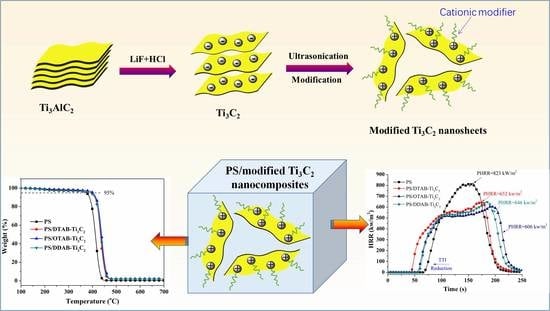
Functionalization of MXene Nanosheets for Polystyrene towards High Thermal Stability and Flame Retardant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
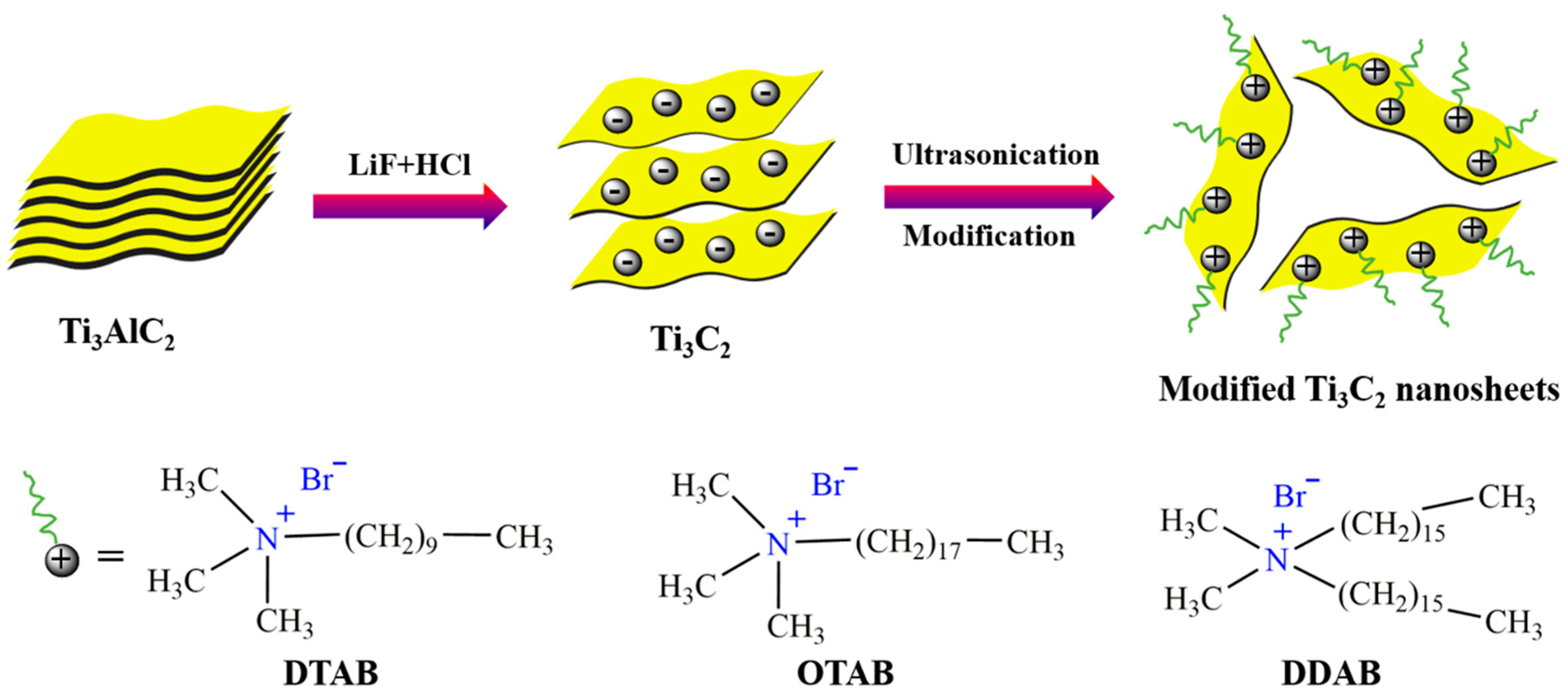
2.2. Preparation of Functionalized MXene Nanosheets
2.3. Preparation of Functionalized MXene Nanosheets/PS Nanocomposites
2.4. Characterizations
3. Results and Discussion
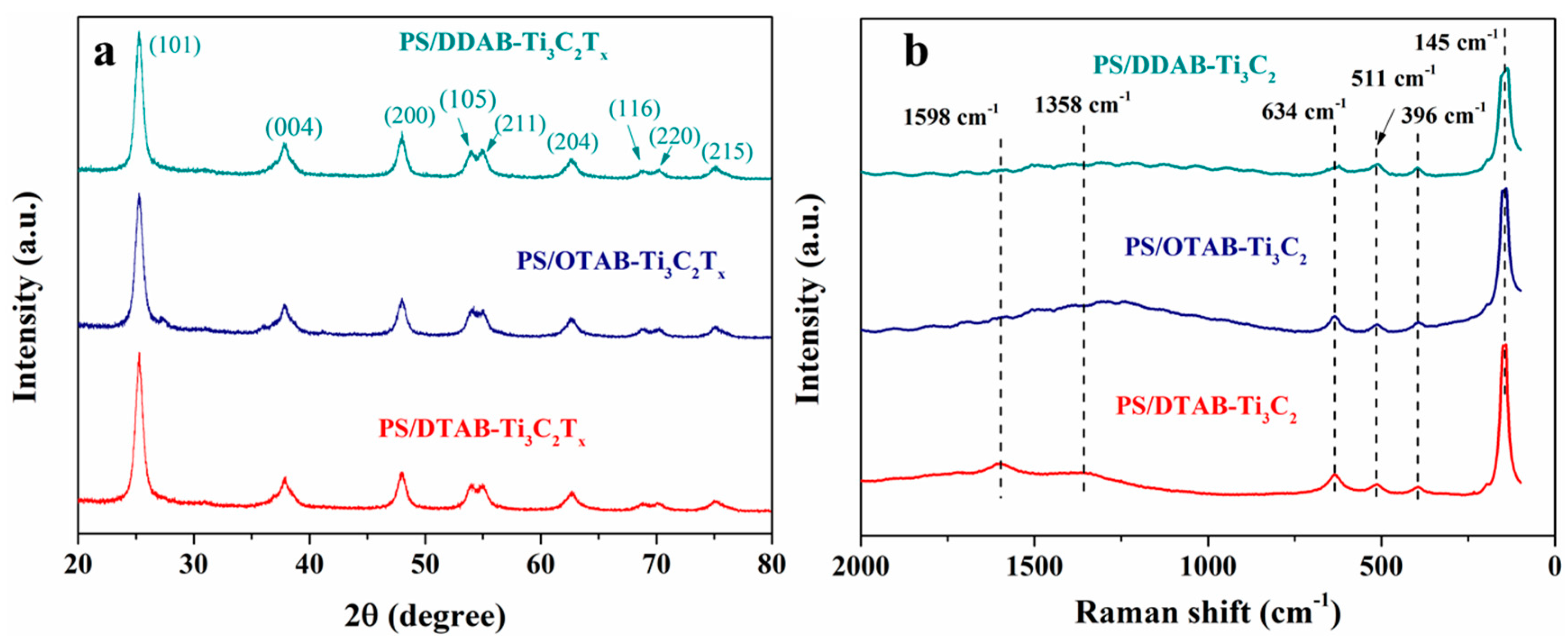
3.1. Characterizations of Functionalized MXene Nanosheets
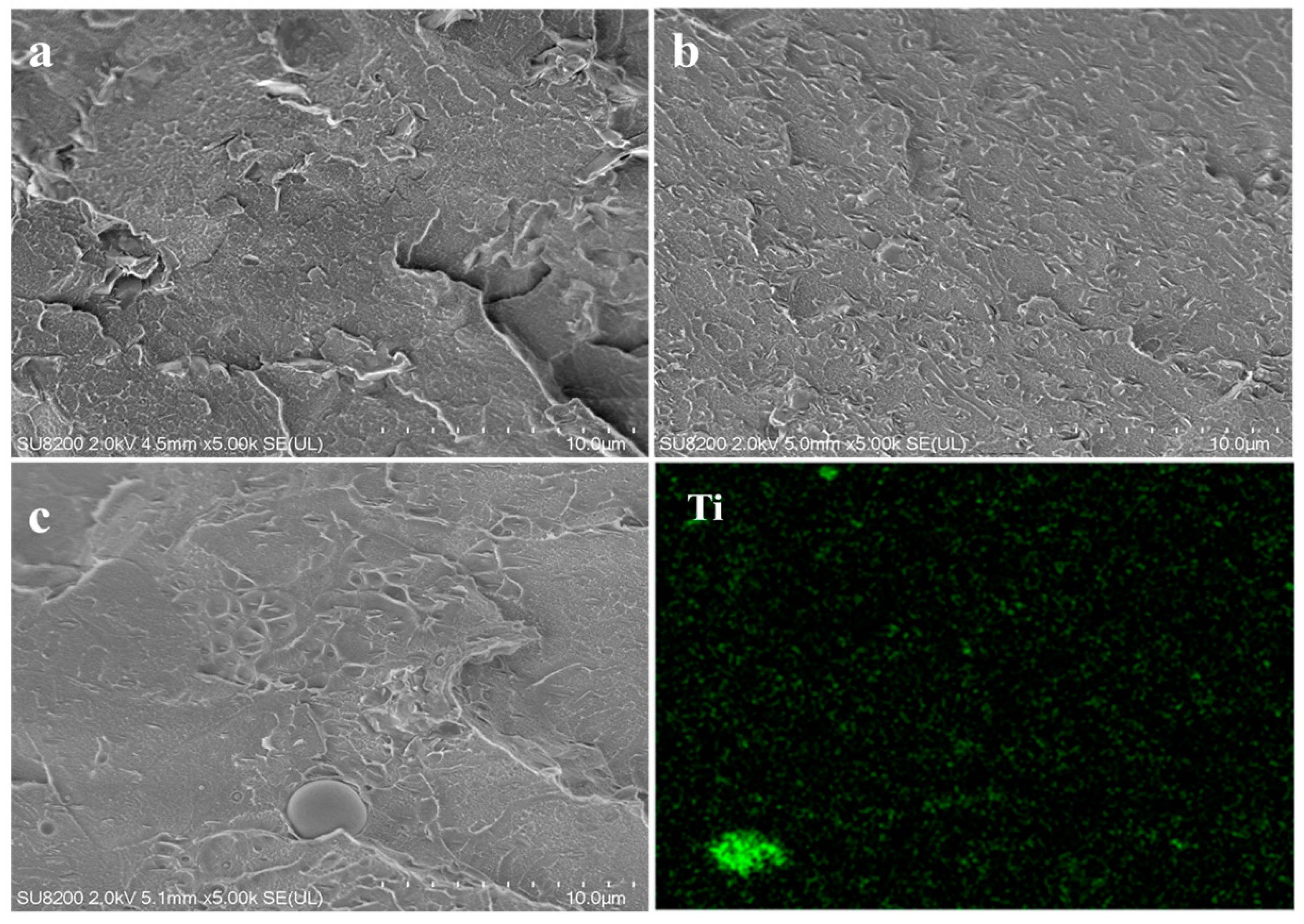
3.2. Morphology and Dispersion
3.3. Thermal Decomposition Behaviors
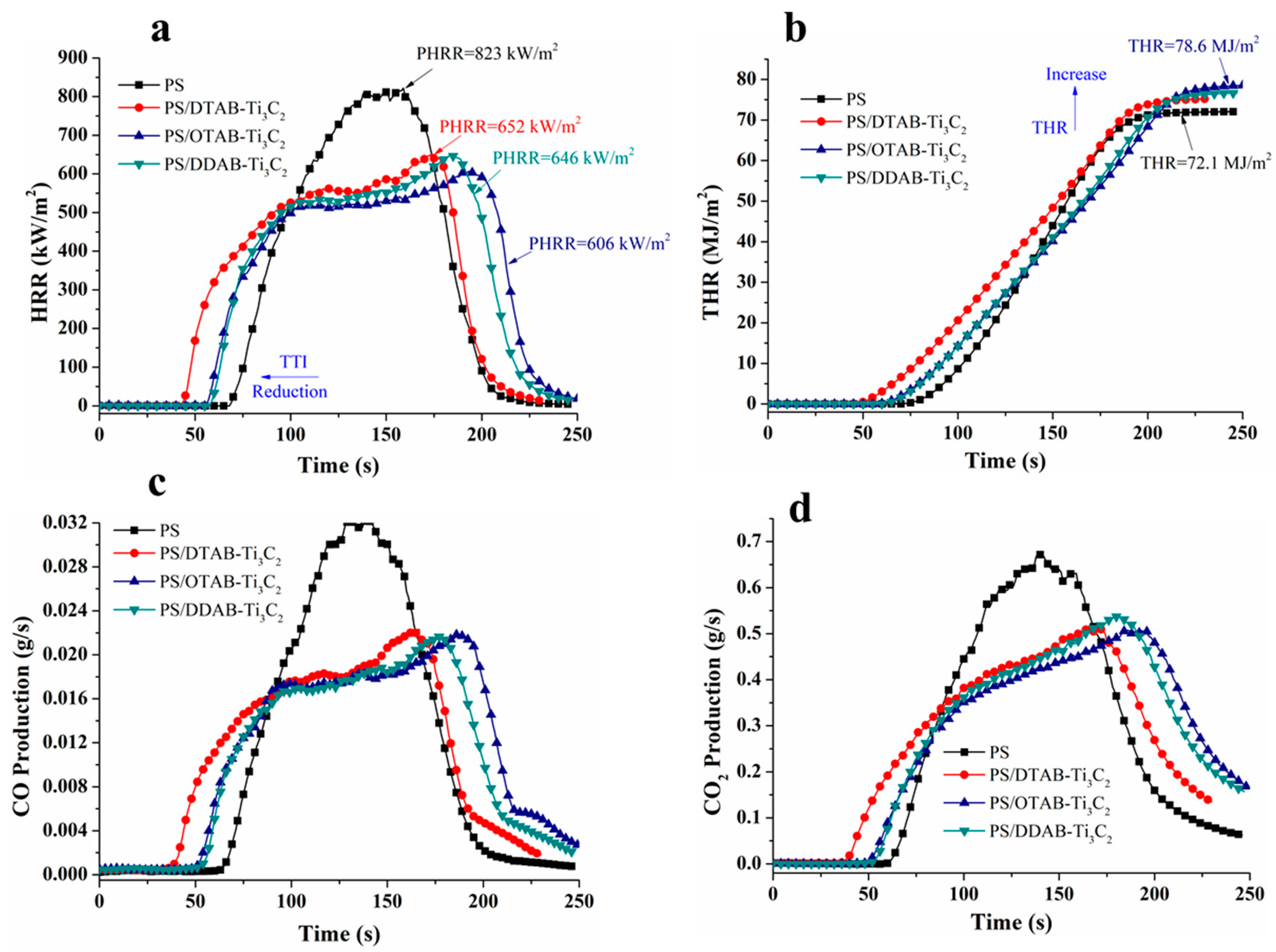
3.4. Flame Retardant Properties
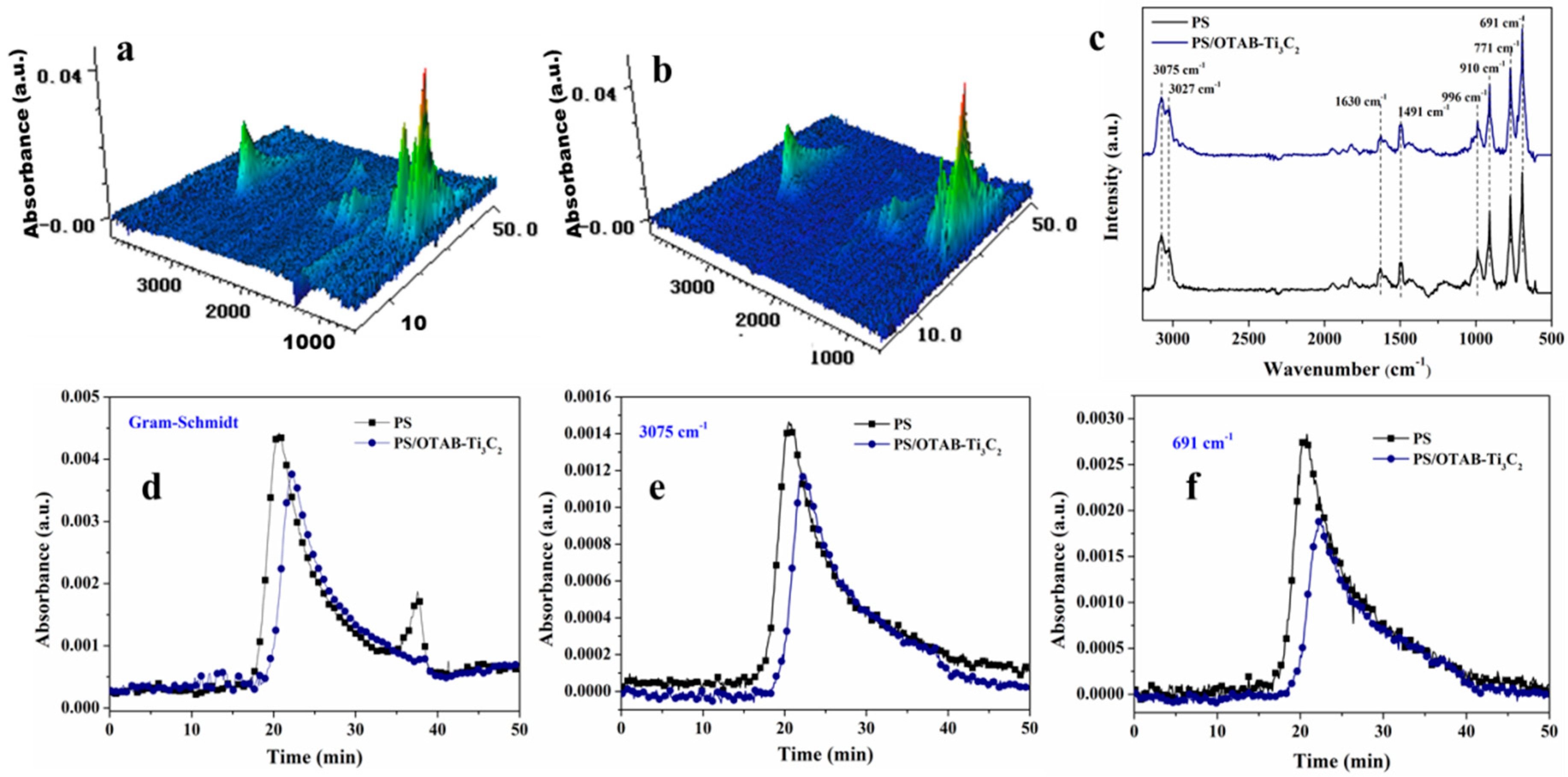
3.5. Volatile Product Analysis
3.6. Residue Analysis
3.7. Comparison of Thermal Stability and Flame Retardancy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tai, Q.; Kan, Y.; Chen, L.; Xing, W.; Hu, Y.; Song, L. Morphologies and thermal properties of flame-retardant polystyrene/α-zirconium phosphate nanocomposites. React. Funct. Polym. 2010, 70, 340–345. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer−layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites†. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Wünsch, J.R. Polystyrene: Synthesis, Production and Applications; iSmithers Rapra Publishing: Shawbury, UK, 2000; Vol. 112. [Google Scholar]
- Tai, Q.; Song, L.; Hu, Y.; Yuen, R.K.K.; Feng, H.; Tao, Y. Novel styrene polymers functionalized with phosphorus–nitrogen containing molecules: Synthesis and properties. Mater. Chem. Phys. 2012, 134, 163–169. [Google Scholar] [CrossRef]
- Tai, Q.; Chen, L.; Song, L.; Nie, S.; Hu, Y.; Yuen, R.K.K. Preparation and thermal properties of a novel flame retardant copolymer. Polym. Degrad. Stab. 2010, 95, 830–836. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame retardant mechanisms of red phosphorus and magnesium hydroxide in high impact polystyrene. Macromol. Chem. Phys. 2004, 205, 2185–2196. [Google Scholar] [CrossRef]
