Biotechnological Preparation of Gelatines from Chicken Feet
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
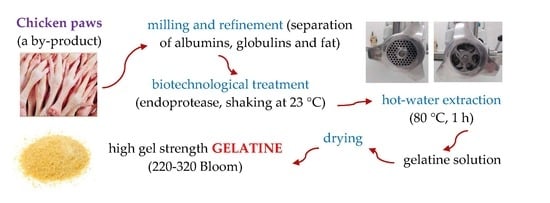
2.2. Strategy
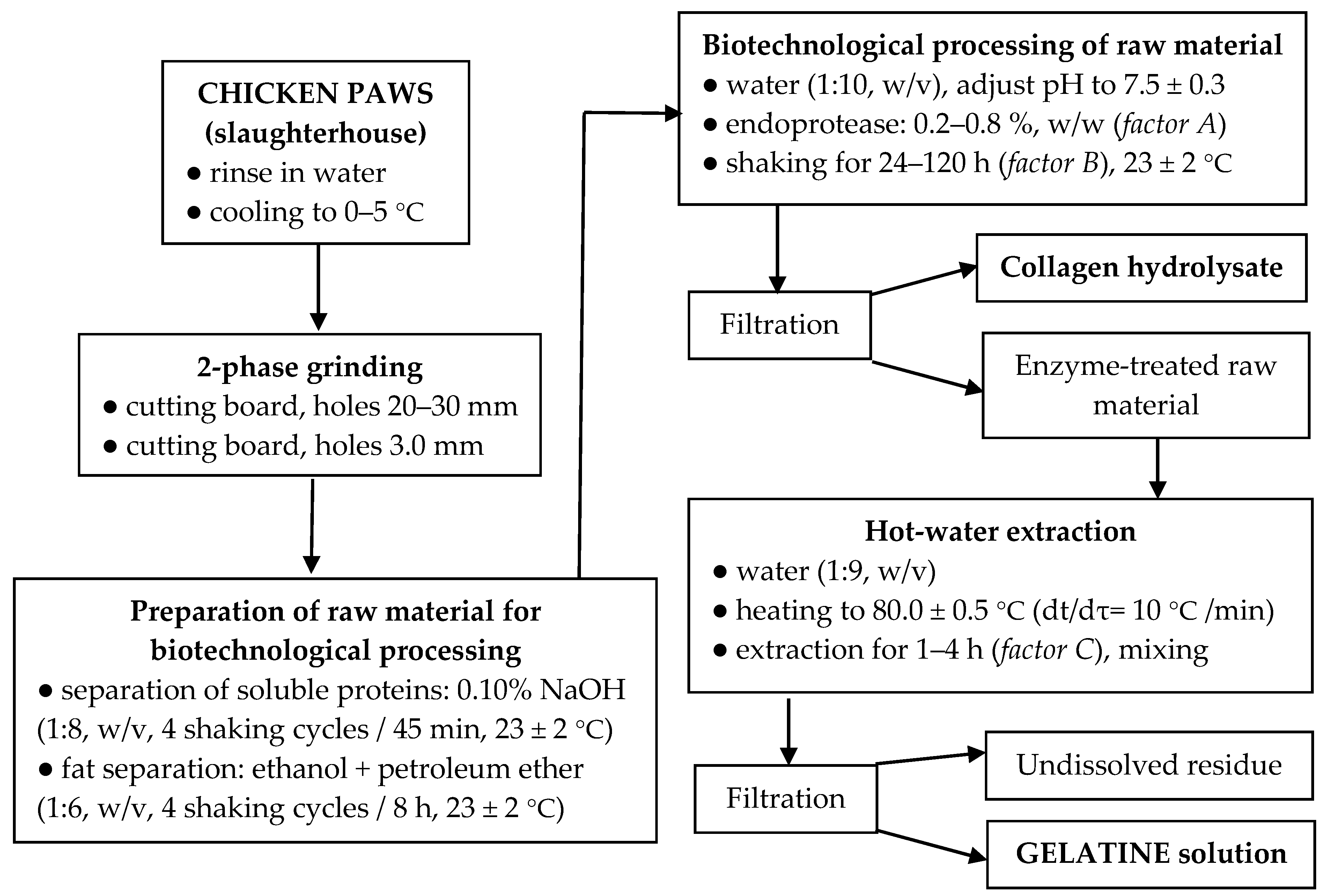
2.3. Grinding and Preparation of Raw Material for Biotechnological Processing
2.4. Biotechnological Processing of Raw Material and Gelatine Extraction
