Catalyst Speciation during ansa-Zirconocene-Catalyzed Polymerization of 1-Hexene Studied by UV-vis Spectroscopy—Formation and Partial Re-Activation of Zr-Allyl Intermediates †
Abstract
1. Introduction
2. Materials and Methods
3. Results
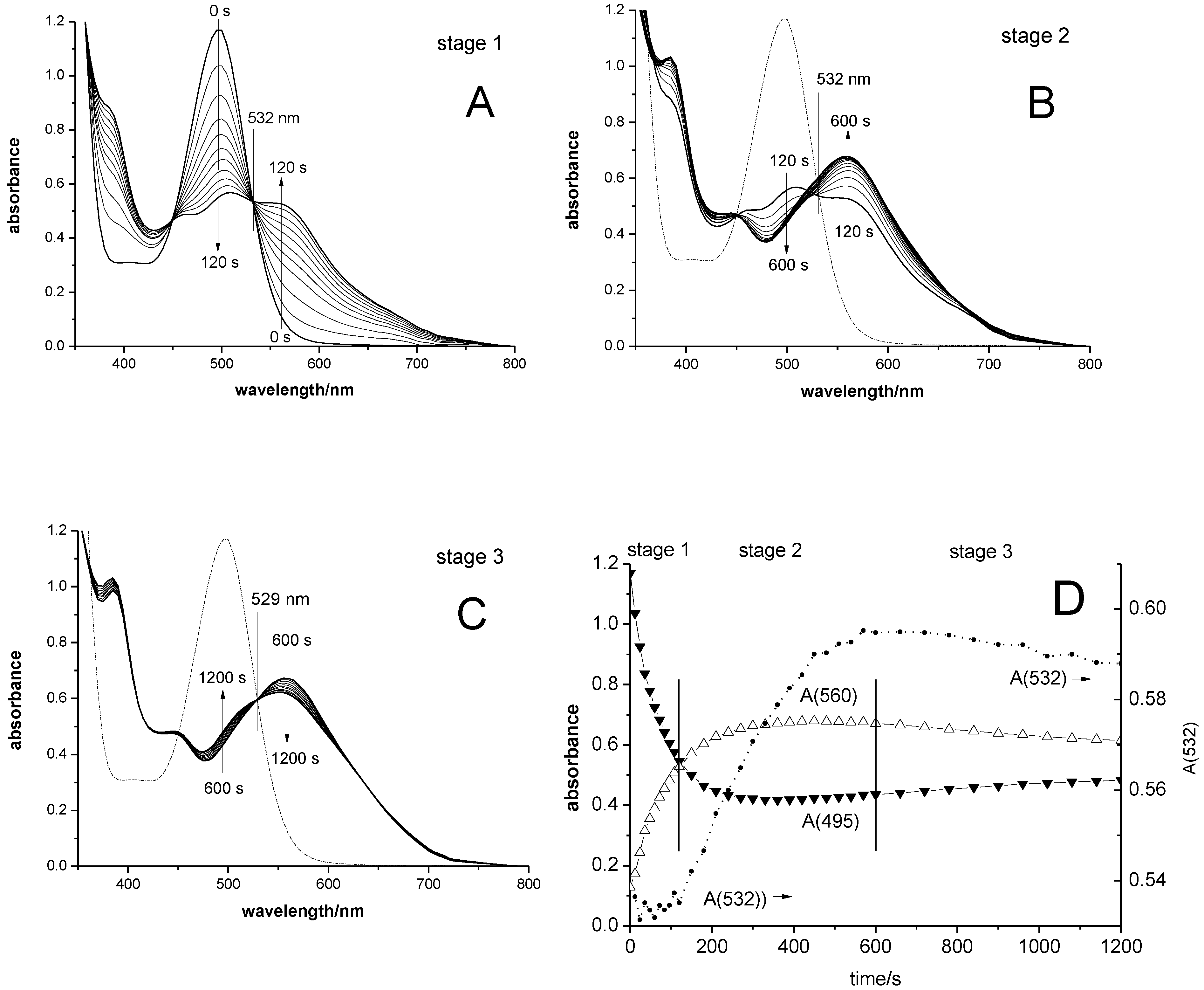
3.1. Qualitative UV-vis and 1H NMR Observations
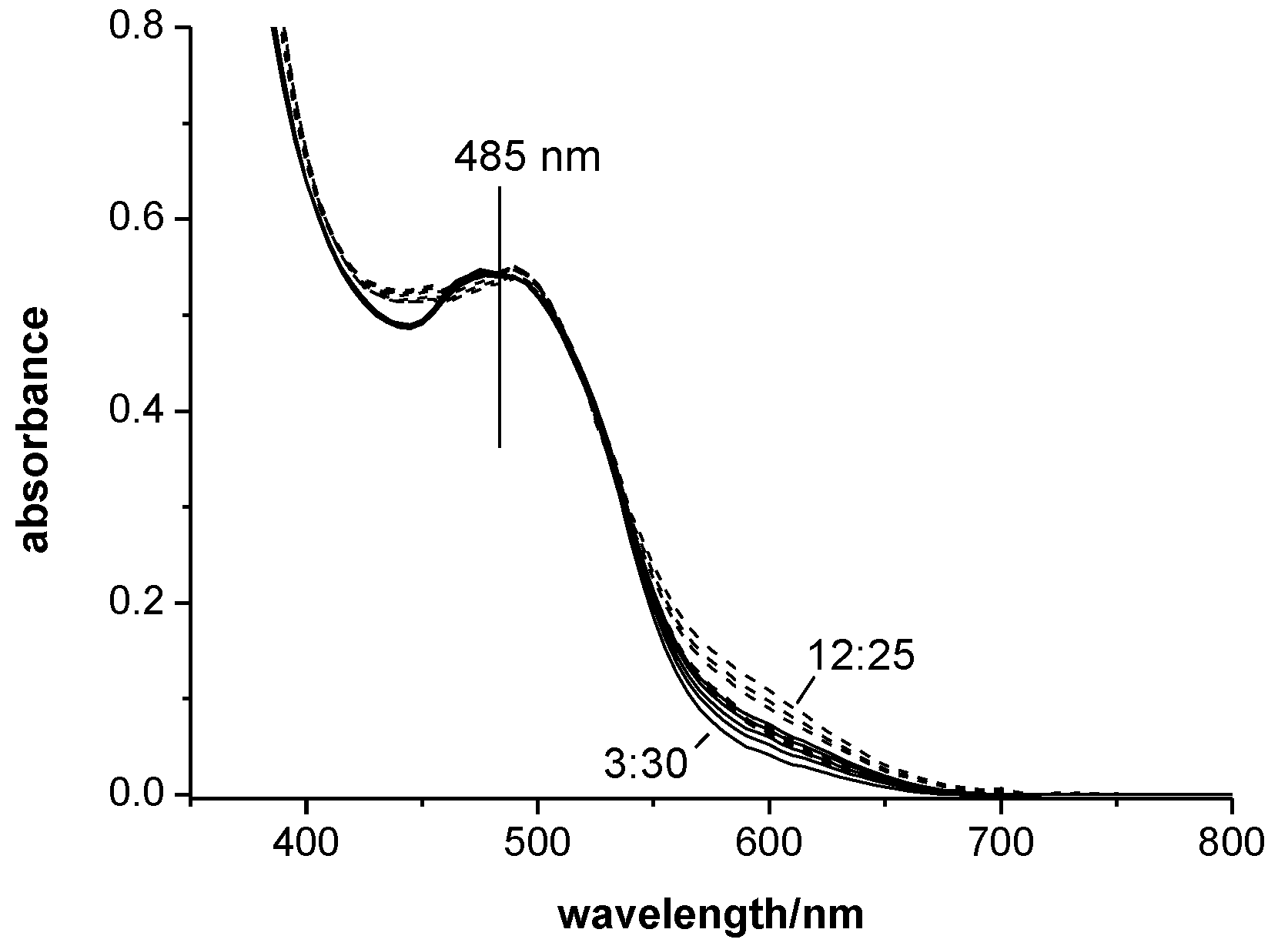
3.2. UV-vis Spectra of SBIZr-Alkyl Cations
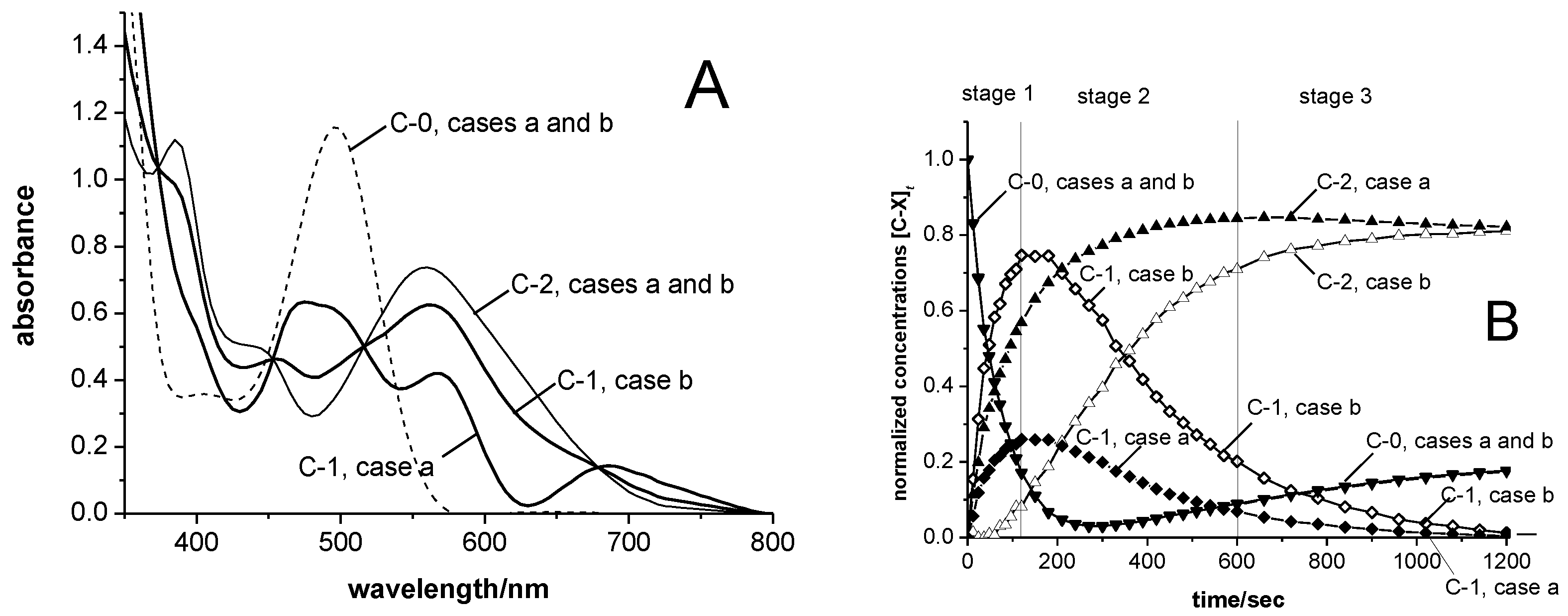
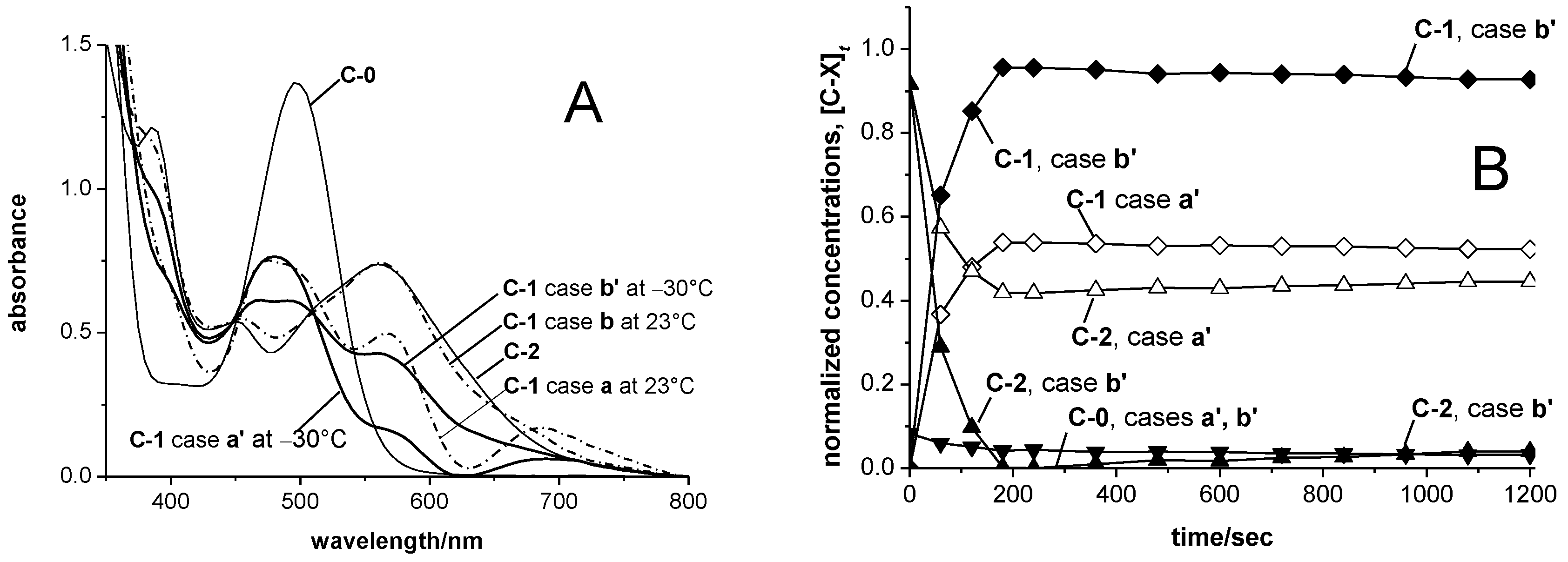
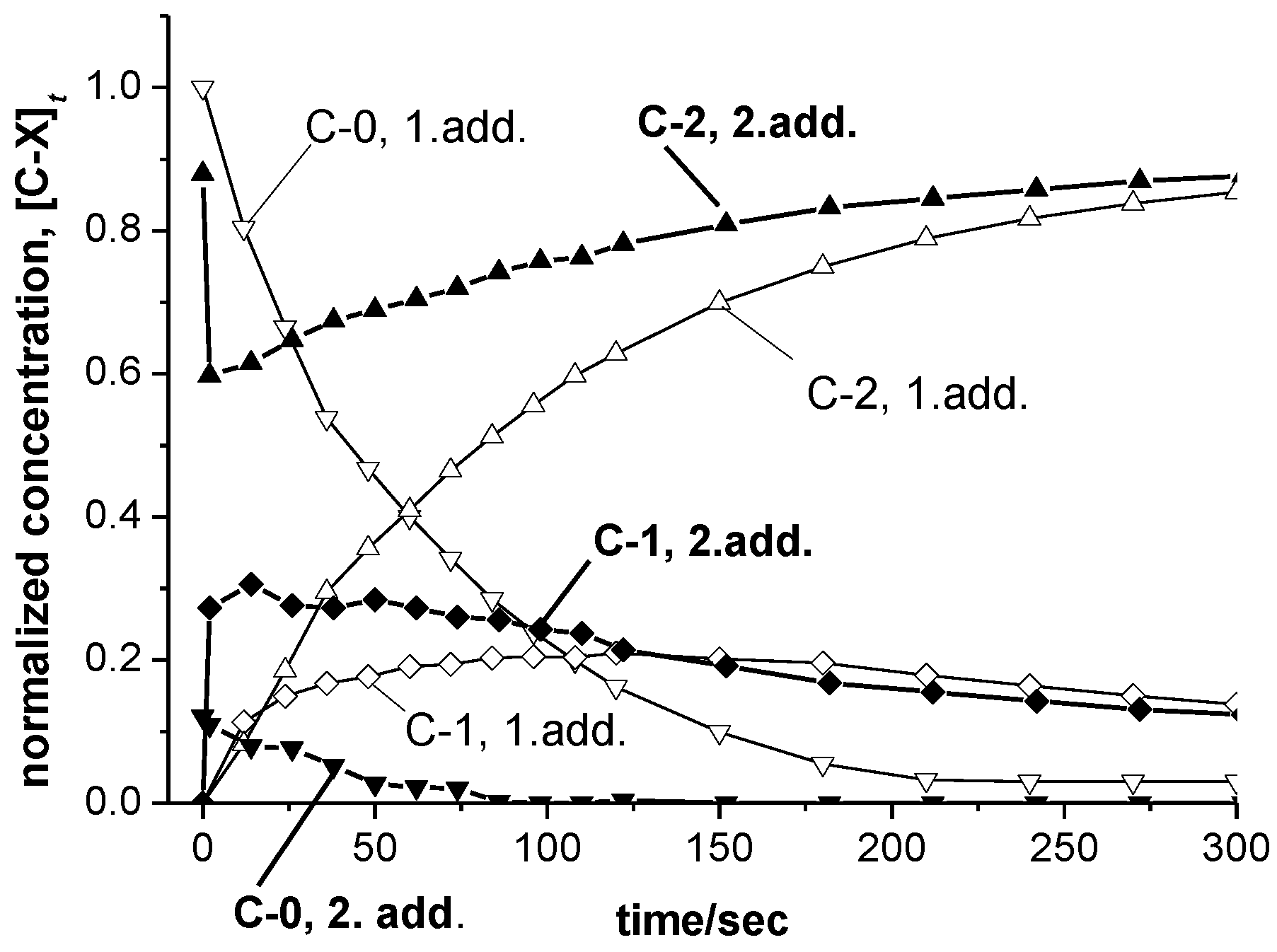
3.3. UV-vis Spectra and Concentration Profiles of Species C-1 and C-2
- For case a, a main absorbance band of species C-1 similar to those of the cations SBIZr-Me+ and SBIZrCH2SiMe3+ would support the plausible assumption that C-1 consists of SBIZr-polymeryl cations. This is connected, however, with the non-trivial notion that species of types C-1 and C-2 should both arise simultaneously from the pre-catalyst C-0. This assumption is compatible with the appearance of the isosbestic point at 532 nm, since during reaction stage 1 species of type C-1 and C-2 arise in constant proportion ([C-2]t<120/[C-1]t<120 = 1.9 ± 0.1), as well as with its disappearance during reaction stage 2, where this proportionality is lost.
- For case b, on the other hand, the virtual absence of species C-2 during stage 1 would be an intuitively attractive explanation for the occurrence of an isosbestic point at 532 nm during that reaction stage. The notion, however, that both the chain-propagating intermediate C-1 and the final species C-2 should be of the SBIZr-allyl+ type, as implied by their closely similar UV-vis spectra in case b, is not in line with generally accepted assumptions concerning catalyst systems of the kind considered here.