- Nyambo, C.; Kandare, E.; Wang, D.; Wilkie, C.A. Flame-retarded polystyrene: Investigating chemical interactions between ammonium polyphosphate and mgal layered double hydroxide. Polym. Degrad. Stab. 2008, 93, 1656–1663. [Google Scholar] [CrossRef]
- Yan, Y.-W.; Huang, J.-Q.; Guan, Y.-H.; Shang, K.; Jian, R.-K.; Wang, Y.-Z. Flame retardance and thermal degradation mechanism of polystyrene modified with aluminum hypophosphite. Polym. Degrad. Stab. 2014, 99, 35–42. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Wang, L.-L.; Wang, M.-Z.; Chun-Yin Yuen, A.; Bo-Yuan Chen, T.; Yang, W.; Pan, T.-Z.; Zhi, Y.-R.; Lu, H.-D. Simultaneous enhancements in the mechanical, thermal stability, and flame retardant properties of poly(1,4-butylene terephthalate) nanocomposites with a novel phosphorus–nitrogen-containing polyhedral oligomeric silsesquioxane. RSC Adv. 2017, 7, 54021–54030. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Camino, G. Metal functionalized poss as fire retardants in polypropylene. Polym. Degrad. Stab. 2006, 91, 2275–2281. [Google Scholar] [CrossRef]
- Blanco, I. The rediscovery of poss: A molecule rather than a filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Song, P.a.; Liu, H.; Shen, Y.; Du, B.; Fang, Z.; Wu, Y. Fabrication of dendrimer-like fullerene (c60)-decorated oligomeric intumescent flame retardant for reducing the thermal oxidation and flammability of polypropylene nanocomposites. J. Mater. Chem. 2009, 19. [Google Scholar] [CrossRef]
- Song, P.; Shen, Y.; Du, B.; Guo, Z.; Fang, Z. Fabrication of fullerene-decorated carbon nanotubes and their application in flame-retarding polypropylene. Nanoscale 2009, 1, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Yang, W.; Yang, W.; Hu, Q.; Si, J.; Lu, H.; Yang, B.; Song, L.; Hu, Y.; Yuen, R.K. Functionalized carbon nanotubes with phosphorus- and nitrogen-containing agents: Effective reinforcer for thermal, mechanical, and flame-retardant properties of polystyrene nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 26266–26274. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Harris, R.; Awad, W.; Douglas, J. Thermal degradation and flammability properties of poly(propylene)/carbon nanotube composites. Macromol. Rapid Commun. 2002, 23, 761–765. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Yang, B.; Lu, H.; Song, L.; Hu, Y. Facile preparation of modified carbon nanotube-reinforced pbt nanocomposites with enhanced thermal, flame retardancy, and mechanical properties. Polym. Compos. 2016, 37, 1812–1820. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Yang, W.; Kan, Y.; Song, L.; Hu, Y.; Lu, H.; Yuen, R.K.K. Effect of organo-modified montmorillonite on flame retardant poly(1,4-butylene terephthalate) composites. Polym. Adv. Technol. 2011, 22, 2564–2570. [Google Scholar] [CrossRef]
- Yang, W.; Hu, Y.; Tai, Q.; Lu, H.; Song, L.; Yuen, R.K.K. Fire and mechanical performance of nanoclay reinforced glass-fiber/pbt composites containing aluminum hypophosphite particles. Compos. Part. A Appl. Sci. Manuf. 2011, 42, 794–800. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R.; Hull, R.; Kandola, B.K. Flammability, degradation and structural characterization of fibre-forming polypropylene containing nanoclay–flame retardant combinations. Polym. Degrad. Stab. 2006, 91, 719–725. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, H.; Yang, W.; Xiao, X.; Hu, Y. Preparation and characterization of bio-nanocomposites based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and coal layered double hydroxide using melt intercalation. Compos. Part. A Appl. Sci. Manuf. 2012, 43, 547–552. [Google Scholar] [CrossRef]
- Nyambo, C.; Songtipya, P.; Manias, E.; Jimenez-Gasco, M.M.; Wilkie, C.A. Effect of mgal-layered double hydroxide exchanged with linear alkyl carboxylates on fire-retardancy of pmma and ps. J. Mater. Chem. 2008, 18, 194–195. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Qiu, S.; Xing, W.; Hu, W.; Song, L.; Lo, S.; Hu, Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Fang, F.