Antibacterial Activity and Cytotoxicity of Immobilized Glucosamine/Chondroitin Sulfate on Polylactic Acid Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PLA Films
2.2. Plasma Treatment
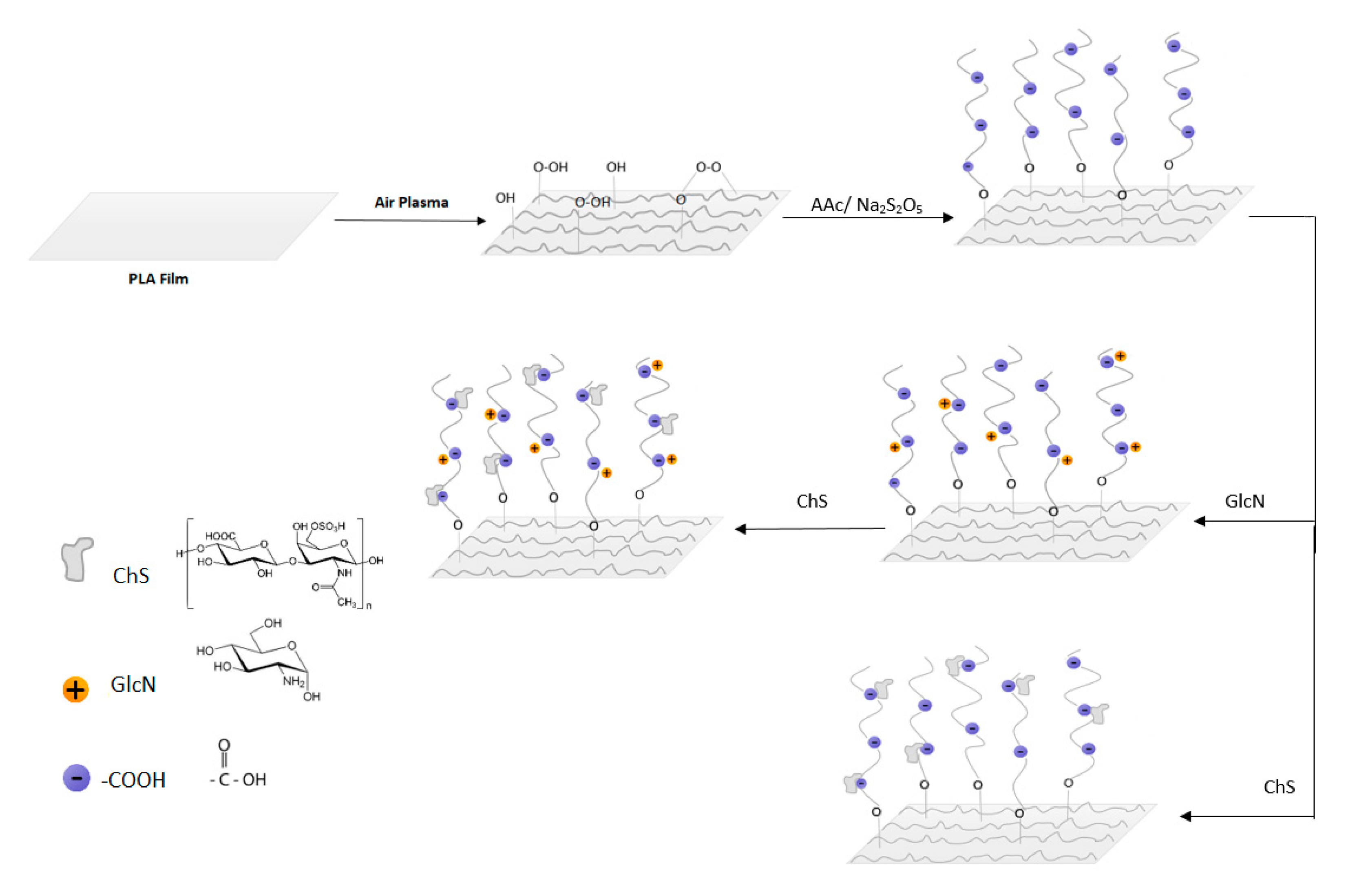
2.3. PAA Grafting and Immobilization of Saccharides
2.4. Characterizations of the Samples
2.5. Cytotoxicity Testing
2.6. Antibacterial Testing
3. Results and Discussion
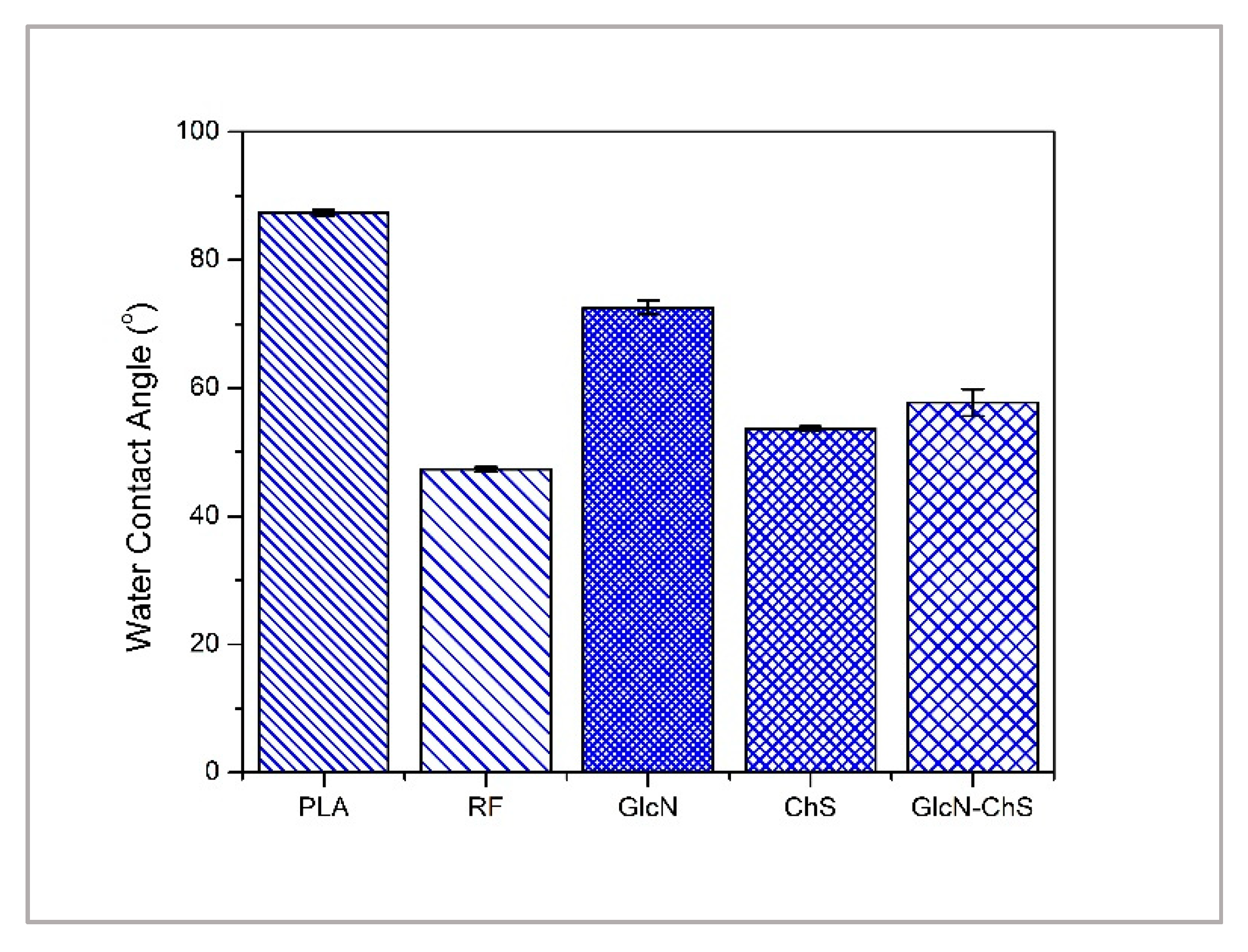
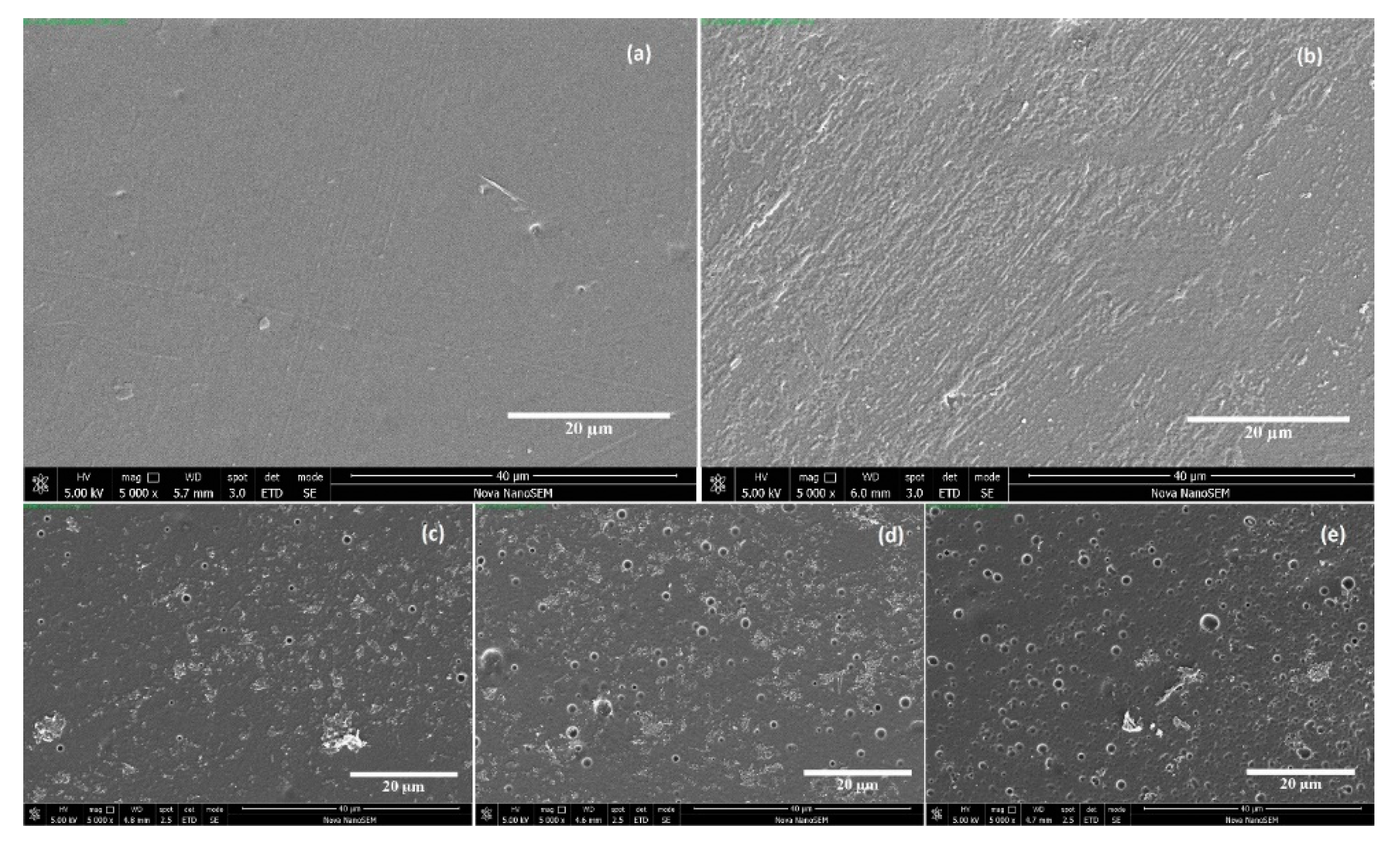
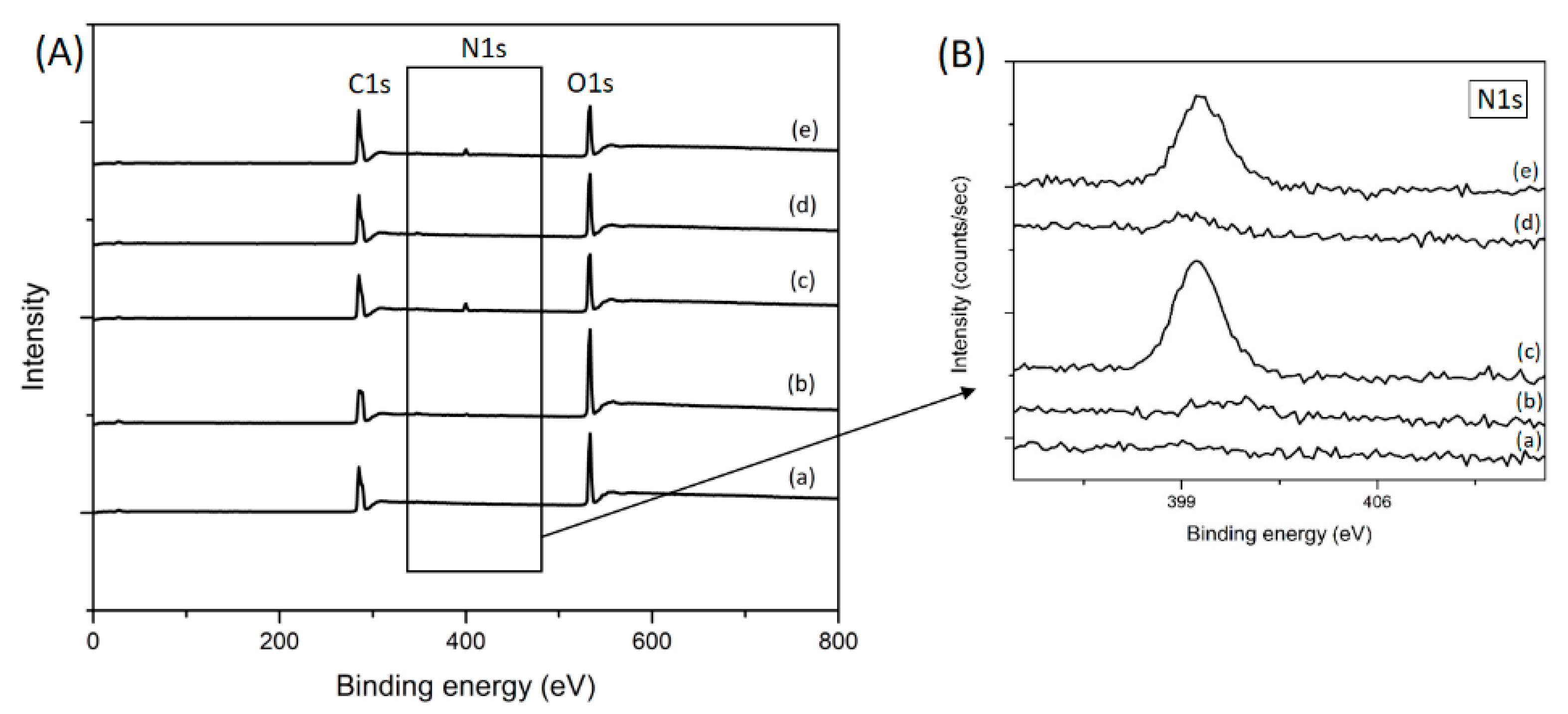
3.1. Surface Chemistry and Morphology
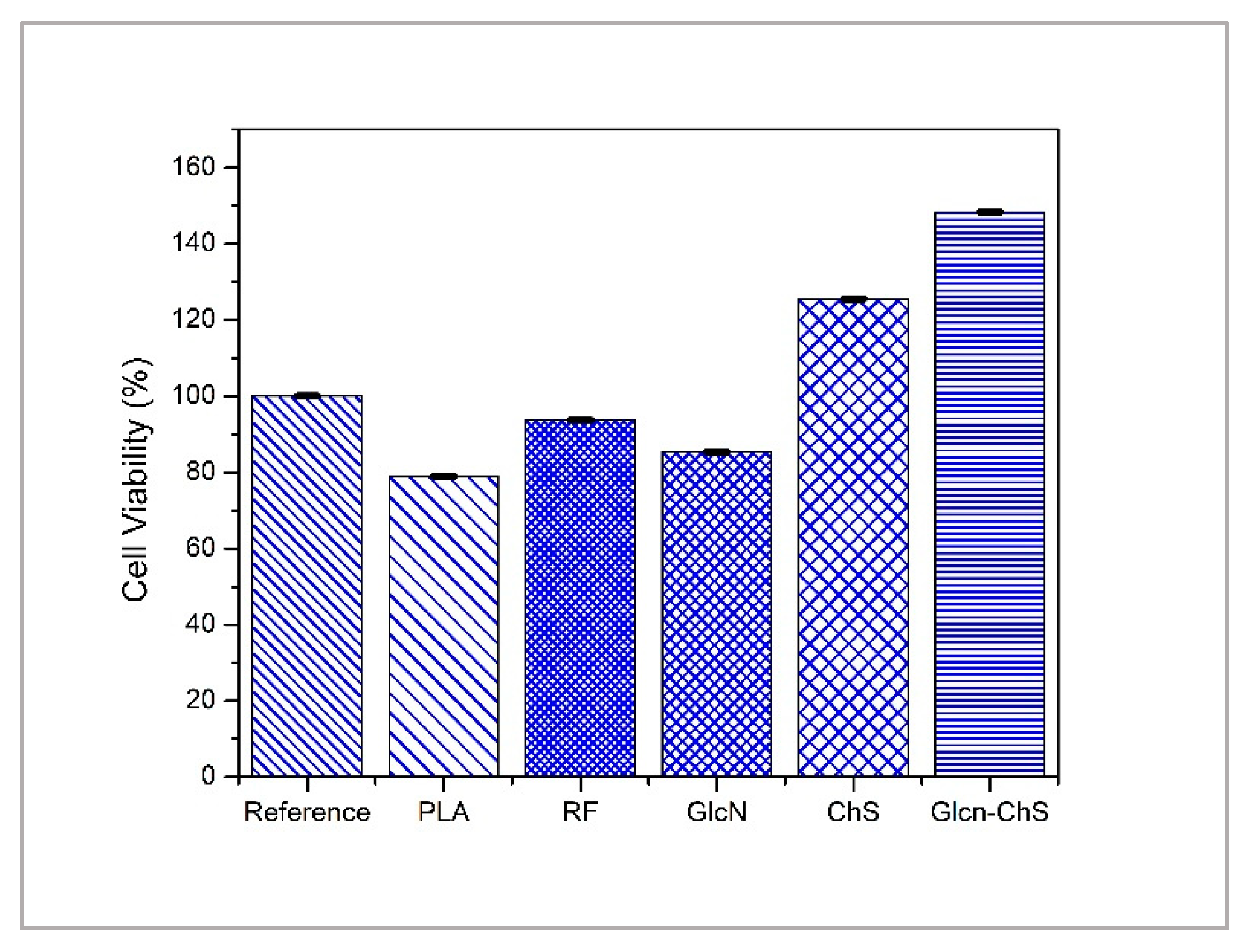
3.2. Cytotoxicity of PLA Films
3.3. Antibacterial Performance of PLA Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vert, M. After soft tissues, bone, drug delivery and packaging, PLA aims at blood. Eur. Polym. J. 2015, 68, 516–525. [Google Scholar] [CrossRef]
- He, C.; Chen, Q.; Yarmolenko, M.; Rogachev, A.; Piliptsou, D.; Jiang, X.; Rogachev, A. Structure and antibacterial activity of PLA-based biodegradable nanocomposite coatings by electron beam deposition from active gas phase. Prog. Org. Coat. 2018, 123, 282–291. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications. In Biomedical Science Engineering Technology; Intech Open: Berlin, Germany, 2012. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Swilem, A.E.; Lehocký, M.; Humpolíček, P.; Kucekova, Z.; Novák, I.; Mičušík, M.; Abd El-Rehim, H.A.; Hegazy, E.A.; Hamed, A.A.; Kousal, J. Description of D-glucosamine immobilization kinetics onto poly(lactic acid) surface via a multistep physicochemical approach for preparation of novel active biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