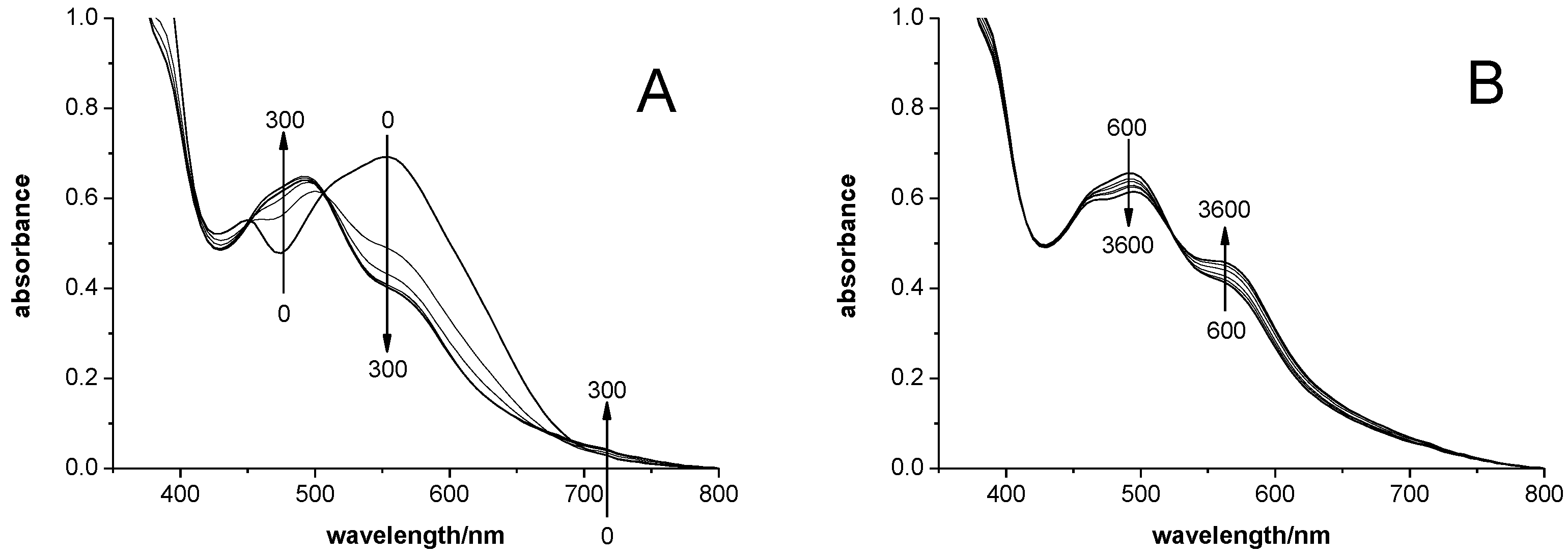
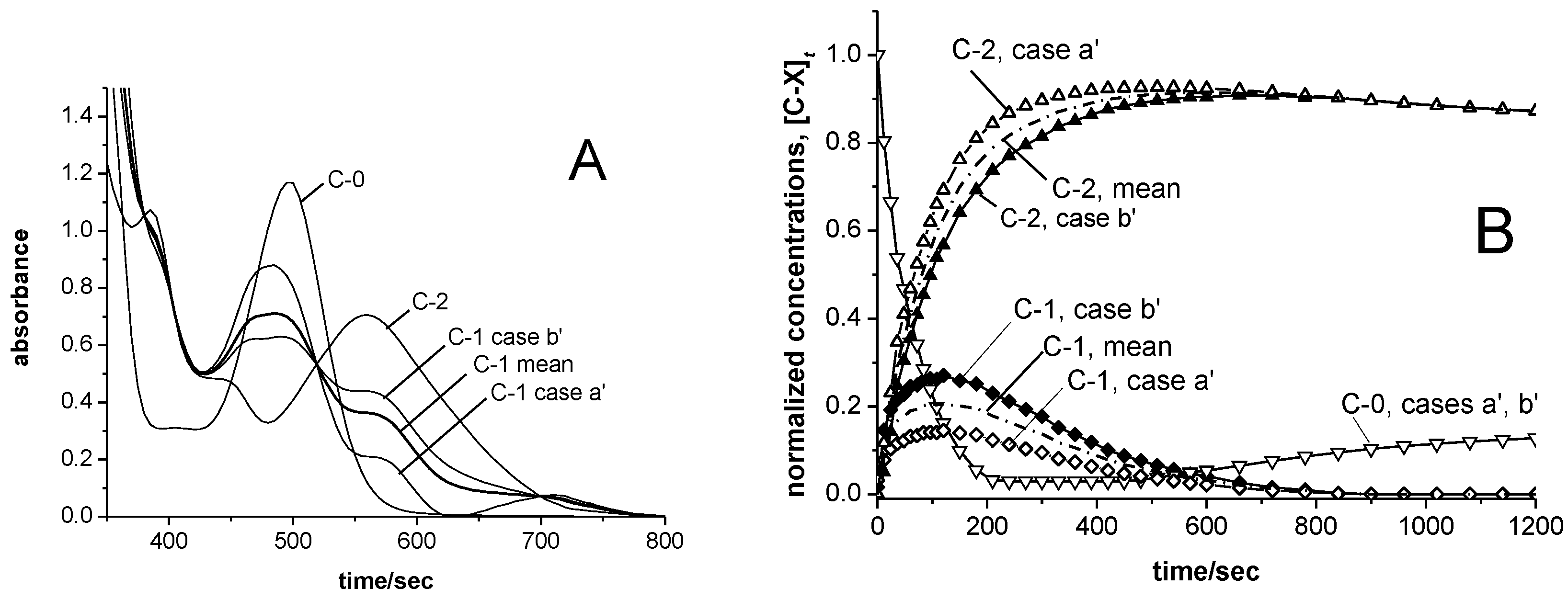
3.4. Catalyst Re-Activation
3.5. Temperature Effects
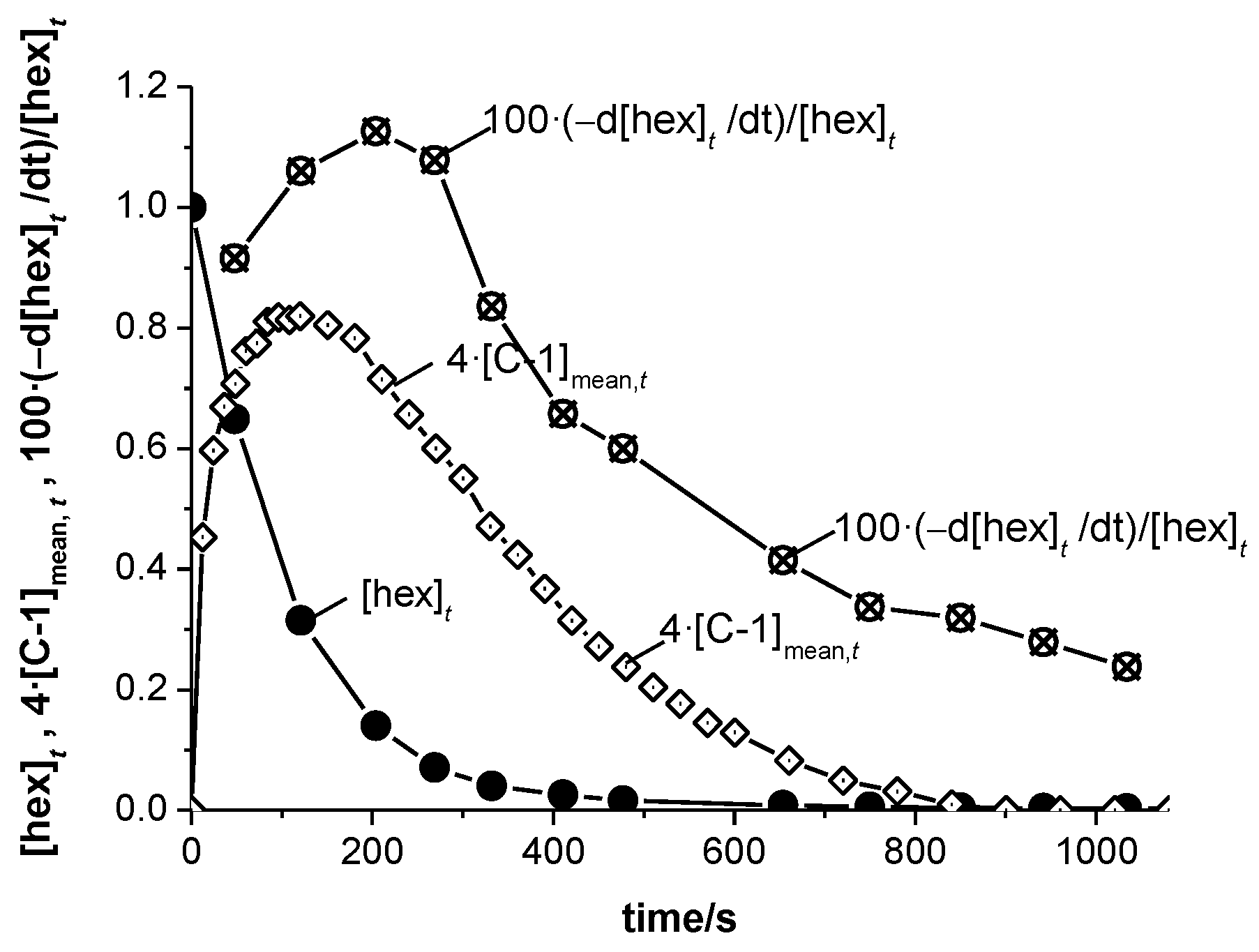
3.6. Reaction Rates and Mechanisms
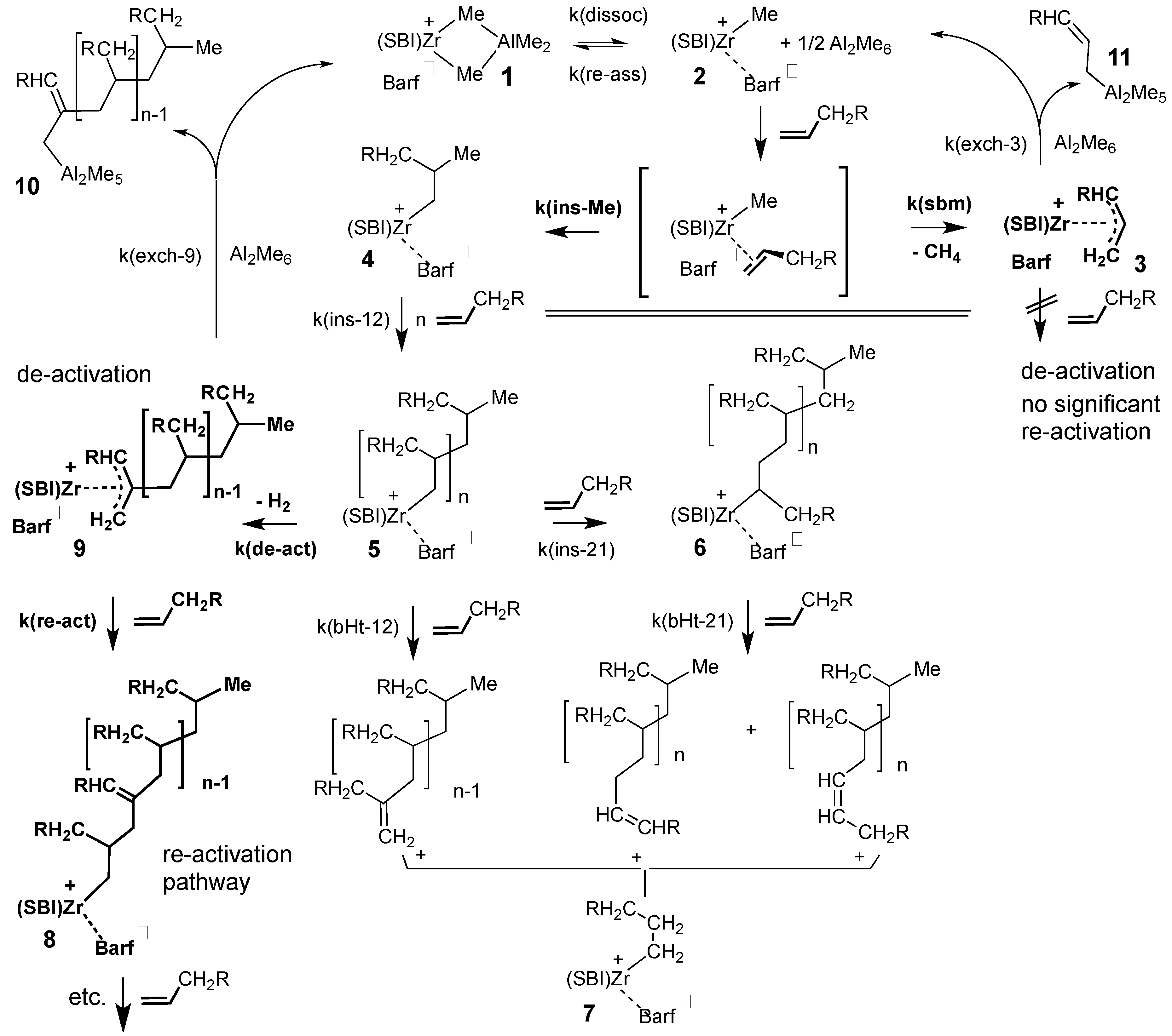
4. Discussion
- cations of composition SBIZr-η3-(1-R-C3H4)+ (R = n-propyl) arising during the initiation reaction between SBIZr-Me+ and 1-hexene by σ-bond metathesis under release of methane,
- polymer-containing cations SBIZr-η3-(1-R-2-pol-C3H3)+ (pol = i-polyhexyl) formed from SBIZr-σ-polyhexyl+ cations, e.g., by loss of H2, during later reaction stages.