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Improved flame resistance and thermo-mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self-assembly in water. Compos. Part. B Eng. 2019, 165, 406–416. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Zhou, K.; Yang, W.; Hu, Y.; Gong, X. The influence of manganese-cobalt oxide/graphene on reducing fire hazards of poly(butylene terephthalate). J. Hazard. Mater. 2014, 278, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, P.; Mou, Y.; Kang, M.; Liu, M.; Song, L.; Lu, A.; Rong, J. Synthesis of the polyethylene glycol solid-solid phase change materials with a functionalized graphene oxide for thermal energy storage. Polym. Test. 2017, 63, 494–504. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Song, L.; Hu, Y. Mos2/polymer nanocomposites: Preparation, properties, and applications. Polym. Rev. 2017, 57, 440–466. [Google Scholar] [CrossRef]
- Zhou, K.; Yang, W.; Tang, G.; Wang, B.; Jiang, S.; Hu, Y.; Gui, Z. Comparative study on the thermal stability, flame retardancy and smoke suppression properties of polystyrene composites containing molybdenum disulfide and graphene. RSC Adv. 2013, 3. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.; Guo, W.; Qiu, S.; Wang, X.; Lo, S.; Hu, Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol–gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Zhang, Y.; Cai, W.; Yao, C.; Hu, Y.; Hu, W. Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (co and hcn) generation and fire hazard of thermoplastic polyurethane. J. Hazard. Mater. 2019, 362, 482–494. [Google Scholar] [CrossRef]
- Yang, W.; Yuen, A.C.Y.; Ping, P.; Wei, R.-C.; Hua, L.; Zhu, Z.; Li, A.; Zhu, S.-E.; Wang, L.-L.; Liang, J.; et al. Pectin-assisted dispersion of exfoliated boron nitride nanosheets for assembled bio-composite aerogels. Compos. Part. A Appl. Sci. Manuf. 2019, 119, 196–205. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Hong Ng, V.M.; Huang, H.; Zhou, K.; Lee, P.S.; Que, W.; Xu, Z.J.; Kong, L.B. Recent progress in layered transition metal carbides and/or nitrides (mxenes) and their composites: Synthesis and applications. J. Mater. Chem. A 2017, 5, 3039–3068. [Google Scholar] [CrossRef]
