Adamantane Functionalized Poly(2-oxazoline)s with Broadly Tunable LCST-Behavior by Molecular Recognition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
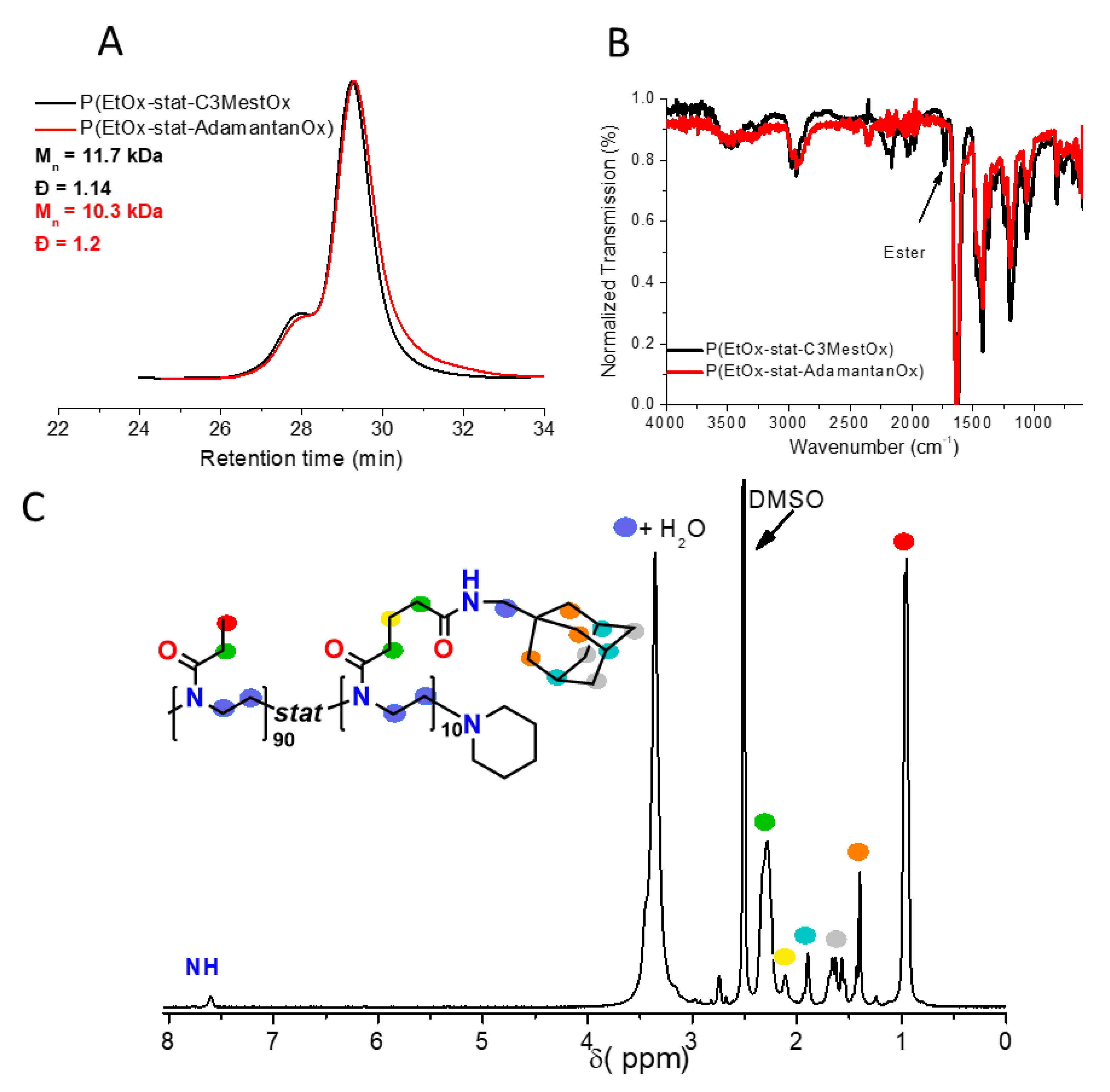
2.3. Synthesis of Poly(2-ethyl-2-oxazoline)90-stat-poly(2-C3Mest-2-oxazoline)10 Copolymer (Poly(EtOx-stat-C3MestOx))
2.4. TBD-Catalyzed Amidation of the Poly(2-ethyl-2-oxazoline)90-stat-poly(2-C3Mest-2-oxazoline)10 Copolymer with 1-adamantanemethylamine (P(EtOx-stat-AdamantanOx))
2.5. Cloud Point Measurements
2.6. 1H NMR Titration Experiments
2.7. Diffusion Ordered NMR Spectra (2D DOSY)
3. Results and Discussion
3.1. Polymer Synthesis
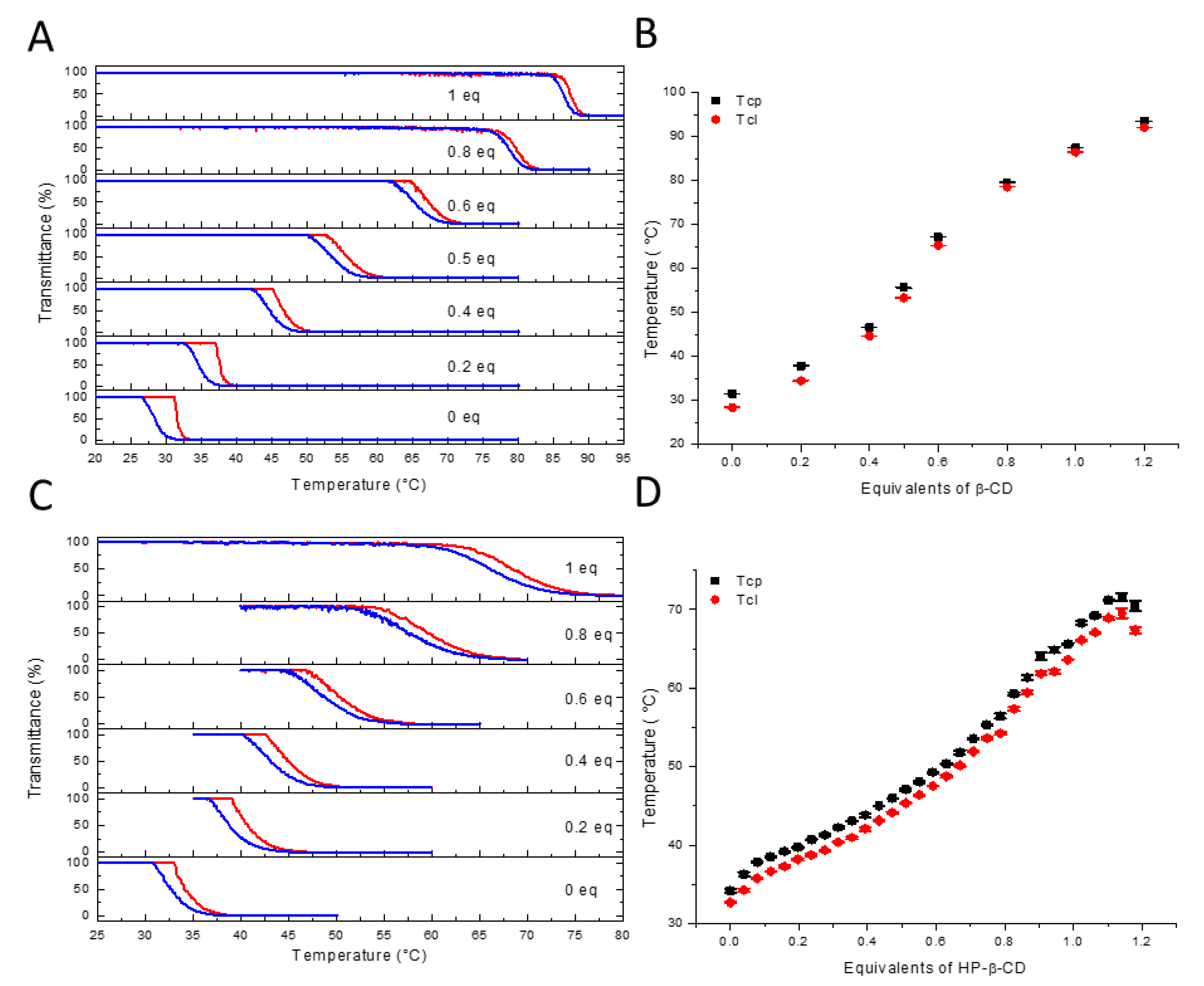
3.2. Tuning Thermoresponsive Behavior by Molecular Recognition
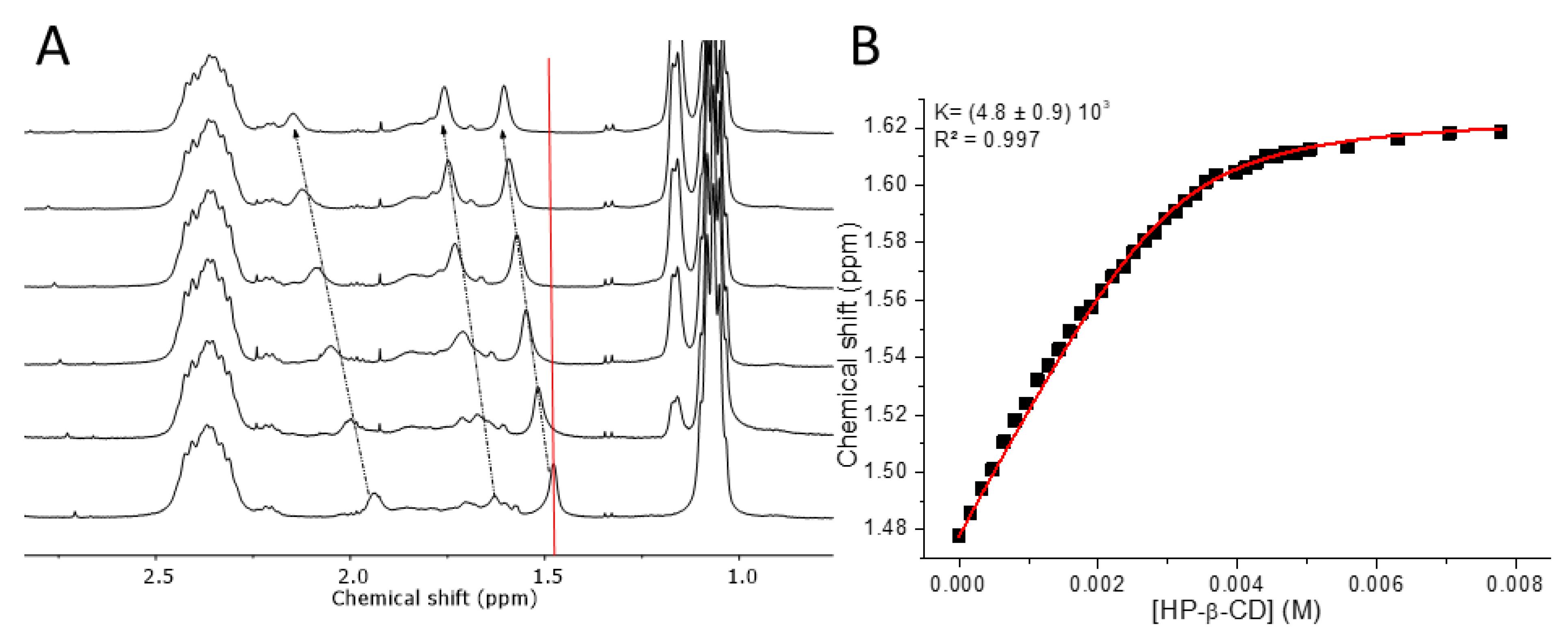
3.3. Molecular Recognition of Adamantane Pendant Groups by Hydroxypropyl-β-CD
3.4. Diffusion Ordered NMR Spectroscopy
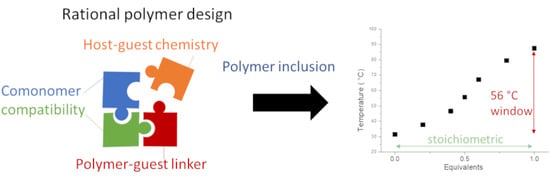
3.5. Rationalization of Polymer Design with Respect to Thermal Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, X.; Chung, J.Y.; Lim, Y.X.; Li, Z.; Loh, X.J. Review of Adaptive Programmable Materials and Their Bioapplications. ACS Appl. Mater. Interfaces 2016, 8, 33351–33370. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Wang, H. Temperature-responsive polymers: Synthesis, properties, and biomedical applications. Nano Res. 2018, 11, 5400–5423. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. PH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Bertrand, O.; Gohy, J.F. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2017, 8, 52–73. [Google Scholar] [CrossRef]
- Bowser, B.H.; Craig, S.L. Empowering mechanochemistry with multi-mechanophore polymer architectures. Polym. Chem. 2018, 9, 3583–3593. [Google Scholar] [CrossRef]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; Van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R.; et al. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Liu, M.; Wang, K.; Tao, L.; Wan, Q.; Wei, Y. Recent progress and advances in redox-responsive polymers as controlled delivery nanoplatforms. Mater. Chem. Front. 2017, 1, 807–822. [Google Scholar] [CrossRef]
- Yan, Q.; Yuan, J.; Cai, Z.; Xin, Y.; Kang, Y.; Yin, Y. Voltage-responsive vesicles based on orthogonal assembly of two homopolymers. J. Am. Chem. Soc. 2010, 132, 9268–9270. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution: Unexpected properties from known building blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Soeriyadi, A.H.; Li, G.Z.; Slavin, S.; Jones, M.W.; Amos, C.M.; Becer, C.R.; Whittaker, M.R.; Haddleton, D.M.; Boyer, C.; Davis, T.P. Synthesis and modification of thermoresponsive poly(oligo(ethylene glycol) methacrylate) via catalytic chain transfer polymerization and thiol-ene michael addition. Polym. Chem. 2011, 2, 815–822. [Google Scholar] [CrossRef]
- Magnusson, J.P.; Khan, A.; Pasparakis, G.; Saeed, A.O.; Wang, W.; Alexander, C. Ion-sensitive “isothermal” responsive polymers prepared in water. J. Am. Chem. Soc. 2008, 130, 10852–10853. [Google Scholar] [CrossRef]
- Platé, N.; Lebedeva, T.; Valuev, L. Lower critical solution temperature in aqueous solutions of N-alkyl-substituted Polyacrylamides. Polym. J. 1999, 31, 21–27. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Morrow, J.P.; Pizzi, D.; Nanayakkara, S.; Davis, T.P.; Saito, K.; Kempe, K. Nonionic Water-Soluble and Cytocompatible Poly(amide acrylate)s. Macromolecules 2020, 53, 693–701. [Google Scholar] [CrossRef]
- Higashi, N.; Sonoda, R.; Koga, T. Thermo-responsive amino acid-based vinyl polymers showing widely tunable LCST/UCST behavior in water. RSC Adv. 2015, 5, 67652–67657. [Google Scholar] [CrossRef]
- Hedir, G.G.; Arno, M.C.; Langlais, M.; Husband, J.T.; O’Reilly, R.K.; Dove, A.P. Poly(oligo(ethylene glycol) vinyl acetate)s: A Versatile Class of Thermoresponsive and Biocompatible Polymers. Angew. Chem. Int. Ed. 2017, 56, 9178–9182. [Google Scholar] [CrossRef]
- Yiu, A.; Simchuk, D.; Hao, J. Facile Synthesis of Novel Thermo-Responsive Polyvalerolactones with Tunable LCSTs. Macromol. Chem. Phys. 2020, 221, 1–6. [Google Scholar] [CrossRef]
- Park, J.S.; Kataoka, K. Comprehensive and accurate control of thermosensitivity of poly(2-alkyl-2-oxazoline)s via well-defined gradient or random copolymerization. Macromolecules 2007, 40, 3599–3609. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef]
- Fu, X.; Xing, C.; Sun, J. Tunable LCST/UCST-Type Polypeptoids and Their Structure-Property Relationship. Biomacromolecules 2020, 21, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, J. Thermoresponsive polypeptoids. Polymers 2020, 12, 2973. [Google Scholar] [CrossRef]