- Olefin insertions into a zirconocene-Me+ bond are known to be much slower than insertions into a zirconocene-polymeryl+ bond [5]; σ-bond metathesis might thus be more competitive here.
- The formation of CH3D from SBIZr-Me+ and toluene-d8, as described in Section 3.2. above, indicates a pronounced tendency of SBIZr-Me+ cations toward σ-bond metathesis.
- The occurrence of Zr-allyl species, which cannot be re-activated by monomer, might contribute to observations that only a fraction of the total zirconocene content of such polymerization catalyst systems seem to be actively involved in chain growth, while major portions remain in some ‘dormant’ state. Based on analyses of a series of polypropylene samples, Busico, Cipullo and coworkers have proposed that the deactivation of propagating centers is caused by 2,1-misinsertions [7]. To which degrees such 2,1-misinsertions and/or the formation of Zr-allyl species contribute to the observed deactivation might be clarified by further studies on the microstructures of the polymer products and by suitable quenching experiments.
- The presence of alkyl or polymeryl substituents at the central position of polymer-carrying Zr-allyl cations, such as complex 9, will undoubtedly cause substantial steric strain with the ligand framework of these SBIZr complexes. Even greater steric strain is to be expected for homologous complexes with extended aromatic ring ligands above and below the equatorial ligand plane, such as dimethylsilyl-bridged bis-benzindenyl or bis-4-phenyl-indenyl zirconium derivatives [49,50]. Catalysts with such extended ligands might thus owe their particularly high activities to a destabilization of polymer-carrying Zr-allyl cations of type 9, i.e., to an increased rate of their re-conversion to Zr-polymeryl species and/or to a suppression of their formation.
- The occurrence of polymer-carrying Zr-allyl cations such as 9, which are easily re-converted to Zr-polymeryl cations by relatively fast monomer insertion, might explain an apparently higher than first-order dependence of polymerization rates on monomer concentrations, repeatedly observed with various zirconocene-based polymerization catalyst systems. As shown by Fait et al. [18], this phenomenon is best explained by changes from an inactive to an active catalyst state induced by higher monomer concentrations. Such a situation would be expected to pertain for zirconocene-catalyzed olefin polymerizations run within certain concentration and temperature regimes, due to a monomer-dependent interconversion between catalytically active Zr-polymeryl and polymer-carrying Zr-allyl cations described above.
- For practical use, zirconocene-based olefin-polymerization catalysts have to be immobilized on some solid support, usually on a suitable silica gel. Frequently, these supported catalyst are subjected, before actual use, to a ‘pre-polymerization’ procedure, i.e., to a treatment with some olefin monomer, in order to package each catalyst grain in a thin polymer layer, which stabilizes it against crumbling, but allows its shrapnel-like expansion into minute fragments during the proper polymerization run [51,52]. In view of our study, such pre-polymerized zirconocene catalyst are likely to be present on their supports in form of their Zr-π-allyl derivatives and should hence follow re-activation kinetics similar to that shown in Figure 8 above, rather than the much slower activation kinetics of a catalyst precursor of type Zr(μ-Me)2AlMe2+.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brintzinger, H.H.; Fischer, D. Development of ansa-Metallocene Catalysts for Isotactic Olefin Polymerization. Adv. Polym. Sci. 2013, 258, 29–42. [Google Scholar]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1345. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, M. The Chemistry of Catalyst Activation: The Case of Group 4 Polymerization Catalysts. Organometallics 2010, 29, 4711–4740. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P. Frontiers of mechanistic studies of coordination polymerization and oligomerization of α-olefins. Coord. Chem. Rev. 2012, 256, 2994–3007. [Google Scholar] [CrossRef]
- Liu, Z.; Somsook, E.; White, C.B.; Kimberly, A.; Rosaaen, K.A.; Landis, C.R. Kinetics of Initiation, Propagation, and Termination for the [rac-(C2H4(1-indenyl)2)ZrMe] [MeB(C6F5)3]-Catalyzed Polymerization of 1-Hexene. J. Am. Chem. Soc. 2001, 123, 11193–11207. [Google Scholar] [CrossRef] [PubMed]
- Landis, C.R.; Rosaaen, K.A.; Sillars, D.R. Direct Observation of Insertion Events at rac-(C2H4(1-indenyl)2)ZrMeB(C6F5)3-Polymeryl Intermediates: Distinction between Continuous and Intermittent Propagation Modes. J. Am. Chem. Soc. 2003, 125, 1710–1711. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Romanelli, V.; Ronca, S.; Togrou, M. Reactivity of Secondary Metal-Alkyls in Catalytic Propene Polymerization: How Dormant Are “Dormant Chains”? J. Am. Chem. Soc. 2005, 127, 1608–1609. [Google Scholar] [CrossRef]
- Song, F.; Lancaster, S.J.; Cannon, R.D.; Schormann, M.; Humphrey, S.M.; Zuccaccia, C.; Macchioni, A.; Bochmann, M. Synthesis, Ion Aggregation, Alkyl Bonding Modes, and Dynamics of 14-Electron Metallocenium Ion Pairs [(SBI)MCH2SiMe3…X-] (M = Zr, Hf): Inner-Sphere (X = MeB(C6F5)3) versus Outer-Sphere (X = B(C6F5)4) Structures and the Implications for “Continuous” or “Intermittent” Alkene Polymerization Mechanisms. Organometallics 2005, 24, 1315–1328. [Google Scholar]
- Babushkin, D.E.; Brintzinger, H.H. Reactive Intermediates Formed During Olefin Polymerization by Methylalumoxane-Activated ansa-Zirconocene Catalysts: Identification of a Chain-Carrying Intermediate by NMR Methods. J. Am. Chem. Soc. 2010, 132, 452–453. [Google Scholar] [CrossRef]
