Study on Stability and Stability Mechanism of Styrene-Acrylic Emulsion Prepared Using Nanocellulose Modified with Long-Chain Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
2.2.1. Preparation of NCC
2.2.2. Preparation of MNCC
2.2.3. Preparation of MNCC Suspension
2.2.4. Preparation of Styrene-Acrylic Emulsion
2.3. Reaction Principle
2.4. Methods
3. Results and Discussion
3.1. Preparation and Analysis of MNCC
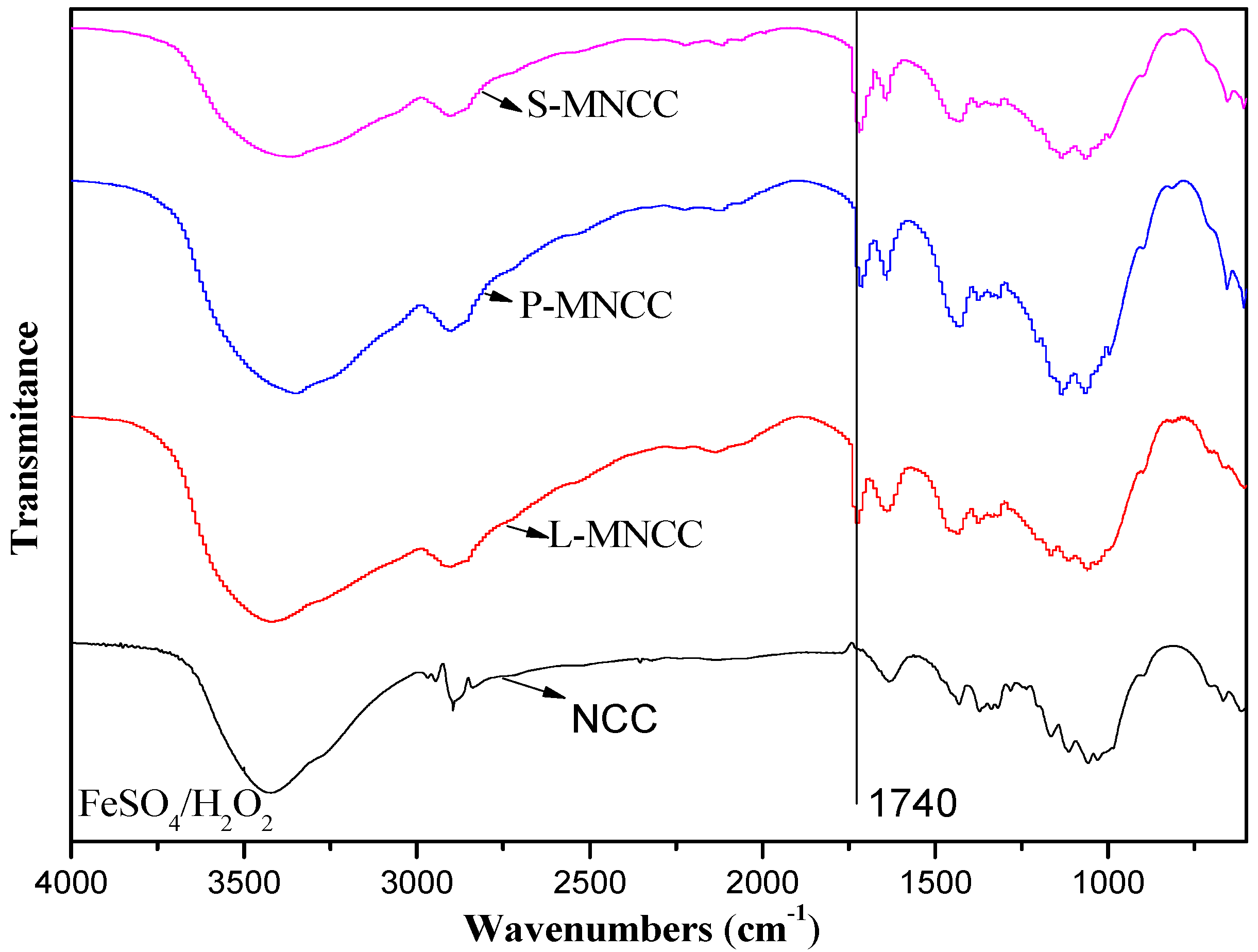
3.1.1. Infrared Characterization of MNCC
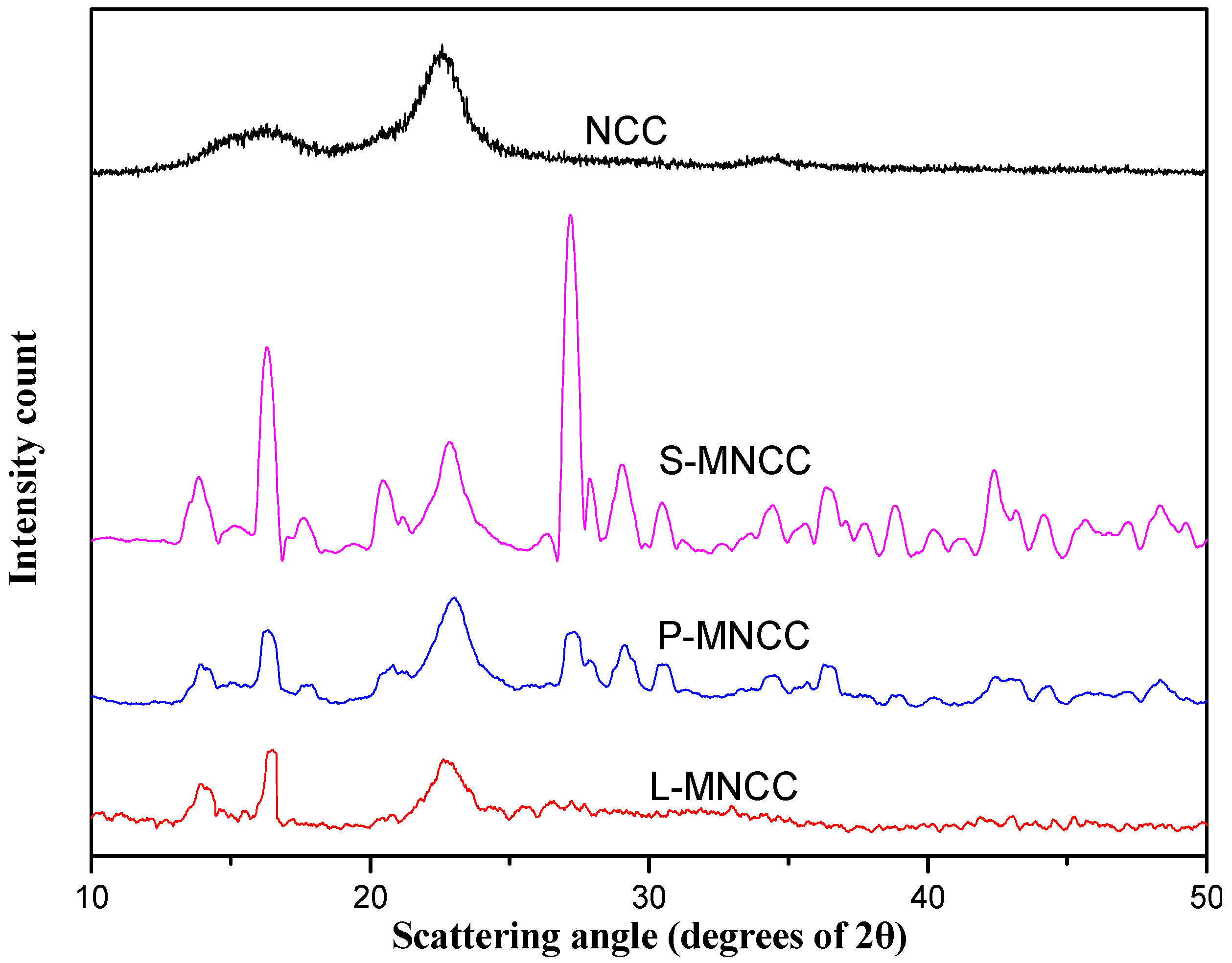
3.1.2. XRD Analysis of MNCC
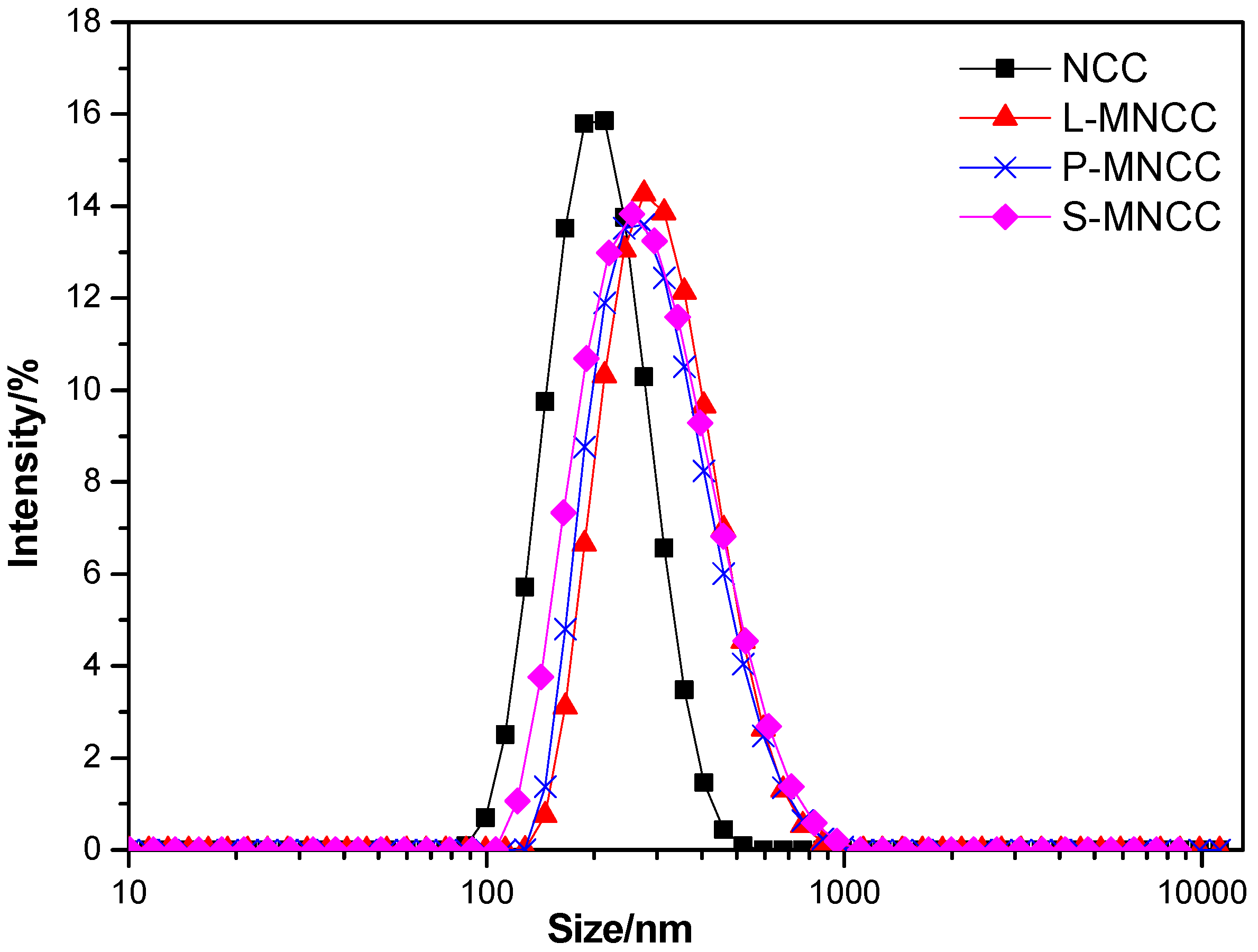
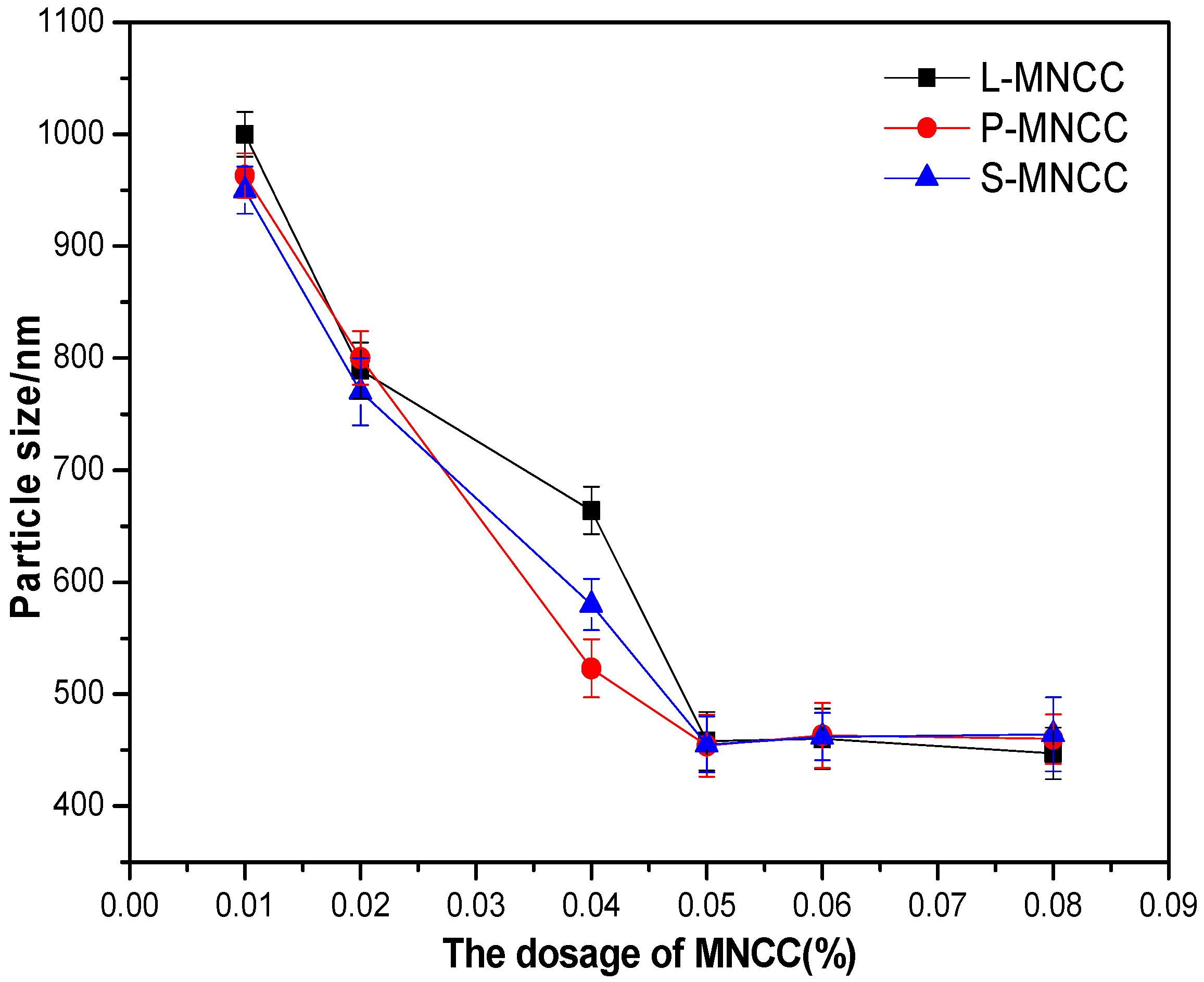
3.1.3. Particle Size Test of MNCC
3.2. Emulsification and Stability Analysis of Styrene-Acrylic Emulsion
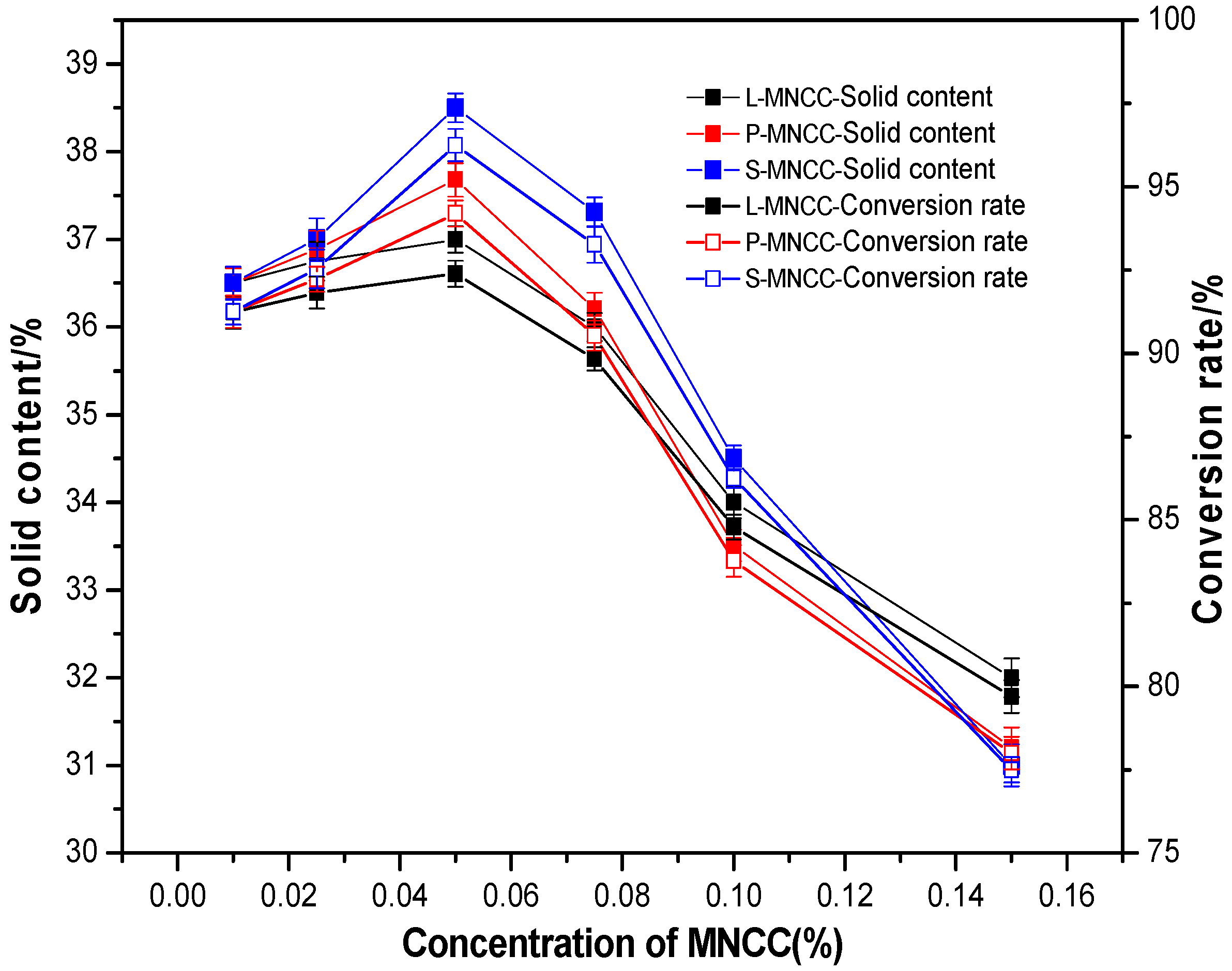
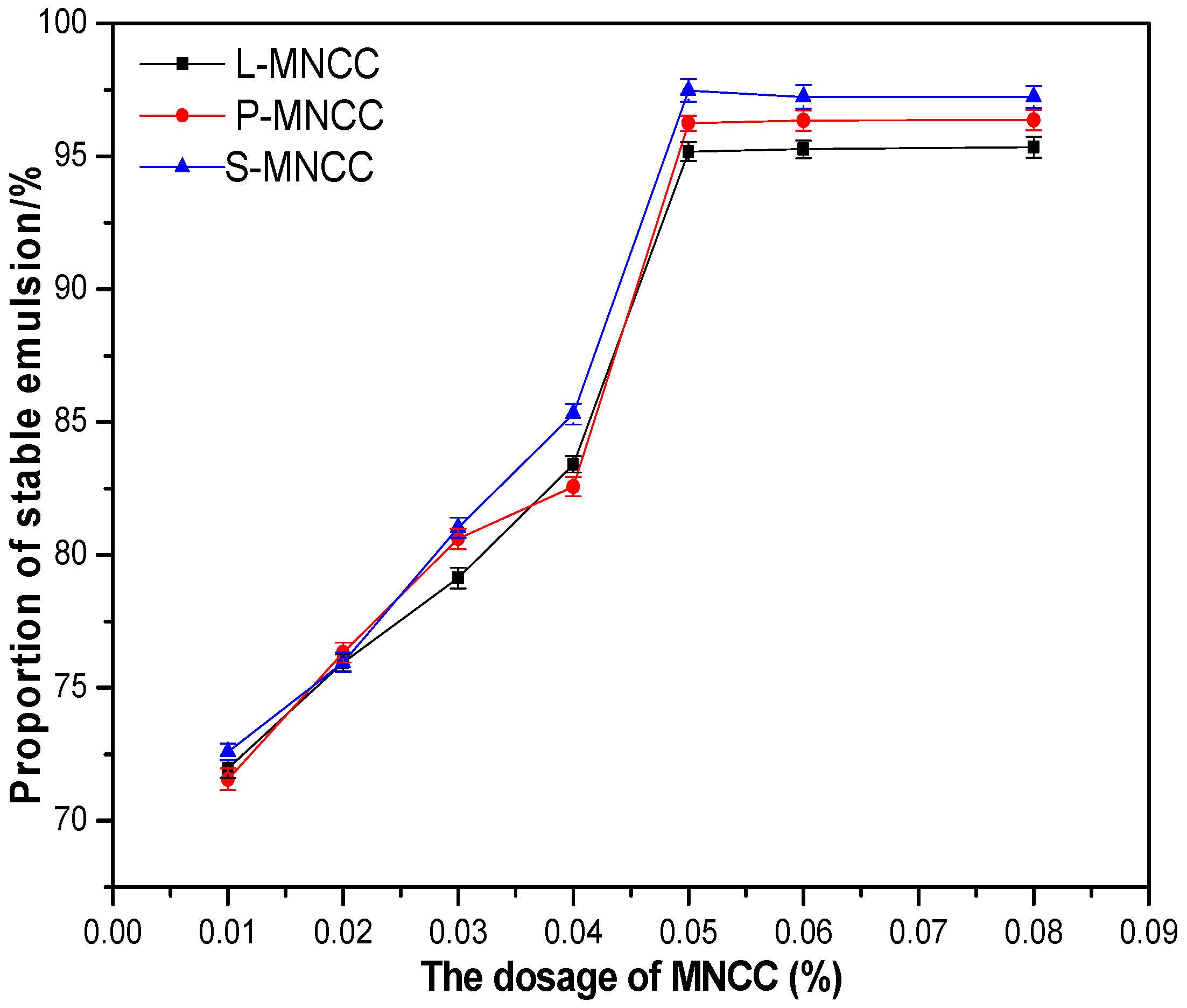
3.2.1. Effect of MNCC Dosage on the Solid Content and Conversion of Emulsion
3.2.2. Effect of MNCC Dosage on Emulsion Particle Size
3.2.3. Effect of pH, Ca2+, and Dilution on the Stability of Styrene-Acrylic Emulsion
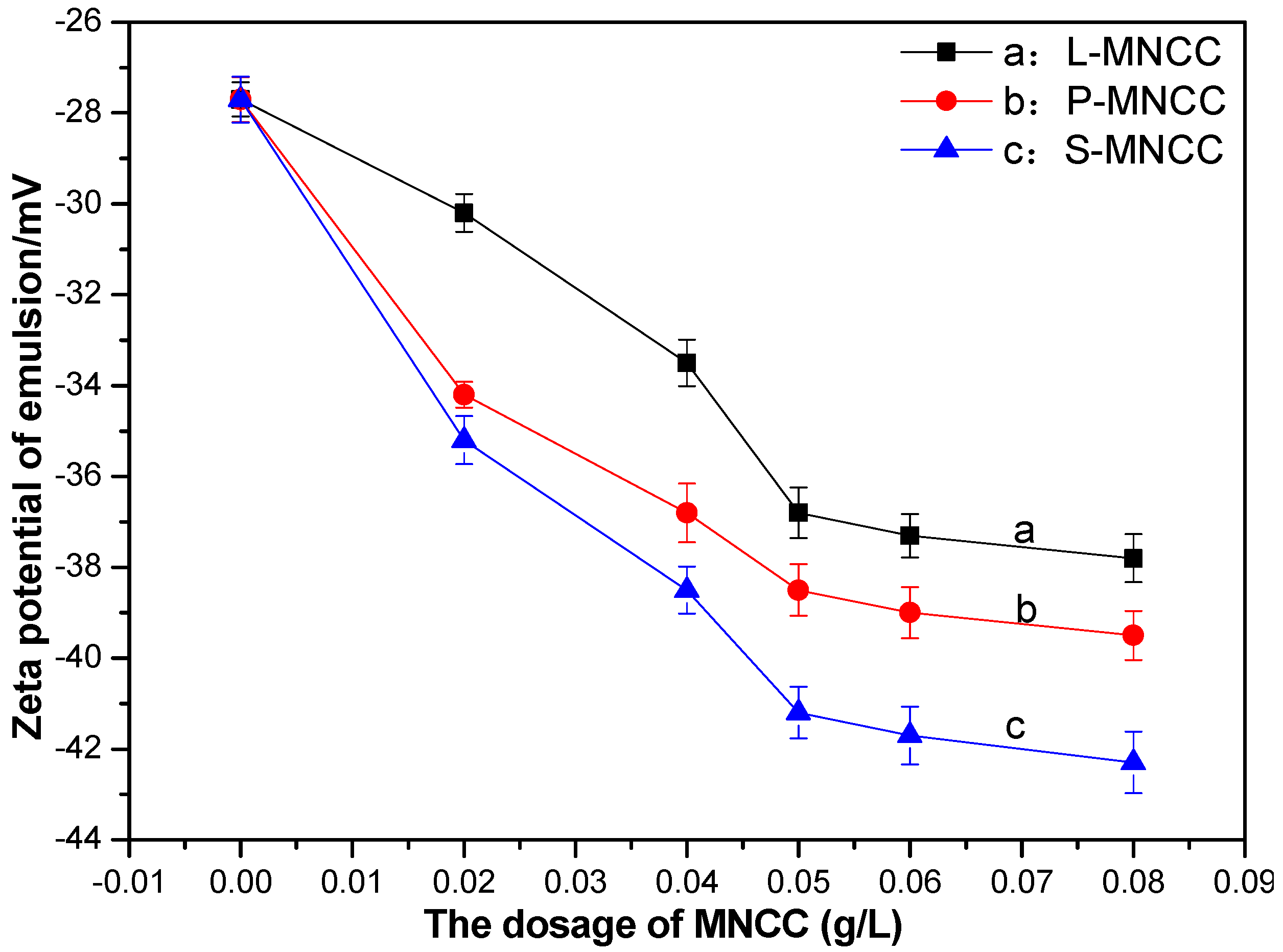
3.2.4. Zeta Electric Potential Test of Styrene-Acrylic Emulsion