- Yu, X.F.; Li, Y.C.; Cheng, J.B.; Liu, Z.B.; Li, Q.Z.; Li, W.Z.; Yang, X.; Xiao, B. Monolayer ti(2)co(2): A promising candidate for nh(3) sensor or capturer with high sensitivity and selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (mxene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive mxene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. U.S.A 2014, 111, 16676–16681. [Google Scholar] [CrossRef]
- Zhi, W.; Xiang, S.; Bian, R.; Lin, R.; Wu, K.; Wang, T.; Cai, D. Study of mxene-filled polyurethane nanocomposites prepared via an emulsion method. Compos. Sci. Technol. 2018, 168, 404–411. [Google Scholar] [CrossRef]
- Yu, B.; Tawiah, B.; Wang, L.Q.; Yin Yuen, A.C.; Zhang, Z.C.; Shen, L.L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated mxene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef]
- Hu, H.; Bai, Z.; Niu, B.; Wu, M.; Hua, T. Binder-free bonding of modularized mxene thin films into thick film electrodes for on-chip micro-supercapacitors with enhanced areal performance metrics. J. Mater. Chem. A 2018, 6, 14876–14884. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. Stability and selective oxidation of aluminum in nano-laminate ti3alc2upon heating in argon. Chem. Mater. 2003, 15, 3716–3720. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2d metal carbides and nitrides (mxenes) for energy storage. Nat. Rev. Mater. 2017, 2. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. Synthesis and characterization of differently substituted phenyl hepta isobutyl-polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym. Compos. 2014, 35, 151–157. [Google Scholar] [CrossRef]