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results and Discussion
3.1. Study of the Influence of Process Factors on the Gelatine Yield and Quality of Prepared Products
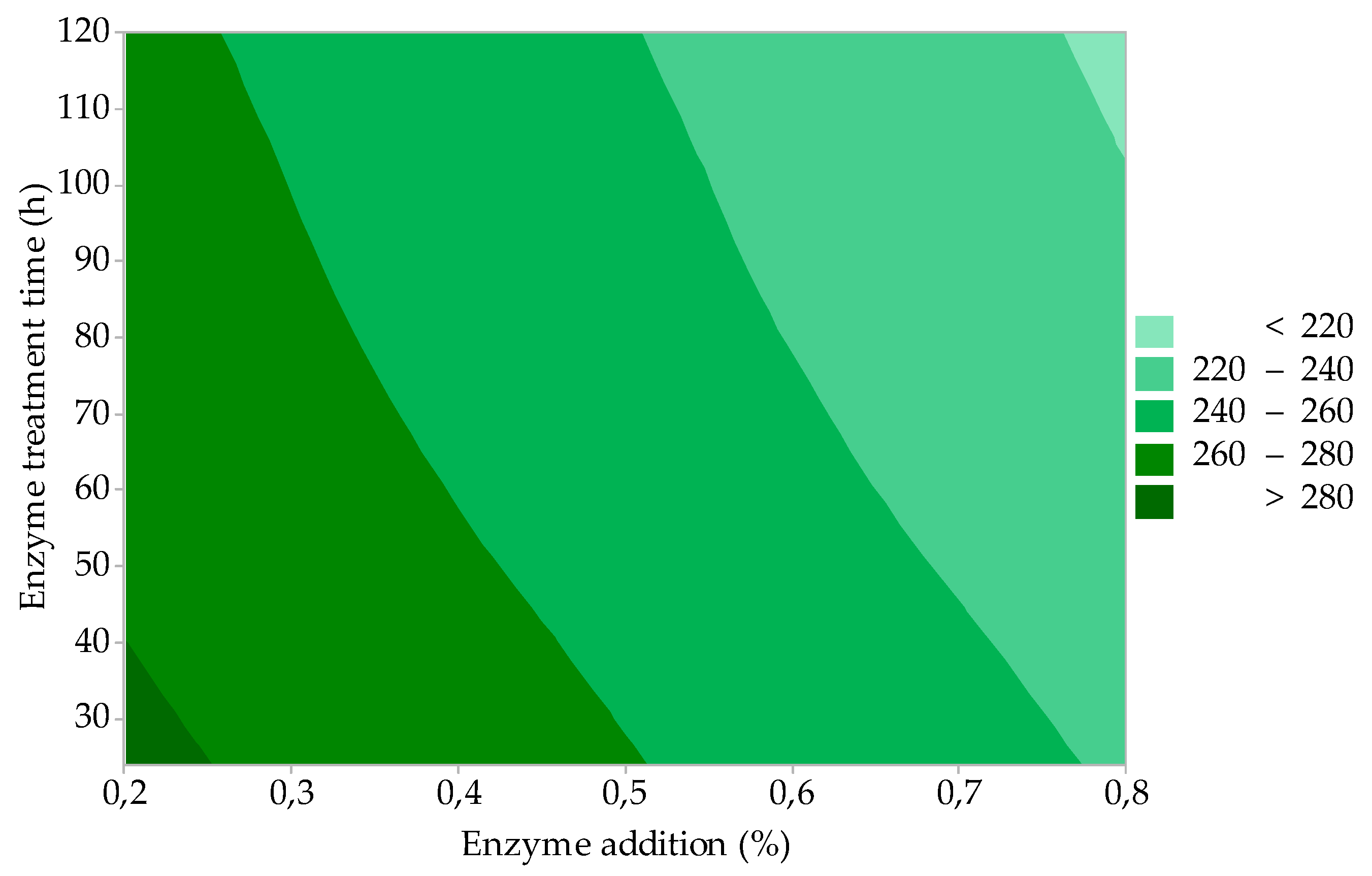
3.1.1. Gelatine Yield
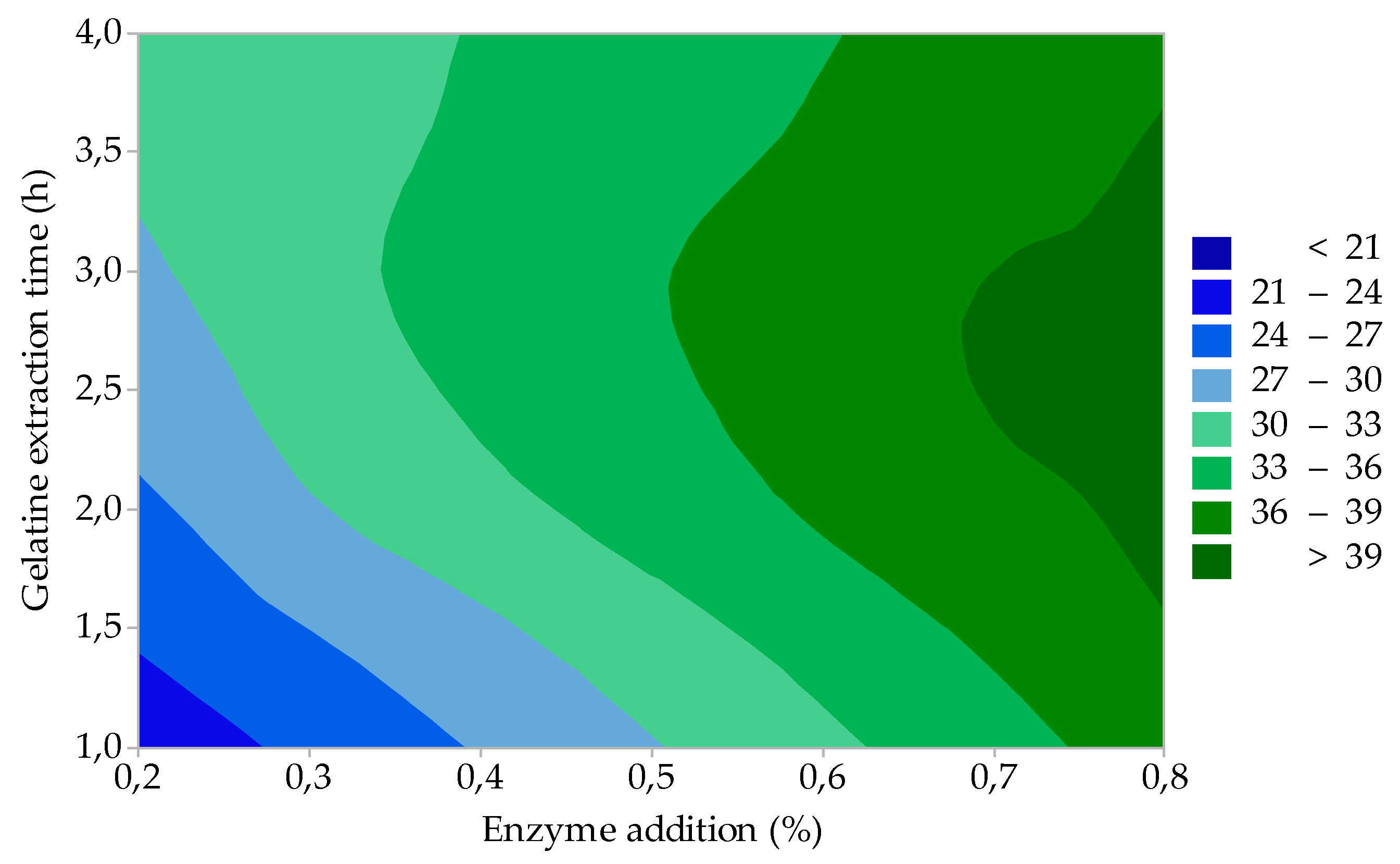
3.1.2. Gelatine Gel Strength
3.1.3. Gelatine Viscosity
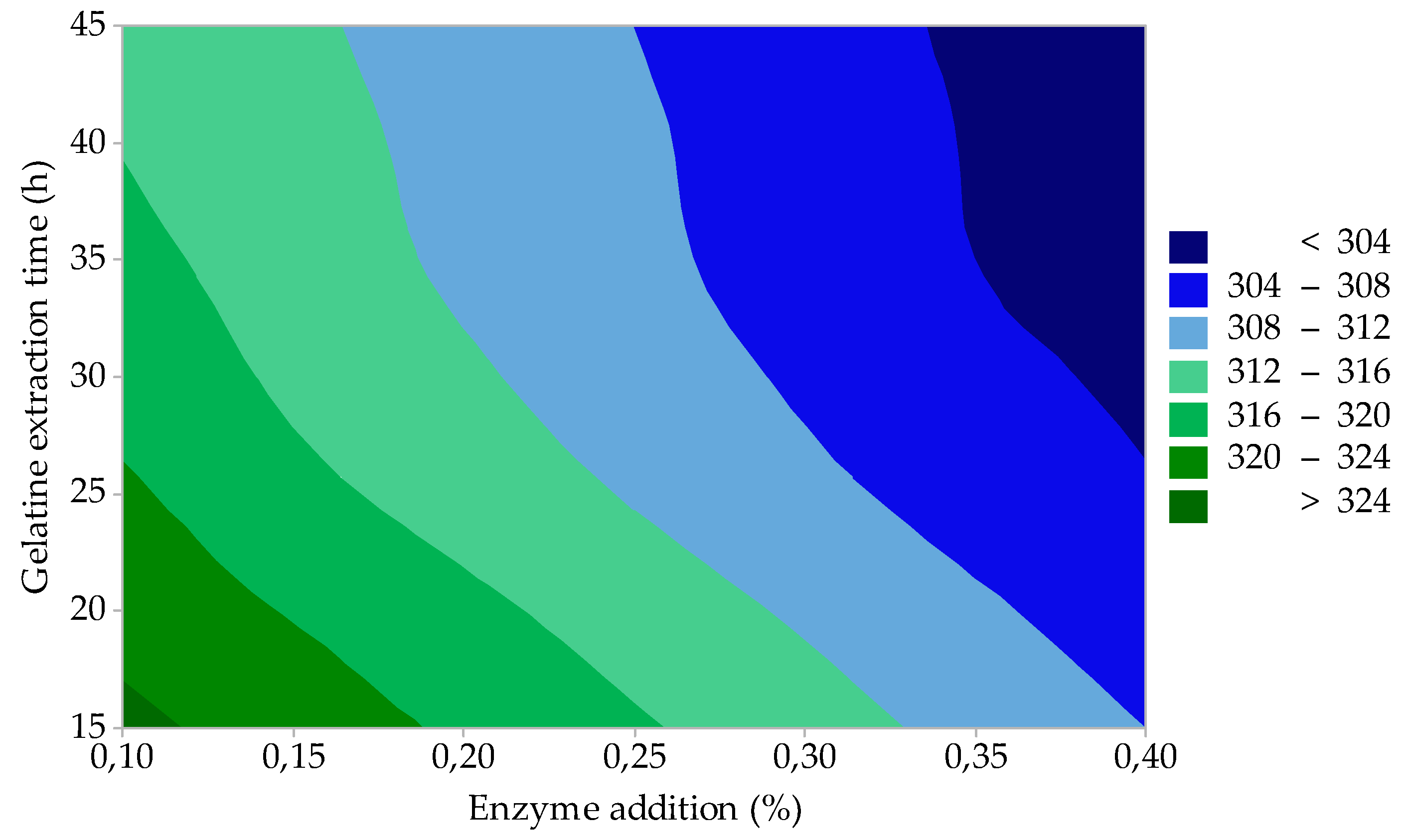
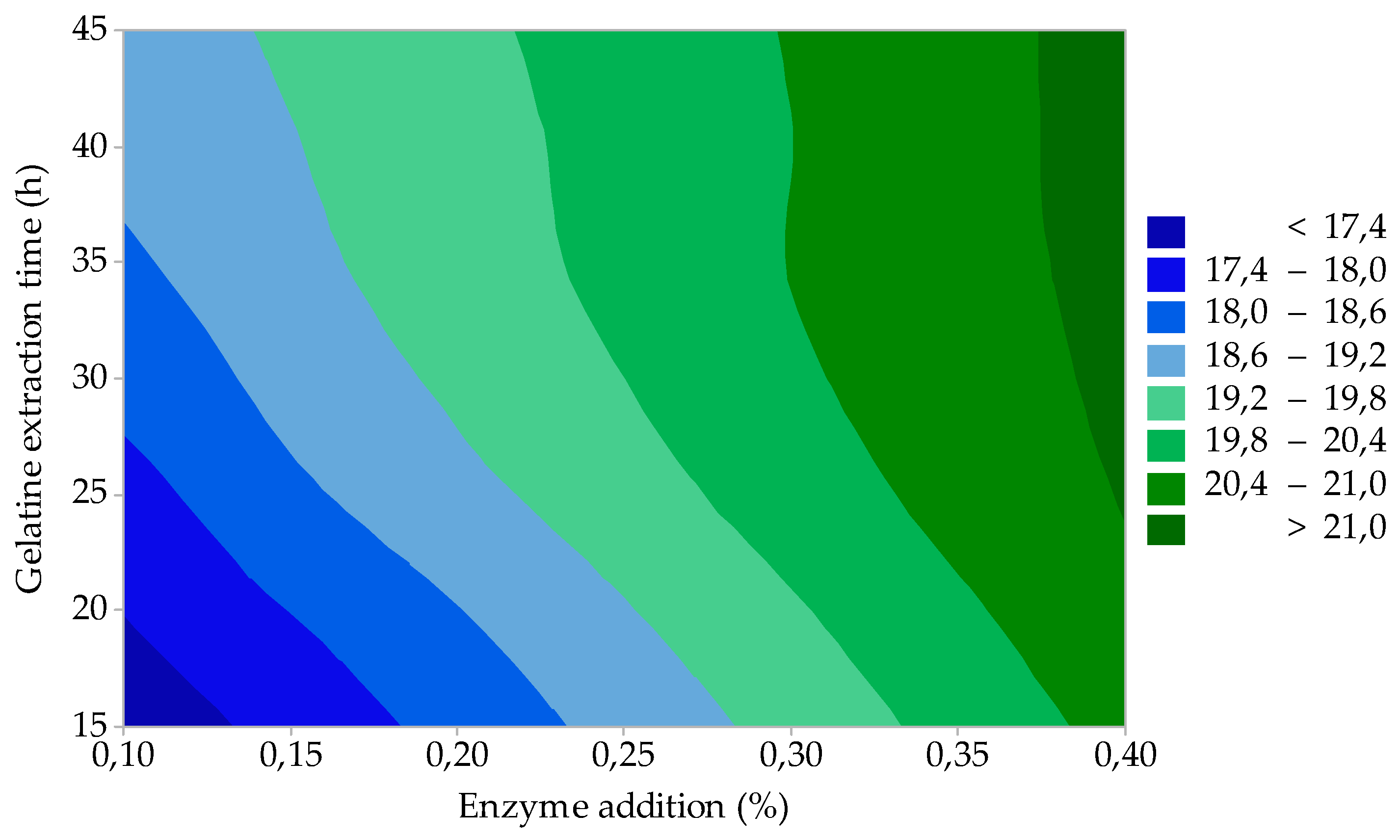
3.2. Process Optimization
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schrieber, R.; Gareis, H. Gelatine Handbook—Theory and Industrial Practice, 1st ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 45–117. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Sheela, A.K. Gelatin Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2012–2018. Available online: http://www.transparencymarketresearch.com/gelatin.html/ (accessed on 22 March 2019).
- Ferraro, V.; Anton, M.; Santé-Lhoutellier, V. The “sisters” α-helices of collagen, elastin and keratin recovered from animal by-products: Functionality, bioactivity and trends of application. Trends Food Sci. Technol. 2016, 51, 65–75. [Google Scholar] [CrossRef]
- The National Agricultural Statistics Service (NASS), United States Department of Agriculture. Poultry Slaughter 2016 Summary. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/pslaan17.pdf/ (accessed on 22 March 2019).
- Eurostat Statistics Explained, Meat Production Statistics for EU-28. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Meat_production_statistics#Poultry_meat/ (accessed on 22 March 2019).