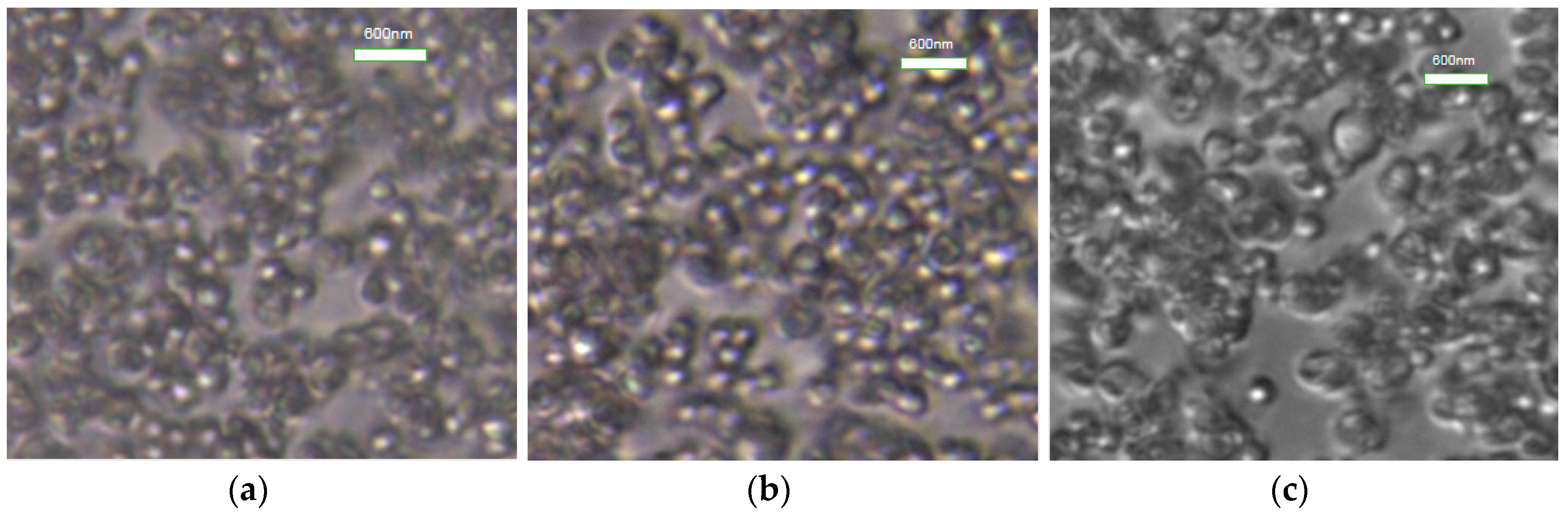
3.2.5. Optical Microscopy Test of Styrene-Acrylic Emulsion
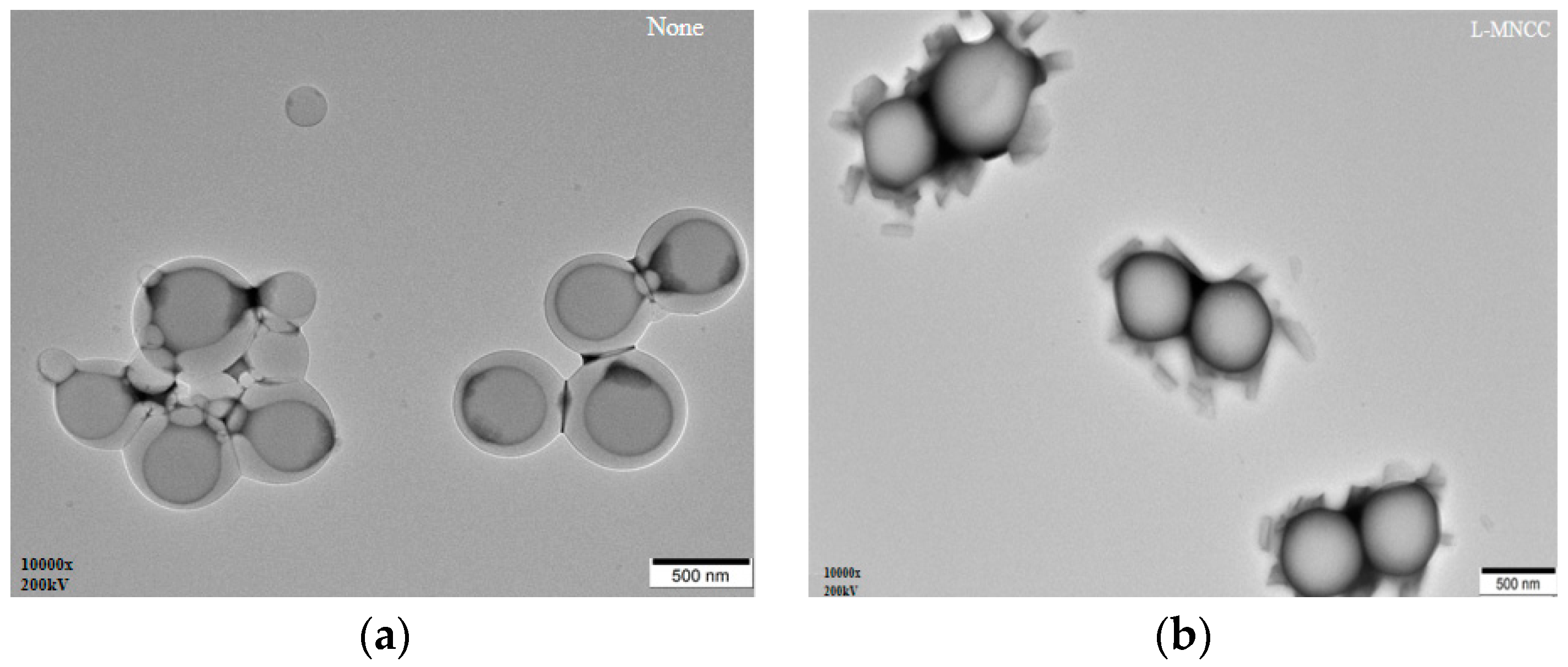
3.2.6. Transmission Electron Microscopy Analysis of the Emulsion
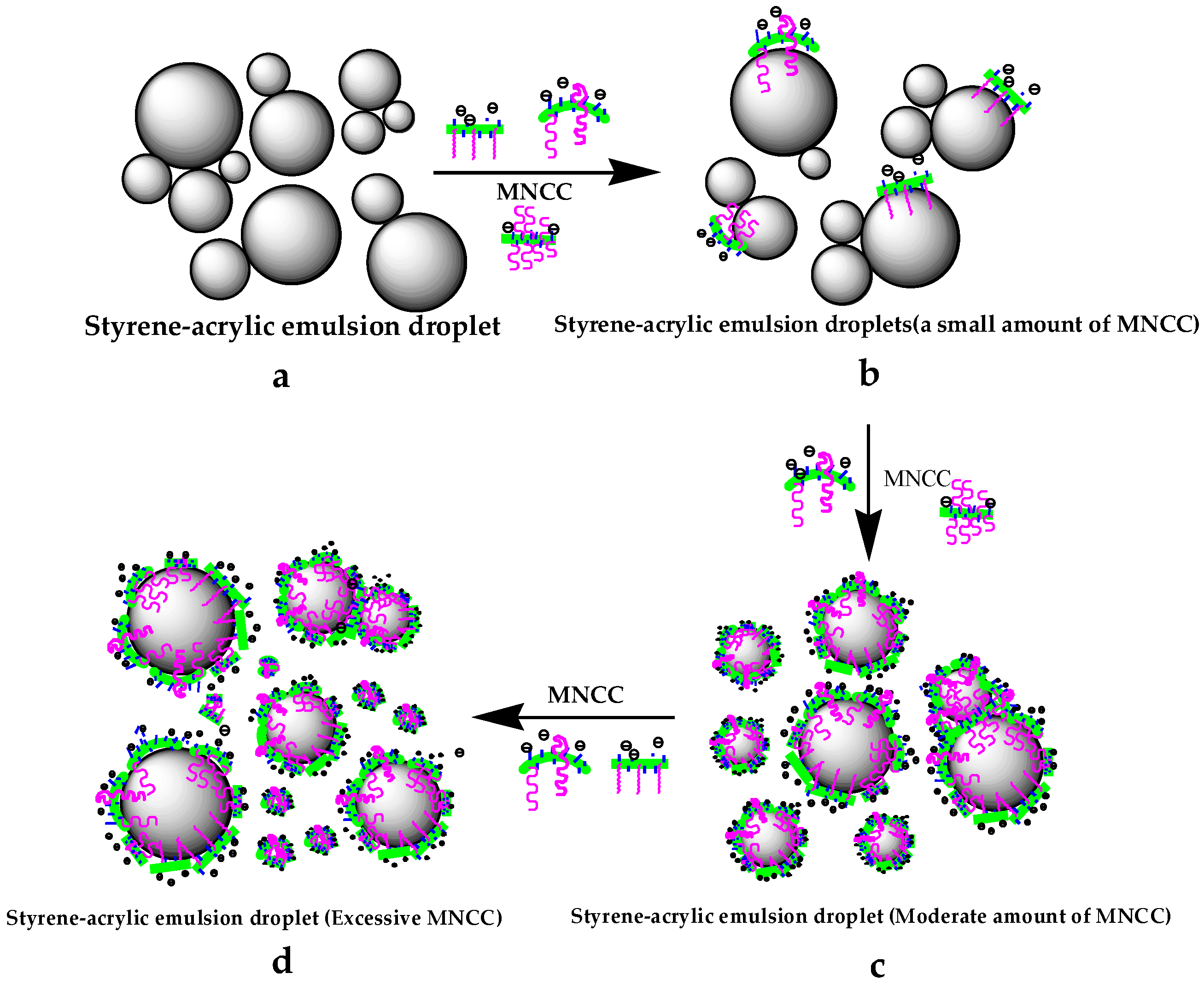
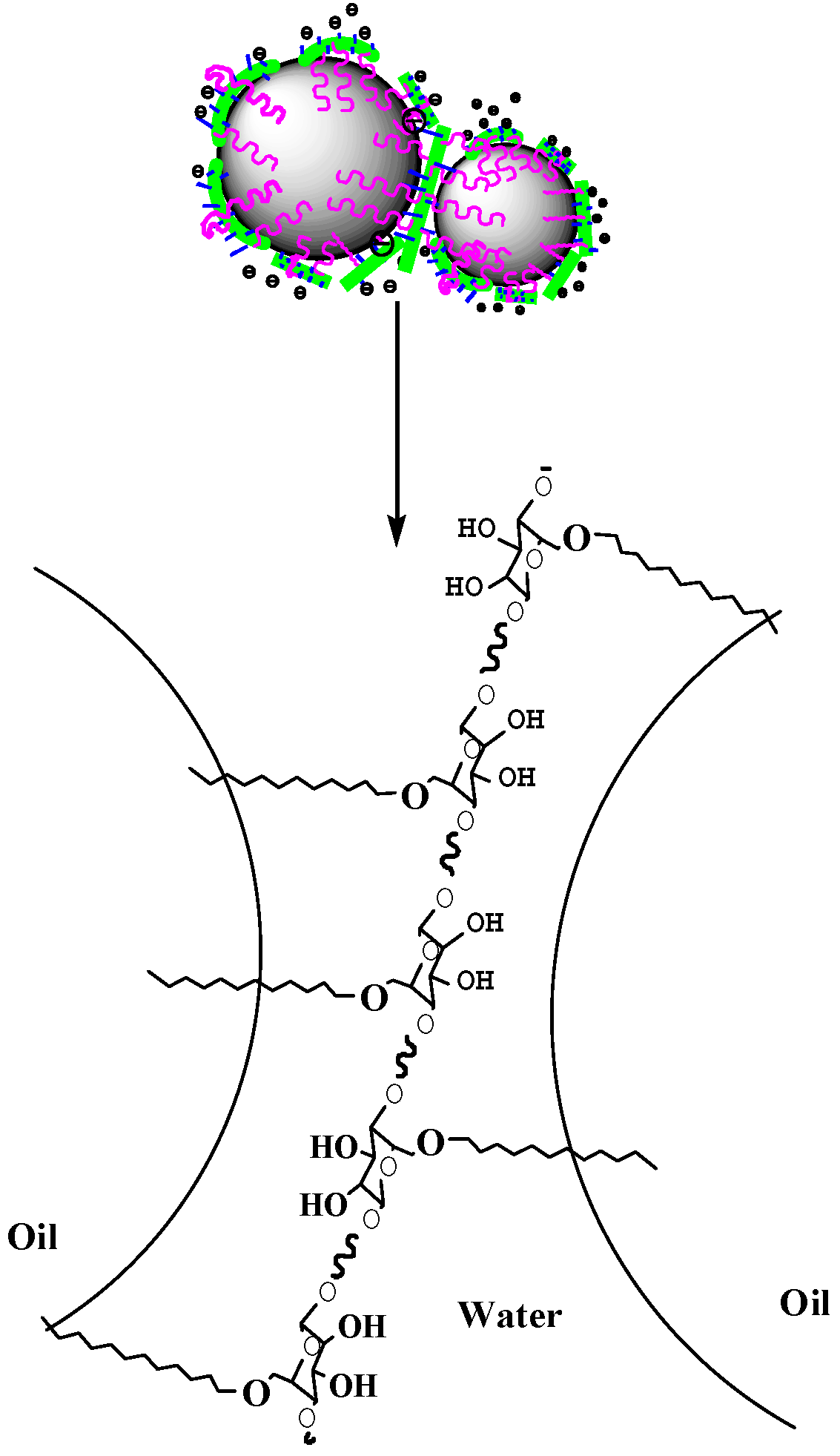
3.2.7. Mechanism Analysis of MNCC-Stabilized Emulsion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mcclements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Gelot, A.; Friesen, W.; Hamza, H.A. Emulsification of oil and water in the presence of finely divided solids and surface-active agents. Colloids Surf. 1984, 12, 271–303. [Google Scholar] [CrossRef]
- Nakache, E.; Dupeyrat, M.; Vignes-Adler, M. Experimental and theoretical study of an interfacial instability at some oil—Water interfaces involving a surface-active agent: I. Physicochemical description and outlines for a theoretical approach. J. Colloid Interface Sci. 1983, 94, 187–200. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of solids in the surface-layers of solutions and suspensions. Proc. R. Soc. Lond. 1903, 72, 156–164. [Google Scholar]