- García, M.G.; Marchese, J.; Ochoa, N.A. Effect of the particle size and particle agglomeration on composite membrane performance. J. Appl. Polym. Sci. 2010, 118, 2417–2424. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.-R.; Yuen, A.C.-Y.; Chen, T.B.-Y.; Chan, M.-C.; Peng, L.-Z.; Yang, W.-J.; Zhu, S.-E.; Yang, B.-H.; Hu, K.-H.; et al. Synthesis of phosphorus-containing silane coupling agent for surface modification of glass fibers: Effective reinforcement and flame retardancy in poly(1,4-butylene terephthalate). Chem. Eng. J. 2017, 321, 257–267. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Pan, H.; Yang, W.; Liew, K.M.; Song, L.; Hu, Y. Synthesis and characterization of mno2 nanosheets based multilayer coating and applications as a flame retardant for flexible polyurethane foam. Compos. Sci. Technol. 2016, 123, 212–221. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Zheng, Y.; Yang, J.; Duan, Z.; Hu, Y. Design of reduced graphene oxide decorated with dopo-phosphanomidate for enhanced fire safety of epoxy resin. J. Colloid. Interface Sci. 2018, 521, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tawiah, B.; Yu, C.; Qian, Y.-F.; Wang, L.-L.; Yuen, A.C.-Y.; Zhu, S.-E.; Hu, E.-Z.; Chen, T.B.-Y.; Yu, B.; et al. Manufacturing, mechanical and flame retardant properties of poly(lactic acid) biocomposites based on calcium magnesium phytate and carbon nanotubes. Compos. Part. A Appl. Sci. Manuf. 2018, 110, 227–236. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.-J.; Tawiah, B.; Zhang, Y.; Wang, L.-L.; Zhu, S.-E.; Chen, T.B.Y.; Yuen, A.C.Y.; Yu, B.; Liu, Y.-F.; et al. Synthesis of anhydrous manganese hypophosphite microtubes for simultaneous flame retardant and mechanical enhancement on poly(lactic acid). Compos. Sci. Technol. 2018, 164, 44–50. [Google Scholar] [CrossRef]
- Faravelli, T.; Pinciroli, M.; Pisano, F.; Bozzano, G.; Dente, M.; Ranzi, E. Thermal degradation of polystyrene. J. Anal. Appl. Pyrol. 2001, 60, 103–121. [Google Scholar] [CrossRef]
- Feng, X.; Xing, W.; Song, L.; Hu, Y.; Liew, K.M. Tio2 loaded on graphene nanosheet as reinforcer and its effect on the thermal behaviors of poly(vinyl chloride) composites. Chem. Eng. J. 2015, 260, 524–531. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling tio2-rgo nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013, 132-133, 452–459. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, Y.; Xu, W.; Huang, D.; Wang, Z.; Hong, M. Fabrication and thermal stability of two-dimensional carbide ti 3 c 2 nanosheets. Ceram. Int. 2016, 42, 8419–8424. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, J.; Zeng, W.; Hu, Y.; Gui, Z. In situ synthesis, morphology, and fundamental properties of polymer/mos2 nanocomposites. Compos. Sci. Technol. 2015, 107, 120–128. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Yuan, B.; Hu, Y.; Song, L. Functionalized graphene oxide for fire safety applications of polymers: A combination of condensed phase flame retardant strategies. J. Mater. Chem. 2012, 22. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, Y. Solvothermal synthesis of organically modified α-zirconium phosphate-based polystyrene nanocomposites and thermal stability. J. Appl. Polym. Sci. 2011, 122, 593–598. [Google Scholar] [CrossRef]
- Matusinovic, Z.; Lu, H.; Wilkie, C.A. The role of dispersion of ldh in fire retardancy: The effect of dispersion on fire retardant properties of polystyrene/ca−al layered double hydroxide nanocomposites. Polym. Degrad. Stab. 2012, 97, 1563–1568. [Google Scholar] [CrossRef]
- Zhi, Y.-R.; Yu, B.; Yuen, A.C.Y.; Liang, J.; Wang, L.-Q.; Yang, W.; Lu, H.-D.; Yeoh, G.-H. Surface manipulation of thermal-exfoliated hexagonal boron nitride with polyaniline for improving thermal stability and fire safety performance of polymeric materials. ACS Omega 2018, 3, 14942–14952. [Google Scholar] [CrossRef]
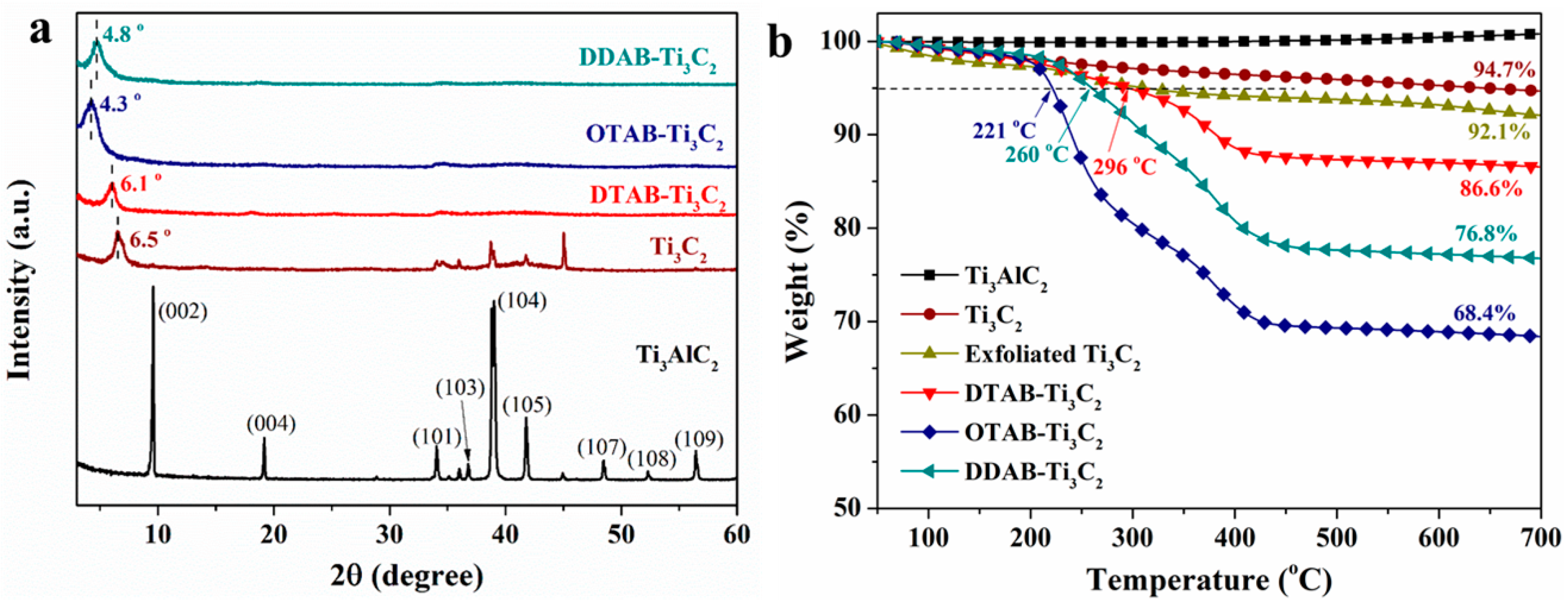
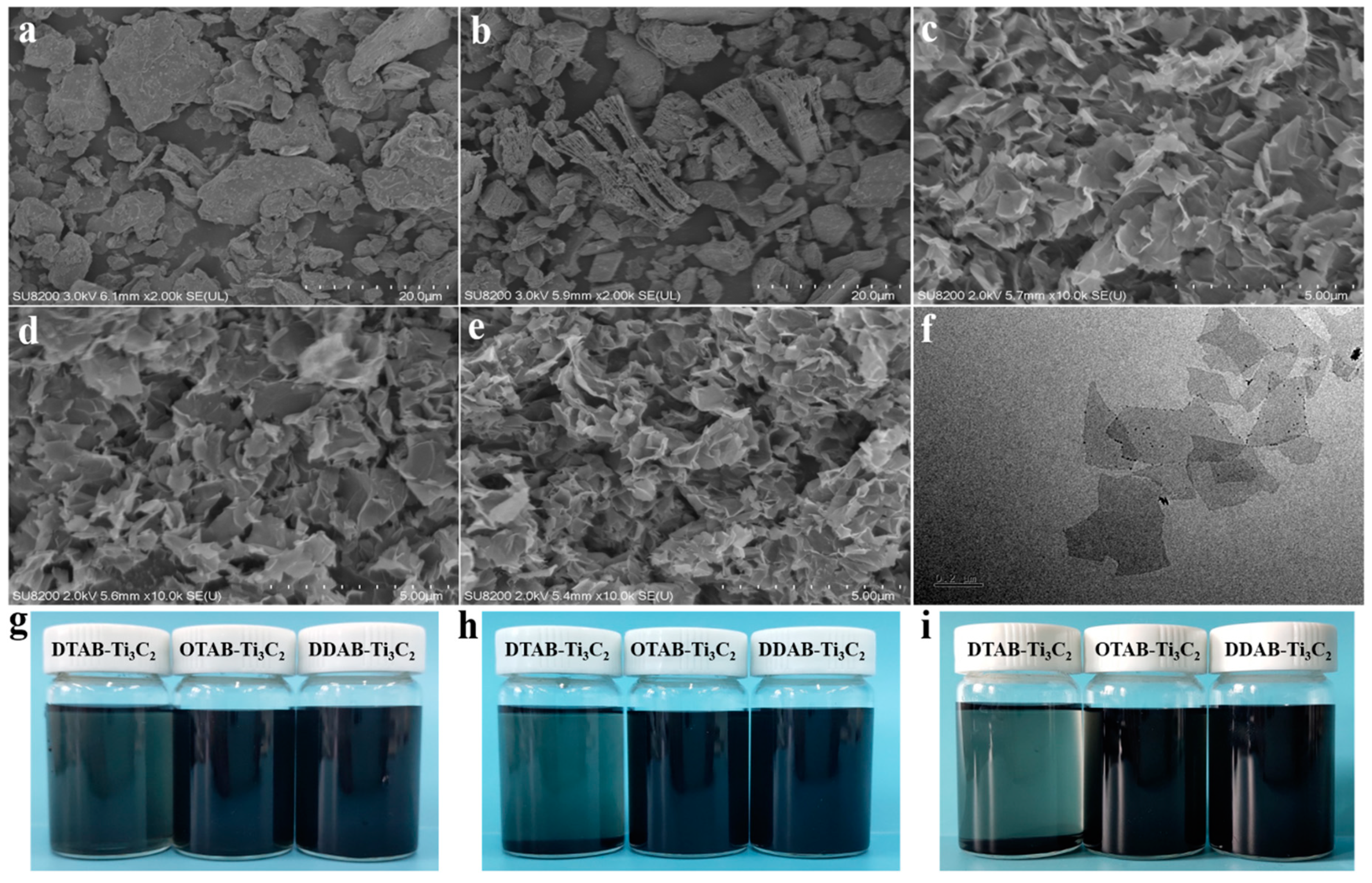
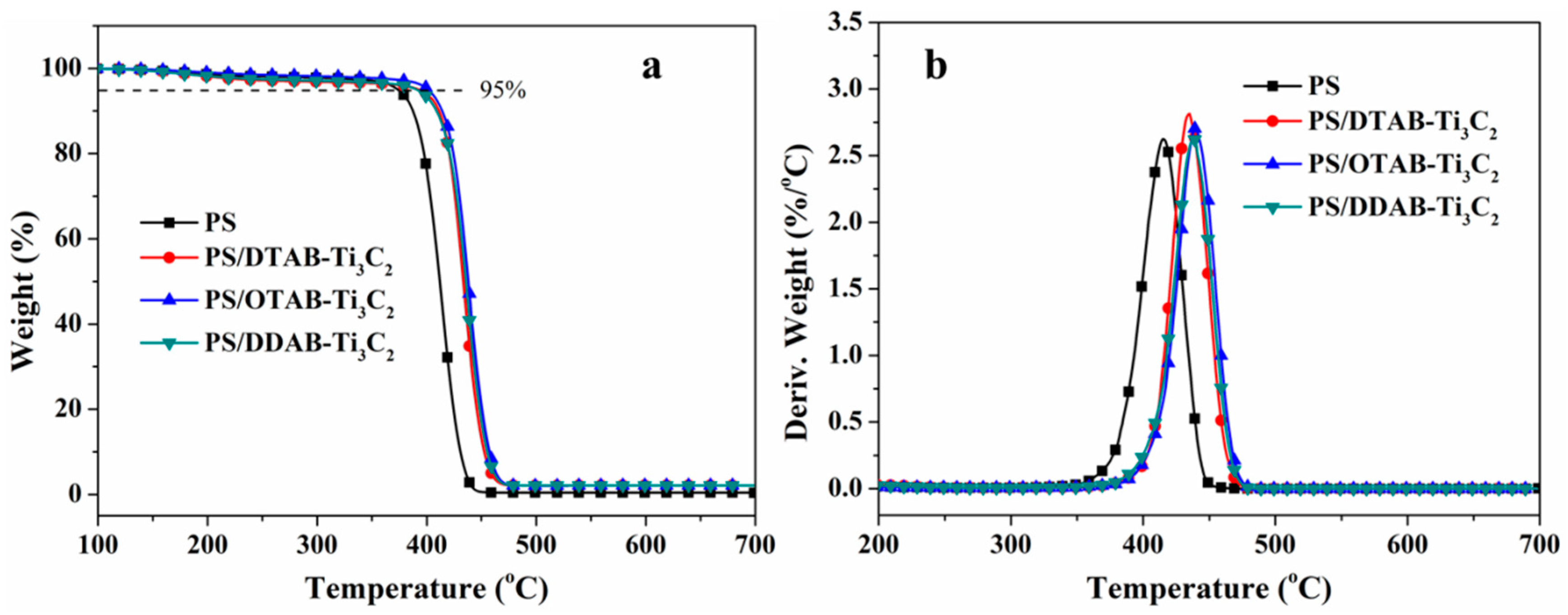

| Sample No. | T5% (°C) | Tmax (°C) | Char Residues (700 °C, wt%) |
|---|---|---|---|
| Neat PS | 374 | 415 | 0.4 |
| PS/DTAB-Ti3C2 | 394 | 435 | 2.1 |
| PS/OTAB-Ti3C2 | 399 | 440 | 2.1 |
| PS/DDAB-Ti3C2 | 397 | 438 | 2.1 |
| Sample No. | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | PCOP (g/s) | PCO2P (g/s) |
|---|---|---|---|---|---|
| Error | ±2 | ±15 | ±0.5 | ±0.005 | ±0.02 |