- Stankevich, K.S.; Danilenko, N.V.; Gadirov, R.M.; Goreninskii, S.I.; Tverdokhlebov, S.I.; Filimonov, V.D. A new approach for the immobilization of poly(acrylic) acid as a chemically reactive cross-linker on the surface of poly(lactic) acid-based biomaterials. Mater. Sci. Eng. C 2017, 71, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Pandiyaraj, K.N.; Ferraria, A.M.; Rego, A.M.; Deshmukh, R.R.; Su, P.; Halleluyah, J.M.; Halim, A.S. Low-pressure plasma enhanced immobilization of chitosan on low-density polyethylene for bio-medical applications. Appl. Surf. Sci. 2015, 328, 1–12. [Google Scholar] [CrossRef]
- Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Pelkova, J.; Martino, A.D.; Karakurt, I.; Saha, P. Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization. Polymers 2019, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Popelka, A.; Novák, I.; Lehocký, M.; Junkar, I.; Mozetič, M.; Kleinová, A.; Janigová, I.; Slouf, M.; Bílek, F.; Chodák, I. A new route for chitosan immobilization onto polyethylene surface. Carbohydr. Polym. 2012, 90, 1501–1508. [Google Scholar] [CrossRef]
- Prat, R.; Shi, M.; Clouet, F. Interactions of Cold Plasmas with Polymers and Their Model Molecules: Degradation vs. Functionalzation. J. Macromol. Sci. Part A 1997, 34, 471–488. [Google Scholar] [CrossRef]
- Bolbasov, E.; Rybachuk, M.; Golovkin, A.; Antonova, L.; Shesterikov, E.; Malchikhina, A.; Novikov, V.A.; Anissimov, Y.G.; Tverdokhlebov, S.I. Surface modification of poly(l-lactide) and polycaprolactone bioresorbable polymers using RF plasma discharge with sputter deposition of a hydroxyapatite target. Mater. Lett. 2014, 132, 281–284. [Google Scholar] [CrossRef]
- Yu, D.; Lin, W.; Lin, C.; Yang, M. Cytocompatibility and Antibacterial Activity of a PHBV Membrane with Surface-Immobilized Water-Soluble Chitosan and Chondroitin-6-sulfate. Macromol. Biosci. 2006, 6, 348–357. [Google Scholar] [CrossRef]
- Stoleru, E.; Dumitriu, R.P.; Munteanu, B.S.; Zaharescu, T.; Tănase, E.E.; Mitelut, A.; Ailiesei, G.L.; Vasile, C. Novel procedure to enhance PLA surface properties by chitosan irreversible immobilization. Appl. Surf. Sci. 2016, 367, 407–417. [Google Scholar] [CrossRef]
- Saxena, S.; Ray, A.R.; Gupta, B. Chitosan immobilization on polyacrylic acid grafted polypropylene monofilament. Carbohydr. Polym. 2010, 82, 1315–1322. [Google Scholar] [CrossRef]
- Chang, S.; Chian, C. Plasma surface modification effects on biodegradability and protein adsorption properties of chitosan films. Appl. Surf. Sci. 2013, 282, 735–740. [Google Scholar] [CrossRef]
- Maslakci, N.N.; Ulusoy, S.; Oksuz, A.U. Investigation of the effects of plasma-treated chitosan electrospun fibers onto biofilm formation. Sens. Actuators B Chem. 2017, 246, 887–895. [Google Scholar] [CrossRef]
- Ando, Y.; Miyamoto, H.; Noda, I.; Sakurai, N.; Akiyama, T.; Yonekura, Y.; Shimazaki, T.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. Calcium phosphate coating containing silver shows high antibacterial activity and low cytotoxicity and inhibits bacterial adhesion. Mater. Sci. Eng. C 2010, 30, 175–180. [Google Scholar] [CrossRef]
- Campoccia, D.; Visai, L.; Renò, F.; Cangini, I.; Rizzi, M.; Poggi, A.; Montanaro, L.; Rimondini, L.A.; Arciola, C.R. Bacterial adhesion to poly-(d,l)lactic acid blended with vitamin E: Toward gentle anti-infective biomaterials. J. Biomed. Mater. Res. Part A 2014, 103, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.J.; Sagers, R.D.; Pitt, W.G. Bacterial adhesion to orthopedic implant polymers. J. Biomed. Mater. Res. 1996, 30, 403–410. [Google Scholar] [CrossRef]
- Hawser, S.; Lociuro, S.; Islam, K. Dihydrofolate reductase inhibitors as antibacterial agents. Biochem. Pharmacol. 2006, 71, 941–948. [Google Scholar] [CrossRef]
- Munteanu, N.S.; Pâslaru, E.; Zemljič, L.F.; Anamaria, S.; Pricope, G.M.; Vasile, C. Chitosan coatings applied to polyethylene surface to obtain food-packaging materials. Cell. Chem. Technol. 2014, 48, 565–575. [Google Scholar]
- Machovsky, M.; Kuritka, I.; Bazant, P.; Vesela, D.; Saha, P. Antibacterial performance of ZnO-based fillers with mesoscale structured morphology in model medical PVC composites. Mater. Sci. Eng. C 2014, 41, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Badaraev, A.; Nemoykina, A.; Bolbasov, E.; Tverdokhlebov, S. PLLA scaffold modification using magnetron sputtering of the copper target to provide antibacterial properties. Resour.-Eff. Technol. 2017, 3, 204–211. [Google Scholar] [CrossRef]
- Silva, F.D.; Cinca, N.; Dosta, S.; Cano, I.; Guilemany, J.; Caires, C.; Lima, A.R.; Silva, C.M.; Oliveira, S.L.; Caires, A.R.; et al. Corrosion resistance and antibacterial properties of copper coating deposited by cold gas spray. Surf. Coat. Technol. 2019, 361, 292–301. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Ortiz, L.; Hitchner, M.; Palmer, T.; Caputo, G.A. Characterization of a Histidine Containing Antimicrobial Peptide with pH Dependent Activity. Biophys. J. 2019, 116, 83a. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Liu, H.; Yang, X.; Shan, Y.; Xu, X.; Shang, S.; Song, Z. Dual-functional antimicrobial coating based on a quaternary ammonium salt from rosin acid with in vitro and in vivo antimicrobial and antifouling properties. Chem. Eng. J. 2019, 374, 564–575. [Google Scholar] [CrossRef]
- Russo, L.; Gloria, A.; Russo, T.; Damora, U.; Taraballi, F.; Santis, R.D.; Ambrosio, L.; Nicotra, F.; Cipolla, L. Glucosamine grafting on poly(ε-caprolactone): A novel glycated polyester as a substrate for tissue engineering. RSC Adv. 2013, 3, 6286. [Google Scholar] [CrossRef]
- Dawlee, S.; Sugandhi, A.; Balakrishnan, B.; Labarre, D.; Jayakrishnan, A. Oxidized Chondroitin Sulfate-Cross-Linked Gelatin Matrixes: A New Class of Hydrogels. Biomacromolecules 2005, 6, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.; Cheng, K.; Hu, C.; Huang, Y.; Young, J. Novel protein-loaded chondroitin sulfate–chitosan nanoparticles: Preparation and characterization. Acta Biomater. 2011, 7, 3804–3812. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Xue, J.; Qian, B.; Chen, H.; Zhu, Y.; Lan, M. Preparation and antifouling property of polyurethane film modified by chondroitin sulfate. Appl. Surf. Sci. 2017, 394, 403–413. [Google Scholar] [CrossRef]
- Burge, K.Y.; Hannah, L.; Eckert, J.V.; Gunasekaran, A.; Chaaban, H. The Protective Influence of Chondroitin Sulfate, a Component of Human Milk, on Intestinal Bacterial Invasion and Translocation. J. Hum. Lact. 2019, 35, 538–549. [Google Scholar] [CrossRef]
- Tóth, I.Y.; Illés, E.; Szekeres, M.; Tombácz, E. Preparation and characterization of chondroitin-sulfate-A-coated magnetite nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2015, 380, 168–174. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016, 152, 21–29. [Google Scholar] [CrossRef]
- Jeong, K.; Ahn, K.; Lee, B.I.; Lee, C.; Kim, S. The mechanism of transglutaminase 2 inhibition with glucosamine: Implications of a possible anti-inflammatory effect through transglutaminase inhibition. J. Cancer Res. Clin. Oncol. 2009, 136, 143–150. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Li, C.; Ji, X.; Feng, J.; Li, P. The antioxidant activity of glucosamine hydrochloride in vitro. Bioorg. Med. Chem. 2006, 14, 1706–1709. [Google Scholar] [CrossRef]
- Rozin, A.P. Glucosamine sulfate—Environmental antibacterial activity. Clin. Rheumatol. 2009, 28, 1221–1223. [Google Scholar] [CrossRef]
- Appelt, H.R.; Oliveira, J.S.; Santos, R.C.; Rodrigues, O.E.; Santos, M.Z.; Heck, E.F.; Rosa, L.C. Synthesis and Antimicrobial Activity of Carbohydrate Based Schiff Bases: Importance of Sugar Moiety. Int. J. Carbohydr. Chem. 2013, 2013, 320892. [Google Scholar] [CrossRef]
- Malik, S.; Singh, M.; Mathur, A. Antimicrobial Activity of Food Grade Glucosamine. Int. J. Biotechnol. Bioeng. Res. 2013, 4, 307–312. [Google Scholar]
- Calamia, V.; Mateos, J.; Fernández-Puente, P.; Lourido, L.; Rocha, B.; Fernández-Costa, C.; Montell, E.; Verges, J.; Ruiz-Romero, J.; Blanco, F.J. A pharmacoproteomic study confirms the synergistic effect of chondroitin sulfate and glucosamine. Sci. Rep. 2014, 4, 5069. [Google Scholar] [CrossRef]
- Lippiello, L.; Woodward, J.; Karpman, R.; Hammad, T.A. In Vivo Chondroprotection and Metabolic Synergy of Glucosamine and Chondroitin Sulfate. Clin. Orthop. Relat. Res. 2000, 381, 229–240. [Google Scholar] [CrossRef]
- Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis. Obstet. Gynecol. 2006, 107, 1415. [CrossRef]
- De Souza Lins Borba, F.K.; Felix, G.L.; Costa, E.V.; Silva, L.; Dias, P.F.; Nogueira, R.D. Fractal analysis of extra-embryonic vessels of chick embryos under the effect of glucosamine and chondroitin sulfates. Microvasc. Res. 2016, 105, 114–118. [Google Scholar] [CrossRef]
- Yue, J.; Yang, M.; Yi, S.; Dong, B.; Li, W.; Yang, Z.; Lu, Z.; Zhang, R.; Yong, J. Chondroitin sulfate and/or glucosamine hydrochloride for Kashin-Beck disease: A cluster-randomized, placebo-controlled study. Osteoarthr. Cartil. 2012, 20, 622–629. [Google Scholar] [CrossRef]
- ISO 22196:2007-Plastics-Measurement of Antibacterial Activity on Plastics Surfaces; ISO: Geneva, Switzerland, 2007.
- ISO 10993-5:2009-Biological Evaluation of Medical Devices; ISO: Geneva, Switzerland, 2009.
- Graeser, A.; Giller, K.; Wiegand, H.; Barella, L.; Saadatmandi, C.B.; Rimbach, G. Synergistic Chondroprotective Effect of α-Tocopherol, Ascorbic Acid, and Selenium as well as Glucosamine and Chondroitin on Oxidant Induced Cell Death and Inhibition of Matrix Metalloproteinase-3—Studies in Cultured Chondrocytes. Molecules 2009, 15, 27–39. [Google Scholar] [CrossRef]
- Lv, C.; Wang, L.; Zhu, X.; Lin, W.; Chen, X.; Huang, Z.; Huang, L.; Yang, S. Glucosamine promotes osteoblast proliferation by modulating autophagy via the mammalian target of rapamycin pathway. Biomed. Pharmacother. 2018, 99, 271–277. [Google Scholar] [CrossRef]
- Bascoul-Colombo, C.; Garaiova, I.; Plummer, S.F.; Harwood, J.L.; Caterson, B.; Hughes, C.E. Glucosamine Hydrochloride but Not Chondroitin Sulfate Prevents Cartilage Degradation and Inflammation Induced by Interleukin-1α in Bovine Cartilage Explants. Cartilage 2015, 7, 70–81. [Google Scholar] [CrossRef]
- Montell, E.; Contreras-Muñoz, P.; Torrent, A.; Varga, M.D.; Rodas, G.; Marotta, M. Mechanisms of action of chondroitin sulfate and glucosamine in muscle tissue: In vitro and in vivo results. a new potential treatment for muscle injuries? Ann. Rheum. Dis. 2018, 77, 1228–1229. [Google Scholar]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Augusta, S.; Gruber, H.F.; Streichsbier, F. Synthesis and antibacterial activity of immobilized quaternary ammonium salts. J. Appl. Polym. Sci. 1994, 53, 1149–1163. [Google Scholar] [CrossRef]
| Sample Type | Composition (%) | Ratio | |||
|---|---|---|---|---|---|
| C | O | N | S | O/C | |
| PLA | 67.8 | 31.9 | - | - | 0.47 |
| RF | 60.6 | 38.2 | 0.9 | - | 0.63 |
| GlcN | 69.4 | 29.6 | 0.5 | - | 0.44 |
| ChS | 66.3 | 28.9 | 4.4 | 0.1 | 0.43 |
| GlcN-ChS | 70.7 | 25.1 | 3.6 | 0.3 | 0.36 |
| Sample | Initial CFU | PLA | RF | GlcN | ChS | GlcN-ChS | |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| S.aureus CCM 4516 N (cfu/cm2) | 2.0 × 106 | 1.8 × 105 | 2.1 × 105 | 1.9 | 8.8 | 1.1 × 101 | |
| E.coli CCM 4517 N (cfu/cm2) | 2.2 × 107 | 2.3 × 106 | 2.2 × 106 | 7.8 | <1 | 7.7 × 101 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakurt, I.; Ozaltin, K.; Vesela, D.; Lehocky, M.; Humpolíček, P.; Mozetič, M. Antibacterial Activity and Cytotoxicity of Immobilized Glucosamine/Chondroitin Sulfate on Polylactic Acid Films. Polymers 2019, 11, 1186. https://doi.org/10.3390/polym11071186
Karakurt I, Ozaltin K, Vesela D, Lehocky M, Humpolíček P, Mozetič M. Antibacterial Activity and Cytotoxicity of Immobilized Glucosamine/Chondroitin Sulfate on Polylactic Acid Films. Polymers. 2019; 11(7):1186. https://doi.org/10.3390/polym11071186
Chicago/Turabian StyleKarakurt, Ilkay, Kadir Ozaltin, Daniela Vesela, Marian Lehocky, Petr Humpolíček, and Miran Mozetič. 2019. "Antibacterial Activity and Cytotoxicity of Immobilized Glucosamine/Chondroitin Sulfate on Polylactic Acid Films" Polymers 11, no. 7: 1186. https://doi.org/10.3390/polym11071186
APA StyleKarakurt, I., Ozaltin, K., Vesela, D., Lehocky, M., Humpolíček, P., & Mozetič, M. (2019). Antibacterial Activity and Cytotoxicity of Immobilized Glucosamine/Chondroitin Sulfate on Polylactic Acid Films. Polymers, 11(7), 1186. https://doi.org/10.3390/polym11071186








