Adsorption of Direct Blue 78 Using Chitosan and Cyclodextrins as Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Chitosan-NaOH Polymer Preparation
2.3. β-CDs-EPI Polymer Preparation
2.4. Dye Solution Preparation
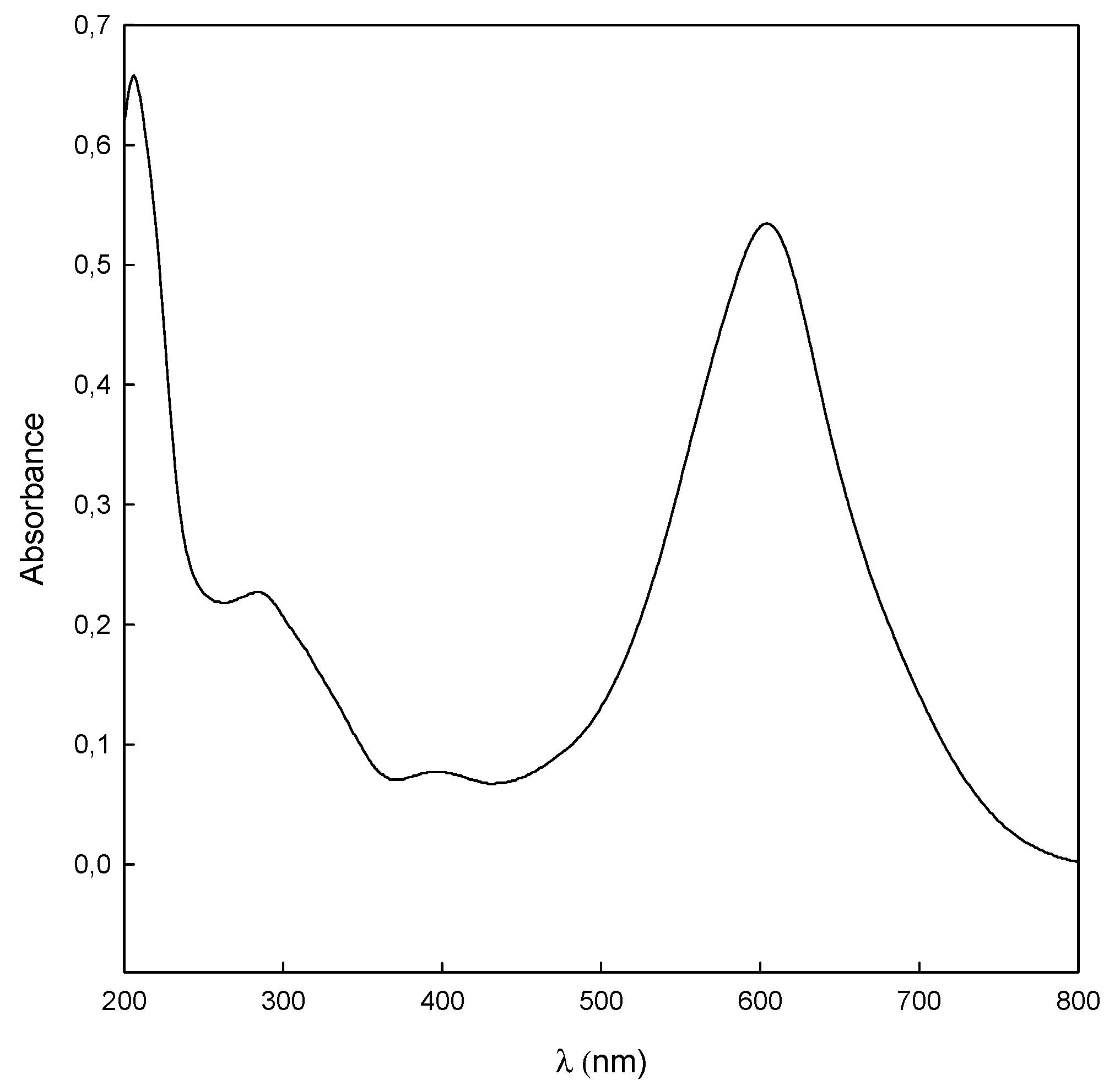
2.5. Analyses and Data Evaluation
2.6. Polymer Characterization
2.6.1. Porosity
2.6.2. Swelling Capacity
2.6.3. Particle Size Distribution
2.7. Adsorption Experiments
2.8. Adsorption Kinetics
2.9. Isotherm Analysis
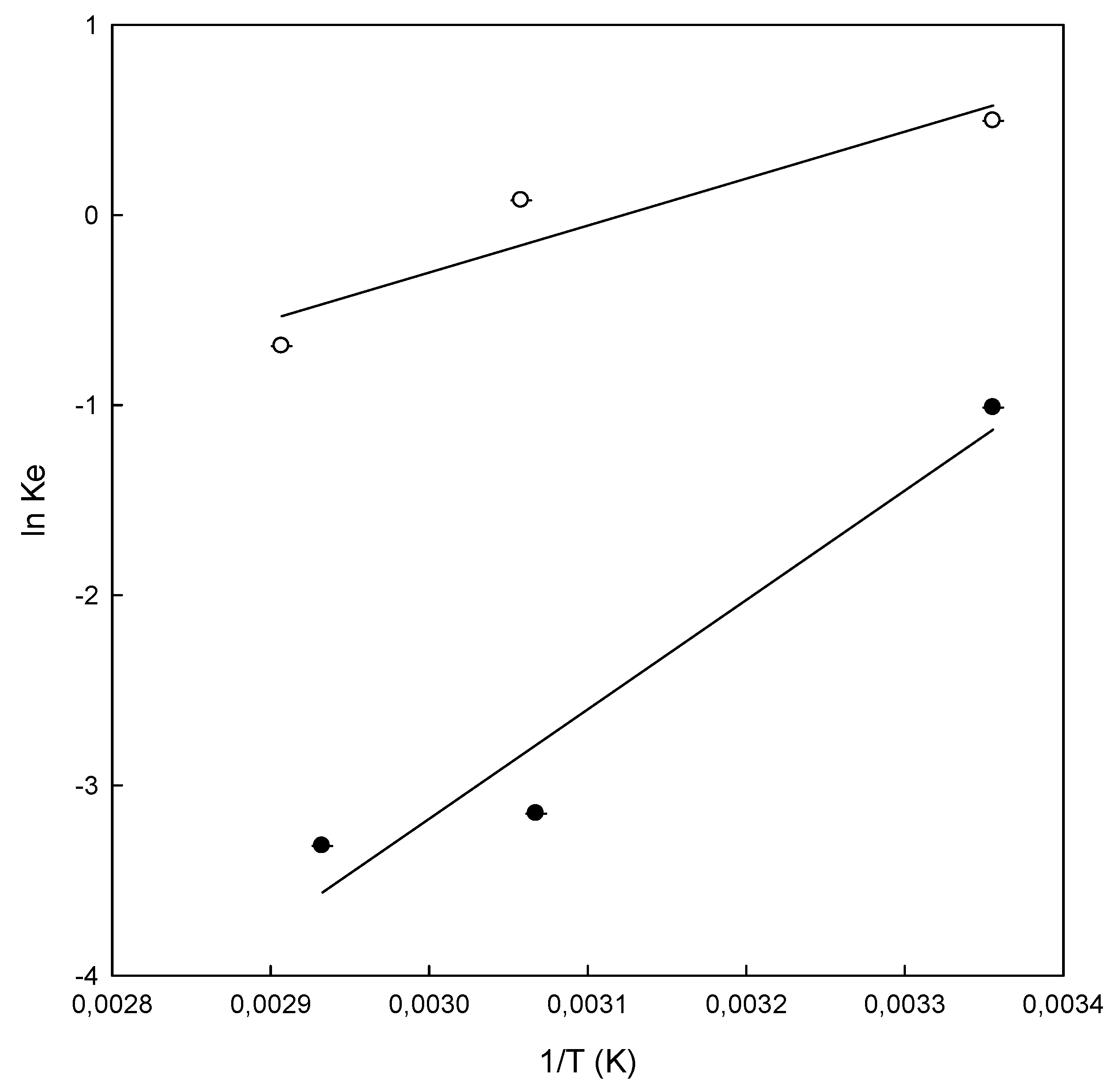
2.10. Thermodynamic Study
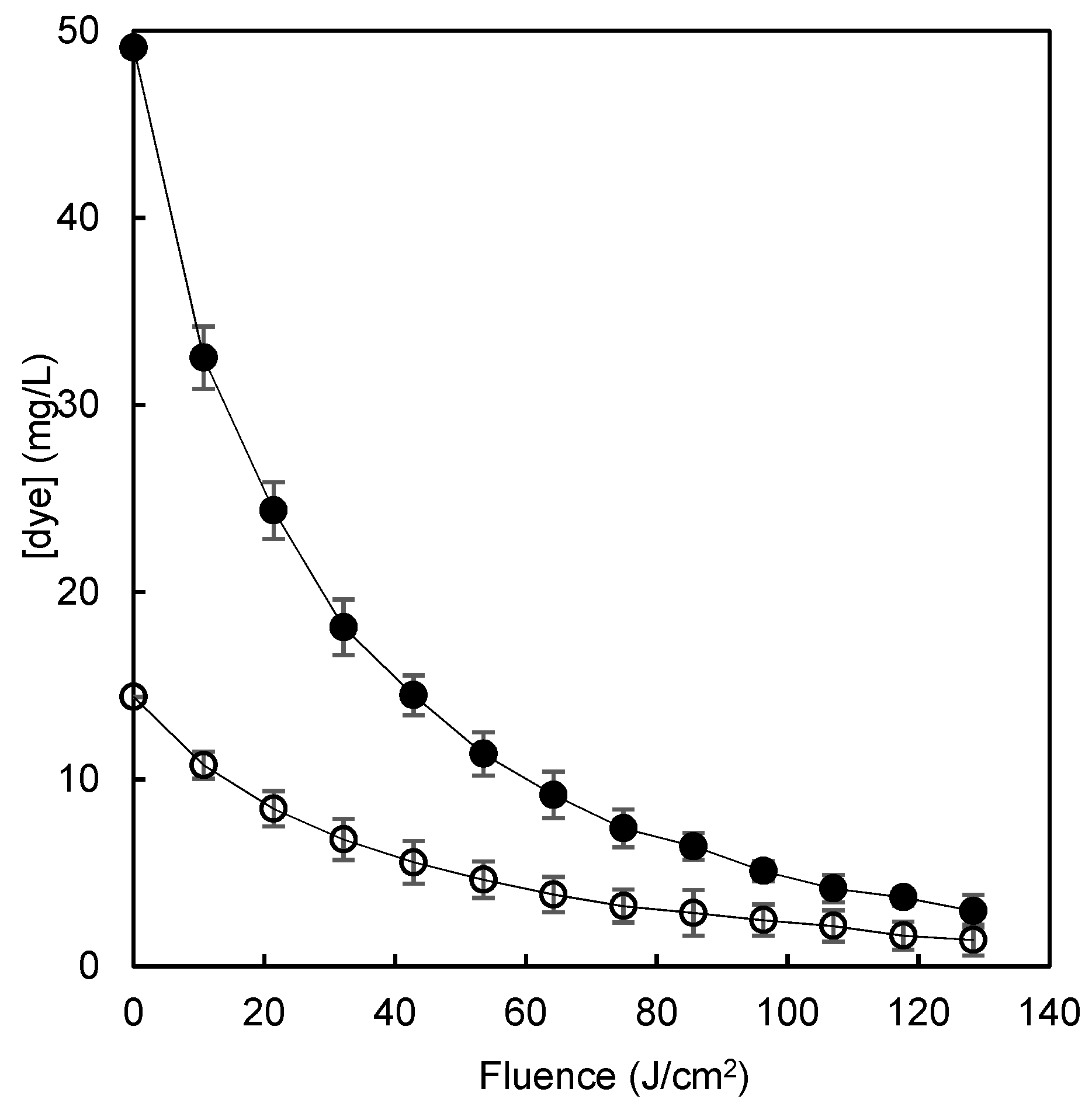
2.11. Advanced Oxidation Process
3. Results
3.1. Polymer Characterization
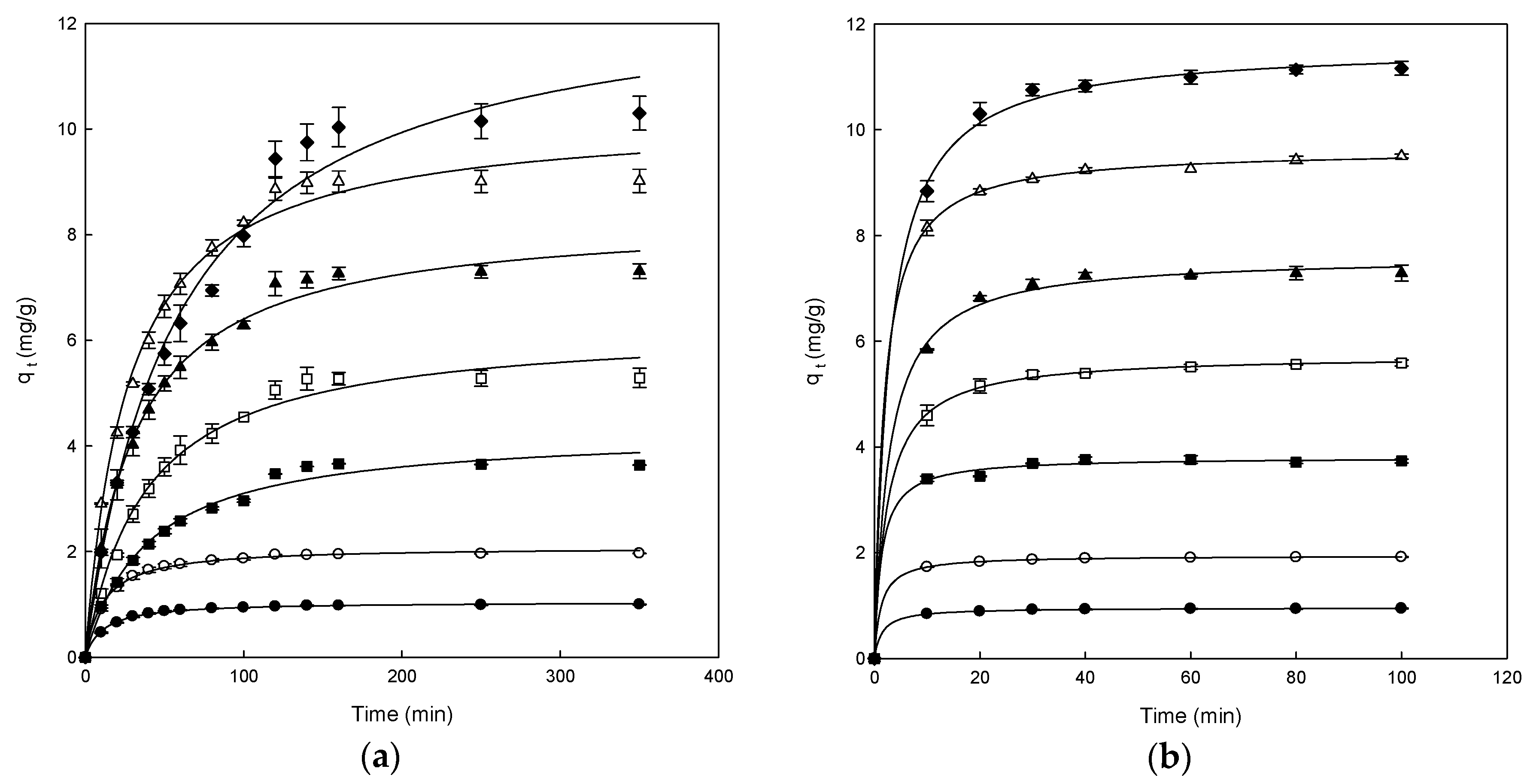
3.2. Effect of Contact Time
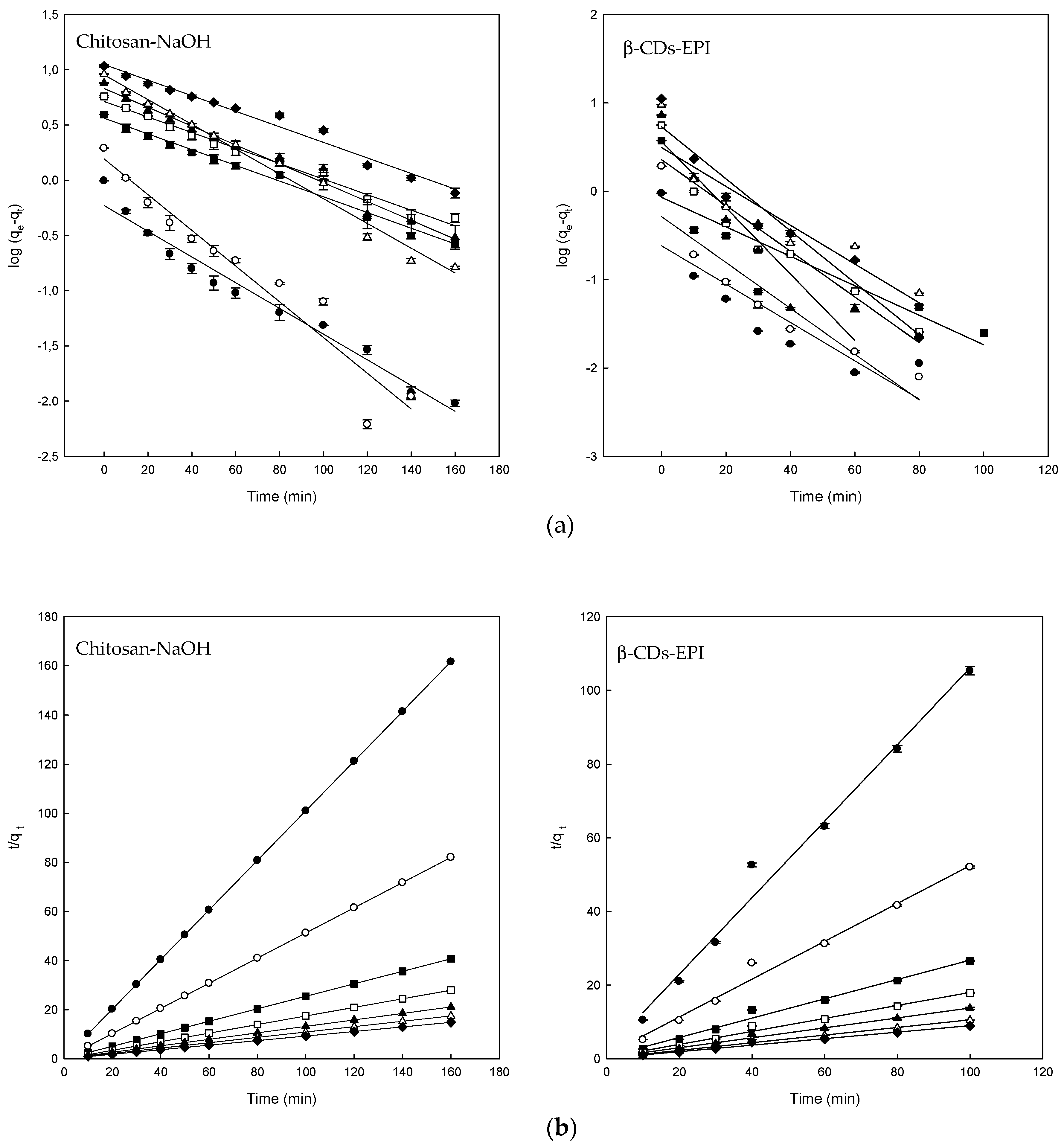
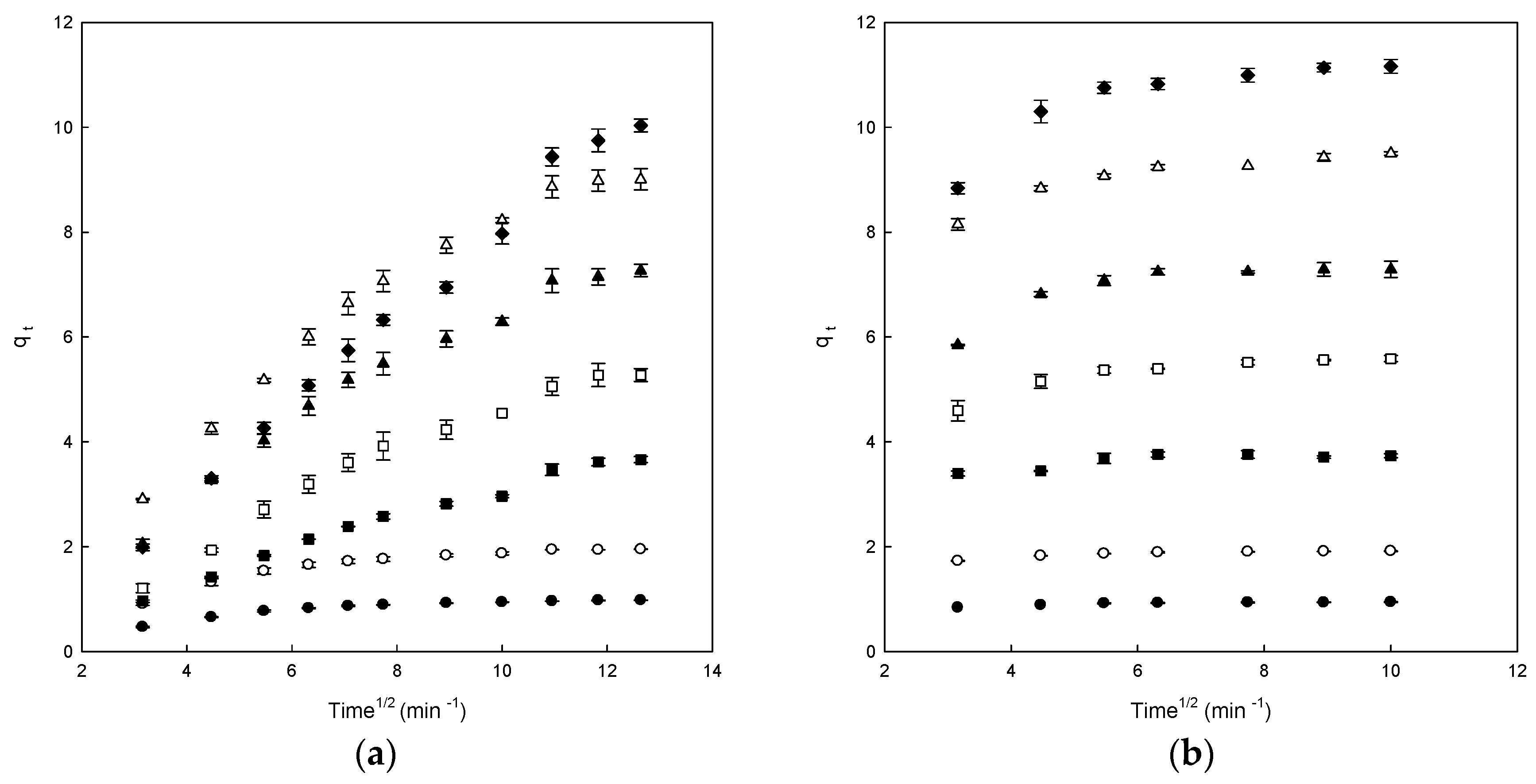
3.3. Adsorption Kinetics
3.4. Adsorption Equilibrium
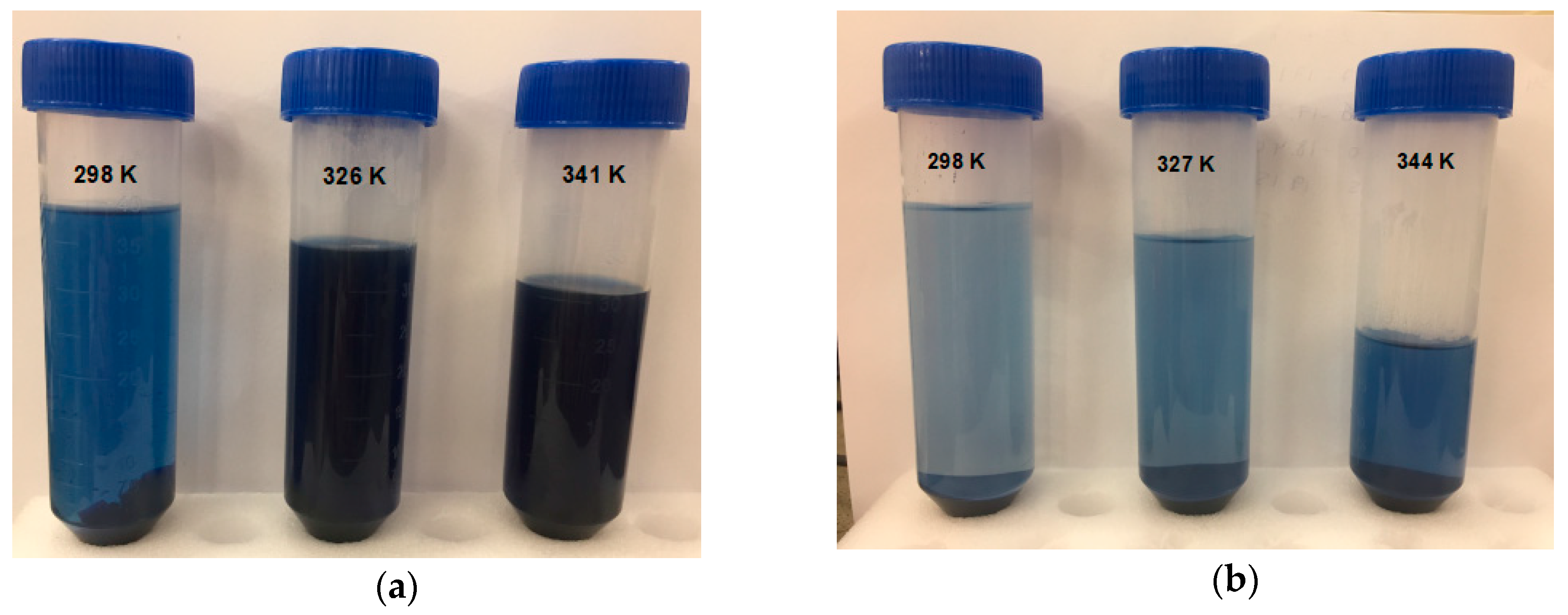
3.5. Thermodynamic Study