| Neat PS | 62 | 823 | 72.1 | 0.0322 | 0.673 |
| PS/DTAB-Ti3C2 | 52 | 652 | 75.2 | 0.0220 | 0.511 |
| PS/OTAB-Ti3C2 | 49 | 606 | 78.6 | 0.0218 | 0.507 |
| PS/DDAB-Ti3C2 | 35 | 646 | 76.6 | 0.0216 | 0.537 |
| Sample No. | Additives Loading (wt.%) | T5% (°C) | PHRR (kW/m2)/Technique | Ref. |
|---|---|---|---|---|
| PS/GNS | 3 | +13 | −17.4%/CC | [28] |
| PS/CTAB-MoS2 | 3 | −114 | −9.6%/MCC | [56] |
| PS/FGO | 3 | −52.7%/CC | [57] | |
| PS/OZrP | 5 | −73 | [58] | |
| Poly(St-co-AEPPA)/ZrP | 3 | −17 | −13.9%/MCC | [1] |
| PS/CaAl LDH-B | 3 | −19.1%/CC | [59] | |
| PS/PANI-BNO | 3 | +5 | −31.3%/CC | [60] |
| PS/DTAB-Ti3C2 | 2 | +20 | −20.8%/CC | This work |
| PS/OTAB-Ti3C2 | 2 | +25 | −26.4%/CC | This work |
| PS/DDAB-Ti3C2 | 2 | +23 | −21.5%/CC | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, J.-Y.; Tawiah, B.; Sun, W.-L.; Lin, B.; Wang, C.; Yuen, A.C.Y.; Yu, B.; Li, A.; Yang, W.; Lu, H.-D.; et al. Functionalization of MXene Nanosheets for Polystyrene towards High Thermal Stability and Flame Retardant Properties. Polymers 2019, 11, 976. https://doi.org/10.3390/polym11060976
Si J-Y, Tawiah B, Sun W-L, Lin B, Wang C, Yuen ACY, Yu B, Li A, Yang W, Lu H-D, et al. Functionalization of MXene Nanosheets for Polystyrene towards High Thermal Stability and Flame Retardant Properties. Polymers. 2019; 11(6):976. https://doi.org/10.3390/polym11060976
Chicago/Turabian StyleSi, Jing-Yu, Benjamin Tawiah, Wei-Long Sun, Bo Lin, Cheng Wang, Anthony Chun Yin Yuen, Bin Yu, Ao Li, Wei Yang, Hong-Dian Lu, and et al. 2019. "Functionalization of MXene Nanosheets for Polystyrene towards High Thermal Stability and Flame Retardant Properties" Polymers 11, no. 6: 976. https://doi.org/10.3390/polym11060976
APA StyleSi, J.-Y., Tawiah, B., Sun, W.-L., Lin, B., Wang, C., Yuen, A. C. Y., Yu, B., Li, A., Yang, W., Lu, H.-D., Chan, Q. N., & Yeoh, G. H. (2019). Functionalization of MXene Nanosheets for Polystyrene towards High Thermal Stability and Flame Retardant Properties. Polymers, 11(6), 976. https://doi.org/10.3390/polym11060976