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of by products and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Ockerman, H.W.; Hansen, C.I. Animal By-Product Processing and Utilization, 1st ed.; CRC Press: London, UK, 2000; pp. 23–83. [Google Scholar]
- Coutand, M.; Cyr, M.; Deydier, E.; Guilet, R.; Clastres, P. Characteristics of industrial and laboratory meat and bone meal ashes and their potential applications. J. Hazard. Mater. 2008, 150, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Pitk, P.; Kaparaju, P.; Vilu, R. Methane potential of sterilized solid slaughterhouse wastes. Bioresour. Technol. 2012, 116, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillœn, M.C.; Montero, M.V.P. Extraction of gelatin from megrim (Lepidorhombus boscii) skins with several organic acids. J. Food Sci. 2001, 66, 213–216. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- Norziah, M.H.; Al-Hassan, A.; Khairulnizam, A.B.; Mordi, M.N.; Norita, M. Characterization of fish gelatin from surimi processing wastes: Thermal analysis and effect of transglutaminase on gel properties. Food Hydrocoll. 2009, 23, 1610–1616. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Improvement of gelatin extraction from bigeye snapper skin using pepsin-aided process in combination with protease inhibitor. Food Hydrocoll. 2008, 22, 615–622. [Google Scholar] [CrossRef]
- Kasankala, L.M.; Xue, Y.; Weilong, Y.; Hong, S.D.; He, Q. Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresour. Technol. 2007, 98, 3338–3343. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Hsu, F.W.; Chang, H.S.; Lin, L.C.; Sakata, R. Effect of different acids on the extraction of pepsin-solubilised collagen containing melanin from silky fowl feet. Food Chem. 2009, 113, 563–567. [Google Scholar] [CrossRef]
- Liu, D.C.; Lin, Y.K.; Chen, M.T. Optimum condition of extracting collagen from chicken feet and its characteristics. Asian-Australas. J. Anim. Sci. 2001, 14, 1638–1644. [Google Scholar] [CrossRef]
- Chakka, A.K.; Muhammed, A.; Sakhare, P.Z.; Bhaskar, N. Poultry processing waste as an alternative source for mammalian gelatin: Extraction and characterization of gelatin from chicken feet using food grade acids. Waste Biomass Valoriz. 2017, 8, 2583–2593. [Google Scholar] [CrossRef]
- Almeida, P.F.; da Silva Lannes, S.C. Extraction and physicochemical characterization of gelatin from chicken by-product. J. Food Process Eng. 2013, 36, 824–833. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Nazlin, F.B.; Howell, K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef]
- Xu, M.; Wei, L.; Xiao, Y.; Bi, H.; Yang, H.; Du, Y. Physicochemical and functional properties of gelatin extracted from Yak skin. Int. J. Biol. Macromol. 2017, 95, 1246–1253. [Google Scholar] [CrossRef]
- Antony, J. Design of Experiments for Engineers and Scientists, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2003; pp. 54–70. [Google Scholar]
- Du, L.; Khiari, Z.; Pietrasik, Z.; Betti, M. Physicochemical and functional properties of gelatins extracted from turkey and chicken heads. Poult. Sci. 2013, 92, 2463–2474. [Google Scholar] [CrossRef]
- Huda, N.; Seow, E.K.; Normawati, M.N.; Nik Aisyah, N.M. Preliminary study on physicochemical properties of duck feet collagen. Int. J. Poult. Sci. 2013, 12, 615–621. [Google Scholar] [CrossRef]
- Mrázek, P.; Mokrejš, P.; Gál, R.; Krejčí, O. Preparation of collagen concentrate from chicken feet. Waste Forum 2018, 4, 444–451. [Google Scholar]
- Mokrejš, P.; Gál, R.; Janáčová, D.; Plšková, M.; Brychtová, M. Chicken paws by-products as an alternative source of proteins. Orient. J. Chem. 2017, 33, 2209–2216. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldrá, F. Handbook of Food Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 357–754. [Google Scholar]
- ISO 1443:1973. Meat and Meat Products—Determination of Total Fat Content; ISO: Geneva, Switzerland, 1973. [Google Scholar]
- ISO 937:1978. Meat and Meat Products—Determination of Nitrogen Content; ISO: Geneva, Switzerland, 1978. [Google Scholar]
- ISO 3496:1994. Meat and Meat Products—Determination of Hydroxyproline Content; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- Vázquez-Ortiz, F.A.; González-Méndez, N.F. Determination of collagen as a quality index in Bologna from Northwestern Mexico. J. Food Compos. Anal. 1996, 9, 269–276. [Google Scholar] [CrossRef]
- Standard Testing Methods for Edible Gelatin. Official Procedure of the Gelatin Manufacturers Institute of America, Inc. Available online: http://www.gelatin-gmia.com/images/GMIA_Official_Methods_of_Gelatin_Revised_2013.pdf/ (accessed on 18 March 2019).
- Stange, K. Angewandte Statistik Teil 2—Mehrdimensionale Probleme; Springer: Heidelberg, Germany, 1971; pp. 469–495. [Google Scholar]
- Widyasari, R.; Rawdkuen, S. Extraction and characterization of gelatin from chicken feet by acid and ultrasound assisted extraction. Food Appl. Biosci. J. 2014, 2, 83–95. [Google Scholar]
- Almeida, P.F.; Calarge, F.A.; Santana, J.C.C. Production of a product similar to gelatin from chicken feet collagen. Eng. Agrícola 2013, 33, 1289–1300. [Google Scholar] [CrossRef]
- Lai, J.Y.; Lin, P.K.; Hsiue, G.H.; Cheng, H.Y.; Huang, S.J.; Li, Y.T. Low Bloom strength gelatin as a carrier for potential use in retinal sheet encapsulation and transplantation. Biomacromolecules 2009, 10, 310–319. [Google Scholar] [CrossRef]
- Lai, J.Y.; Lu, P.L.; Chen, K.H.; Tabata, Y.; Hsiue, G.H. Effect of charge and molecular weight on the functionality of gelatin carriers for corneal endothelial cell therapy. Biomacromolecules 2006, 7, 1836–1844. [Google Scholar] [CrossRef]
- Chou, S.F.; Luo, L.J.; Lai, J.Y.; Ma, D.H.K. On the importance of Bloom number of gelatin to the development of biodegradable in situ gelling copolymers for intracameral drug delivery. Int. J. Pharm. 2016, 511, 30–43. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
- Lai, J.Y. The role of Bloom index of gelatin on the interaction with retinal pigment epithelial cells. Int. J. Mol. Sci. 2009, 10, 3442–3456. [Google Scholar] [CrossRef]
- Wang, C.S.; Virgilio, N.; Wood-Adams, P.; Heuzey, M.C. A mechanism for the synergistic gelation properties of gelatin B and xanthan gum aqueous mixtures. Carbohydr. Polym. 2017, 175, 484–492. [Google Scholar] [CrossRef]

| Exp. No. | Factors under Study | Collagen Hydrolysate | Gelatine | Summary of the Process | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||||
| Enzyme Addition (%) | Enzyme Treatment (h) | Extraction Time (h) | HY (%) | AshHa ± SD (%) | GY (%) | AshGa ± SD (%) | F ± SD (Bloom) | η ± SD (mPa·s) | pH ± SD | Y∑ (%) | MBE (%) | |
| 1 | 0.2 | 24 | 1 | 9.8 | 20.6 ± 0.1 | 20.1 | 1.35 ± 0.02 | 295 ± 3 | 6.9 ± 0.1 | 6.2 ± 0.1 | 29.9 | 2.0 |
| 2 | 0.2 | 24 | 4 | 10.0 | 21.8 ± 0.2 | 27.4 | 0.61 ± 0.03 | 273 ± 3 | 6.5 ± 0.1 | 6.4 ± 0.1 | 37.4 | 4.9 |
| 3 | 0.2 | 120 | 1 | 10.7 | 23.9 ± 0.1 | 24.1 | 1.66 ± 0.01 | 266 ± 2 | 5.9 ± 0.2 | 6.4 ± 0.1 | 34.8 | 5.9 |
| 4 | 0.2 | 120 | 4 | 11.0 | 24.7 ± 0.3 | 33.5 | 0.88 ± 0.03 | 263 ± 2 | 5.2 ± 0.1 | 6.0 ± 0.2 | 44.5 | 3.8 |
| 5 | 0.8 | 24 | 1 | 11.2 | 27.2 ± 0.1 | 36.5 | 0.93 ± 0.02 | 241 ± 2 | 5.1 ± 0.2 | 6.4 ± 0.1 | 47.7 | 2.5 |
| 6 | 0.8 | 24 | 4 | 11.1 | 28.3 ± 0.2 | 37.9 | 0.77 ± 0.02 | 235 ± 3 | 4.7 ± 0.1 | 6.4 ± 0.1 | 49.0 | 2.6 |
| 7 | 0.8 | 120 | 1 | 11.4 | 28.6 ± 0.1 | 38.3 | 1.61 ± 0.01 | 228 ± 2 | 3.7 ± 0.1 | 6.3 ± 0.2 | 49.7 | 1.6 |