- Liu, H.; Gu, X.; Hu, M.; Hu, Y.; Wang, C. Facile fabrication of nanocomposite microcapsules by combining layer-by-layer self-assembly and Pickering emulsion templating. RSC Adv. 2014, 4, 16751–16758. [Google Scholar] [CrossRef]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Bohn, A.; Fink, H.-P.; Fink, H. Cellulose: Fascinating biopolymer and sustainable raw material. Anew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Chmielarz, P. Cellulose-based graft copolymers prepared by simplified electrochemically mediated ATRP. Express Polym. Lett. 2017, 11, 140–151. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Winuprasith, T.; Suphantharika, M. Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: Preparation, characterization, and evaluation as an emulsion stabilizer. Food Hydrocoll. 2013, 32, 383–394. [Google Scholar] [CrossRef]
- Laitinen, O.; Ojala, J.; Sirviö, J.A.; Liimatainen, H. Sustainable stabilization of oil in water emulsions by cellulose nanocrystals synthesized from deep eutectic solvents. Cellulose 2017, 24, 1679–1689. [Google Scholar] [CrossRef]
- Sun, W.; Sun, D.; Wei, Y.; Liu, S.; Zhang, S. Oil-in-water emulsions stabilized by hydrophobically modified hydroxyethyl cellulose: Adsorption and thickening effect. J. Colloid Interface Sci. 2007, 311, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Luo, S.; McDonald, A.G.; Agarwal, U.P.; Hirth, K.C.; Matuana, L.M.; Sabo, R.C.; Stark, N.M. Preparation and Characterization of the Nanocomposites from Chemically Modified Nanocellulose and Poly(lactic acid). J. Renew. Mater. 2017, 5, 410–422. [Google Scholar] [CrossRef]
- Cunha, A.G.; Mougel, J.-B.; Berglund, L.A.; Capron, I.; Cathala, B. Preparation of Double Pickering Emulsions Stabilized by Chemically Tailored Nanocelluloses. Langmuir 2014, 30, 9327–9335. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, E.; Kashimoto, A.; Fukuda, K.; Hotta, H.; Suzuki, T.; Kitsuki, T. Thickening properties and emulsification mechanisms of new derivatives of polysaccharides in aqueous solution. J. Colloid Interface Sci. 2005, 282, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Fantin, M.; Chmielarz, P.; Wang, Y.; Lorandi, F.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Harnessing the Interaction between Surfactant and Hydrophilic Catalyst to Control eATRP in Miniemulsion. Macromolecules 2017, 50, 3726–3732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lorandi, F.; Fantin, M.; Chmielarz, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Miniemulsion ARGET ATRP via Interfacial and Ion-Pair Catalysis: From ppm to ppb of Residual Copper. Macromolecules 2017, 50, 8417–8425. [Google Scholar] [CrossRef] [PubMed]
- Naseem, S.; Wu, C.M.; Xu, T.Z.; Lai, C.C.; Rwei, S.P. Oil-Water Separation of Electrospun Cellulose Triacetate Nanofiber Membranes Modified by Electrophoretically Deposited TiO2/Graphene Oxide. Polymers 2018, 10, 746. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Rapid synthesis of graft copolymers from natural cellulose fibers. Carbohyd. Polym. 2013, 98, 820–828. [Google Scholar] [CrossRef]
- Rahman, M.L.; Rohani, N.; Mustapa, N.; Yusoff, M.M. Synthesis of polyamidoxime chelating ligand from polymer-grafted corn-cob cellulose for metal extraction. J. Appl. Polym. Sci. 2014, 131, 40833. [Google Scholar] [CrossRef]
- Lee, H.J.; Ramaraj, B.; Yoon, K.R. Esterification on solid support by surface-initiated ring-opening polymerization of -caprolactone from benzylic hydroxyl-functionalized Wang resin bead. J. Appl. Polym. Sci. 2010, 111, 839–844. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.; Galotto, M. Improvement of Polylactide Properties through Cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.A.; Longo, M.L. Dissolution Behavior of Lipid Monolayer-Coated, Air-Filled Microbubbles: Effect of Lipid Hydrophobic Chain Length. Langmuir 2002, 18, 9225–9233. [Google Scholar] [CrossRef]
- Ershova, O.; Da Costa, E.V.; Fernandes, A.J.S.; Domingues, M.R.; Evtuguin, D.V.; Sixta, H.; Costa, E.V. Effect of urea on cellulose degradation under conditions of alkaline pulping. Cellulose 2012, 19, 2195–2204. [Google Scholar] [CrossRef]
- Stachurski, M. The zeta potential of emulsion droplets of the aliphatic hydrocarbons in aqueous solutions. Colloids Surf. 1985, 15, 255–259. [Google Scholar] [CrossRef]
- Pickering, U.S. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, H.; Wang, Z.; Zhu, X. Polymerization Processes of Emulsifier-free Pickering Emulsion Stabilized by Nanocrystalline Cellulose. BioResources 2018, 13, 4132–4144. [Google Scholar] [CrossRef]


| Monomers and Reactants | Ratio of the Reaction (wt%) | Mass of Reactants (g) |
|---|---|---|
| styrene | 20 | 10 |
| butyl acrylate | 20 | 10 |
| ammonium persulfate | 1 | 0.5 |
| MNCC | 0–0.15 | 0–0.075 |
| hydroquinone | 0.001 | 0.0005 |
| water | ≈58.95 | 29.48 |
| Ca2+ stability test (A) | Dosage of L-MNCC | 0.01% | 0.025% | 0.05% | 0.075% | 0.1% |
| Stability | Layered, oil occupies 1/3 | Layered, oil occupies 1/4 | Not layered, stable | Not layered, stable | Not layered, stable | |
| pH stability test (B) | pH | 3 | 5 | 7 | 9 (NaOH) | 11 (NaOH) |
| Stability | Not layered, Stable | Not layered, stable | Not layered, stable | Layered, Slight flocculation | Layered, flocculated | |
| Dilution stability test (C) | Dilution multiple | 1000 | 100 | 50 | 10 | 5 |
| Stability | Not layered, stable | Not layered, stable | Not layered, stable | Not layered, stable | Not layered, stable | |
| Centrifugal oscillation stability (D) | Centrifugal rate | 5000 r/min | 4000 r/min | 3000 r/min | 2000 r/min | 1000 r/min |
| Stability | Layered, oil occupies 1/4 | Not layered, stable | Not layered, stable | Not layered, stable | Not layered, stable |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yang, H.; Lu, J.; Lang, J.; Gao, H. Study on Stability and Stability Mechanism of Styrene-Acrylic Emulsion Prepared Using Nanocellulose Modified with Long-Chain Fatty Acids. Polymers 2019, 11, 1131. https://doi.org/10.3390/polym11071131
Zhang H, Yang H, Lu J, Lang J, Gao H. Study on Stability and Stability Mechanism of Styrene-Acrylic Emulsion Prepared Using Nanocellulose Modified with Long-Chain Fatty Acids. Polymers. 2019; 11(7):1131. https://doi.org/10.3390/polym11071131
Chicago/Turabian StyleZhang, Heng, Hongyan Yang, Junliang Lu, Jinyan Lang, and Hongkun Gao. 2019. "Study on Stability and Stability Mechanism of Styrene-Acrylic Emulsion Prepared Using Nanocellulose Modified with Long-Chain Fatty Acids" Polymers 11, no. 7: 1131. https://doi.org/10.3390/polym11071131
APA StyleZhang, H., Yang, H., Lu, J., Lang, J., & Gao, H. (2019). Study on Stability and Stability Mechanism of Styrene-Acrylic Emulsion Prepared Using Nanocellulose Modified with Long-Chain Fatty Acids. Polymers, 11(7), 1131. https://doi.org/10.3390/polym11071131





