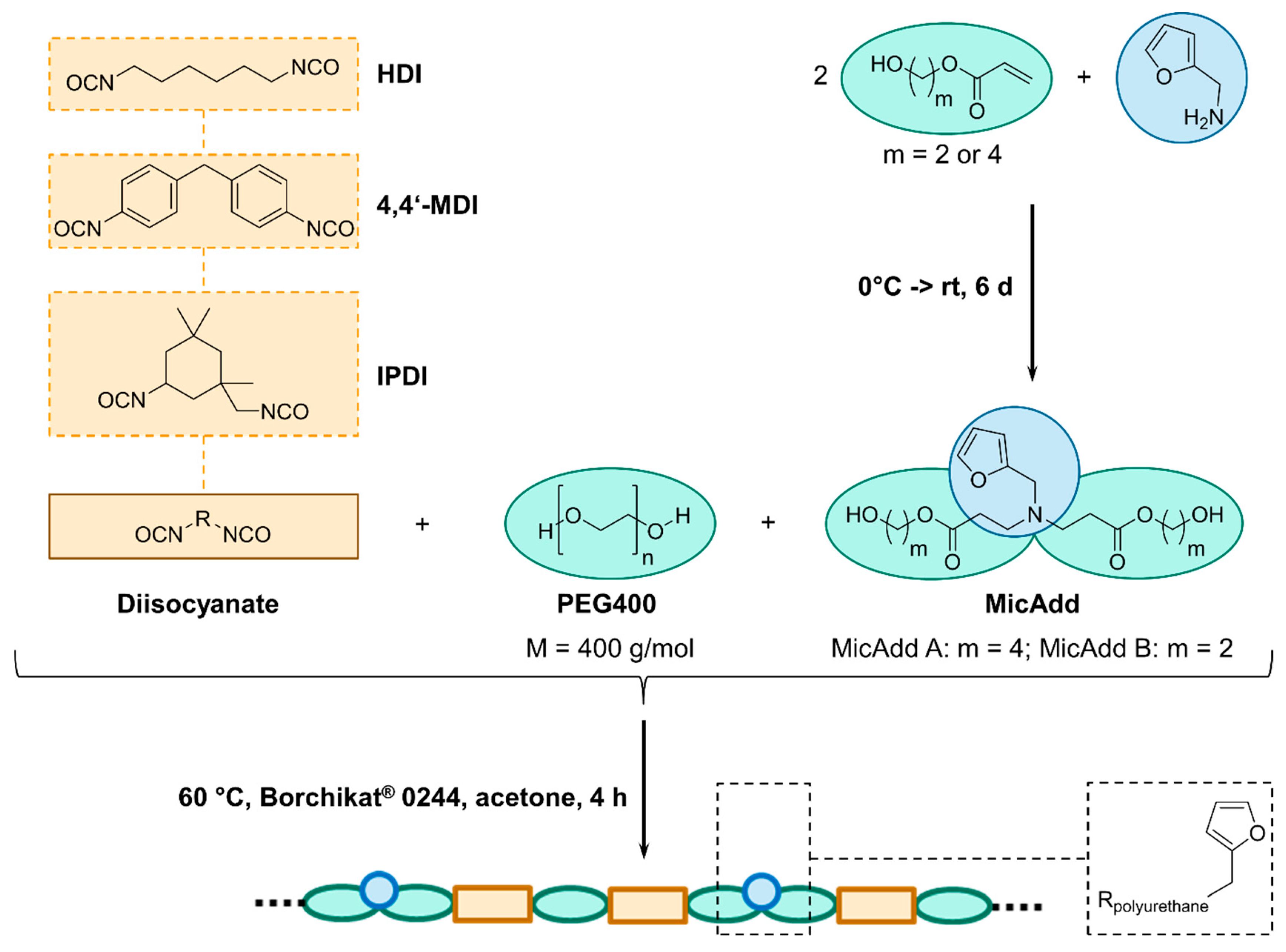
2.3. General Procedure for Synthesis of Michael Products
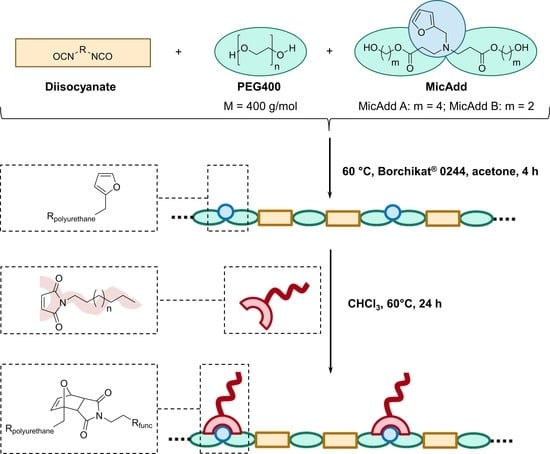
2.4. General Procedure for Synthesis of Linear Polyurethanes
2.5. General Procedure for Diels–Alder Reaction of Linear Polyurethanes
3. Results and discussion
3.1. Synthesis of Furfurylated Products via Michael Addition
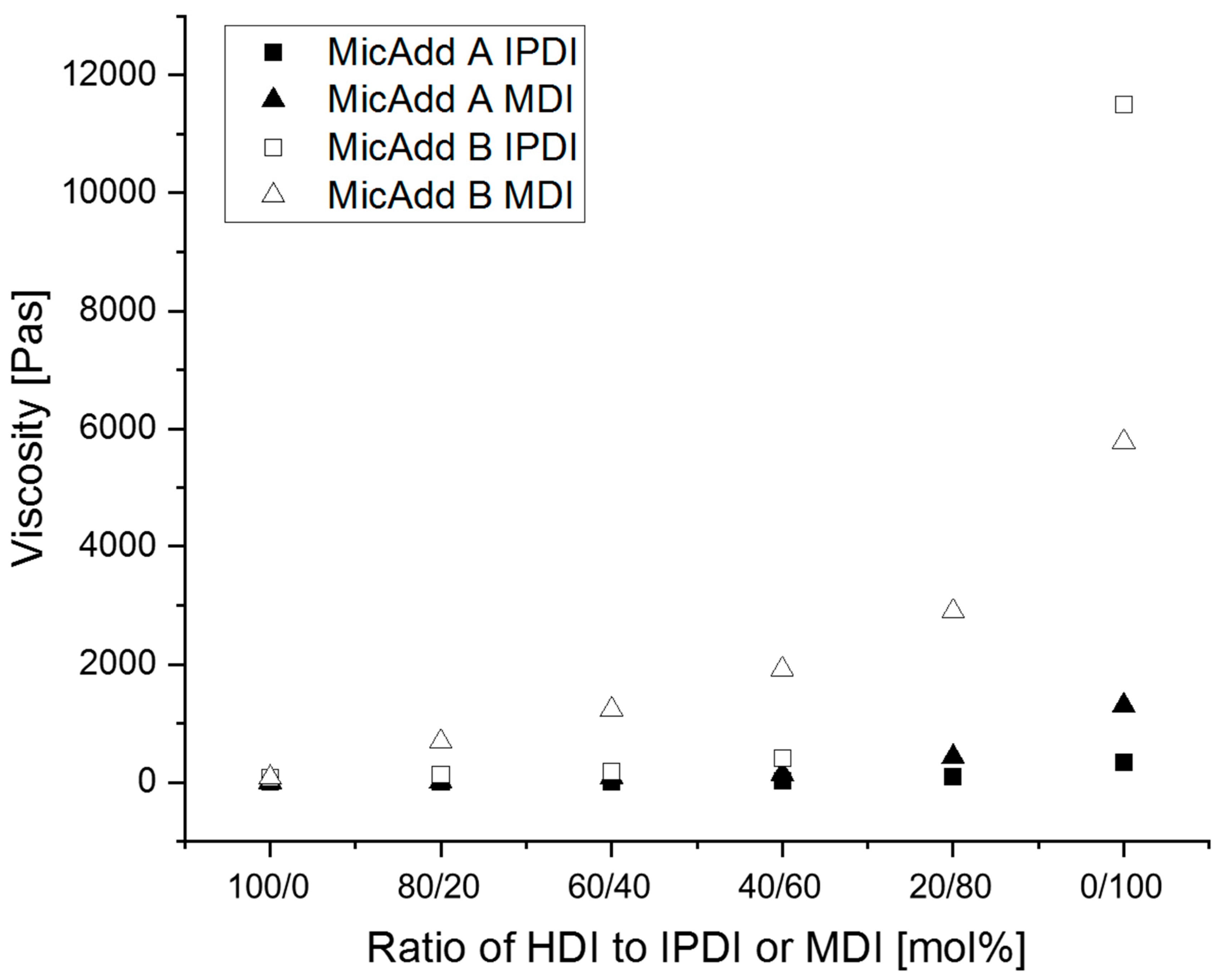
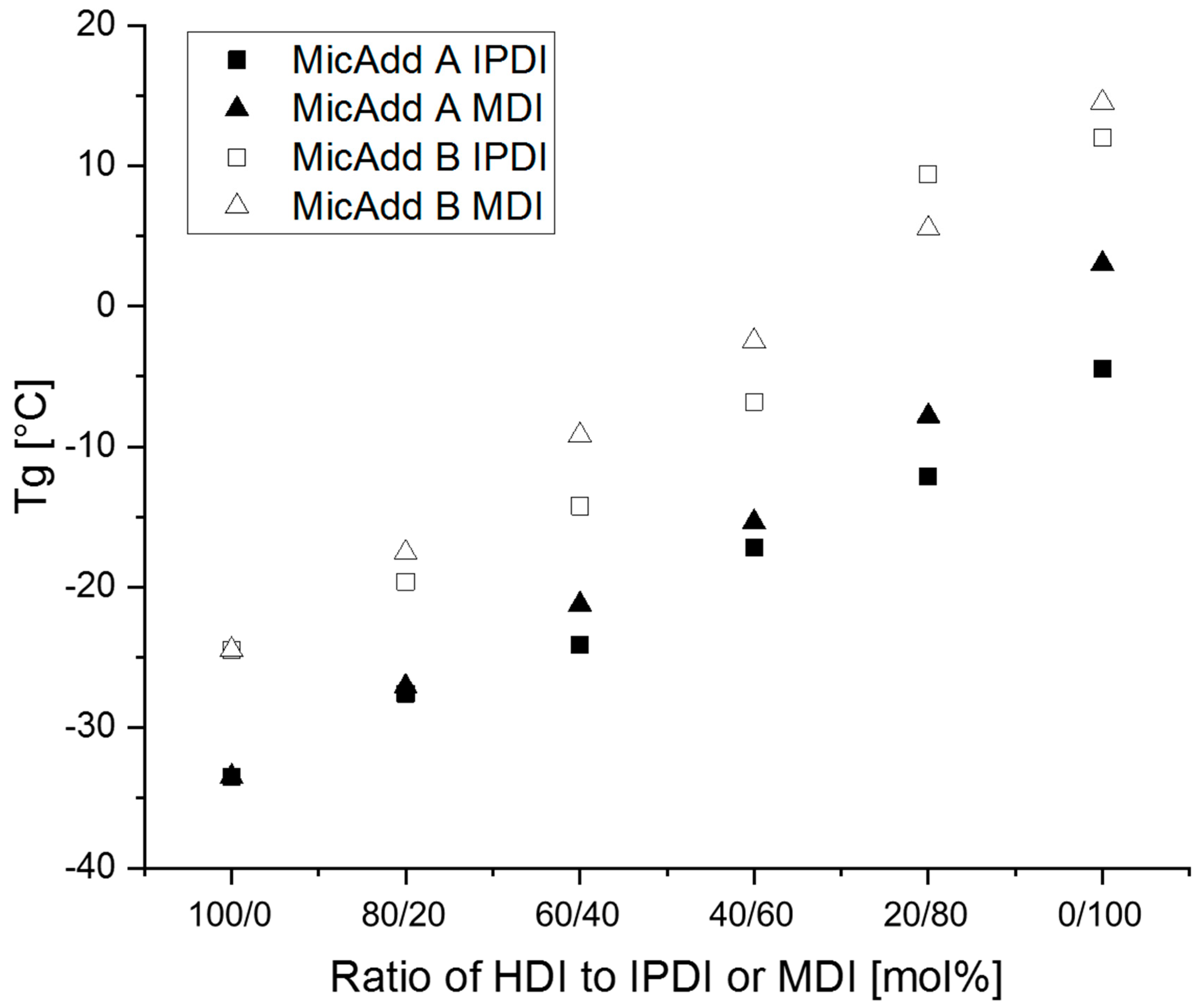
3.2. Synthesis of Linear Polyurethanes (PUs)
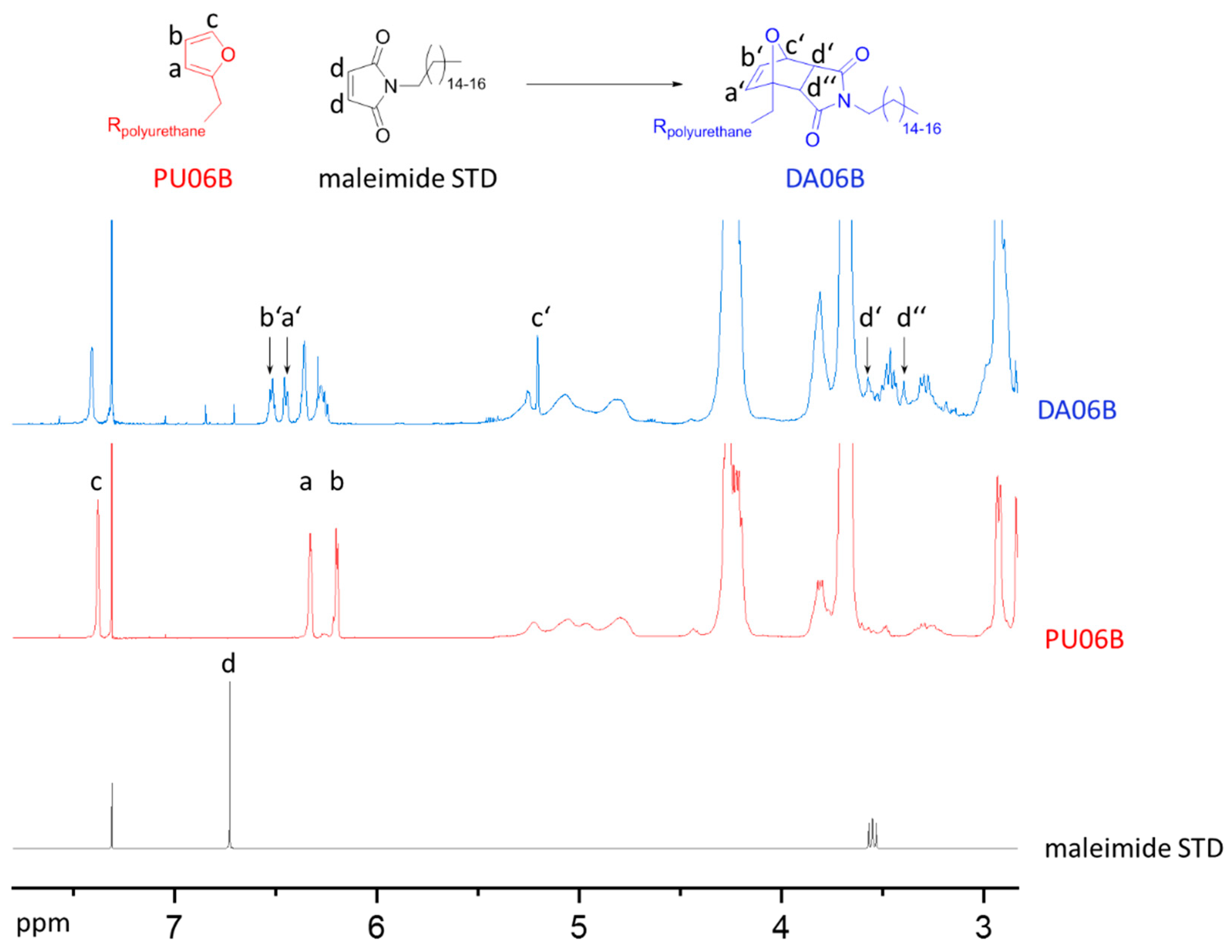
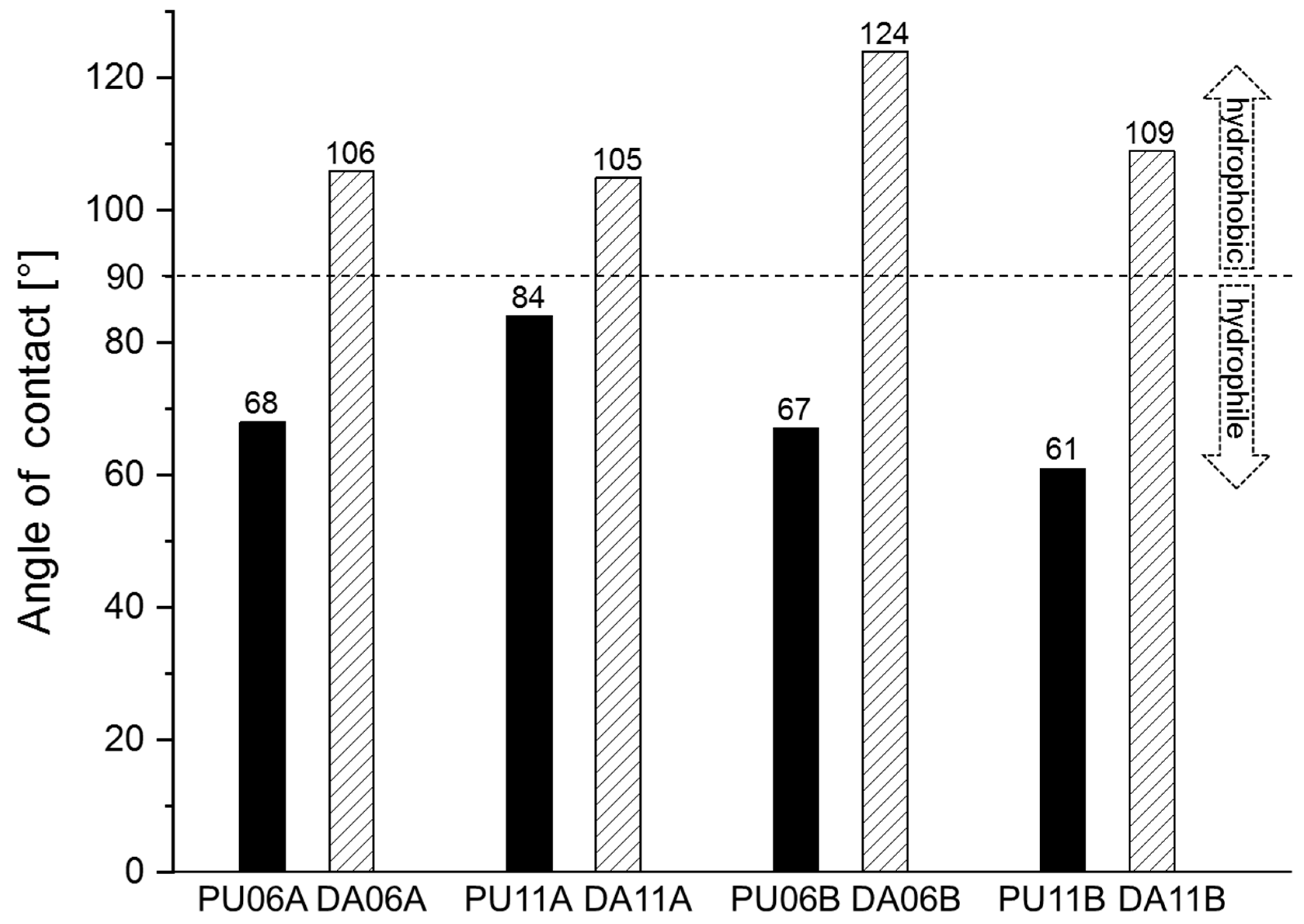
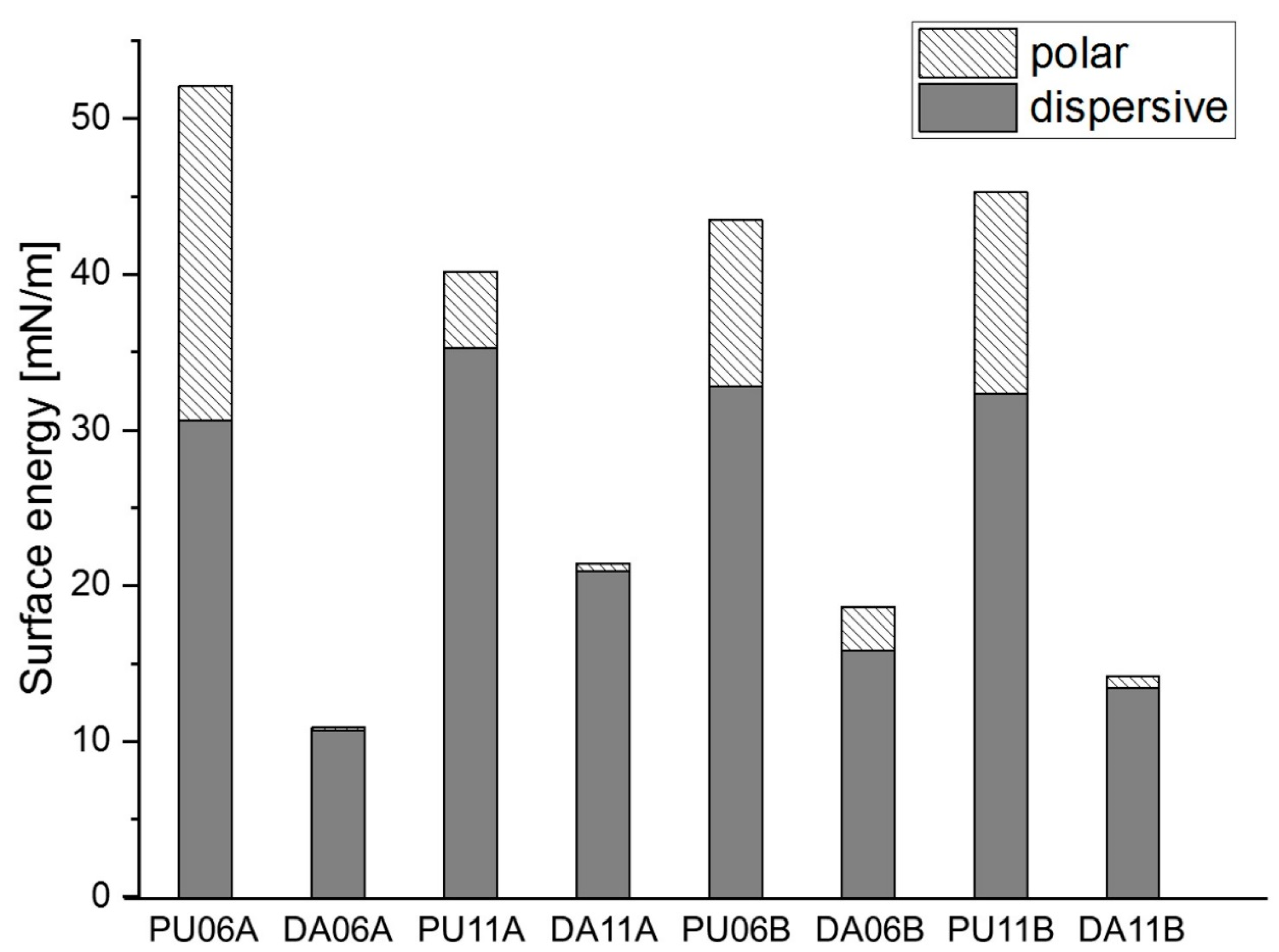
3.3. Diels–Alder Reaction of N-Alkylated Maleimides with Linear PUs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Butruk, B.; Ziętek, P.; Ciach, T. Simple method of fabrication of hydrophobic coatings for polyurethanes. Cent. Eur. J. Chem. 2011, 9, 1039. [Google Scholar] [CrossRef]
- Gunashekar, S.; Abu-Zahra, N. Characterization of functionalized polyurethane foam for lead ion removal from water. Int. J. Polym. Sci. 2014, 2014, 570309. [Google Scholar] [CrossRef]
- Gunashekar, S.; Abu-Zahra, N. Synthesis of functionalized polyurethane foam using BES chain extender for lead ion removal from aqueous solutions. J. Cell. Plast. 2015, 51, 453. [Google Scholar] [CrossRef]