- Jana, S.; Uchman, M. Poly(2-oxazoline)-based stimulus-responsive (Co)polymers: An overview of their design, solution properties, surface-chemistries and applications. Prog. Polym. Sci. 2020, 106, 101252. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering-A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Park, J.R.; Van Guyse, J.F.R.; Podevyn, A.; Bolle, E.C.L.; Bock, N.; Linde, E.; Celina, M.; Hoogenboom, R.; Dargaville, T.R. Influence of side-chain length on long-term release kinetics from poly(2-oxazoline)-drug conjugate networks. Eur. Polym. J. 2019, 120, 109217. [Google Scholar] [CrossRef]
- Kim, Y.J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Vanparijs, N.; Nuhn, L.; De Geest, B.G. Transiently thermoresponsive polymers and their applications in biomedicine. Chem. Soc. Rev. 2017, 46, 1193–1239. [Google Scholar] [CrossRef]
- Vancoillie, G.; Van Guyse, J.F.R.; Voorhaar, L.; Maji, S.; Frank, D.; Holder, E.; Hoogenboom, R. Understanding the effect of monomer structure of oligoethylene glycol acrylate copolymers on their thermoresponsive behavior for the development of polymeric sensors. Polym. Chem. 2019, 10, 5778–5789. [Google Scholar] [CrossRef]
- Sun, W.; An, Z.; Wu, P. UCST or LCST? Composition-Dependent Thermoresponsive Behavior of Poly(N-acryloylglycinamide-co-diacetone acrylamide). Macromolecules 2017, 50, 2175–2182. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Thijs, H.M.L.; Jochems, M.J.H.C.; van Lankvelt, B.M.; Fijten, M.W.M.; Schubert, U.S. Tuning the LCST of poly(2-oxazoline)s by varying composition and molecular weight: Alternatives to poly(N-isopropylacrylamide)? Chem. Commun. 2008, 2008, 5758–5760. [Google Scholar] [CrossRef]
- Villano, L.D.; Kommedal, R.; Fijten, M.W.M.; Schubert, U.S.; Hoogenboom, R.; Kelland, M.A. A study of the kinetic hydrate inhibitor performance and seawater biodegradability of a series of poly(2-alkyl-2-oxazoline)s. Energy Fuels 2009, 23, 3665–3673. [Google Scholar] [CrossRef]
- Taylor, L.D.; Cerankowski, L.D. Preparation Of Films Exhibiting A Balanced Temperature Dependence To Permeation By Aqueous Solutions—A Study Of Lower Consolute Behavior. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 2551–2570. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Zorn, A.-M.M.; Keul, H.; Barner-Kowollik, C.; Moeller, M. Copolymers of 2-hydroxyethylacrylate and 2-methoxyethyl acrylate by nitroxide mediated polymerization: Kinetics, SEC-ESI-MS analysis and thermoresponsive properties. Polym. Chem. 2012, 3, 335–342. [Google Scholar] [CrossRef]
- Steinhauer, W.; Hoogenboom, R.; Keul, H.; Moeller, M. Copolymerization of 2-hydroxyethyl acrylate and 2-methoxyethyl acrylate via RAFT: Kinetics and thermoresponsive properties. Macromolecules 2010, 43, 7041–7047. [Google Scholar] [CrossRef]
- Popescu, D.; Hoogenboom, R.; Keul, H.; Moeller, M. Thermoresponsive polyacrylates obtained via a cascade of enzymatic transacylation and FRP or NMP. Polym. Chem. 2010, 1, 878–890. [Google Scholar] [CrossRef]
- de la Rosa, V.R.; Woisel, P.; Hoogenboom, R. Supramolecular control over thermoresponsive polymers. Mater. Today 2015, 19, 44–55. [Google Scholar] [CrossRef]
- De La Rosa, V.R.; Nau, W.M.; Hoogenboom, R. Tuning temperature responsive poly(2-alkyl-2-oxazoline)s by supramolecular host-guest interactions. Org. Biomol. Chem. 2015, 13, 3048–3057. [Google Scholar] [CrossRef]
- Ji, X.; Chen, J.; Chi, X.; Huang, F. PH-responsive supramolecular control of polymer thermoresponsive behavior by pillararene-based host-guest interactions. ACS Macro Lett. 2014, 3, 110–113. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Zhang, Q.; Luo, Z.; Deng, Y.; Yang, W.; Dong, S.; Wang, Q.A.; Han, C. Supramolecular control over pillararene-based LCST phase behaviour. New J. Chem. 2018, 42, 8330–8333. [Google Scholar] [CrossRef]
- Bigot, J.; Bria, M.; Caldwell, S.T.; Cazaux, F.; Cooper, A.; Charleux, B.; Cooke, G.; Fitzpatrick, B.; Fournier, D.; Lyskawa, J.; et al. LCST: A powerful tool to control complexation between a dialkoxynaphthalene-functionalised poly(N-isopropylacrylamide) and CBPQT 4+ in water. Chem. Commun. 2009, 2009, 5266–5268. [Google Scholar] [CrossRef] [PubMed]
- Sambe, L.; de La Rosa, V.R.; Belal, K.; Stoffelbach, F.; Lyskawa, J.; Delattre, F.; Bria, M.; Cooke, G.; Hoogenboom, R.; Woisel, P. Programmable Polymer-Based Supramolecular Temperature Sensor with a Memory Function. Angew. Chem. 2014, 126, 5144–5148. [Google Scholar] [CrossRef]
- Kretschmann, O.; Steffens, C.; Ritter, H. Cyclodextrin complexes of polymers bearing adamantyl groups: Host-guest interactions and the effect of spacers on water solubility. Angew. Chem. Int. Ed. 2007, 46, 2708–2711. [Google Scholar] [CrossRef]
- Wintgens, V.; Charles, M.; Allouache, F.; Amiel, C. Triggering the thermosensitive properties of hydrophobically modified poly(N-isopropylacrylamide) by complexation with cyclodextrin polymers. Macromol. Chem. Phys. 2005, 206, 1853–1861. [Google Scholar] [CrossRef]
- Jia, Y.G.; Zhu, X.X. Thermoresponsiveness of copolymers bearing cholic acid pendants induced by complexation with β-cyclodextrin. Langmuir 2014, 30, 11770–11775. [Google Scholar] [CrossRef]
- Maatz, G.; Maciollek, A.; Ritter, H. Cyclodextrin-induced host-guest effects of classically prepared poly(NIPAM) bearing azo-dye end groups. Beilstein J. Org. Chem. 2012, 8, 1929–1935. [Google Scholar] [CrossRef]
- Reinelt, S.; Steinke, D.; Ritter, H. End-group-functionalized poly(N,N-diethylacrylamide) via free-radical chain transfer polymerization: Influence of sulfur oxidation and cyclodextrin on self-organization and cloud points in water. Beilstein J. Org. Chem. 2014, 10, 680–691. [Google Scholar] [CrossRef]
- de la Rosa, V.R.; Hoogenboom, R. Solution Polymeric Optical Temperature Sensors with Long-Term Memory Function Powered by Supramolecular Chemistry. Chem. Eur. J. 2015, 21, 1302–1311. [Google Scholar] [CrossRef]
- Burkhart, A.; Ritter, H. Influence of cyclodextrin on the UCST- and LCST-Behavior of poly(2-methacrylamido-caprolactam)-CO-(N,N-dimethylacrylamide). Beilstein J. Org. Chem. 2014, 10, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Kellett, K.; Duggan, B.M.; Gilson, M.K. Facile synthesis of a diverse library of mono-3-substituted β-cyclodextrin analogues. Supramol. Chem. 2019, 31, 251–259. [Google Scholar] [CrossRef]
- Hanessian, S.; Benalil, A.; Laferriere, C. The synthesis of functionalized cyclodextrins as scaffolds and templates for molecular diversity, catalysis, and inclusion phenomena. J. Org. Chem. 1995, 60, 4786–4797. [Google Scholar] [CrossRef]
- Munro, I.C.; Newberne, P.M.; Young, V.R.; Bär, A. Safety assessment of gamma-cyclodextrin. Regul. Toxicol. Pharmacol. 2004, 39 (Suppl. 1), 3–13. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Bouten, P.J.M.; Hertsen, D.; Vergaelen, M.; Monnery, B.D.; Catak, S.; Van Hest, J.C.M.; Van Speybroeck, V.; Hoogenboom, R. Synthesis of poly(2-oxazoline)s with side chain methyl ester functionalities: Detailed understanding of living copolymerization behavior of methyl ester containing monomers with 2-alkyl-2-oxazolines. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2649–2661. [Google Scholar] [CrossRef]
- Bouten, P.J.M.; Lava, K.; Van Hest, J.C.M.; Hoogenboom, R. Thermal properties of methyl ester-containing poly(2-oxazoline)s. Polymers 2015, 7, 1998–2008. [Google Scholar] [CrossRef]
- Van Guyse, J.F.; Mees, M.A.; Vergaelen, M.; Baert, M.; Verbraeken, B.; Martens, P.J.; Hoogenboom, R. Amidation of Methyl Ester Side Chain bearing Poly(2-oxazoline)s with Tyramine: A Quest for a Selective and Quantitative Approach. Polym. Chem. 2019, 10, 954–962. [Google Scholar] [CrossRef]
- Connell, M.A.; Bowyer, P.J.; Adam Bone, P.; Davis, A.L.; Swanson, A.G.; Nilsson, M.; Morris, G.A. Improving the accuracy of pulsed field gradient NMR diffusion experiments: Correction for gradient non-uniformity. J. Magn. Reson. 2009, 198, 121–131. [Google Scholar] [CrossRef]
- Sinnaeve, D. The Stejskal-Tanner equation generalized for any gradient shape-An overview of most pulse sequences measuring free diffusion. Concepts Magn. Reson. Part A Bridg. Educ. Res. 2012, 40 A, 39–65. [Google Scholar] [CrossRef]
- Mees, M.A.; Hoogenboom, R. Functional Poly(2-oxazoline)s by Direct Amidation of Methyl Ester Side Chains. Macromolecules 2015, 48, 3531–3538. [Google Scholar] [CrossRef]
- Van Guyse, J.F.R.; Xu, X.; Hoogenboom, R. Acyl guanidine functional poly(2-oxazoline)s as reactive intermediates and stimuli-responsive materials. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2616–2624. [Google Scholar] [CrossRef]
- Monnery, B.D.; Jerca, V.V.; Sedlacek, O.; Verbraeken, B.; Cavill, R.; Hoogenboom, R. Defined High Molar Mass Poly(2-Oxazoline)s. Angew. Chem. 2018, 130, 15626–15630. [Google Scholar] [CrossRef]
- Walach, W.; Oleszko-Torbus, N.; Utrata-Wesolek, A.; Bochenek, M.; Kijeńska-Gawrońska, E.; Górecka, Z.; Świȩszkowski, W.; Dworak, A. Processing of (Co)poly(2-oxazoline)s by electrospinning and extrusion from melt and the postprocessing properties of the (co)polymers. Polymers 2020, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Do, J.L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, G.; Artavia, G. Use of the Adamantane Structure in Medicinal Chemistry. Curr. Med. Chem. 2010, 17, 2967–2978. [Google Scholar] [CrossRef]
- Dolin, R.; Reichman, R.C.; Madore, H.P.; Maynard, R.; Linton, P.N.; Webber-Jones, J. A Controlled Trial of Amantadine and Rimantadine in the Prophylaxis of Influenza a Infection. N. Engl. J. Med. 1982, 307, 580–584. [Google Scholar] [CrossRef]
- Van Guyse, J.F.R.; Verjans, J.; Vandewalle, S.; De Bruycker, K.; Du Prez, F.E.; Hoogenboom, R. Full and Partial Amidation of Poly(methyl acrylate) as Basis for Functional Polyacrylamide (Co)Polymers. Macromolecules 2019, 52, 5102–5109. [Google Scholar] [CrossRef]
- Ito, D.; Ogura, Y.; Sawamoto, M.; Terashima, T. Acrylate-Selective Transesterification of Methacrylate/Acrylate Copolymers: Postfunctionalization with Common Acrylates and Alcohols. ACS Macro Lett. 2018, 7, 997–1002. [Google Scholar] [CrossRef]
- Eftink, M.R.; Andy, M.L.; Bystrom, K.; Perlmutter, H.D.; Kristol, D.S. Cyclodextrin Inclusion Complexes: Studies of the Variation in the Size of Alicyclic Guests. J. Am. Chem. Soc. 1989, 111, 6765–6772. [Google Scholar] [CrossRef]
- Kwak, E.S.; Gomez, F.A. Determination of the binding of β-cyclodextrin derivatives to adamantane carboxylic acids using capillary electrophoresis. Chromatographia 1996, 43, 659–662. [Google Scholar] [CrossRef]
- Harries, D.; Rau, D.C.; Parsegian, V.A. Solutes probe hydration in specific association of cyclodextrin and adamantane. J. Am. Chem. Soc. 2005, 127, 2184–2190. [Google Scholar] [CrossRef]
- Cromwell, W.C.; Byström, K.; Eftink, M.R. Cyclodextrin-adamantanecarboxylate inclusion complexes: Studies of the variation in cavity size. J. Phys. Chem. 1985, 89, 326–332. [Google Scholar] [CrossRef]
- Palepu, R.; Reinsborough, V.C. β-Cyclodextrin Inclusion of Adamantane Derivatives in Solution. Aust. J. Chem. 1990, 43, 2119–2123. [Google Scholar] [CrossRef]
- Gelb, R.I.; Schwartz, L.M. Complexation of admantane-ammonium substrates by beta-cyclodextrin and its O-methylated derivatives. J. Incl. Phenom. Macrocycl. Chem. 1989, 7, 537–543. [Google Scholar] [CrossRef]
- Granadero, D.; Bordello, J.; Pérez-Alvite, M.J.; Novo, M.; Al-Soufi, W. Host-guest complexation studied by fluorescence correlation spectroscopy: Adamantane-cyclodextrin inclusion. Int. J. Mol. Sci. 2010, 11, 173–188. [Google Scholar] [CrossRef]
- Bergeron, R.J.; Channing, M.A.; Gibeily, G.J.; Pillor, D.M. Disposition requirements for binding in aqueous solution of polar substrates in the cyclohexaamylose cavity. J. Am. Chem. Soc. 1977, 99, 5146–5151. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Schneider, H.J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1785. [Google Scholar] [CrossRef]
- Bakirci, H.; Zhang, X.; Nau, W.M. Induced circular dichroism and structural assignment of the cyclodextrin inclusion complexes of bicyclic azoalkanes. J. Org. Chem. 2005, 70, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Nau, W.M.; Zhang, X. An exceedingly long-lived fluorescent state as a distinct structural and dynamic probe for supramolecular association: An exploratory study of host-guest complexation by cyclodextrins. J. Am. Chem. Soc. 1999, 121, 8022–8032. [Google Scholar] [CrossRef]
- Schönbeck, C.; Holm, R. Exploring the Origins of Enthalpy-Entropy Compensation by Calorimetric Studies of Cyclodextrin Complexes. J. Phys. Chem. B 2019, 123, 6686–6693. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1917. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.C.; Cottington, R.L. Viscosity of deuterium oxide and water in the range 5 to 125 C. J. Res. Natl. Bur. Stand. 1949, 42, 573–578. [Google Scholar] [CrossRef]
- Park, J.-R.; Sarwat, M.; Bolle, E.C.L.; de Laat, M.A.; Van Guyse, J.F.R.; Podevyn, A.; Hoogenboom, R.; Dargaville, T.R. Drug–polymer conjugates with dynamic cloud point temperatures based on poly(2-oxazoline) copolymers. Polym. Chem. 2020, 11, 5191–5199. [Google Scholar] [CrossRef]
- Osawa, S.; Osada, K.; Hiki, S.; Dirisala, A.; Ishii, T.; Kataoka, K. Polyplex Micelles with Double-Protective Compartments of Hydrophilic Shell and Thermoswitchable Palisade of Poly(oxazoline)-Based Block Copolymers for Promoted Gene Transfection. Biomacromolecules 2016, 17, 354–361. [Google Scholar] [CrossRef]




| Compound | Df (m2/s) | Rh (nm) |
|---|---|---|
| P(EtOx-stat-AdamantanOx) (25 °C) | 5.41 × 10−11 | 3.68 |
| P(EtOx-stat-AdamantanOx) (42 °C) | 8.34 × 10−11 | 3.52 |
| β-CD (25 °C) | 2.66 × 10−10 | 0.75 |
| β-CD (42 °C) | 3.83 × 10−10 | 0.77 |
| Complex (25 °C) | 4.68 × 10−11 | 4.26 |
| Complex (42 °C) | 4.42 × 10−10 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Guyse, J.F.R.; Bera, D.; Hoogenboom, R. Adamantane Functionalized Poly(2-oxazoline)s with Broadly Tunable LCST-Behavior by Molecular Recognition. Polymers 2021, 13, 374. https://doi.org/10.3390/polym13030374
Van Guyse JFR, Bera D, Hoogenboom R. Adamantane Functionalized Poly(2-oxazoline)s with Broadly Tunable LCST-Behavior by Molecular Recognition. Polymers. 2021; 13(3):374. https://doi.org/10.3390/polym13030374
Chicago/Turabian StyleVan Guyse, Joachim F. R., Debaditya Bera, and Richard Hoogenboom. 2021. "Adamantane Functionalized Poly(2-oxazoline)s with Broadly Tunable LCST-Behavior by Molecular Recognition" Polymers 13, no. 3: 374. https://doi.org/10.3390/polym13030374
APA StyleVan Guyse, J. F. R., Bera, D., & Hoogenboom, R. (2021). Adamantane Functionalized Poly(2-oxazoline)s with Broadly Tunable LCST-Behavior by Molecular Recognition. Polymers, 13(3), 374. https://doi.org/10.3390/polym13030374