| 8 | 0.8 | 120 | 4 | 11.5 | 29.4 ± 0.1 | 39.1 | 1.32 ± 0.01 | 206 ± 2 | 3.1 ± 0.1 | 6.1 ± 0.1 | 50.6 | 0.7 |
| 9 | 0.5 | 72 | 2.5 | 10.9 | 25.7 ± 0.2 | 35.4 | 1.53 ± 0.02 | 249 ± 3 | 6.5 ± 0.3 | 6.1 ± 0.1 | 46.2 | 2.6 |
| Degree of Freedom | Sum of Squares | Mean Squares | F-Value | P-Value | |
|---|---|---|---|---|---|
| Response: Gelatine yield, GY (%) = 16.35 + 19.46 A + 0.0341 B + 1.575 C; R2 = 88.65 | |||||
| Regression | 3 | 338.71 | 112.905 | 13.01 | 0.008 |
| Factor A (Enzyme addition) | 1 | 272.61 | 272.611 | 31.42 | 0.002 |
| Factor B (Enzyme treatment time) | 1 | 21.45 | 21.451 | 2.47 | 0.177 |
| Factor C (Gelatine extraction time) | 1 | 44.65 | 44.651 | 5.15 | 0.073 |
| Error | 5 | 43.38 | 8.676 | ||
| Total | 8 | 382.10 | |||
| Response: Gelatine gel strength, F (Bloom) = 315.85 – 77.92 A – 0.2109 B – 4.42 C; R2 = 97.20 | |||||
| Regression | 3 | 5542.4 | 1847.46 | 57.87 | 0.000 |
| Factor A (Enzyme addition) | 1 | 4371.1 | 4371.13 | 136.92 | 0.000 |
| Factor B (Enzyme treatment time) | 1 | 820.1 | 820.12 | 25.69 | 0.004 |
| Factor C (Gelatine extraction time) | 1 | 351.1 | 351.13 | 11.00 | 0.021 |
| Error | 5 | 159.6 | 31.93 | ||
| Total | 8 | 5702.0 | |||
| Response: Gelatine viscosity, η (mPa·s) = 8.37–3.29 A—0.0138 B—0.175 C; R2 = 87.18 | |||||
| Regression | 3 | 11.87 | 3.955 | 11.33 | 0.011 |
| Factor A (Enzyme addition) | 1 | 7.80 | 7.801 | 22.35 | 0.005 |
| Factor B (Enzyme treatment time) | 1 | 3.51 | 3.511 | 10.16 | 0.025 |
| Factor C (Gelatine extraction time) | 1 | 0.55 | 0.551 | 1.58 | 0.264 |
| Error | 5 | 1.75 | 0.349 | ||
| Total | 8 | 13.61 | |||
| Exp. No. | Factors under Study | Gelatine | |||||
|---|---|---|---|---|---|---|---|
| A | C | ||||||
| Enzyme Addition (%) | Extraction Time (min) | GY (%) | AshG a ± SD (%) | F ± SD (Bloom) | η ± SD (mPa·s) | pH ± SD | |
| 10 | 0.1 | 15 | 17.0 | 1.31 | 325 | 7.3 | 7.3 |
| 11 | 0.1 | 45 | 18.9 | 1.23 | 315 | 7.2 | 7.2 |
| 12 | 0.4 | 15 | 20.6 | 1.94 | 308 | 6.9 | 7.2 |
| 13 | 0.4 | 45 | 21.2 | 1.58 | 301 | 6.8 | 6.9 |
| 14 | 0.25 | 30 | 19.8 | 1.45 | 310 | 6.9 | 7.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokrejš, P.; Mrázek, P.; Gál, R.; Pavlačková, J. Biotechnological Preparation of Gelatines from Chicken Feet. Polymers 2019, 11, 1060. https://doi.org/10.3390/polym11061060
Mokrejš P, Mrázek P, Gál R, Pavlačková J. Biotechnological Preparation of Gelatines from Chicken Feet. Polymers. 2019; 11(6):1060. https://doi.org/10.3390/polym11061060
Chicago/Turabian StyleMokrejš, Pavel, Petr Mrázek, Robert Gál, and Jana Pavlačková. 2019. "Biotechnological Preparation of Gelatines from Chicken Feet" Polymers 11, no. 6: 1060. https://doi.org/10.3390/polym11061060
APA StyleMokrejš, P., Mrázek, P., Gál, R., & Pavlačková, J. (2019). Biotechnological Preparation of Gelatines from Chicken Feet. Polymers, 11(6), 1060. https://doi.org/10.3390/polym11061060