Selectively Biodegradable Polyesters: Nature-Inspired Construction Materials for Future Biomedical Applications
Abstract
1. Introduction
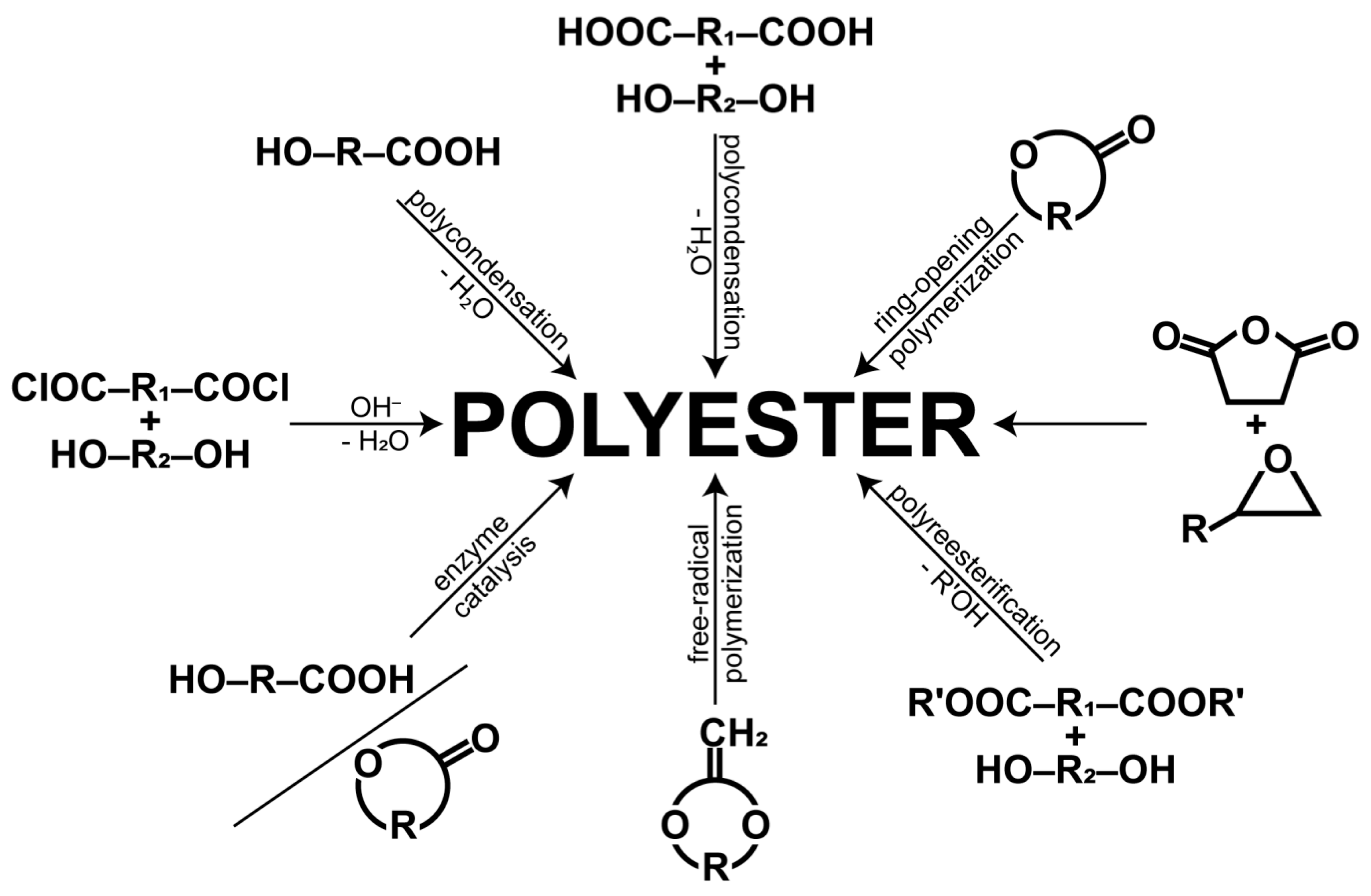
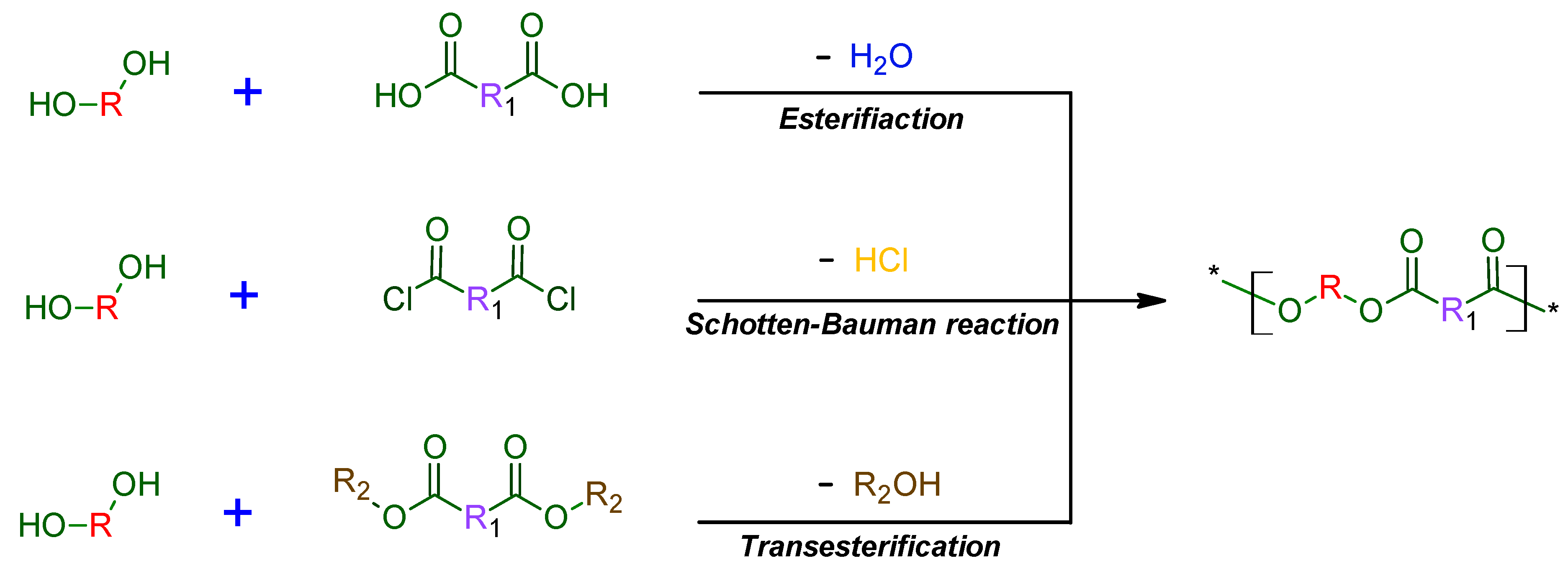
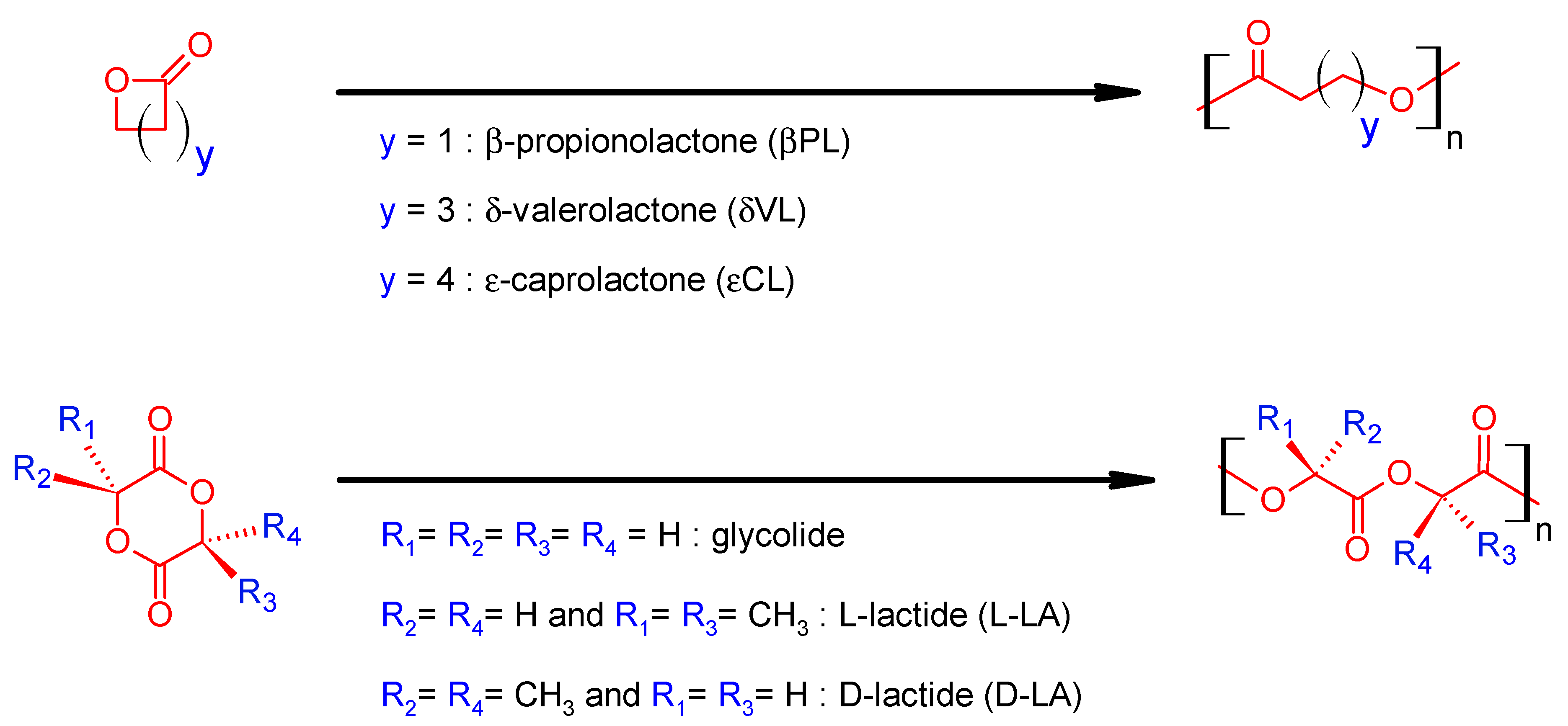
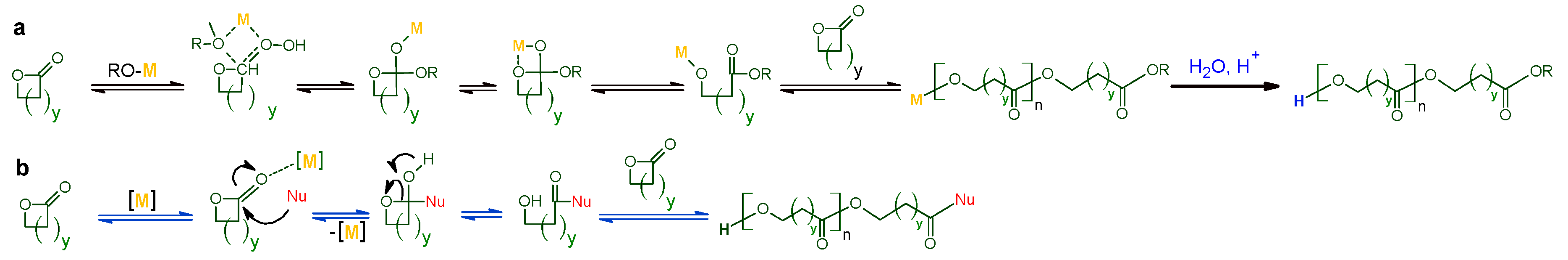
1.1. Synthesis and Mechanisms
1.2. Drug Release from Polyesters in General
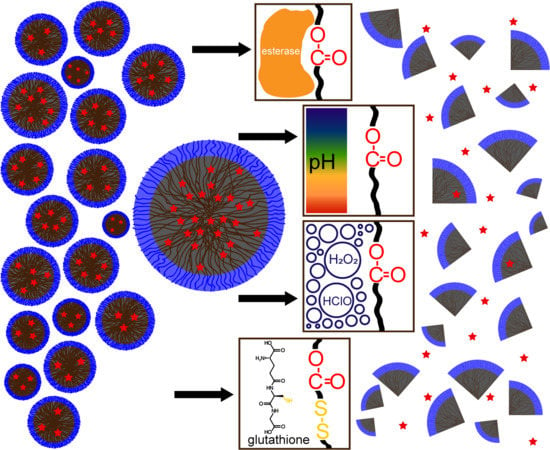
2. Polyesters with Stimuli-Sensitive Linkages
2.1. pH-Labile Polyesters
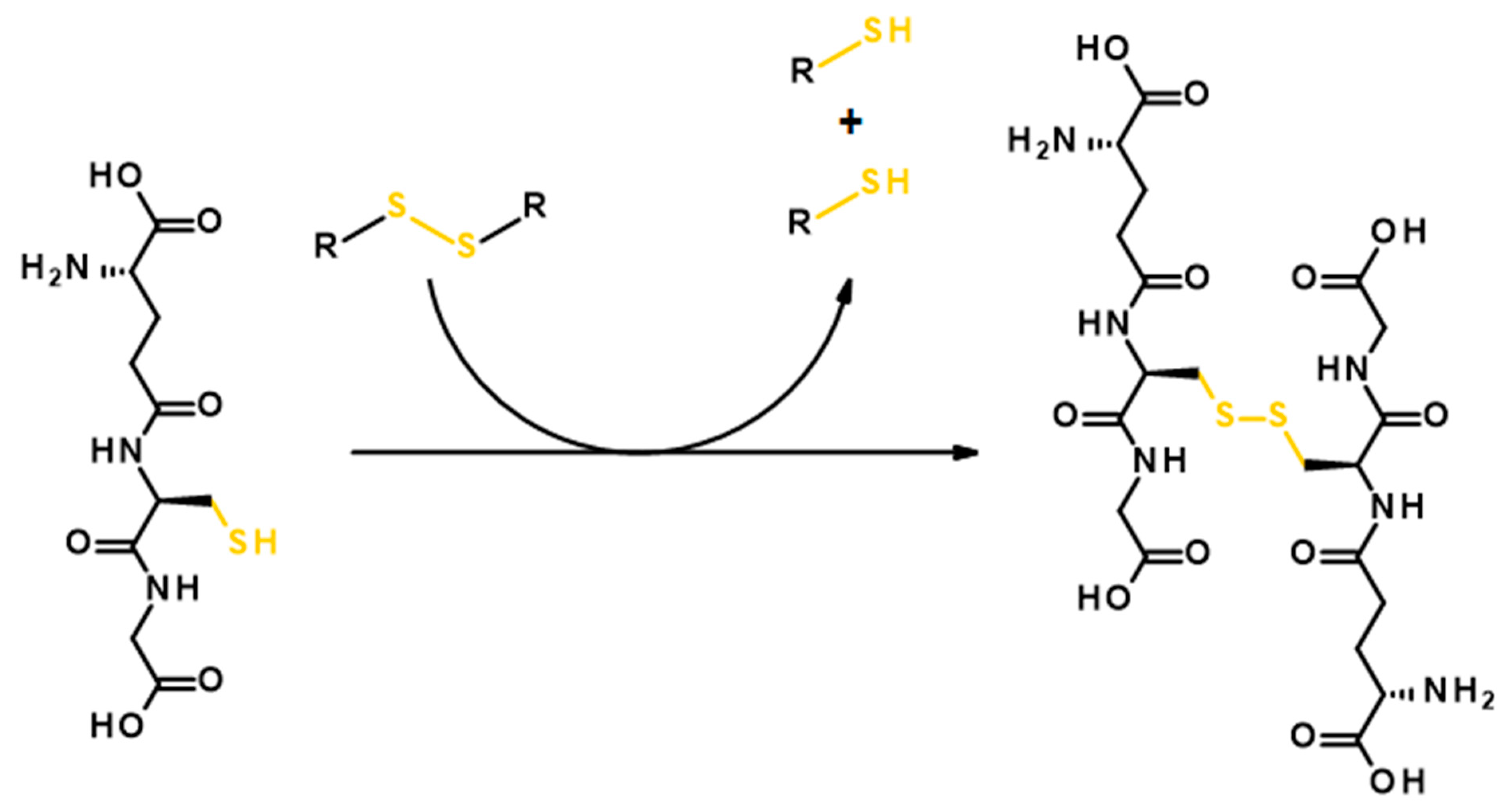
2.2. Reductively Labile Polyesters
2.3. Reactive Oxygen Species (ROS)-Labile Polyesters
2.3.1. Poly(propylene sulfide)s
2.3.2. Selenium-Containing Polyesters
2.3.3. Aryl Boronic Esters Containing Polyesters
2.3.4. Polyoxalates
2.4. Enzymatically Labile Polyesters
3. Conclusions
Funding
Conflicts of Interest
References
- Sivaram, S.J.R. Wallace hume carothers and the birth of rational polymer synthesis. Resonance 2017, 22, 339–353. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. Handbook of Polyester Drug Delivery Systems; Pan Stanford Publishing: Singapore, 2017. [Google Scholar]
- Albertsson, A.-C.; Varma, I.K. Aliphatic polyesters. In Biopolymers Online; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Ding, D.; Pan, X.; Zhang, Z.; Li, N.; Zhu, J.; Zhu, X. A degradable copolymer of 2-methylene-1,3-dioxepane and vinyl acetate by photo-induced cobalt-mediated radical polymerization. Polym. Chem. 2016, 7, 5258–5264. [Google Scholar] [CrossRef]
- Sun, L.F.; Zhuo, R.X.; Liu, Z.L. Synthesis and enzymatic degradation of 2-methylene-1,3-dioxepane and methyl acrylate copolymers. J. Polym. Sci. A Polym. Chem. 2003, 41, 2898–2904. [Google Scholar] [CrossRef]
- Edlund, U.; Albertsson, A.C. Polyesters based on diacid monomers. Adv. Drug Deliv. Rev. 2003, 55, 585–609. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S. Mechanism of aliphatic polyester formation. In Biopolymers; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Jäger, A.; Gromadzki, D.; Jäger, E.; Giacomelli, F.C.; Kozlowska, A.; Kobera, L.; Brus, J.; Říhová, B.; El Fray, M.; Ulbrich, K.; et al. Novel “soft” biodegradable nanoparticles prepared from aliphatic based monomers as a potential drug delivery system. Soft Matter 2012, 8, 4343–4354. [Google Scholar] [CrossRef]
- Jäger, E.; Jäger, A.; Etrych, T.; Giacomelli, F.C.; Chytil, P.; Jigounov, A.; Putaux, J.-L.; Říhová, B.; Ulbrich, K.; Štěpánek, P. Self-assembly of biodegradable copolyester and reactive hpma-based polymers into nanoparticles as an alternative stealth drug delivery system. Soft Matter 2012, 8, 9563–9575. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. In Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Carothers, W.H. Polymers and polyfunctionality. Trans. Faraday Soc. 1936, 32, 39–49. [Google Scholar] [CrossRef]
- Carothers, W.H. Studies on polymerization and ring formation. I. An introduction to the general theory of condensation polymers. J. Am. Chem. Soc. 1929, 51, 2548–2559. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Papadimitriou, S.A.; Karavas, E.; Avgoustakis, K. Novel biodegradable polyester poly(propylene succinate): Synthesis and application in the preparation of solid dispersions and nanoparticles of a water-soluble drug. AAPS Pharmscitech 2009, 10, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D.; Karavelidis, V.; Karavas, E. Novel biodegradable polyesters. Synthesis and application as drug carriers for the preparation of raloxifene hcl loaded nanoparticles. Molecules 2009, 14, 2410–2430. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, B.-H. Microbial succinic acid, its polymer poly(butylene succinate), and applications. In Plastics from Bacteria: Natural Functions and Applications; Chen, G.G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 347–388. [Google Scholar]
- Yang, J.; Zhang, S.; Liu, X.; Cao, A. A study on biodegradable aliphatic poly(tetramethylene succinate): The catalyst dependences of polyester syntheses and their thermal stabilities. Polym. Degrad. Stab. 2003, 81, 1–7. [Google Scholar] [CrossRef]
- Luo, S.; Li, F.; Yu, J.; Cao, A. Synthesis of poly(butylene succinate-co-butylene terephthalate) (pbst) copolyesters with high molecular weights via direct esterification and polycondensation. J. Appl. Polym. Sci. 2010, 115, 2203–2211. [Google Scholar] [CrossRef]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and properties of poly(butylene succinate): Efficiency of different transesterification catalysts. J. Polym. Sci. A Polym. Chem. 2011, 49, 5301–5312. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S. Synthesis of poly(alkylene succinate) biodegradable polyesters i. Mathematical modelling of the esterification reaction. Polymer 2006, 47, 4851–4860. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S. Synthesis of poly(alkylene succinate) biodegradable polyesters, part ii: Mathematical modelling of the polycondensation reaction. Polymer 2008, 49, 3677–3685. [Google Scholar] [CrossRef]
- Gallardo, A.; San Román, J.; Dijkstra, P.J.; Feijen, J. Random polyester transesterification: Prediction of molecular weight and mw distribution. Macromolecules 1998, 31, 7187–7194. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef]
- Jérôme, R.; Lecomte, P. 4-new developments in the synthesis of aliphatic polyesters by ring-opening polymerisation. In Biodegradable Polymers for Industrial Applications; Smith, R., Ed.; Woodhead Publishing: Cambridge, UK, 2005; pp. 77–106. [Google Scholar]
- Lecomte, P.; Jérôme, C. Recent developments in ring-opening polymerization of lactones. In Synthetic Biodegradable Polymers; Rieger, B., Künkel, A., Coates, G.W., Reichardt, R., Dinjus, E., Zevaco, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 173–217. [Google Scholar]
- Penczek, S.; Cypryk, M.; Duda, A.; Kubisa, P.; Słomkowski, S. Living ring-opening polymerizations of heterocyclic monomers. Prog. Polym. Sci. 2007, 32, 247–282. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Jain, R.; Shah, N.H.; Malick, A.W.; Rhodes, C.T. Controlled drug delivery by biodegradable poly(ester) devices: Different preparative approaches. Drug Dev. Ind. Pharm. 1998, 24, 703–727. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. Off. J. Controll. Release Soc. 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (pla)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. Plga-based nanoparticles: An overview of biomedical applications. J. Controll. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Idris, S.B.; Dånmark, S.; Finne-Wistrand, A.; Arvidson, K.; Albertsson, A.-C.; Bolstad, A.I.; Mustafa, K. Biocompatibility of polyester scaffolds with fibroblasts and osteoblast-like cells for bone tissue engineering. J. Bioact. Compat. Polym. 2010, 25, 567–583. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of pla and plga microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Pamula, E.; Dobrzynski, P.; Szot, B.; Kretek, M.; Krawciow, J.; Plytycz, B.; Chadzinska, M. Cytocompatibility of aliphatic polyesters—In vitro study on fibroblasts and macrophages. J. Biomed. Mater. Res. Part A 2008, 87, 524–535. [Google Scholar] [CrossRef]
- Knight, P.T.; Kirk, J.T.; Anderson, J.M.; Mather, P.T. In vivo kinetic degradation analysis and biocompatibility of aliphatic polyester polyurethanes. J. Biomed. Mater. Res. Part A 2010, 94, 333–343. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.M.; Spenlehauer, G.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Sokolsky-Papkov, M.; Agashi, K.; Olaye, A.; Shakesheff, K.; Domb, A.J. Polymer carriers for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Bartus, C.; William Hanke, C.; Daro-Kaftan, E. A decade of experience with injectable poly-l-lactic acid: A focus on safety. Off. Publ. Am. Soc. Dermatol. Surg. 2013, 39, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Bakhru, S.H.; Furtado, S.; Morello, A.P.; Mathiowitz, E. Oral delivery of proteins by biodegradable nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 811–821. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv.Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. Plga nanoparticles containing various anticancer agents and tumour delivery by epr effect. Adv. Drug Deliv.Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Cao, A.; Wang, J. In vitro evaluation of biodegradable poly(butylene succinate) as a novel biomaterial. Macromol. Biosci. 2005, 5, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, W.; Li, Q.; Li, Y.; Cao, A. Novel biodegradable aliphatic poly(butylene succinate-co-cyclic carbonate)s bearing functionalizable carbonate building blocks: Ii. Enzymatic biodegradation and in vitro biocompatibility assay. Biomacromolecules 2004, 5, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Bechthold, I.; Bretz, K.; Kabasci, S.; Kopitzky, R.; Springer, A. Succinic acid: A new platform chemical for biobased polymers from renewable resources. Chem. Eng. Technol. 2008, 31, 647–654. [Google Scholar] [CrossRef]
- Jäger, E.; Donato, R.K.; Perchacz, M.; Jäger, A.; Surman, F.; Höcherl, A.; Konefał, R.; Donato, K.Z.; Venturini, C.G.; Bergamo, V.Z.; et al. Biocompatible succinic acid-based polyesters for potential biomedical applications: Fungal biofilm inhibition and mesenchymal stem cell growth. RSC Adv. 2015, 5, 85756–85766. [Google Scholar] [CrossRef]
- Jäger, A.; Jäger, E.; Giacomelli, F.C.; Nallet, F.; Steinhart, M.; Putaux, J.-L.; Konefał, R.; Spěváček, J.; Ulbrich, K.; Štěpánek, P. Structural changes on polymeric nanoparticles induced by hydrophobic drug entrapment. Physicochem. Eng. Asp. 2018, 538, 238–249. [Google Scholar] [CrossRef]
- Jäger, A.; Jäger, E.; Syrová, Z.; Mazel, T.; Kováčik, L.; Raška, I.; Höcherl, A.; Kučka, J.; Konefal, R.; Humajova, J.; et al. Poly(ethylene oxide monomethyl ether)-block-poly(propylene succinate) nanoparticles: Synthesis and characterization, enzymatic and cellular degradation, micellar solubilization of paclitaxel, and in vitro and in vivo evaluation. Biomacromolecules 2018, 19, 2443–2458. [Google Scholar] [CrossRef]
- Van Dijkhuizen-Radersma, R.; Roosma, J.R.; Kaim, P.; Métairie, S.; Péters, F.L.A.M.A.; de Wijn, J.; Zijlstra, P.G.; de Groot, K.; Bezemer, J.M. Biodegradable poly(ether-ester) multiblock copolymers for controlled release applications. J. Biomed. Mater. Res. 2003, 67, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Van Dijkhuizen-Radersma, R.; Roosma, J.R.; Sohier, J.; Péters, F.L.A.M.A.; van den Doel, M.; van Blitterswijk, C.A.; de Groot, K.; Bezemer, J.M. Biodegradable poly(ether-ester) multiblock copolymers for controlled release applications: An in vivo evaluation. J. Biomed. Mater. Res. 2004, 71, 118–127. [Google Scholar] [CrossRef]
- Wang, L.-C.; Chen, J.-W.; Liu, H.-L.; Chen, Z.-Q.; Zhang, Y.; Wang, C.-Y.; Feng, Z.-G. Synthesis and evaluation of biodegradable segmented multiblock poly(ether ester) copolymers for biomaterial applications. Polym. Int. 2004, 53, 2145–2154. [Google Scholar] [CrossRef]
- Lindström, A.; Albertsson, A.-C.; Hakkarainen, M. Quantitative determination of degradation products an effective means to study early stages of degradation in linear and branched poly(butylene adipate) and poly(butylene succinate). Polym. Degrad. Stab. 2004, 83, 487–493. [Google Scholar] [CrossRef]
- Bremer, J.; Osmundsen, H. Chapter 5 fatty acid oxidation and its regulation. In New Comprehensive Biochemistry; Numa, S., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 7, pp. 113–154. [Google Scholar]
- Domb, A.J.; Maniar, M. Absorbable biopolymers derived from dimer fatty acids. J. Polym. Sci. A Polym. Chem. 1993, 31, 1275–1285. [Google Scholar] [CrossRef]
- Jain, J.P.; Sokolsky, M.; Kumar, N.; Domb, A.J. Fatty acid based biodegradable polymer. Polym. Rev. 2008, 48, 156–191. [Google Scholar] [CrossRef]
- Jäger, E.; Jäger, A.; Chytil, P.; Etrych, T.; Říhová, B.; Giacomelli, F.C.; Štěpánek, P.; Ulbrich, K. Combination chemotherapy using core-shell nanoparticles through the self-assembly of hpma-based copolymers and degradable polyester. J. Controll. Release 2013, 165, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Burkersroda, F.v.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
- Ge, H.; Hu, Y.; Yang, S.; Jiang, X.; Yang, C. Preparation, characterization, and drug release behaviors of drug-loaded ε-caprolactone/l-lactide copolymer nanoparticles. J. Appl. Polym. Sci. 2000, 75, 874–882. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, C.; Dong, C.-M. Poly(l-lactide)-b-poly(ethylene oxide) copolymers with different arms: Hydrophilicity, biodegradable nanoparticles, in vitro degradation, and drug-release behavior. J. Biomed. Mater. Res. 2009, 88, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2009, 6, 3021–3032. [Google Scholar]
- Kang Moo, H.; Hyun Su, M.; Sang Cheon, L.; Hong Jae, L.; Sungwon, K.; Kinam, P. A new hydrotropic block copolymer micelle system for aqueous solubilization of paclitaxel. J. Controll. Release 2008, 126, 122–129. [Google Scholar] [CrossRef]
- Mahmud, A.; Patel, S.; Molavi, O.; Choi, P.; Samuel, J.; Lavasanifar, A. Self-associating poly(ethylene oxide)-b-poly(α-cholesteryl carboxylate-ε-caprolactone) block copolymer for the solubilization of stat-3 inhibitor cucurbitacin i. Biomacromolecules 2009, 10, 471–478. [Google Scholar] [CrossRef]
- Patel, S.K.; Lavasanifar, A.; Choi, P. Roles of nonpolar and polar intermolecular interactions in the improvement of the drug loading capacity of peo-b-pcl with increasing pcl content for two hydrophobic cucurbitacin drugs. Biomacromolecules 2009, 10, 2584–2591. [Google Scholar] [CrossRef]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1446. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Šubr, V.R. Polymeric anticancer drugs with ph-controlled activation. Adv. Drug Deliv. Rev. 2004, 56, 1023–1050. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.-Y.; Qiao, S.-L.; Fan, G.; Fan, Y.-S.; Chen, Y.; Wang, H. One-pot synthesis of ph-sensitive poly(rgd-co-β-amino ester)s for targeted intracellular drug delivery. Polym. Chem. 2014, 5, 844–853. [Google Scholar] [CrossRef]
- Yi, Y.; Lin, G.; Chen, S.; Liu, J.; Zhang, H.; Mi, P. Polyester micelles for drug delivery and cancer theranostics: Current achievements, progresses and future perspectives. Mater. Sci. Eng. C 2018, 83, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-B.; Mahmud, A.; Uludağ, H.; Lavasanifar, A. Multifunctional polymeric micelles for enhanced intracellular delivery of doxorubicin to metastatic cancer cells. Pharm. Res. 2008, 25, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-B.; Ma, Z.; Lai, R.; Lavasanifar, A. The therapeutic response to multifunctional polymeric nano-conjugates in the targeted cellular and subcellular delivery of doxorubicin. Biomaterials 2010, 31, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.M.; Hurley, J.P.; Salmaso, S.; Kale, A.; Tolcheva, E.; Levchenko, T.S.; Torchilin, V.P. “Smart” drug delivery systems: Double-targeted ph-responsive pharmaceutical nanocarriers. Bioconj. Chem. 2006, 17, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Nishiyama, N.; Fukushima, S.; Koyama, H.; Yasuhiro, M.; Kataoka, K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular ph-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced In Vivo antitumor efficacy. Bioconj. Chem. 2005, 16, 122–130. [Google Scholar] [CrossRef]
- Almutairi, A.; Guillaudeu, S.J.; Berezin, M.Y.; Achilefu, S.; Fréchet, J.M.J. Biodegradable ph-sensing dendritic nanoprobes for near-infrared fluorescence lifetime and intensity imaging. J. Am. Chem. Soc. 2008, 130, 444–445. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, J.; Su, Y.; Wu, J.; Zhu, L.; Zhu, X.; Yan, D.; Zhu, B. Design and synthesis of thermo-responsive hyperbranched poly(amine-ester)s as acid-sensitive drug carriers. Polym. Chem. 2011, 2, 1661–1670. [Google Scholar] [CrossRef]
- Honglawan, A.; Ni, H.; Weissman, D.; Yang, S. Synthesis of random copolymer based ph-responsive nanoparticles as drug carriers for cancer therapeutics. Polym. Chem. 2013, 4, 3667–3675. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Ph-responsive copolymer assemblies for controlled release of doxorubicin. Bioconj. Chem. 2005, 16, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Goodwin, A.P.; Fréchet, J.M.J. Acetals as ph-sensitive linkages for drug delivery. Bioconj. Chem. 2004, 15, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Heller, J. Controlled drug release from poly(ortho esters). J. Controll. Release 1985, 446, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Palumbo, R.N.; Ji, W.; Wang, C. Poly(ortho ester amides): Acid-labile temperature-responsive copolymers for potential biomedical applications. Biomacromolecules 2009, 10, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Srinophakun, T.; Boonmee, J. Preliminary study of conformation and drug release mechanism of doxorubicin-conjugated glycol chitosan, via cis-aconityl linkage, by molecular modeling. Int. J. Mol. Sci. 2011, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Lee, E.A.; Park, T.G. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J. Controll. Release 2002, 82, 17–27. [Google Scholar] [CrossRef]
- Heffernan, M.J.; Murthy, N. Polyketal nanoparticles: A new ph-sensitive biodegradable drug delivery vehicle. Bioconj. Chem. 2005, 16, 1340–1342. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Barr, J. Poly(ortho esters)from concept to reality. Biomacromolecules 2004, 5, 1625–1632. [Google Scholar] [CrossRef]
- Van Den Mooter, G.; Maris, B.; Samyn, C.; Augustijns, P.; Kinget, R. Use of azo polymers for colon-specific drug delivery. J. Pharm. Sci. 1997, 86, 1321–1327. [Google Scholar] [CrossRef]
- Mutlu, H.; Geiselhart, C.M.; Barner-Kowollik, C. Untapped potential for debonding on demand: The wonderful world of azo-compounds. Mater. Horiz. 2018, 5, 162–183. [Google Scholar] [CrossRef]
- Coelho, P.J.; Castro, M.C.R.; Fernandes, S.S.M.; Fonseca, A.M.C.; Raposo, M.M.M. Enhancement of the photochromic switching speed of bithiophene azo dyes. Tetrahedron Lett. 2012, 53, 4502–4506. [Google Scholar] [CrossRef]
- Brown, J.P. Reduction of polymeric azo and nitro dyes by intestinal bacteria. Appl. Environ. Microbiol. 1981, 41, 1283–1286. [Google Scholar] [PubMed]
- Eom, T.; Yoo, W.; Kim, S.; Khan, A. Biologically activatable azobenzene polymers targeted at drug delivery and imaging applications. Biomaterials 2018, 185, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Samyn, C.; Kalala, W.; Van den Mooter, G.; Kinget, R. Synthesis and in vitro biodegradation of poly(ether-ester) azo polymers designed for colon targeting. Int. J. Pharm. 1995, 121, 211–216. [Google Scholar] [CrossRef]
- Lu, L.; Chen, G.; Qiu, Y.; Li, M.; Liu, D.; Hu, D.; Gu, X.; Xiao, Z.J.S.B. Nanoparticle-based oral delivery systems for colon targeting: Principles and design strategies. Sci. Bull. 2016, 61, 670–681. [Google Scholar] [CrossRef]
- Rajpurohit, H.; Sharma, P.; Sharma, S.; Bhandari, A. Polymers for colon targeted drug delivery. Indian J. Pharm. Sci. 2010, 72, 689–696. [Google Scholar] [CrossRef]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Glutathione responsive polymers and their application in drug delivery systems. Polym. Chem. 2017, 8, 97–126. [Google Scholar] [CrossRef]
- Ling, X.; Tu, J.; Wang, J.; Shajii, A.; Kong, N.; Feng, C.; Zhang, Y.; Yu, M.; Xie, T.; Bharwani, Z.; et al. Glutathione-responsive prodrug nanoparticles for effective drug delivery and cancer therapy. ACS Nano 2019, 13, 357–370. [Google Scholar] [CrossRef]
- Song, N.; Liu, W.; Tu, Q.; Liu, R.; Zhang, Y.; Wang, J. Preparation and in vitro properties of redox-responsive polymeric nanoparticles for paclitaxel delivery. Colloids Surfaces B Biointerfaces 2011, 87, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tan, L.; He, C.; Liu, B.; Xu, Y.; Zhu, Z.; Shao, Z.; Gong, B.; Shen, Y.-M. Redox-responsive micelles self-assembled from dynamic covalent block copolymers for intracellular drug delivery. Acta Biomat. 2015, 17, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Namgung, R.; Kim, J.; Singha, K.; Kim, W.J. Bioreducible polymers for gene silencing and delivery. Acc. Chem. Res. 2012, 45, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Bauhuber, S.; Hozsa, C.; Breunig, M.; Göpferich, A. Delivery of nucleic acids via disulfide-based carrier systems. Adv. Mater. 2009, 21, 3286–3306. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Yang, W.; Cheng, Y. Disulfide cross-linked low generation dendrimers with high gene transfection efficacy, low cytotoxicity, and low cost. J. Am. Chem. Soc. 2012, 134, 17680–17687. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ding, A.-X.; Zhang, K.-X.; Gong, B.; Lu, Z.-L.; He, L. Degradable polyesters via ring-opening polymerization of functional valerolactones for efficient gene delivery. Org. Biomol. Chem. 2017, 15, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Cai, X.; Zha, G.; Luo, Q.; Sun, R.; Li, X.; Shen, Z. Reduction-triggered release of paclitaxel from in situ formed biodegradable core-cross-linked micelles. J. Mater. Chem. B 2015, 3, 3024–3031. [Google Scholar] [CrossRef]
- Yameen, B.; Vilos, C.; Choi, W.I.; Whyte, A.; Huang, J.; Pollit, L.; Farokhzad, O.C. Drug delivery nanocarriers from a fully degradable peg-conjugated polyester with a reduction-responsive backbone. Chem. Eur. J. 2015, 21, 11325–11329. [Google Scholar] [CrossRef]
- Vo, C.D.; Kilcher, G.; Tirelli, N. Polymers and sulfur: What are organic polysulfides good for? Preparative strategies and biological applications. Macromol. Rapid Commun. 2009, 30, 299–315. [Google Scholar] [CrossRef]
- Song, C.-C.; Du, F.-S.; Li, Z.-C. Oxidation-responsive polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 3413–3426. [Google Scholar] [CrossRef]
- Napoli, A.; Valentini, M.; Tirelli, N.; Müller, M.; Hubbell, J.A. Oxidation-responsive polymeric vesicles. Nat. Mater. 2004, 3, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Rehor, A.; Tirelli, N.; Hubbell, J.A. A new living emulsion polymerization mechanism: Episulfide anionic polymerization. Macromolecules 2002, 35, 8688–8693. [Google Scholar] [CrossRef]
- Allen, B.L.; Johnson, J.D.; Walker, J.P. Encapsulation and enzyme-mediated release of molecular cargo in polysulfide nanoparticles. ACS Nano 2011, 5, 5263–5272. [Google Scholar] [CrossRef] [PubMed]
- Hirosue, S.; Kourtis, I.C.; van der Vlies, A.J.; Hubbell, J.A.; Swartz, M.A. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and t cell activation. Vaccine 2010, 28, 7897–7906. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Meyer, T.A.; Nelson, C.E.; Duvall, C.L. Poly(ps-b-dma) micelles for reactive oxygen species triggered drug release. J. Controll. Release 2012, 162, 591–598. [Google Scholar] [CrossRef]
- Poole, K.M.; Nelson, C.E.; Joshi, R.V.; Martin, J.R.; Gupta, M.K.; Haws, S.C.; Kavanaugh, T.E.; Skala, M.C.; Duvall, C.L. Ros-responsive microspheres for on demand antioxidant therapy in a model of diabetic peripheral arterial disease. Biomaterials 2015, 41, 166–175. [Google Scholar] [CrossRef]
- Zhang, J.; Tokatlian, T.; Zhong, J.; Ng, Q.K.T.; Patterson, M.; Lowry, W.E.; Carmichael, S.T.; Segura, T. Physically associated synthetic hydrogels with long-term covalent stabilization for cell culture and stem cell transplantation. Adv. Mater. 2011, 23, 5098–5103. [Google Scholar] [CrossRef]
- Kim, K.; Lee, C.-S.; Na, K. Light-controlled reactive oxygen species (ros)-producible polymeric micelles with simultaneous drug-release triggering and endo/lysosomal escape. Chem. Commun. 2016, 52, 2839–2842. [Google Scholar] [CrossRef]
- Caucheteux, S.M.; Mitchell, J.P.; Ivory, M.O.; Hirosue, S.; Hakobyan, S.; Dolton, G.; Ladell, K.; Miners, K.; Price, D.A.; Kan-Mitchell, J.; et al. Polypropylene sulfide nanoparticle p24 vaccine promotes dendritic cell-mediated specific immune responses against hiv-1. J. Investig. Dermatol. 2016, 136, 1172–1181. [Google Scholar] [CrossRef]
- Cao, W.; Wang, L.; Xu, H. Selenium/tellurium containing polymer materials in nanobiotechnology. Nano Today 2015, 10, 717–736. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual redox responsive assemblies formed from diselenide block copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, S.; Cao, W.; Li, Y.; Sun, Z.; Wang, Z.; Xu, H. Red light responsive diselenide-containing block copolymer micelles. J. Mater. Chem. B 2013, 1, 740–743. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, M.; Du, F.-S.; Li, Z.-C. Ros-responsive poly(ε-caprolactone) with pendent thioether and selenide motifs. Polym. Chem. 2018, 9, 3762–3773. [Google Scholar] [CrossRef]
- Wang, C.; An, X.; Pang, M.; Zhang, Z.; Zhu, X.; Zhu, J.; Du Prez, F.E.; Pan, X. Dynamic diselenide-containing polyesters from alcoholysis/oxidation of γ-butyroselenolactone. Polym. Chem. 2018, 9, 4044–4051. [Google Scholar] [CrossRef]
- Broaders, K.E.; Grandhe, S.; Fréchet, J.M.J. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J. Am. Chem. Soc. 2011, 133, 756–758. [Google Scholar] [CrossRef] [PubMed]
- De Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef]
- Jäger, E.; Höcherl, A.; Janoušková, O.; Jäger, A.; Hrubý, M.; Konefał, R.; Netopilik, M.; Pánek, J.; Šlouf, M.; Ulbrich, K.; et al. Fluorescent boronate-based polymer nanoparticles with reactive oxygen species (ros)-triggered cargo release for drug-delivery applications. Nanoscale 2016, 8, 6958–6963. [Google Scholar] [CrossRef]
- Lee, D.; Khaja, S.; Velasquez-Castano, J.C.; Dasari, M.; Sun, C.; Petros, J.; Taylor, W.R.; Murthy, N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765. [Google Scholar] [CrossRef]
- Kim, S.; Seong, K.; Kim, O.; Kim, S.; Seo, H.; Lee, M.; Khang, G.; Lee, D. Polyoxalate nanoparticles as a biodegradable and biocompatible drug delivery vehicle. Biomacromolecules 2010, 11, 555–560. [Google Scholar] [CrossRef]
- Lee, D.; Bae, S.; Ke, Q.; Lee, J.; Song, B.; Karumanchi, S.A.; Khang, G.; Choi, H.S.; Kang, P.M. Hydrogen peroxide-responsive copolyoxalate nanoparticles for detection and therapy of ischemia–reperfusion injury. J. Controll. Release 2013, 172, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Bae, S.; Hong, D.; Lim, H.; Yoon, J.H.; Hwang, O.; Park, S.; Ke, Q.; Khang, G.; Kang, P.M. H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents. Sci. Rep. 2013, 3, 2233. [Google Scholar] [CrossRef] [PubMed]
- Höcherl, A.; Jäger, E.; Jäger, A.; Hrubý, M.; Konefał, R.; Janoušková, O.; Spěváček, J.; Jiang, Y.; Schmidt, P.W.; Lodge, T.P.; et al. One-pot synthesis of reactive oxygen species (ros)-self-immolative polyoxalate prodrug nanoparticles for hormone dependent cancer therapy with minimized side effects. Polym. Chem. 2017, 8, 1999–2004. [Google Scholar] [CrossRef]
- Dongwon, L.; Madhuri, D.; Venkata, E.; Niren, M.; Junhua, Y.; Robert, D. Detection of hydrogen peroxide with chemiluminescent micelles. Int. J. Nanomed. 2008, 3, 471–476. [Google Scholar]
- Liang, X.; Duan, J.; Li, X.; Zhu, X.; Chen, Y.; Wang, X.; Sun, H.; Kong, D.; Li, C.; Yang, J. Improved vaccine-induced immune responses via a ros-triggered nanoparticle-based antigen delivery system. Nanoscale 2018, 10, 9489–9503. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Lee, H.J.; Park, S.; Kim, Y.; Jeong, B. Fast degradable polycaprolactone for drug delivery. Biomacromolecules 2018, 19, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.S.; Santos, T.C.; Reis, R.L. 4-controlling the degradation of natural polymers for biomedical applications. In Natural-Based Polymers for Biomedical Application; Reis, R.L., Neves, N.M., Mano, J.F., Gomes, M.E., Marques, A.P., Azevedo, H.S., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 106–128. [Google Scholar]
- Azevedo, H.S.; Reis, R.L. Understanding the enzymatic degradation of biodegradable polymers and strategies to control their degradation rate. In Biodegradable Systems in Tissue Engineering and Regenerative Medicine; Reis, R.L., Román, J.S., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 186–210. [Google Scholar]
- Buchholz, V.; Agarwal, S.; Greiner, A. Synthesis and enzymatic degradation of soft aliphatic polyesters. Macromol. Biosci. 2016, 16, 207–213. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J.; Stryer, L. Biochemistry, 5th ed.; W.H. Freeman: New York, NY, USA, 2015. [Google Scholar]
- Trousil, J.; Filippov, S.K.; Hrubý, M.; Mazel, T.; Syrová, Z.; Cmarko, D.; Svidenská, S.; Matějková, J.; Kováčik, L.; Porsch, B.; et al. System with embedded drug release and nanoparticle degradation sensor showing efficient rifampicin delivery into macrophages. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 307–315. [Google Scholar] [CrossRef]
- Brulé, E.; Robert, C.; Thomas, C.M. Sequence-controlled ring-opening polymerization: Synthesis of new polyester structures. In Sequence-Controlled Polymers: Synthesis, Self-Assembly, and Properties; American Chemical Society: Washington, DC, USA, 2014; Volume 1170, pp. 349–368. [Google Scholar]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Trousil, J.; Syrová, Z.; Dal, N.-J.K.; Rak, D.; Konefał, R.; Pavlova, E.; Matějková, J.; Cmarko, D.; Kubíčková, P.; Pavliš, O.; et al. Rifampicin nanoformulation enhances treatment of tuberculosis in zebrafish. Biomacromolecules 2019, 20, 1798–1815. [Google Scholar] [CrossRef]



| ROS-Sensitive Materials | Chemical Structure and Oxidation | References |
|---|---|---|
| Poly(propylene sulfide)s |  | [109,110,111,112,113,114,115,116,117,118,119] |
| Selenium containing polyesters | 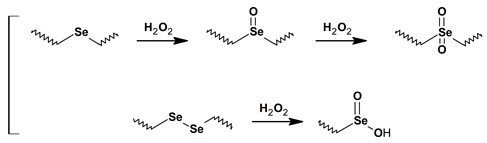 | [120,121,122,123,124] |
| Arylboronic ester containing polyesters |  | [125,126,127] |
| Polyoxalates |  | [128,129,130,131,132,133,134,135] |
| Polyester | Sensitive Linkage | Biomedical Application | References |
|---|---|---|---|
| pH-sensitive | Ketal | Anti-inflammatory drug (Dexamethasone); | [80] |
| Orthoester | Injectable drug release device, Cell scaffold; | [77] | |
| Cis-aconityl | Chemotherapy (Doxorubicin); | [79] | |
| Reductive-labile | Azo groups | Colon therapy (Imaging); | [86] |
| Disulfide | Chemotherapy (Paclitaxel); | [99] | |
| Propylene sulfide | Chemotherapy (Doxorubicin); | [109] | |
| Propylene sulfide | Vaccination (antigens); | [105] | |
| Propylene sulfide | Chemotherapy (Paclitaxel); | [107] | |
| ROS-labile | Selenide, diselenide | Chemotherapy (Doxorubicin); | [112,113] |
| Aryl boronic esters | Chemotherapy (Paclitaxel); | [118] | |
| Oxalate | Chemotherapy (Dyethylstilbestrol); | [123] | |
| Oxalate | Antioxidant and anti-inflammatory (Vanilyl alcohol); | [122] | |
| Enzymatically-labile | Ester bond | Antituberculotic (Rifampicin); | [134] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbánek, T.; Jäger, E.; Jäger, A.; Hrubý, M. Selectively Biodegradable Polyesters: Nature-Inspired Construction Materials for Future Biomedical Applications. Polymers 2019, 11, 1061. https://doi.org/10.3390/polym11061061
Urbánek T, Jäger E, Jäger A, Hrubý M. Selectively Biodegradable Polyesters: Nature-Inspired Construction Materials for Future Biomedical Applications. Polymers. 2019; 11(6):1061. https://doi.org/10.3390/polym11061061
Chicago/Turabian StyleUrbánek, Tomáš, Eliézer Jäger, Alessandro Jäger, and Martin Hrubý. 2019. "Selectively Biodegradable Polyesters: Nature-Inspired Construction Materials for Future Biomedical Applications" Polymers 11, no. 6: 1061. https://doi.org/10.3390/polym11061061
APA StyleUrbánek, T., Jäger, E., Jäger, A., & Hrubý, M. (2019). Selectively Biodegradable Polyesters: Nature-Inspired Construction Materials for Future Biomedical Applications. Polymers, 11(6), 1061. https://doi.org/10.3390/polym11061061