- Chen, C.-H.; Shih, W.-C.; Hilty, C. In Situ Determination of Tacticity, Deactivation, and Kinetics in [rac-(C2H4(1-Indenyl)2)ZrMe][B(C6F5)4] and [Cp2ZrMe][B(C6F5)4]-Catalyzed Polymerization of 1-Hexene Using 13C Hyperpolarized NMR. J. Am. Chem. Soc. 2015, 137, 6965–6971. [Google Scholar] [CrossRef]
- Richardson, D.E.; Alameddin, N.G.; Ryan, M.F.; Hayes, T.; Eyler, J.R.; Siedle, A.R. Intrinsic Ancillary Ligand Effects in Cationic Zirconium Polymerization Catalysts: Gas-Phase Reactions of [L2ZrCH3]+ Cations with Alkenes. J. Am. Chem. Soc. 1996, 118, 11244–11253. [Google Scholar] [CrossRef]
- Karol, F.J.; Kao, S.-C.; Wasserman, E.P.; Brady, R.C. Use of copolymerization studies with metallocene catalysts to probe the nature of the active sites. New J. Chem. 1997, 21, 797–805. [Google Scholar]
- Resconi, L. On the mechanisms of growing-chain-end isomerization and transfer reactions in propylene polymerization with isospecific, C2-symmetric zirconocene catalysts. J. Mol. Catal. A 1999, 146, 167–178. [Google Scholar] [CrossRef]
- Resconi, L.; Camurati, I.; Sudmeijer, O. Chain transfer reactions in propylene polymerization with zirconocene catalysts. Top. Catal. 1999, 7, 145–163. [Google Scholar] [CrossRef]
- Horton, A.D. Pentamethylcyclopentadienyl Ligand Activation in a Cationic Zirconocene Complex: Formation of an Unusual Pendant Allyl Ligand with 1,3-Butadiene. Organometallics 1992, 11, 3271–3275. [Google Scholar] [CrossRef]
- Feichtinger, D.; Plattner, D.A.; Chen, P. Ziegler-Natta-like Olefin Oligomerization by Alkylzirconocene Cations in an Electrospray Ionization Tandem Mass Spectrometer. J. Am. Chem. Soc. 1998, 120, 7125–7126. [Google Scholar] [CrossRef]
- Margl, P.M.; Woo, T.K.; Ziegler, T. Potential Catalyst Deactivation Reaction in Homogeneous Ziegler-Natta Polymerization of Olefins: Formation of an Allyl Intermediate. Organometallics 1998, 17, 4997–5002. [Google Scholar] [CrossRef]
- Fait, A.; Resconi, L.; Guerra, G.; Corradini, P. A Possible Interpretation of the Nonlinear Propagation Rate Laws for Insertion Polymerizations: A Kinetic Model Based on a Single-Center, Two-State Catalyst. Macromolecules 1999, 32, 2104–2109. [Google Scholar] [CrossRef]
- Lieber, S.; Prosenc, M.-H.; Brintzinger, H.H. Zirconocene Allyl Complexes: Dynamics in Solution, Reaction with Aluminum Alkyls, B(C6F5)3-Induced Propene Insertion, and Density-Functional Calculations on Possible Formation and Reaction Pathways. Organometallics 2000, 19, 377–387. [Google Scholar] [CrossRef]
- Erker, G. Homogeneous Single-Component Betaine Ziegler−Natta Catalysts Derived from (Butadiene)zirconocene Precursors. Acc. Chem. Res. 2001, 34, 309–317. [Google Scholar] [CrossRef]
- Yoder, J.C.; Bercaw, J.E. Chain Epimerization during Propylene Polymerization with Metallocene Catalysts: Mechanistic Studies Using a Doubly Labeled Propylene. J. Am. Chem. Soc. 2002, 124, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Al-Humydi, A.; Garrison, J.C.; Mohammed, M.; Youngs, W.J.; Collins, S. Propene polymerization using ansa-metallocenium ions: Catalyst deactivation processes during monomer consumption and molecular structures of the products formed. Polyhedron 2005, 24, 1234–1249. [Google Scholar] [CrossRef]
- Vatamanu, M.; Stojcevic, G.; Baird, M.C. Detection of an (η1-Alkene Intermediate of the Type [Cp2Zr(Me)(η1-alkene)]+: The Role of Such Species in Metallocene Catalyst Deactivation to Allylic Species. J. Am. Chem. Soc. 2008, 130, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Vatamanu, M. Synthesis, Structures, and Dynamic Features of d0 Zirconocene-Allyl Complexes. Organometallics 2014, 33, 3683–3694. [Google Scholar] [CrossRef]
- Vatamanu, M. Observation of zirconium allyl species formed during zirconocene-catalyzed propene polymerization and mechanistic insights. J. Catal. 2015, 323, 112–120. [Google Scholar] [CrossRef]
- Landis, C.R.; Christianson, M.D. Metallocene-catalyzed alkene polymerization and the observation of Zr-allyls. Proc. Natl. Acad. Sci. USA 2006, 103, 15349–15354. [Google Scholar] [CrossRef] [PubMed]
- Bryliakov, K.P.; Talsi, E.P.; Semikolenova, N.V.; Zakharov, V.A.; Brand, J.; Alonso-Moreno, C.; Bochmann, M. Formation and structures of cationic zirconium complexes in ternary systems rac-(SBI)ZrX2/AlBui3/[CPh3][B(C6F5)4] (X = Cl, Me). J. Organomet. Chem. 2007, 692, 859–868. [Google Scholar] [CrossRef][Green Version]
- Babushkin, D.E.; Panchenko, V.N.; Brintzinger, H.H. Zirconium Allyl Complexes as Participants in Zirconocene-Catalyzed α-Olefin Polymerizations. Angew. Chem. Int. Ed. 2014, 53, 9645–9649. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, V.N.; Babushkin, D.E.; Brintzinger, H.H. Zirconium-Allyl Complexes as Resting States in Zirconocene-Catalyzed α-Olefin Polymerization. Macromol. Rapid Commun. 2015, 36, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Babushkin, D.E.; Brintzinger, H.H. Activation of Dimethyl Zirconocene by Methylaluminoxane (MAO) - Size Estimate for Me-MAO- Anions by Pulsed Field-Gradient NMR. J. Am. Chem. Soc. 2002, 124, 12869–12873. [Google Scholar] [CrossRef]
- Bochmann, M.; Lancaster, S.J. Monomer-Dimer Equilibria in Homo- and Heterodinuclear Cationic Alkylzirconium Complexes and Their Role in Polymerization Catalysis. Angew. Chem. Int. Ed. Engl. 1994, 33, 1634–1637. [Google Scholar] [CrossRef]
- Tritto, I.; Donetti, R.; Sacchi, M.C.; Locatelli, P.; Zannoni, G. Evidence of Zircononium-Polymeryl Ion Pairs from 13C NMR in Situ 13C2H4 Polymerization with Cp2Zr(13CH3)2-Based Catalysts. Macromolecules 1999, 32, 264–269. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Semikolenova, N.V.; Zakharov, V.A.; Talsi, E.P. Mechanism of dimethylzirconocene activation with methylaluminoxane: NMR monitoring of intermediates at high Al/Zr ratios. Macromol. Chem. Phys. 2000, 201, 558–567. [Google Scholar] [CrossRef]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure-Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef] [PubMed]
- Theurkauff, G.; Bondon, A.; Dorcet, V.; Carpentier, J.-F.; Kirillov, E. Heterobi- and -trimetallic Ion Pairs of Zirconocene-Based Isoselective Olefin Polymerization Catalysts with AlMe3. Angew. Chem. Int. Ed. 2015, 54, 6343–6346. [Google Scholar] [CrossRef]
- Theurkauff, G.; Bader, M.; Marquet, N.; Bondon, A.; Roisnel, T.; Guegan, J.-P.; Amar, A.; Boucekkine, A.; Carpentier, J.-F.; Kirillov, E. Discrete Ionic Complexes of Highly Isoselective Zirconocenes. Solution Dynamics, Trimethylaluminum Adducts, and Implications in Propylene Polymerization. Organometallics 2016, 35, 258–276. [Google Scholar] [CrossRef]
- Zhou, J.; Lancaster, S.J.; Walker, D.A.; Beck, S.; Thornton-Pett, M.; Bochmann, M. Synthesis, structures, and reactivity of weakly coordinating anions with delocalized borate structure: The assessment of anion effects in metallocene polymerization catalysts. J. Am. Chem. Soc. 2001, 123, 223–237. [Google Scholar] [CrossRef]
- Wieser, U. Spektroskopische Untersuchungen an Zirkonocen-Katalysatorsystemen; UFO, Atelier für Gestaltung und Verl.: Allensbach, Germany, 2002; p. 66. [Google Scholar]
- Jaumot, J.; Gargallo, R.; de Juan, A.; Tauler, R. A graphical user-friendly interface for MCR-ALS: A new tool for multivariate curve resolution in MATLAB. Chemom. Intell. Lab. Syst. 2005, 76, 101–110. [Google Scholar] [CrossRef]
- Jaumot, J.; de Juan, A.; Tauler, R. MCR-ALS GUI 2.0: New features and applications. Chemom. Intell. Lab. Syst. 2015, 140, 1–12. [Google Scholar] [CrossRef]
- Tauler, R.; Smilde, A.; Kowalski, B.R. Selectivity, local rank, three-way data analysis and ambiguity in multivariate curve resolution. J. Chemom. 1995, 9, 31–58. [Google Scholar] [CrossRef]
- Tauler, R. Calculation of maximum and minimum band boundaries of feasible solutions for species profiles obtained by multivariate curve resolution. J. Chemom. 2001, 15, 627–646. [Google Scholar] [CrossRef]
- Jaumot, J.; Tauler, R. MCR-BANDS: A user friendly MATLAB program for the evaluation of rotation ambiguities in Multivariate Curve Resolution. Chemom. Intell. Lab. Syst. 2010, 103, 96–107. [Google Scholar] [CrossRef]
- Zhang, X.; Tauler, R. Measuring and comparing the resolution performance and the extent of rotation ambiguities of some bilinear modeling methods. Chemom. Intell. Lab. Syst. 2015, 147, 47–57. [Google Scholar] [CrossRef]
- Neymeyr, K.; Sawall, M.; Hess, D. Pure component spectral recovery and constrained matrix factorizations: Concepts and applications. J. Chemom. 2010, 24, 67–74. [Google Scholar] [CrossRef]
- Sawall, M.; Rahimdoust, N.; Selent, D.; Kubisc, C.; Schröder, H.; Selent, D.; Hess, D.; Abdollahi, H.; Franke, R.; Börner, A.; et al. Soft constraints for reducing the intrinsic rotational ambiguity of the area of feasible solutions. Chemom. Intell. Lab. Syst. 2015, 149, 140–150. [Google Scholar] [CrossRef]
- Thompson, M.E.; Baxter, S.M.; Bulls, A.R.; Burger, B.J.; Nolan, M.C.; Santarsiero, B.D.; Schaefer, W.P.; Bercaw, J.E. “σ-Bond Metathesis” for C-H Bonds of Hydrocarbons and Sc-R (R = H, alkyl, aryl) Bonds of Permethylscandocene Derivatives. Evidence for Noninvolvement of the tt System in Electrophilic Activation of Aromatic and Vinylic C-H Bonds. J. Am. Chem. Soc. 1987, 109, 203–219. [Google Scholar] [CrossRef]
- Waterman, R. σ-Bond Metathesis: A 30-Year Retrospective. Organometallics 2013, 32, 7249–7263. [Google Scholar] [CrossRef]
- Spaleck, W.; Küber, F.; Winter, A.; Rohrmann, J.; Bachmann, B.; Antberg, M.; Dolle, V.; Paulus, E. The Influence of Aromatic Substituents on the Polymerization Behavior of Bridged Zirconocene Catalysts. Organometallics 1994, 13, 954–963. [Google Scholar] [CrossRef]
- Stehling, U.; Diebold, J.; Kirsten, R.; Röll, W.; Brintzinger, H.H.; Langhauser, F. ansa-Zirconocene Polymerization Catalysts with Annelated Ring Ligands - Effects on Catalytic Activity and Polymer Chain Length. Organometallics 1994, 13, 964–970. [Google Scholar] [CrossRef]
- Hlatky, G.G. Heterogeneous Single-Site Catalysts for Olefin Polymerization. Chem. Rev. 2000, 100, 1347–1376. [Google Scholar] [CrossRef]
- Fink, G.; Steinmetz, B.; Zechlin, J.; Przybyla, C.; Tesche, B. Propene Polymerization with Silica-Supported Metallocene/MAO Catalysts. Chem. Rev. 2000, 100, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchenko, V.N.; Babushkin, D.E.; Bercaw, J.E.; Brintzinger, H.H.
Catalyst Speciation during ansa-Zirconocene-Catalyzed Polymerization of 1-Hexene Studied by UV-vis Spectroscopy—Formation and Partial Re-Activation of Zr-Allyl Intermediates
Panchenko VN, Babushkin DE, Bercaw JE, Brintzinger HH.
Catalyst Speciation during ansa-Zirconocene-Catalyzed Polymerization of 1-Hexene Studied by UV-vis Spectroscopy—Formation and Partial Re-Activation of Zr-Allyl Intermediates
Panchenko, Valentina N., Dmitrii E. Babushkin, John E. Bercaw, and Hans H. Brintzinger.
2019. "Catalyst Speciation during ansa-Zirconocene-Catalyzed Polymerization of 1-Hexene Studied by UV-vis Spectroscopy—Formation and Partial Re-Activation of Zr-Allyl Intermediates
Panchenko, V. N., Babushkin, D. E., Bercaw, J. E., & Brintzinger, H. H.
(2019). Catalyst Speciation during ansa-Zirconocene-Catalyzed Polymerization of 1-Hexene Studied by UV-vis Spectroscopy—Formation and Partial Re-Activation of Zr-Allyl Intermediates