3.6. Advanced Oxidation Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Biochem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Nakkeeran, E.; Varjani, S.J.; Dixit, V.; Kalaiselvi, A. Synthesis, characterization and application of zinc oxide nanocomposite for dye removal from textile industrial wastewater. Indian J. Exp. Biol. 2018, 56, 498–503. [Google Scholar]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef]
- Mahmoud, H.R.; Ibrahim, S.M.; El-Molla, S.A. Textile dye removal from aqueous solutions using cheap MgO nanomaterials: Adsorption kinetics, isotherm studies and thermodynamics. Adv. Powder Technol. 2016, 27, 223–231. [Google Scholar] [CrossRef]
- Livani, M.J.; Ghorbani, M. Fabrication of NiFe2O4 magnetic nanoparticles loaded on activated carbon as novel nanoadsorbent for Direct Red 31 and Direct Blue 78 adsorption. Environ. Technol. 2018, 39, 2977–2993. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Goscianska, J.; Marciniak, M.; Pietrzak, R. Ordered mesoporous carbons modified with cerium as effective adsorbents for azo dyes removal. Sep. Purif. Technol. 2015, 154, 236–245. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, M. Removal of cationic dye methylene blue (MB) from aqueous solution by ground raw and base modified pine cone powder. Environ. Earth Sci. 2014, 71, 1507–1519. [Google Scholar] [CrossRef]
- Crini, G.; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Sargın, I.; Kaya, M.; Arslan, G.; Baran, T.; Ceter, T. Preparation and characterisation of biodegradable pollen–chitosan microcapsules and its application in heavy metal removal. Bioresour. Technol. 2015, 177, 1–7. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.S.; Meng, X.H.; Yu, L.J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Navarro, P.; Gabaldón, J.A.; Gómez-López, V.M. Degradation of an azo dye by a fast and innovative pulsed light/H2O2 advanced oxidation process. Dyes Pigm. 2017, 136, 887. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Lucas-Abellán, C.; Mercader-Ros, M.T.; López-Miranda, S.; Gómez-López, V.M.; Semeraro, P.; Cosma, P.; Fini, P. Adsorption properties of β-and hydroxypropyl-β-cyclodextrins cross-linked with epichlorohydrin in aqueous solution. A sustainable recycling strategy in textile dyeing process. Polymers 2019, 11, 252. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, G.; Wu, C.; Sun, J.; Song, R.; Huang, W. Porous chitosan doped with graphene oxide as highly effective adsorbent for methyl orange and amido black 10B. Carbohyd. Polym. 2015, 115, 686–693. [Google Scholar] [CrossRef]
- Shi, X.N.; Wang, W.B.; Wang, A.Q. Effect of surfactant on porosity and swelling behaviors of guar gum-g-poly (sodium acrylate-co-styrene)/attapulgite superabsorbent hydrogels. Colloid Surf. B 2011, 88, 279–286. [Google Scholar] [CrossRef]
- Ho, Y. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process. Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of CI Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Ho, Y.; Porter, J.; McKay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N. Adsorption of CI Basic Blue 9 on cyclodextrin-based material containing carboxylic groups. Dyes Pigm. 2006, 70, 204–211. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- McKay, G. Adsorption of dyestuffs from aqueous solutions with activated carbon I: Equilibrium and batch contact-time studies. J. Chem. Technol. Biotechnol. 1982, 32, 759–772. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Hernández, J.A.G.; Lucas-Abellán, C.; Mercader-Ros, M.T.; Serrano-Martínez, A.; Núñez-Delicado, E.; Cosma, P.; Fini, P. Removing of Direct Red 83: 1 using α-and HP-α-CDs polymerized with epichlorohydrin: Kinetic and equilibrium studies. Dyes Pigm. 2018, 149, 736–746. [Google Scholar] [CrossRef]
- Temkin, M. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim. URS 1940, 12, 327–356. [Google Scholar]
- Oladoja, N.; Aboluwoye, C.; Oladimeji, Y. Kinetics and isotherm studies on methylene blue adsorption onto ground palm kernel coat. Turkish J. Eng. Environ. Sci. 2009, 32, 303–312. [Google Scholar]
- Subramani, S.; Thinakaran, N. Isotherm, kinetic and thermodynamic studies on the adsorption behaviour of textile dyes onto chitosan. Process Saf. Environ. Prot. 2017, 106, 1–10. [Google Scholar] [CrossRef]


| Polymers Properties | Chitosan-NaOH Polymer | β-CDs-EPI Polymer |
|---|---|---|
| Swelling capacity (Qeg, g/g) | 0.80 ± 0.01 | 0.63 ± 0.01 |
| Porosity (%) | 37.6 ± 0.3 | 54.1 ± 1.1 |
| Density (g/cm3) | 1.4 ± 0.1 | 1.5 ± 0.2 |
| Particle size distribution D[4:3] (µm) | 350 | 586 |
| PFOM 1 | Chitosan-NaOH | β-CDs-EPI | ||||||
| Co (mg/L) | qeexp | qecal | k1 (min−1) | R2 | qeexp | qecal | k1 (min−1) | R2 |
| 25 | 0.990 | 0.590 | 0.026 | 0.971 | 0.950 | 0.242 | 0.049 | 0.751 |
| 50 | 1.950 | 1.560 | 0.037 | 0.934 | 1.920 | 0.517 | 0.059 | 0.950 |
| 100 | 3.930 | 3.640 | 0.016 | 0.980 | 3.760 | 0.850 | 0.038 | 0.736 |
| 150 | 5.730 | 5.150 | 0.016 | 0.986 | 5.590 | 2.280 | 0.059 | 0.919 |
| 200 | 7.570 | 6.780 | 0.020 | 0.983 | 7.290 | 3.650 | 0.086 | 0.890 |
| 250 | 9.170 | 9.000 | 0.026 | 0.954 | 9.500 | 3.120 | 0.050 | 0.839 |
| 300 | 10.800 | 11.140 | 0.016 | 0.979 | 11.160 | 5.370 | 0.067 | 0.938 |
| PSOM 2 | Chitosan-NaOH | β-CDs-EPI | ||||||
| Co (mg/L) | qeexp | qecal | K2 (min−1) | R2 | qeexp | qecal | K2 (min−1) | R2 |
| 25 | 0.990 | 1.000 | 0.045 | 0.999 | 0.950 | 0.950 | 0.0135 | 0.999 |
| 50 | 1.950 | 1.950 | 0.093 | 0.999 | 1.920 | 1.920 | 1.920 | 0.999 |
| 100 | 3.930 | 3.930 | 0.285 | 0.999 | 3.760 | 3.730 | 3.730 | 0.999 |
| 150 | 5.730 | 5.730 | 0.430 | 0.999 | 5.590 | 5.620 | 5.620 | 0.999 |
| 200 | 7.570 | 7.570 | 0.534 | 0.999 | 7.290 | 7.300 | 7.300 | 0.999 |
| 250 | 9.170 | 9.160 | 0.644 | 0.999 | 9.500 | 9.520 | 9.520 | 0.999 |
| 300 | 10.800 | 10.790 | 0.864 | 0.999 | 11.160 | 11.240 | 11.240 | 0.999 |
| IDM 3 | Chitosan-NaOH | β-CDs-EPI | ||||||
| Co (mg/L) | qeexp | qecal | Ki (mg/g min1/2) | R2 | qeexp | qecal | Ki (mg/g min1/2) | R2 |
| 25 | 0.990 | 0.475 | 0.045 | 0.804 | 0.950 | 0.827 | 0.0135 | 0.750 |
| 50 | 1.950 | 0.930 | 0.093 | 0.806 | 1.920 | 1.700 | 0.0241 | 0.771 |
| 100 | 3.930 | 0.233 | 0.285 | 0.981 | 3.760 | 3.315 | 0.0495 | 0.627 |
| 150 | 5.730 | 0.260 | 0.430 | 0.962 | 5.590 | 4.495 | 0.124 | 0.761 |
| 200 | 7.570 | 1.000 | 0.534 | 0.959 | 7.290 | 5.850 | 0.170 | 0.633 |
| 250 | 9.170 | 1.610 | 0.644 | 0.952 | 9.500 | 7.940 | 0.170 | 0.814 |
| 300 | 10.800 | −0.522 | 0.864 | 0.991 | 11.160 | 8.732 | 0.279 | 0.699 |
| Isotherm | Parameter | Chitosan-Naoh | β-CDS-EPI |
|---|---|---|---|
| Freundlich | KF | 2.0 | 0.87 |
| nF | 1.88 | 1.2 | |
| R2 | 0.956 | 0.954 | |
| Langmuir | qmax | 12.30 | 23.47 |
| KL | 2.187 | 0.819 | |
| aL | 0.178 | 0.035 | |
| R2 | 0.981 | 0.516 | |
| RL | 0.183–0.018 | 0.534–0.087 | |
| