A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification
Abstract
1. Introduction
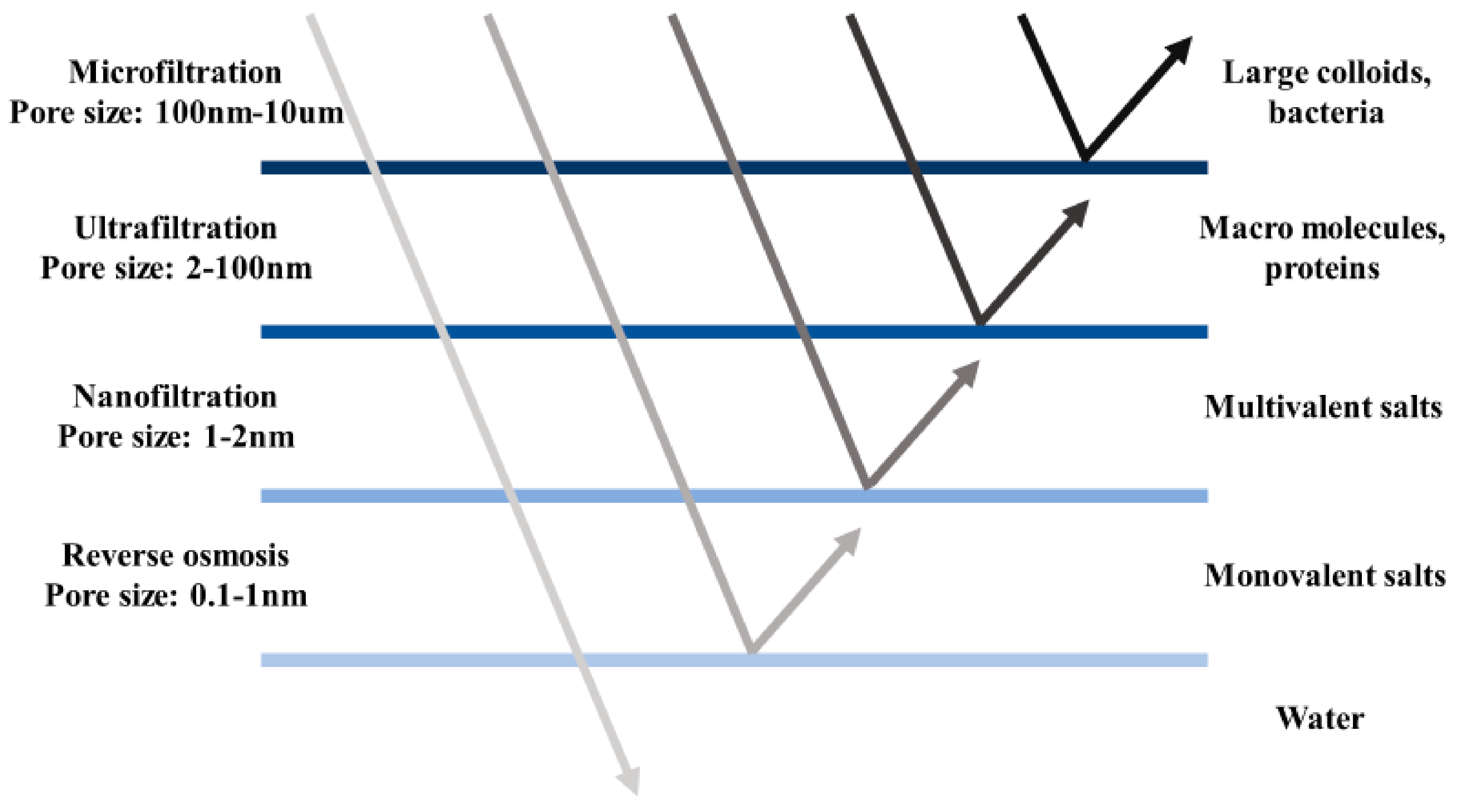
2. Reverse Osmosis and Nanofiltration Membranes
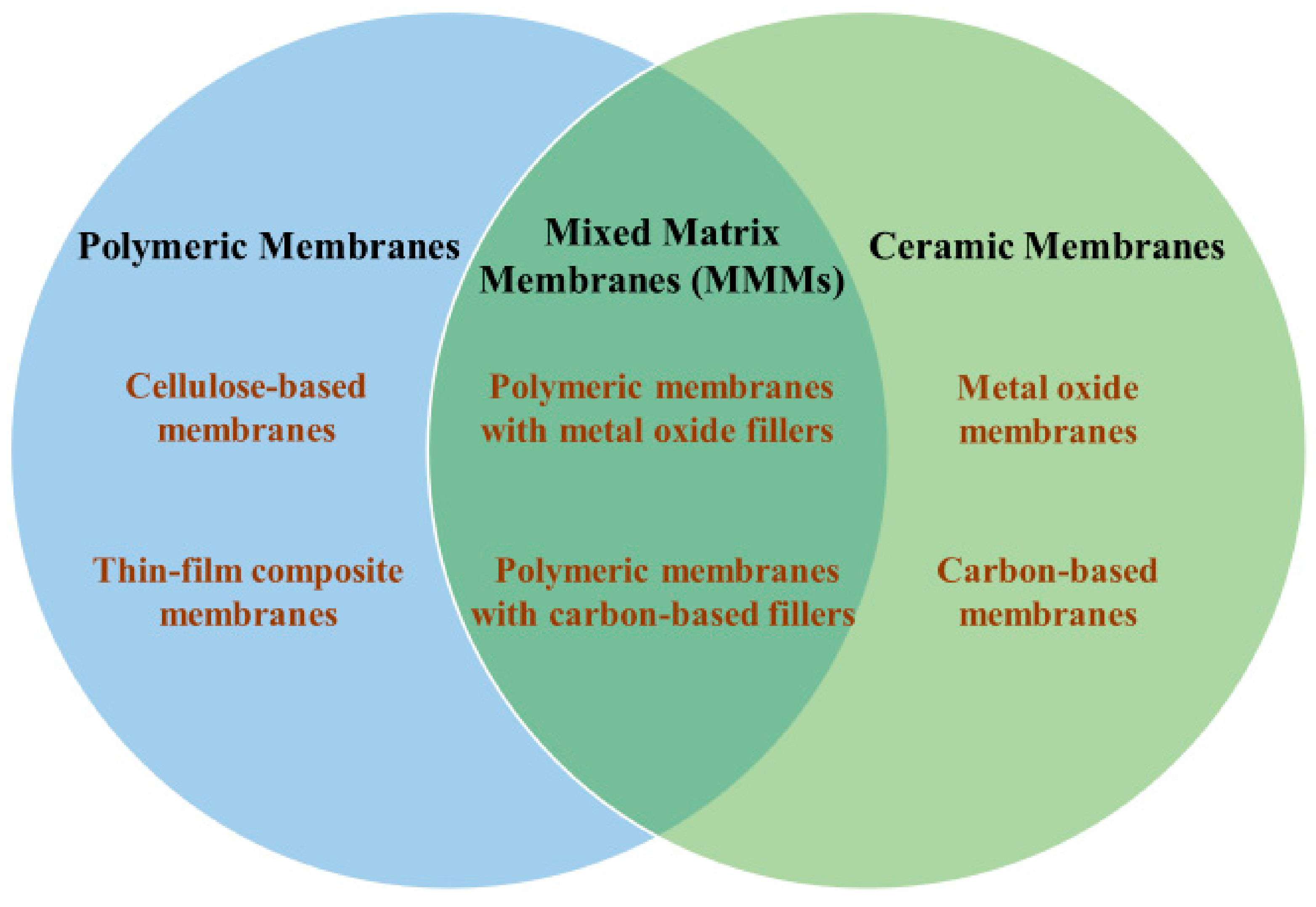
2.1. Polymeric Membranes
2.1.1. Cellulose-Based Membranes
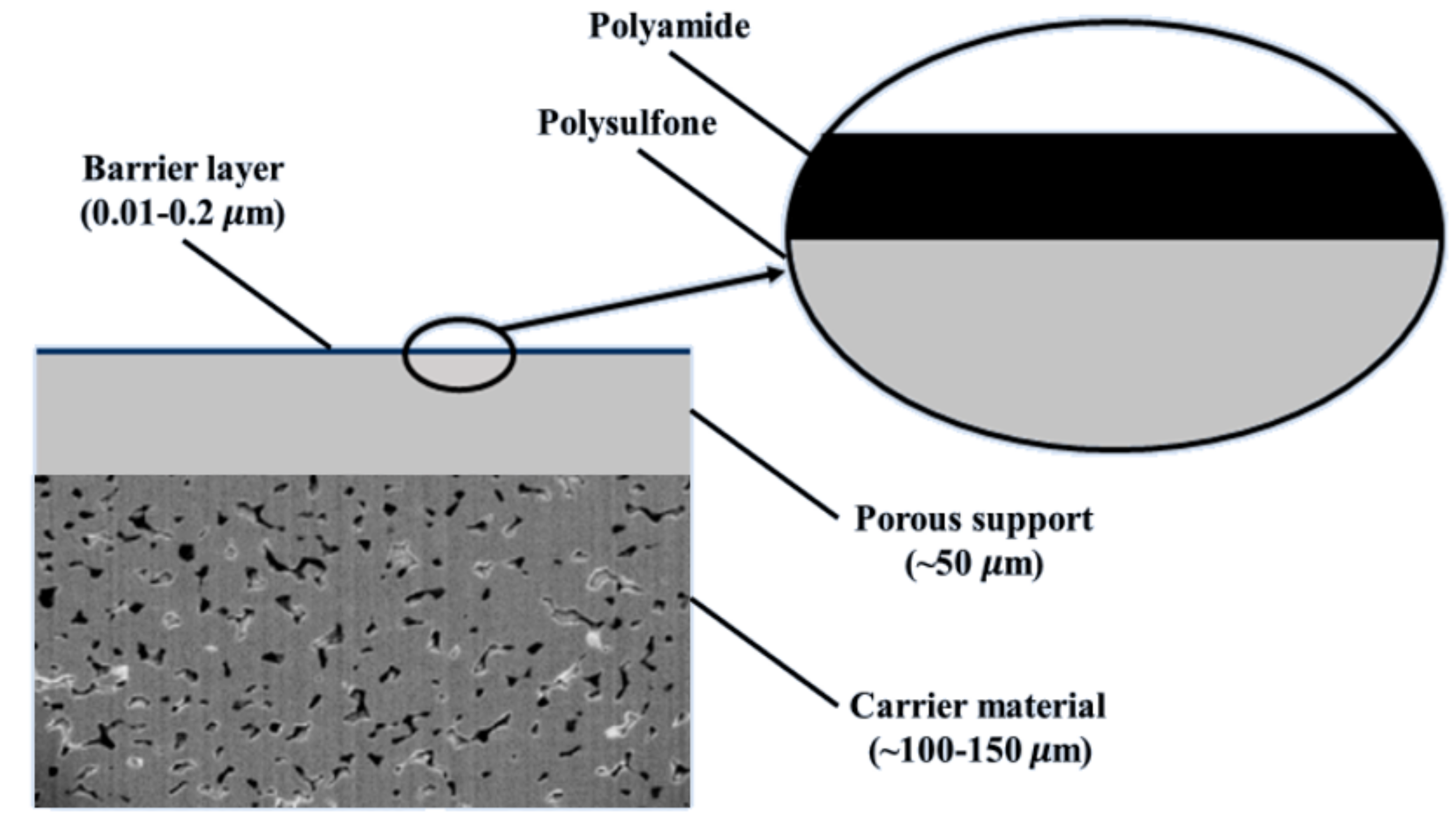
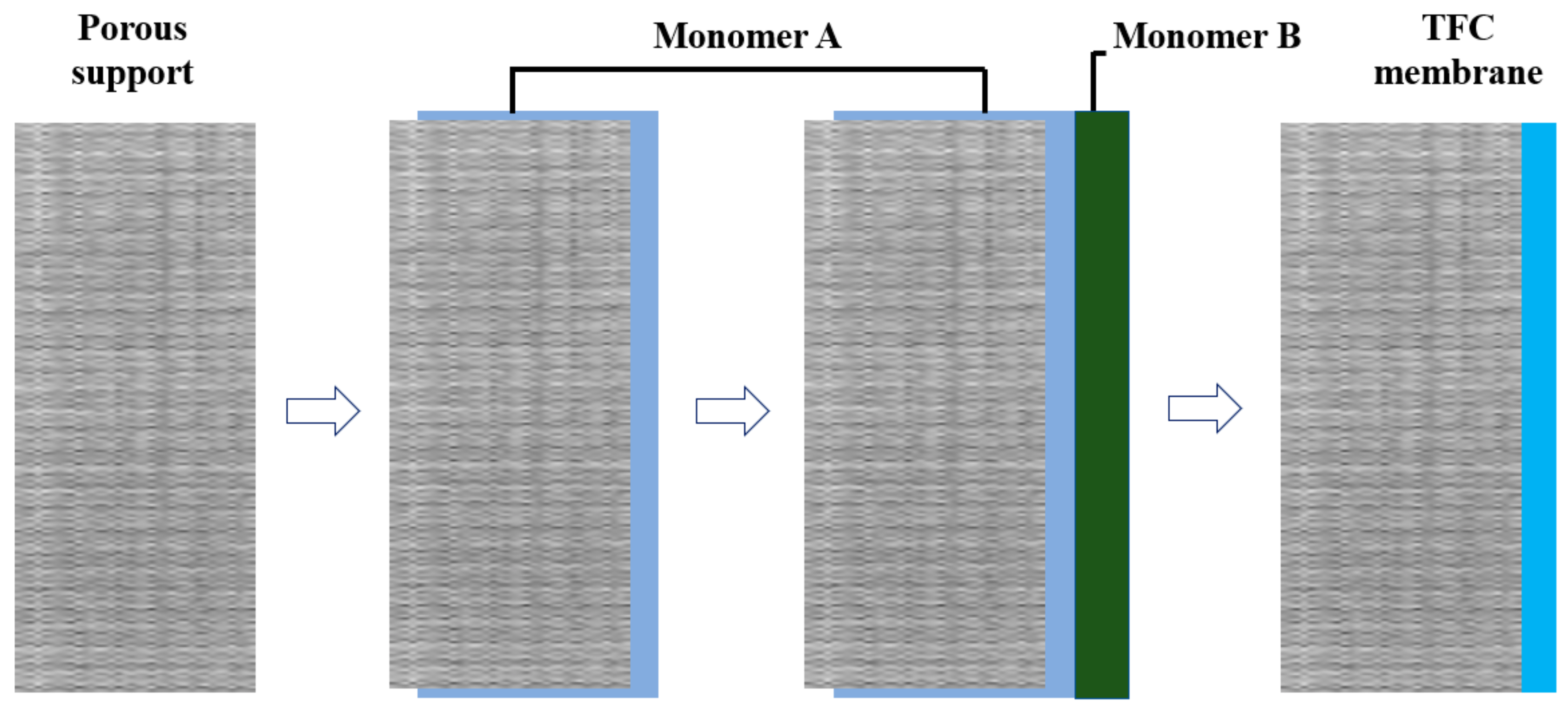
2.1.2. Thin-Film Composite Membranes
2.2. Ceramic Membranes
2.2.1. Metal Oxide Membranes
2.2.2. Carbon-Based Membranes
2.3. Mixed Matrix Membranes
3. Challenges and Future Perspectives
4. Conclusions
Funding
Conflicts of Interest
References
- Satapathy, S.K.; Kanungo, S. Environment friendly industrial growth for sustainability. Int. J. Life Sci. Earth Sci. 2018, 1, 1–14. [Google Scholar]
- Guo, L.; Jin, H.; Ge, Z.; Lu, Y.; Cao, C. Industrialization prospects for hydrogen production by coal gasification in supercritical water and novel thermodynamic cycle power generation system with no pollution emission. Sci. China Technol. Sci. 2015, 58, 1989–2002. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Yu, D.; Chen, X.; Cheng, R.; Min, S.; Wang, J.; Xiao, Q.; Wang, J. Overview of membrane technology applications for industrial wastewater treatment in China to increase water supply. Resour. Conserv. Recycl. 2015, 105, 1–10. [Google Scholar] [CrossRef]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Chaudhary, H.J. Microbial biotechnology as an emerging industrial wastewater treatment process for arsenic mitigation: A critical review. J. Clean. Prod. 2017, 151, 427–438. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Chen, W.; Mo, J.; Du, X.; Zhang, Z.; Zhang, W. Biomimetic dynamic membrane for aquatic dye removal. Water Res. 2019, 151, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Yamjala, K.; Nainar, M.S.; Ramisetti, N.R. Methods for the analysis of azo dyes employed in food industry. —A review. Food Chem. 2016, 192, 813–824. [Google Scholar] [CrossRef]
- Hansen, É.; Rodrigues, M.A.S.; Aragão, M.E.; de Aquim, P.M. Water and wastewater minimization in a petrochemical industry through mathematical programming. J. Clean. Prod. 2018, 172, 1814–1822. [Google Scholar] [CrossRef]
- Lively, R.P.; Sholl, D.S. From water to organics in membrane separations. Nat. Mater. 2017, 16, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Di, J.; Zhang, X. Uncertainty analysis of primary water pollutant control in China’s pulp and paper industry. J. Environ. Manag. 2016, 169, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Das, P.; Datta, S. Removal of Ranitidine from Pharmaceutical Waste Water Using Activated Carbon (AC) Prepared from Waste Lemon Peel. In Waste Water Recycling and Management; Springer: Singapore, 2019; pp. 123–141. [Google Scholar]
- Tortora, F.; Innocenzi, V.; Prisciandaro, M.; de Michelis, I.; Vegliò, F.; di Celso, G.M. Removal of tetramethyl ammonium hydroxide from synthetic liquid wastes of electronic industry through micellar enhanced ultrafiltration. J. Dispers. Sci. Technol. 2018, 39, 207–213. [Google Scholar] [CrossRef]
- Moslehyani, A.; Goh, P.S. Recent Progresses of Ultrafiltration (UF) Membranes and Processes in Water Treatment. In Membrane Separation Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–110. [Google Scholar]
- Davis, R.H. Microfiltration in Pharmaceutics and Biotechnology. In Current Trends and Future Developments on (Bio-)Membranes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–67. [Google Scholar]
- Zhao, D.; Yu, S. A review of recent advance in fouling mitigation of NF/RO membranes in water treatment: Pretreatment, membrane modification, and chemical cleaning. Desalin. Water Treat. 2015, 55, 870–891. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Ali, Z.; Al Sunbul, Y.; Pacheco, F.; Ogieglo, W.; Wang, Y.; Genduso, G.; Pinnau, I. Defect-free highly selective polyamide thin-film composite membranes for desalination and boron removal. J. Membr. Sci. 2019, 578, 85–94. [Google Scholar] [CrossRef]
- Hadi, P.; Yang, M.; Ma, H.; Huang, X.; Walker, H.; Hsiao, S.B. Biofouling-resistant nanocellulose layer in hierarchical polymeric membranes: Synthesis, characterization and performance. J. Membr. Sci. 2019, 579, 162–171. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid SM, S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Melbiah, J.S.B.; Joseph, P.; Rana, D.; Nagendran, A.; Gandhi, N.N.; Mohan, D.R. Customized antifouling polyacrylonitrile ultrafiltration membranes for effective removal of organic contaminants from aqueous stream. J. Chem. Technol. Biotechnol. 2019, 94, 859–868. [Google Scholar] [CrossRef]
- Vasanth, D.; Prasad, A.D. Ceramic Membrane: Synthesis and Application for Wastewater Treatment—A Review. In Water Resources and Environmental Engineering II; Springer: Singapore, 2019; pp. 101–106. [Google Scholar]
- Wills, J.; Moazzem, S.; Jegatheesan, V. Treating Car Wash Wastewater by Ceramic Ultrafiltration Membranes for Reuse Purposes. In Water Scarcity and Ways to Reduce the Impact; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–73. [Google Scholar]
- Saikia, J.; Sarmah, S.; Bora, J.J.; Das, B.; Goswamee, L.R. Preparation and characterization of low cost flat ceramic membranes from easily available potters’ clay for dye separation. Bull. Mater. Sci. 2019, 42, 104. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Song, B.; Cao, W.; Liu, H.-Y.; Huan, S.-Y.; Peng, P. Novel “loose” GO/MoS2 composites membranes with enhanced permeability for effective salts and dyes rejection at low pressure. J. Membr. Sci. 2019, 574, 112–123. [Google Scholar] [CrossRef]
- Thomas, M.; Corry, B. A computational assessment of the permeability and salt rejection of carbon nanotube membranes and their application to water desalination. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150020. [Google Scholar] [CrossRef]
- Glater, J. The early history of reverse osmosis membrane development. Desalination 1998, 117, 297–309. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane. Adv. Chem. Ser. 1963, 38, 117–132. [Google Scholar]
- Holloway, R.W.; Achilli, A.; Cath, T.Y. The osmotic membrane bioreactor: A critical review. Environ. Sci. Water Res. Technol. 2015, 1, 581–605. [Google Scholar] [CrossRef]
- Shaulsky, E.; Karanikola, V.; Straub, A.P.; Deshmukh, A.; Zucker, I.; Elimelech, M. Asymmetric membranes for membrane distillation and thermo-osmotic energy conversion. Desalination 2019, 452, 141–148. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Chou, W.-L.; Yu, D.-G.; Yang, M.-C. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2015, 16, 600–607. [Google Scholar] [CrossRef]
- Ye, S.H.; Watanabe, J.; Iwasaki, Y.; Ishihara, K. In situ modification on cellulose acetate hollow fiber membrane modified with phospholipid polymer for biomedical application. J. Membr. Sci. 2005, 249, 133–141. [Google Scholar] [CrossRef]
- Goossens, I.; van Haute, A. The influence of mineral fillers on the membrane properties of high flux asymmetric cellulose acetate reverse osmosis membranes. Desalination 1976, 18, 203–214. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Kumar, S.A. Effect of additives concentration on performance of cellulose acetate and polyethersulfone blend membranes. J. Porous Mater. 2010, 17, 515–522. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, K.-S.; Jeong, M.-H.; Lee, J.-S. Highly chlorine-resistant end-group crosslinked sulfonated-fluorinated poly (arylene ether) for reverse osmosis membrane. J. Membr. Sci. 2011, 12, 512–519. [Google Scholar] [CrossRef]
- Park, J.; Choi, W.; Kim, S.H.; Chun, B.H.; Bang, J.; Lee, K.B. Enhancement of Chlorine Resistance in Carbon Nanotube Based Nanocomposite Reverse Osmosis Membranes. Desalin. Water Treat. 2010, 15, 198–204. [Google Scholar] [CrossRef]
- Park, H.B.; Freeman, B.D.; Zhang, Z.-B.; Sankir, M.; McGrath, J.E. Highly Chlorine-Tolerant Polymers for Desalination. Angew. Chem. Int. Ed. 2008, 47, 6019–6024. [Google Scholar] [CrossRef]
- Fujiwara, N.; Numata, K.; Kumano, A.; Ogino, Y.; Nagai, M.; Iwahashi, H. The effect of heavy metal ions on the oxidation of cellulose triacetate membranes. Desalination 1994, 96, 431–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Yan, H.; Pan, G.; Guo, M.; Na, H.; Liu, Y. Highly chlorine-resistant multilayer reverse osmosis membranes based on sulfonated poly (arylene ether sulfone) and poly (vinyl alcohol). Desalination 2014, 336, 58–63. [Google Scholar] [CrossRef]
- Shintani, T.; Matsuyama, H.; Kurata, N.; Ohara, T. Development of a chlorine-resistant polyamide nanofiltration membrane and its field-test results. J. Appl. Polym. Sci. 2007, 106, 4174–4179. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Gohil, J.M.; Suresh, A.K. Chlorine attack on reverse osmosis membranes: Mechanisms and mitigation strategies. J. Membr. Sci. 2017, 541, 108–126. [Google Scholar] [CrossRef]
- Yan, F.; Chen, H.; Lü, Y.; Lü, Z.; Yu, S.; Liu, M.; Gao, C. Improving the water permeability and antifouling property of thin-film composite polyamide nanofiltration membrane by modifying the active layer with triethanolamine. J. Membr. Sci. 2016, 513, 108–116. [Google Scholar] [CrossRef]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A Novel Approach Toward Fabrication of High Performance Thin Film Composite Polyamide Membranes. Sci. Rep. 2016, 6, 22069. [Google Scholar] [CrossRef] [PubMed]
- Simcik, M.; Ruzicka, M.; Karaszova, M.; Sedlakova, Z.; Vejrazka, J.; Veselý, M.; Capek, P.; Friess, K.; Izák, P. Polyamide thin-film composite membranes for potential raw biogas purification: Experiments and modeling. Sep. Purif. Technol. 2016, 167, 163–173. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Synthetic membrane characterisation—A review: Part I. Membr. Technol. 2017, 2017, 7–12. [Google Scholar] [CrossRef]
- Khajouei, M.; Peyravi, M.; Jahanshahi, M. The Potential of Nanoparticles for Upgrading Thin Film Nanocomposite Membranes—A Review. J. Membr. Sci. Res. 2017, 3, 2–12. [Google Scholar]
- Giwa, A.; Akther, N.; Dufour, V.; Hasan, S.W. A critical review on recent polymeric and nano-enhanced membranes for reverse osmosis. RSC Adv. 2016, 6, 8134–8163. [Google Scholar] [CrossRef]
- Guclu, S.; Erkoc-Ilter, S.; Koseoglu-Imer, D.Y.; Unal, S.; Menceloglu, Y.Z.; Ozturk, I.; Koyuncu, I. Interfacially polymerized thin-film composite membranes: Impact of support layer pore size on active layer polymerization and seawater desalination performance. Sep. Purif. Technol. 2019, 212, 438–448. [Google Scholar]
- Zhao, Y.; Dai, L.; Zhang, Q.; Zhou, S.; Zhang, S. Chlorine-resistant sulfochlorinated and sulfonated polysulfone for reverse osmosis membranes by coating method. J. Colloid Interface Sci. 2019, 541, 434–443. [Google Scholar] [CrossRef]
- Borisov, I.; Ovcharova, A.; Bakhtin, D.; Bazhenov, S.; Volkov, A.; Ibragimov, R.; Gallyamov, R.; Bondarenko, G.; Mozhchil, R.; Bildyukevich, A.; et al. Development of Polysulfone Hollow Fiber Porous Supports for High Flux Composite Membranes: Air Plasma and Piranha Etching. Fibers 2017, 5, 6. [Google Scholar] [CrossRef]
- Kim, E.-S.; Kim, Y.J.; Yu, Q.; Deng, B. Preparation and characterization of polyamide thin-film composite (TFC) membranes on plasma-modified polyvinylidene fluoride (PVDF). J. Membr. Sci. 2009, 334, 71–81. [Google Scholar] [CrossRef]
- Yang, S.; Zhen, H.; Su, B. Polyimide thin film composite (TFC) membranes via interfacial polymerization on hydrolyzed polyacrylonitrile support for solvent resistant nanofiltration. RSC Adv. 2017, 7, 42800–42810. [Google Scholar] [CrossRef]
- Yasukawa, M.; Mishima, S.; Tanaka, Y.; Takahashi, T.; Matsuyama, H. Thin-film composite forward osmosis membrane with high water flux and high pressure resistance using a thicker void-free polyketone porous support. Desalination 2017, 402, 1–9. [Google Scholar] [CrossRef]
- Mahdavi, H.; Moslehi, M. A new thin film composite nanofiltration membrane based on PET nanofiber support and polyamide top layer: Preparation and characterization. J. Polym. Res. 2016, 23, 257. [Google Scholar] [CrossRef]
- Alsvik, I.L.; Hägg, M.-B. Preparation of thin film composite membranes with polyamide film on hydrophilic supports. J. Memb. Sci. 2013, 428, 225–231. [Google Scholar] [CrossRef]
- Tijing, L.D.; Yao, M.; Ren, J.; Park, C.-H.; Kim, C.S.; Shon, H.K. Nanofibers for Water and Wastewater Treatment: Recent Advances and Developments; Springer: Singapore, 2019; pp. 431–468. [Google Scholar]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux nanofiltration membranes based on interfacially polymerized polyamide barrier layer on polyacrylonitrile nanofibrous scaffolds. J. Membr. Sci. 2009, 326, 484–492. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Synthesis of high-performance thin film composite (TFC) membranes by controlling the preparation conditions: Technical notes. J. Water Process. Eng. 2019, 30, 100542. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Fang, W.; Wang, R.; Krantz, W.B. Effects of the support on the characteristics and permselectivity of thin film composite membranes. J. Membr. Sci. 2019, 580, 12–23. [Google Scholar] [CrossRef]
- Yu, C.; Li, H.; Zhang, X.; Lü, Z.; Yu, S.; Liu, M.; Gao, C. Polyamide thin-film composite membrane fabricated through interfacial polymerization coupled with surface amidation for improved reverse osmosis performance. J. Membr. Sci. 2018, 566, 87–95. [Google Scholar] [CrossRef]
- Raaijmakers, M.J.T.; Benes, N.E. Current trends in interfacial polymerization chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Habili, E.M. Preparation and modification of thin film PA membranes with improved antifouling property using acrylic acid and UV irradiation. J. Membr. Sci. 2013, 430, 158–166. [Google Scholar] [CrossRef]
- Varin, K.J.; Lin, N.H.; Cohen, Y. Biofouling and cleaning effectiveness of surface nanostructured reverse osmosis membranes. J. Membr. Sci. 2013, 446, 472–481. [Google Scholar] [CrossRef]
- Yu, S.; Yao, G.; Dong, B.; Zhu, H.; Peng, X.; Liu, J.; Liu, M.; Gao, C. Improving fouling resistance of thin-film composite polyamide reverse osmosis membrane by coating natural hydrophilic polymer sericin. Sep. Purif. Technol. 2013, 118, 285–293. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, W.; Du, Y.; Li, M.; Wang, L.; Zhang, X. Toward Enhancing the Chlorine Resistance of Reverse Osmosis Membranes: An Effective Strategy via an End-capping Technology. Environ. Sci. Technol. 2019, 53, 1296–1304. [Google Scholar] [CrossRef]
- Lu, P.; Li, W.; Yang, S.; Liu, Y.; Wang, Q.; Li, Y. Layered double hydroxide-modified thin–film composite membranes with remarkably enhanced chlorine resistance and anti-fouling capacity. Sep. Purif. Technol. 2019, 220, 231–237. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, G.; Xiong, C.; Long, S.; Wang, X.; Yang, J. Preparation and characterization of thin-film-composite reverse-osmosis polyamide membrane with enhanced chlorine resistance by introducing thioether units into polyamide layer. J. Membr. Sci. 2018, 564, 473–482. [Google Scholar] [CrossRef]
- Tin, M.M.M.; Murakami, H.; Nakagoe, O.; Sano, H.; Zheng, G.; Tanabe, S. Enhancement of Chlorine Resistance on RO Membrane by Surface Modification with Epoxy Glue. Chem. Lett. 2018, 47, 682–685. [Google Scholar] [CrossRef]
- Bing, S.; Wang, J.; Xu, H.; Zhao, Y.; Zhou, Y.; Zhang, L.; Gao, C.; Hou, L. Polyamide thin-film composite membrane modified with persulfate for improvement of perm-selectivity and chlorine-resistance. J. Membr. Sci. 2018, 555, 318–326. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2012, 110, 111–131. [Google Scholar] [CrossRef]
- Xiong, S.; Xu, S.; Zhang, S.; Phommachanh, A.; Wang, Y. Highly permeable and antifouling TFC FO membrane prepared with CD-EDA monomer for protein enrichment. J. Membr. Sci. 2019, 527, 281–290. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Fabrication and characterization of thin-film composite (TFC) nanofiltration membranes incorporated with cellulose nanocrystals (CNCs) for enhanced desalination performance and dye removal. Chem. Eng. J. 2018, 358, 1519–1528. [Google Scholar] [CrossRef]
- Shen, L.; Hung, W.-S.; Zuo, J.; Zhang, X.; Lai, J.-Y.; Wang, Y. High-performance thin-film composite polyamide membranes developed with green ultrasound-assisted interfacial polymerization. J. Membr. Sci. 2018, 570, 112–119. [Google Scholar] [CrossRef]
- Hao, X.; Gao, S.; Tian, J.; Sun, Y.; Cui, F.; Tang, C.Y. Calcium-Carboxyl Intrabridging during Interfacial Polymerization: A Novel Strategy to Improve Antifouling Performance of Thin Film Composite Membranes. Environ. Sci. Technol. 2019, 58, 4371–4379. [Google Scholar] [CrossRef]
- Huang, M.; Meng, L.; Li, B.; Niu, F.; Lv, Y.; Deng, Q.; Li, J. Fabrication of innovative forward osmosis membranes via multilayered interfacial polymerization on electrospun nanofibers. J. Appl. Polym. Sci. 2019, 136, 47247. [Google Scholar] [CrossRef]
- He, M.; Yuan, T.; Dong, W.; Li, P.; Niu, Q.J.; Meng, J. High-performance acid-stable polysulfonamide thin-film composite membrane prepared via spinning-assist multilayer interfacial polymerization. J. Mater. Sci. 2019, 54, 886–900. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Z.; Wang, H.; Jiang, H.; Li, Y.; Wu, C. An acid-stable positively charged polysulfonamide nanofiltration membrane prepared by interfacial polymerization of polyallylamine and 1,3-benzenedisulfonyl chloride for water treatment. RSC Adv. 2019, 9, 2042–2054. [Google Scholar] [CrossRef]
- Wang, R.; Shi, X.; Xiao, A.; Zhou, W.; Wang, Y. Interfacial polymerization of covalent organic frameworks (COFs) on polymeric substrates for molecular separations. J. Membr. Sci. 2018, 566, 197–204. [Google Scholar] [CrossRef]
- Ma, R.; Ji, Y.-L.; Weng, X.-D.; An, Q.-F.; Gao, C.-J. High-flux and fouling-resistant reverse osmosis membrane prepared with incorporating zwitterionic amine monomers via interfacial polymerization. Desalination 2016, 381, 100–110. [Google Scholar] [CrossRef]
- Cheng, J.; Shi, W.; Zhang, L.; Zhang, R. A novel polyester composite nanofiltration membrane formed by interfacial polymerization of pentaerythritol (PE) and trimesoyl chloride (TMC). Appl. Surf. Sci. 2017, 416, 152–159. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.-M.; Xu, Z.-L.; Cao, Y.; Dong, Z.-Q.; Shi, X.-L. Preparation, characterization and solvent resistance of γ-Al2O3/α-Al2O3 inorganic hollow fiber nanofiltration membrane. J. Membr. Sci. 2016, 503, 69–80. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, G.; Ji, S. Covalent crosslinked assembly of tubular ceramic-based multilayer nanofiltration membranes for dye desalination. AiChE J. 2013, 59, 3834–3842. [Google Scholar] [CrossRef]
- Song, Y.; Wang, D.K.; Birkett, G.; Martens, W.; Duke, M.C.; Smart, S.; da Costa, J.C.D. Mixed matrix carbon molecular sieve and alumina (CMS-Al2O3) membranes. Scientific reports, 6, 30703. Mixed Matrix Carbon Molecular Sieve and Alumina (CMS-Al2O3) Membranes. Sci. Rep. 2016, 6, 30703. [Google Scholar] [CrossRef]
- Ren, C.; Fang, H.; Gu, J.; Winnubst, L.; Chen, C. Preparation and characterization of hydrophobic alumina planar membranes for water desalination. J. Eur. Ceram. Soc. 2015, 35, 723–730. [Google Scholar] [CrossRef]
- Yacou, C.; Smart, S.; da Costa, J.C.D. Mesoporous TiO2 based membranes for water desalination and brine processing. Sep. Purif. Technol. 2015, 147, 166–171. [Google Scholar] [CrossRef]
- Da, X.; Chen, X.; Sun, B.; Wen, J.; Qiu, M.; Fan, Y. Preparation of zirconia nanofiltration membranes through an aqueous sol–gel process modified by glycerol for the treatment of wastewater with high salinity. J. Membr. Sci. 2016, 504, 29–39. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, T.; Chen, X.; Qiu, M.; Fan, Y. Fabrication of TiO2-doped ZrO2 nanofiltration membranes by using a modified colloidal sol-gel process and its application in simulative radioactive effluent. J. Membr. Sci. 2016, 514, 476–486. [Google Scholar] [CrossRef]
- Elma, M.; Wang, D.K.; Yacou, C.; da Costa, J.C.D. Interlayer-free P123 carbonised template silica membranes for desalination with reduced salt concentration polarisation. J. Membr. Sci. 2015, 475, 376–383. [Google Scholar] [CrossRef]
- Elma, M.; Yacou, C.; Diniz da Costa, J.; Wang, D. Performance and Long Term Stability of Mesoporous Silica Membranes for Desalination. Membranes 2013, 3, 136–150. [Google Scholar] [CrossRef]
- Elma, M.; Wang, D.K.; Yacou, C.; Motuzas, J.; da Costa, J.C.D. High performance interlayer-free mesoporous cobalt oxide silica membranes for desalination applications. Desalination 2015, 365, 308–315. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, X.; Liu, C.; Zhou, C.; Huang, A. Tuning interlayer spacing of graphene oxide membranes with enhanced desalination performance. Desalination 2019, 460, 56–63. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, M.; Ding, H.; Fu, K.; Fan, Y. A reduced graphene oxide nanofiltration membrane intercalated by well-dispersed carbon nanotubes for drinking water purification. Nanoscale 2016, 8, 5696–5705. [Google Scholar] [CrossRef]
- Han, R.; Wu, P. High-performance graphene oxide nanofiltration membrane with continuous nanochannels prepared by the in situ oxidation of MXene. J. Mater. Chem. A 2019, 7, 6475–6481. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zeng, W.-J.; Jiang, T.-T.; Chen, X.; Zhang, X.-L. Incorporating attapulgite nanorods into graphene oxide nanofiltration membranes for efficient dyes wastewater treatment. Sep. Purif. Technol. 2019, 214, 21–30. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, M.; Wang, J.; Liu, G.; Liu, H.; Jiang, Y. Bioinspired Modification of Layer-Stacked Molybdenum Disulfide (MoS2) Membranes for Enhanced Nanofiltration Performance. ACS Omega 2019, 4, 4012–4022. [Google Scholar] [CrossRef]
- Da, X.; Wen, J.; Lu, Y.; Qiu, M.; Fan, Y. An aqueous sol–gel process for the fabrication of high-flux YSZ nanofiltration membranes as applied to the nanofiltration of dye wastewater. Sep. Purif. Technol. 2015, 152, 37–45. [Google Scholar] [CrossRef]
- Voigt, I.; Richter, H.; Stahn, M.; Weyd, M.; Puhlfürß, P.; Prehn, V.; Günther, C. Scale-up of ceramic nanofiltration membranes to meet large scale applications. Sep. Purif. Technol. 2019, 215, 329–334. [Google Scholar] [CrossRef]
- Pang, R.; Zhang, K. Fabrication of hydrophobic fluorinated silica-polyamide thin film nanocomposite reverse osmosis membranes with dramatically improved salt rejection. J. Colloid Interface Sci. 2018, 510, 127–132. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.; Li, S.; Jin, B.; Yue, Q.; Wang, Z. Cerium oxide doped nanocomposite membranes for reverse osmosis desalination. Chemosphere 2019, 218, 974–983. [Google Scholar] [CrossRef]
- Shqau, K.; Mottern, M.L.; Yu, D.; Verweij, H. Preparation and Properties of Porous alpha-Al2O3 Membrane Supports. J. Am. Ceram. Soc. 2006, 89, 1790–1794. [Google Scholar] [CrossRef]
- Ghorbani, M.; Abdizadeh, H.; Golobostanfard, M.R. Hierarchical porous ZnO films synthesized by sol–gel method using triethylenetetramine stabilizer. SN Appl. Sci. 2019, 1, 267. [Google Scholar] [CrossRef]
- Bekkari, R.; Jaber, B.; Labrim, H.; Ouafi, M.; Zayyoun, N.; Laânab, L. Effect of Solvents and Stabilizer Molar Ratio on the Growth Orientation of Sol-Gel-Derived ZnO Thin Films. Int. J. Photoenergy 2019, 2019, 3164043. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Lin, Y.; Cai, Y.; Qiu, M.; Fan, Y. Preparation of high-flux γ-alumina nanofiltration membranes by using a modified sol–gel method. Microporous Mesoporous Mater. 2015, 214, 195–203. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Wang, S.; Xu, C.; Yang, K.; Xu, M. Removal of Pb(II) from wastewater using Al2O3-NaA zeolite composite hollow fiber membranes synthesized from solid waste coal fly ash. Chemosphere 2018, 206, 278–284. [Google Scholar] [CrossRef]
- Da Silva, D.G.; Vasconcelos, W.L.; da Silva, D.G.; Vasconcelos, W.L. Effect of sol-gel processing parameters on structure of zirconia. Cerâmica 2019, 65, 17–21. [Google Scholar] [CrossRef]
- Han, Y.; Huang, J.; Li, W.; Qiao, X.; Gou, M.; Yan, J.; Zhang, T. Synthesis and Characterization of ZrO2 Membranes for Nanofiltration via Sol–Gel Combined with In-Suit Polymerization Method. Nanosci. Nanotechnol. Lett. 2019, 11, 182–192. [Google Scholar] [CrossRef]
- Martin-Orue, C.; Bouhallab, S.; Garem, A. Nanofiltration of amino acid and peptide solutions: Mechanisms of separation. J. Membr. Sci. 1998, 142, 225–233. [Google Scholar] [CrossRef]
- Elmarraki, Y.; Cretin, M.; Persin, M.; Sarrazin, J.; Larbot, A. Elaboration and properties of TiO2–ZnAl2O4 ultrafiltration membranes. Mater. Res. Bull. 2001, 36, 227–237. [Google Scholar] [CrossRef]
- Li, X.; Forouzandeh, F.; Fürstenhaupt, T.; Banham, D.; Feng, F.; Ye, S.; Kwok, D.Y.; Birss, V. New insights into the surface properties of hard-templated ordered mesoporous carbons. Carbon 2018, 127, 707–717. [Google Scholar] [CrossRef]
- Xu, X.; Tan, H.; Wang, Z.; Wang, C.; Pan, L.; Kaneti, Y.V.; Yang, T.; Yamauchi, Y. Extraordinary capacitive deionization performance of highly-ordered mesoporous carbon nano-polyhedra for brackish water desalination. Environ. Sci. Nano 2019, 6, 981–989. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; Bezerra, C.d.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Deng, Y.; Ok, Y.S.; Mohan, D.; Pittman, C.U.; Dou, X. Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ. Res. 2019, 169, 434–444. [Google Scholar] [CrossRef]
- Libbrecht, W.; Verberckmoes, A.; Thybaut, J.W.; van der Voort, P.; de Clercq, J. Soft templated mesoporous carbons: Tuning the porosity for the adsorption of large organic pollutants. Carbon 2017, 116, 528–546. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Jame, S.A.; Zhou, Z. Electrochemical carbon nanotube filters for water and wastewater treatment. Nanotechnol. Rev. 2016, 5, 41–50. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.-H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, J.; Zhu, C.; Zeng, X.C.; Francisco, J.S. Water desalination through rim functionalized carbon nanotubes. J. Mater. Chem. A 2019, 7, 3583–3591. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, W.; Zhang, X. Carbon Nanotubes for Advancing Separation Membranes. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yang, G.; Xie, Z.; Cran, M.; Ng, D.; Gray, S. Enhanced desalination performance of poly (vinyl alcohol)/carbon nanotube composite pervaporation membranes via interfacial engineering. J. Membr. Sci. 2019, 579, 40–51. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Water desalination via novel positively charged hybrid nanofiltration membranes filled with hyperbranched polyethyleneimine modified MWCNT. J. Ind. Eng. Chem. 2019, 69, 127–140. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A. Graphene-Based Materials for Water Purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ahmed, M.; Giwa, A.; Hasan, S.W. Challenges and Opportunities of Graphene-Based Materials in Current Desalination and Water Purification Technologies. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, C.; Guo, Y.; Shen, L.; Ji, C.; Bao, N. Scalable concentration process of graphene oxide dispersions via cross-flow membrane filtration. Chem. Eng. Sci. 2019, 200, 127–137. [Google Scholar] [CrossRef]
- Poniatowska, A.; Trzaskowski, M.; Ciach, T. Production and properties of top-down and bottom-up graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 315–324. [Google Scholar] [CrossRef]
- Ma, B.; Ren, S.; Wang, P.; Jia, C.; Guo, X. Precise control of graphene etching by remote hydrogen plasma. Nano Res. 2019, 12, 137–142. [Google Scholar] [CrossRef]
- McDonnell, S.J.; Wallace, R.M. UV-Ozone Functionalization of 2D Materials. JOM 2019, 71, 224–237. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Zakeri, M.; Nasef, M.M.; Miyake, M.; Mozarmnia, P.; Bazilah, N.A.; Emelin, N.F.; Ahmad, A. Highly durable polybenzimidazole composite membranes with phosphonated graphene oxide for high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2019, 412, 238–245. [Google Scholar] [CrossRef]
- O’Hern, S.C.; Boutilier, M.S.H.; Idrobo, J.-C.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective Ionic Transport through Tunable Subnanometer Pores in Single-Layer Graphene Membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef]
- Makhija, G.; Sharma, V.; Singh, S.; Sharma, N.; Vyas, R.; Sachdev, K. Investigation on the suitability of water/polyethylene glycol solutions for GO layer deposition in GO/Ag/GO films for transparent conducting electrode. Appl. Nanosci. 2019, 1–13. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, F.; Zhang, P.; An, Z.; Zhao, Y.; Chen, L. The photodegradation of methylene blue in water with PVDF/GO/ZnO composite membrane. Mater. Sci. Eng. C 2019, 96, 684–692. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast Mass Transport Through Sub-2-Nanometer Carbon Nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, H.; Wang, H.; Rassu, P.; Wang, Z.; Song, P.; Rao, D. Free-standing graphene oxide membrane with tunable channels for efficient water pollution control. J. Hazard. Mater. 2019, 366, 659–668. [Google Scholar] [CrossRef]
- Rozaini, M.N.H.; Semail, N.-F.; Saad, B.; Kamaruzaman, S.; Abdullah, W.N.; Rahim, N.A.; Miskam, M.; Loh, S.H.; Yahaya, N. Molecularly imprinted silica gel incorporated with agarose polymer matrix as mixed matrix membrane for separation and preconcentration of sulfonamide antibiotics in water samples. Talanta 2019, 199, 522–531. [Google Scholar] [CrossRef]
- Zhu, H.; Yuan, J.; Zhao, J.; Liu, G.; Jin, W. Enhanced CO2/N2 separation performance by using dopamine/polyethyleneimine-grafted TiO2 nanoparticles filled PEBA mixed-matrix membranes. Sep. Purif. Technol. 2019, 214, 78–86. [Google Scholar] [CrossRef]
- Forman, E.M.; Baniani, A.; Fan, L.; Ziegler, K.J.; Zhou, E.; Zhang, F.; Lively, R.P.; Vasenkov, S. Ethylene diffusion in crystals of zeolitic imidazole Framework-11 embedded in polymers to form mixed-matrix membranes. Microporous Mesoporous Mater. 2019, 274, 163–170. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Dashti, A.; Hajilary, N.; Shirazian, S. Organic/Silica Nanocomposite Membranes Applicable to Green Chemistry. In Sustainable Polymer Composites and Nanocomposites; Springer International Publishing: Cham, Switzerland, 2019; pp. 629–652. [Google Scholar]
- Ayaz, M.; Muhammad, A.; Younas, M.; Khan, A.L.; Rezakazemi, M. Enhanced Water Flux by Fabrication of Polysulfone/Alumina Nanocomposite Membrane for Copper(II) Removal. Macromol. Res. 2019, 27, 565–571. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Kim, S.H.; Kim, S.S. Hybrid Organic/Inorganic Reverse Osmosis (RO) Membrane for Bactericidal Anti-Fouling. 1. Preparation and Characterization of TiO2 Nanoparticle Self-Assembled Aromatic Polyamide Thin-Film-Composite (TFC) Membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.-Y.; Sohn, B.-H.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Effect of added NaX nano-zeolite into polyamide as a top thin layer of membrane on water flux and salt rejection in a reverse osmosis process. J. Membr. Sci. 2011, 375, 88–95. [Google Scholar] [CrossRef]
- Huang, H.; Qu, X.; Ji, X.; Gao, X.; Zhang, L.; Chen, H.; Hou, L. Acid and multivalent ion resistance of thin film nanocomposite RO membranes loaded with silicalite-1 nanozeolites. J. Mater. Chem. A 2013, 1, 11343. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Ji, G.; Gu, X. CFD simulation of hollow fiber supported NaA zeolite membrane modules. Sep. Purif. Technol. 2019, 213, 1–10. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Majumder, M.; Chopra, N.; Andrews, R.; Hinds, B.J. Enhanced flow in carbon nanotubes. Nature 2005, 438, 44. [Google Scholar] [CrossRef]
- Kotsalis, E.M.; Walther, J.H.; Koumoutsakos, P. Multiphase water flow inside carbon nanotubes. Int. J. Multiph. Flow 2004, 30, 995–1010. [Google Scholar] [CrossRef]
- Ratto, T.V.; Holt, J.K.; Szmodis, W. Membranes with Embedded Nanotubes for Selective Permeability. U.S. Patent 7993524B2, 25 June 2011. [Google Scholar]
- Badalyan, H.G.; Faltajanyan, S.H. Influence of Ionizing Radiation on the Structure of a Lyotropic Liquid Crystal. J. Contemp. Phys. Armen. Acad. Sci. 2019, 54, 65–70. [Google Scholar] [CrossRef]
- Gan, H.X.; Zhou, H.; Lee, H.J.; Lin, Q.; Tong, Y.W. Toward a Better Understanding of the Nature-Inspired Aquaporin Biomimetic Membrane. Langmuir 2019, 35, 7285–7293. [Google Scholar] [CrossRef]
- Tsuru, T.; Nakao, S.; Kimura, S. Calculation of ion rejection by extended Nernst-Planck equation with charged reverse osmosis membranes for single and mixed electrolyte solutions. J. Chem. Eng. Jpn. 1991, 24, 511–517. [Google Scholar] [CrossRef]
- Oatley-Radcliffe, D.L.; Williams, S.R.; Barrow, M.S.; Williams, P.M. Critical appraisal of current nanofiltration modelling strategies for seawater desalination and further insights on dielectric exclusion. Desalination 2014, 343, 154–161. [Google Scholar] [CrossRef]
- Tarquim, A.J.; Walker, W.S.; Delgado, G.G. Sea Water Reverse Osmosis System to Reduce Concentrate Volume Prior to Disposal. U.S. Patent 20170203979A1, 30 March 2017. [Google Scholar]
- Ślȩzak, A. Irreversible thermodynamic model equations of the transport across a horizontally mounted membrane. Biophys. Chem. 1989, 34, 91–102. [Google Scholar] [CrossRef]
- Yaroshchuk, A.; Bruening, M.L.; Zholkovskiy, E. Modelling nanofiltration of electrolyte solutions. Adv. Colloid Interface Sci. 2019, 268, 39–63. [Google Scholar] [CrossRef]
- Jang, E.-S.; Mickols, W.; Sujanani, R.; Helenic, A.; Dilenschneider, T.J.; Kamcev, J.; Paul, D.R.; Freeman, B.D. Influence of concentration polarization and thermodynamic non-ideality on salt transport in reverse osmosis membranes. J. Membr. Sci. 2019, 572, 668–675. [Google Scholar] [CrossRef]
- Bandini, S.; Vezzani, D. Nanofiltration modeling: The role of dielectric exclusion in membrane characterization. Chem. Eng. Sci. 2003, 58, 3303–3326. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Buekenhoudt, A.; Degrève, J.; van der Bruggen, B. Coupled series–parallel resistance model for transport of solvent through inorganic nanofiltration membranes. Sep. Purif. Technol. 2009, 70, 46–52. [Google Scholar] [CrossRef]
- Park, J.; Jeong, K.; Baek, S.; Park, S.; Ligaray, M.; Chong, T.H.; Cho, K.H. Modeling of NF/RO membrane fouling and flux decline using real-time observations. J. Membr. Sci. 2019, 576, 66–77. [Google Scholar] [CrossRef]
- Tai, Z.S.; Aziz, M.H.A.; Othman, M.H.D.; Dzahir, M.I.H.M.; Hashim, N.A.; Koo, K.N.; Hubadillah, S.K.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Ceramic Membrane Distillation for Desalination. Sep. Purif. Rev. 2019, 1–40. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Mello, A.C.C.; Borges, C.P.; Campos, J.C. Oilfield produced water treatment by ceramic membranes: Preliminary process cost estimation. Desalination 2015, 360, 81–86. [Google Scholar] [CrossRef]
| Membrane | Manufacturer | Selective Layer | Maximum Temperature (°C) | pH Range | Salt Rejection (%) |
|---|---|---|---|---|---|
| SW30HRLE-400 | Dow Filmtec, USA | PA TFC | 45 | 2–11 | 99.8 NaCl |
| NF270-400/34i | Dow Filmtec, USA | PA TFC | 45 | 3–10 | >97 NaCl |
| SWC4+ | Hydranautics, USA | PA TFC | 45 | 3–10 | >99.7 NaCl |
| TM820C-370 | Toray, USA | PA TFC | 45 | 2–11 | >99.5 NaCl |
| HB10255 | Toyobo, Japan | CTA hollow fiber | 40 | 3–8 | >99.4 NaCl |
| TS40 | Microdyn-Nadir, USA | Polypiperazineamide | 45 | 1–12 | 40 NaCl >98.5 MgSO4 |
| TS80 | Microdyn-Nadir, USA | PA TFC | 45 | 1–12 | 80 NaCl >98.5 MgSO4 |
| AD-90 | GE-Osmonics, USA | TFC | 50 | 4–11 | >99.5 NaCl 95% Boron |
| AG4040C | GE-Osmonics, USA | TFC | 50 | 4–11 | >99 NaCl |
| HL2540FM | GE-Osmonics, USA | TFC | 50 | 3–9 | >96 MgSO4 |
| CK4040FM | GE-Osmonics, USA | CA | 30 | 5–6.5 | >94 MgSO4 |
| 8040-SW-400-34 | Koch, USA | Proprietary PA TFC | 45 | 4–11 | >99.5 NaCl |
| 4040-HR | Koch, USA | Proprietary PA TFC | 45 | 4–11 | >99.2 NaCl |
| MPS-34 2540 A2X | Koch, USA | Proprietary composite NF | 50 | 0–14 | 35 NaCl 95 Glucose 97 Sucrose |
| NFX | Synder, USA | Proprietary PA TFC | 50 | 2–11 | 40 NaCl >99 MgSO4 >99 Lactose |
| NFW | Synder, USA | Proprietary PA TFC | 50 | 2–11 | 20 NaCl >97 MgSO4 >98.5 Lactose |
| Membrane | Processing Method | Performance Evaluation | Reference |
|---|---|---|---|
| Cellulose acetate | Blending with polyethersulfone and polyethylene glycol | Such blended membranes had higher porosity (permeability) and chlorine tolerance compared with virgin cellulose acetate membranes. | [36] |
| Sulfonated poly | Made with high fluorine contents | Sulfonated-fluorinated poly membranes displayed long-term stability (>30 days) under high acidic chlorine condition. | [37] |
| Aromatic polyamide | Adding 0.1–1 wt% multi-walled carbon nanotubes | The carbon nanotube based polyamide membranes had good selectivity and longer lifetime during desalination process. | [38] |
| Sulfonated poly | Membranes were prepared by direct copolymerization method | Water permeability and contact angle remained unaffected when exposed to high level of chlorine and wide range of pH (4–10). | [39] |
| Cellulose triacetate | Adding sodium hexametaphosphate (SHMP) as masking agent | SHMP inhibited oxidation degradation of cellulose triacetate membranes by chlorine. | [40] |
| Sulfonated cardo poly | Extra layer of formaldehyde-cross-linked polyvinyl alcohol was coated on membrane surface | The coated layer improved NaCl rejection from 91.2% to 96.8% and the membrane showed better chlorine resistance in RO operation. | [41] |
| Polyamide | Membrane synthesized by interfacial polymerization of N-phenylethylenediamine and 1,3,5-benzenetricarbonyl trichloride | When immersed in NaOCl solution, the membrane exhibited higher chlorine tolerance than a commercial polyamide membrane. | [42] |
| Monomer A | Monomer B | Performance Evaluation | Reference |
|---|---|---|---|
| Ethylenediamine | Cyclodextrins | Membrane had a water flux up to 28 L/m2 h (LMH) and good antifouling properties with flux reduction <20%. | [75] |
| Piperazine | 1,3,5-Benzene-tricarbonyl trichoride | High salt rejection (98% for Na2SO4 and 97.5% for MgSO4) with enhanced water permeability. | [76] |
| m-Phenylenediamine | Trimesoyl chloride | Membrane exhibited large free volume, high water flux, and low reverse salt flux. | [77] |
| Hexylene glycol | 1,3,5-Benzene-tricarbonyl trichoride | Both flux stability and fouling reversibility improved for Ca2+ modified membranes. | [78] |
| 1,3-Phenylenediamine | 1,3,5-Benzene-tricarbonyl trichoride | Membranes with two PA layers showed much higher flux and selectivity than commercial TFC membranes. | [79] |
| Piperazine | 2,4,6-Trischlorosulfonylphenol | Membrane had a flux of 13.98 LMH and good rejections for CuSO4 and H2SO4. | [80] |
| Polyallylamine | 1,3-Benzenedisulfonyl chloride | Membrane was positively charged and had selectivities greater than 90% for heavy metal ions. | [81] |
| p-Phenylenediamine | 1,3,5-Triformylphloroglucinol | Membrane presented a stable rejection to Congo red of 99.5% and a high flux up to 50 LMH. | [82] |
| n-Aminoethyl piperazine propane sulfonate | Trimesoyl chloride | Compared with pristine membrane, the flux increased by 82% while the NaCl rejection remained above 98%. | [83] |
| Pentaerythritol | Trimesoyl chloride | Membrane had a high rejection of Na2SO4 (98.1%) but a low water flux of 6.1 LMH. | [84] |
| Membrane. | Application | Salt Rejection (%) | Flux/Permeability | Reference |
|---|---|---|---|---|
| γ-Al2O3 | Desalination | 97.1 Fe3+, 90.9 Al3+, 85 Mg2+, 84.1 Ca2+, 30.7 Na+, 27.3 NH4+ | 17.4 LMH/bar | [85] |
| PVA-Al2O3 | Dye wastewater treatment, Desalination | 96 Congo red dye 3 NaCl | 25 LMH | [86] |
| CMS-Al2O3 | Desalination | 93 NaCl | 25 kg m−2 h−1, 3.5 wt% NaCl, 75 °C | [87] |
| Al2O3 (FAS grafted) | Desalination | >99.5 NaCl | 19.1 LMH, 2 wt% NaCl, 80 °C | [88] |
| TiO2 | Desalination | 99 NaCl | 6 kg m−2 h−1, 10 wt% NaCl, 75 °C | [89] |
| ZrO2 | High salinity water treatment | >90 PEG 1000 68, 24.92 wt% NaCl | 13 LMH/bar | [90] |
| TiO2-ZrO2 | Radioactive waste treatment | 99.6 Co2+, 99.2 Sr2+, 75.5 Cs+ | 40 LMH/bar | [91] |
| SiO2 | Desalination | 99.5 NaCl | 6.6 kg m−2 h−1, 3.5 wt% NaCl, 22 °C | [92] |
| SiO2 | Desalination | 99.6 NaCl | 9.5 kg m−2 h−1, 3.5 wt% NaCl, 22 °C | [93] |
| CoO-SiO2 | Desalination | 99.7 NaCl | 7.7 kg m−2 h−1, 3.5 wt% NaCl, 22 °C | [94] |
| Ax-GO | Desalination | 99.9 NaCl | 19.7 kg m−2 h−1, 3.5 wt% NaCl, 90 °C | [95] |
| CNT-rGO | Drinking water purification | 97.3 Methyl orange | 20–30 LMH/bar | [96] |
| TiO2-GO | Dye wastewater treatment | >97 Organic dyes | 89.6 LMH/bar | [97] |
| APT-GO | Dye wastewater treatment | ~100 Rhodamine blue | 13.3 LMH, 7.5 mg L−1 RhB | [98] |
| MoS2 | Dye wastewater treatment | 100 Methylene blue | 135.3 LMH/bar | [99] |
| YSZ | Dye wastewater treatment | >98 NaCl | 28 LMH/bar | [100] |
| Suitable Retention Mechanisms | Model | Model Evaluation | Reference |
|---|---|---|---|
| UF | Irreversible thermodynamic model | The model can be used to predict the performance for single electrolyte solution but not for mixed electrolyte solutions. | [156] |
| RO/UF | Extended Nernst-Planck model | Single-ion rejection calculated from the model matched with that obtained from irreversible thermodynamic model, and there is little difference between mixed-ion rejection and experimental data. | [153] |
| NF | Solution-diffusion-electromigration model | Easily modeled chloride and sulfate selectivities with transmission coefficient simplified to zero. | [157] |
| RO | Merten and Lonsdale transport model | The model gave concentration polarization corrected salt transport coefficients whose effects were significant at high feed pressures. | [158] |
| RO/NF | Donnan steric pore model and dielectric exclusion | Dielectric exclusion was considered as the primary effect when analyzed mass transfer of electrolytes and neutral solutes. | [159] |
| NF | Coupled series-parallel resistance model | This model was developed specifically for organic solvents permeating through ceramic membranes and a good fit to experimental data was obtained for different solvents. | [160] |
| RO/NF | Pore blockage-cake filtration model | Model had similar results and coefficient of determination as Faridirad model, but with lower Akaike information criteria values. | [161] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. https://doi.org/10.3390/polym11081252
Yang Z, Zhou Y, Feng Z, Rui X, Zhang T, Zhang Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers. 2019; 11(8):1252. https://doi.org/10.3390/polym11081252
Chicago/Turabian StyleYang, Zi, Yi Zhou, Zhiyuan Feng, Xiaobo Rui, Tong Zhang, and Zhien Zhang. 2019. "A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification" Polymers 11, no. 8: 1252. https://doi.org/10.3390/polym11081252
APA StyleYang, Z., Zhou, Y., Feng, Z., Rui, X., Zhang, T., & Zhang, Z. (2019). A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers, 11(8), 1252. https://doi.org/10.3390/polym11081252