- Demira, M.M.; Yilgorb, I.; Yilgorb, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Xiong, X.; Yu, J.; Guo, Z.-X. Amine-functionalized thermoplastic polyurethane electrospun fibers prepared by co-electrospinning with 3-aminopropyltriethoxysilane and preparation of conductive fiber mats. Polymer 2012, 53, 5190–5196. [Google Scholar] [CrossRef]
- Choi, J.; Moon, D.S.; Ryu, S.G.; Lee, B.; Lee, K.J. Highly functionalized thermoplastic polyurethane from surface click reactions. J. Appl. Polym. Sci. 2018, 135, 46519. [Google Scholar] [CrossRef]
- Breucker, L.; Landfester, K.; Taden, A. Phosphonic acid-functionalized polyurethane dispersions with improved adhesion properties. ACS Appl. Mater. Interfaces 2015, 7, 24641–24648. [Google Scholar] [CrossRef] [PubMed]
- Beniah, G.; Fortman, D.J.; Heath, W.H.; Dichtel, W.R.; Torkelson, J.M. Non-Isocyanate polyurethane thermoplastic elastomer: amide-based chain extender yields enhanced nanophase separation and properties in polyhydroxyurethane. Macromolecules 2017, 50, 4425–4434. [Google Scholar] [CrossRef]
- Pichaiyut, S.; Nakason, C.; Vennemann, N. Thermoplastic elastomers-based natural rubber and thermoplastic polyurethane blends. Iran. Polym. J. 2012, 21, 65–79. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Q.; Li, Z.; Li, Z.; Qin, J.; Ye, C. New azobenzene-containing polyurethanes: post-functional strategy and second-order nonlinear optical properties. Dyes Pigments 2008, 78, 199–206. [Google Scholar] [CrossRef]
- Li, Z.A.; Zeng, Q.; Li, Z.; Dong, S.; Zhu, Z.; Li, Q.; Ye, C.; Di, C.A.; Liu, Y.; Qin, J. An attempt to modify nonlinear optical effects of polyurethanes by adjusting the structure of the chromophore moieties at the molecular level using “click” chemistry. Macromolecules 2006, 39, 8544–8546. [Google Scholar] [CrossRef]
- Fournier, D.; Prez, F.D. “Click” chemistry as a promising tool for side-chain functionalization of polyurethanes. Macromolecules 2008, 41, 4622–4630. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Lu, L.; Yu, X.; Liu, L. Surface hydrophobic modification of polyurethanes by diaryl carbene chemistry: Synthesis and characterization. Appl. Surf. Sci. 2018, 435, 346–351. [Google Scholar] [CrossRef]
- Yu, X.; Yang, P.; Zhang, Z.; Wang, L.; Liu, L.; Wang, Y. Self-healing polyurethane nanocomposite films with recoverable surface hydrophobicity. J. Appl. Polym. Sci. 2018, 135, 46421. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, W.; Mun, S.C.; Ahn, S.; Park, J.J.; Kim, Y.Y.; Kim, E.; Park, O.O.; Wang, D.H. Hydrophilic polyurethane acrylate and its physical property for efficient fabrication of organic photovoltaic cells via stamping transfer. Org. Electron. 2016, 31, 295–302. [Google Scholar] [CrossRef]
- Kojio, K.; Mitsui, Y.; Furukawa, M. Synthesis and properties of highly hydrophilic polyurethane based on diisocyanate with ether group. Polymer 2009, 50, 3693–3697. [Google Scholar] [CrossRef]
- Schmidt, P.; Eschig, S. An Industrial Applicable Method for the Synthesis of N-alkylated Maleimides Based on Fatty Amines. Eur. J. Lipid Sci. Technol. 2019, 121, 1800320. [Google Scholar] [CrossRef]
- Hoare, T.; Timko, B.P.; Santamaria, J.; Goya, G.F.; Irusta, S.; Lau, S.; Stefanescu, C.F.; Lin, D.; Langer, R.; Kohane, D.S. Magnetically triggered nanocomposite membranes: A versatile platform for triggered drug release. Nano Lett. 2011, 11, 1395–1400. [Google Scholar] [CrossRef]
- Gutierrez, L.; Gomez, L.; Irusta, S.; Arruebo, M.; Santamaria, J. Comparative study of the synthesis of silica nanoparticles in micromixer–microreactor and batch reactor systems. Chem. Eng. J. 2011, 171, 674–683. [Google Scholar] [CrossRef]
- Yang, G.; Song, J.; Hou, X. Fabrication of highly hydrophobic two-component thermosetting polyurethane surfaces with silica nanoparticles. Appl. Surf. Sci. 2018, 439, 772–779. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Froidevaux, V.; Borne, M.; Laborbe, E.; Auvergne, R.; Gandini, A.; Boutevin, B. Study of the Diels–Alder and retro-Diels–Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 2015, 5, 37742–37754. [Google Scholar] [CrossRef]
- Iredale, R.J.; Ward, C.; Hamerton, I. Modern advances in bismaleimide resin technology: a 21st century perspective on the chemistry of addition polyimides. Prog. Polym. Sci. 2017, 69, 1–21. [Google Scholar] [CrossRef]
- Buono, P.; Duval, A.; Averous, L.; Habibi, Y. Lignin-Based Materials Through Thiol–Maleimide “Click” Polymerization. ChemSusChem 2017, 10, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: a tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Vijjamarri, S.; Streed, S.; Serum, E.M.; Sibi, M.P.; Du, G. Polymers from bioderived resources: Synthesis of poly(silylether)s from furan derivatives catalyzed by a salen–Mn (V) complex. ACS Sustain. Chem. Eng. 2018, 6, 2491–2497. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Wang, B.; Liu, F.; Liu, Y.; Liu, F. Recyclable biobased materials based on Diels–Alder cycloaddition. J. Appl. Polym. Sci. 2019, 35, 47352. [Google Scholar] [CrossRef]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives. ARKIVOC 2005, 2001, 17–54. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, X. The self-healing cross-linked polyurethane by Diels–Alder polymerization. Adv. Polym. Technol. 2018, 37, 1987–1993. [Google Scholar] [CrossRef]
- Davidson, J.R.; Appuhamillage, G.A.; Thompson, C.M.; Voit, W.; Smaldone, R.A. Design paradigm utilizing reversible Diels–Alder reactions to enhance the mechanical properties of 3D printed materials. ACS Appl. Mater. Interfaces 2016, 8, 16961–16966. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Deng, L.; Jiang, K.; Zhao, S.; Gao, Y.; Sun, R.; Wong, C. Thermally reversible and self-healing novolac epoxy resins based on Diels–Alder chemistry. J. Appl. Polym. Sci. 2015, 132, 42167. [Google Scholar] [CrossRef]
- Chen, X.; Wudl, F.; Mal, A.K.; Shen, H.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Gandini, A. The application of the Diels-Alder reaction to polymer syntheses based on furan/maleimide reversible couplings. Polímeros 2005, 15, 95–101. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.J.D.; Coelho, D. Reversible click chemistry at the service of macromolecular materials. Polym. Chem. 2011, 2, 1713–1719. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Tseng, S.J.; Tang, S.C. Synthesis and characterization of the novel transfection reagent poly (amino ester glycol urethane). Biomacromolecules. Biomacromolecules 2007, 8, 50–58. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owense, D.K. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte der Benetzungstheorie und ihre Anwendung auf die Untersuchung und Veränderung der Oberflächeneigenschaften von Polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]


| Samples | Ratio of Isocyanates (mol%) | Amount of Furan Groups (mmol/g) | ||||
|---|---|---|---|---|---|---|
| with MicAdd A | with MicAdd B | HDI | IPDI | MDI | PUxxA | PUxxB |
| PU01A | PU01B | 100 | - | - | 0.894 | 0.942 |
| PU02A | PU02B | 80 | 20 | - | 0.875 | 0.924 |
| PU03A | PU03B | 60 | 40 | - | 0.858 | 0.905 |
| PU04A | PU04B | 40 | 60 | - | 0.842 | 0.887 |
| PU05A | PU05B | 20 | 80 | - | 0.828 | 0.867 |
| PU06A | PU06B | - | 100 | - | 0.815 | 0.854 |
| PU07A | PU07B | 80 | - | 20 | 0.868 | 0.913 |
| PU08A | PU08B | 60 | - | 40 | 0.845 | 0.887 |
| PU09A | PU09B | 40 | - | 60 | 0.822 | 0.861 |
| PU10A | PU10B | 20 | - | 80 | 0.801 | 0.839 |
| PU11A | PU11B | - | - | 100 | 0.780 | 0.816 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, P.; Eschig, S. Hydrophobilization of Furan-Containing Polyurethanes via Diels–Alder Reaction with Fatty Maleimides. Polymers 2019, 11, 1274. https://doi.org/10.3390/polym11081274
Schmidt P, Eschig S. Hydrophobilization of Furan-Containing Polyurethanes via Diels–Alder Reaction with Fatty Maleimides. Polymers. 2019; 11(8):1274. https://doi.org/10.3390/polym11081274
Chicago/Turabian StyleSchmidt, Philipp, and Steven Eschig. 2019. "Hydrophobilization of Furan-Containing Polyurethanes via Diels–Alder Reaction with Fatty Maleimides" Polymers 11, no. 8: 1274. https://doi.org/10.3390/polym11081274
APA StyleSchmidt, P., & Eschig, S. (2019). Hydrophobilization of Furan-Containing Polyurethanes via Diels–Alder Reaction with Fatty Maleimides. Polymers, 11(8), 1274. https://doi.org/10.3390/polym11081274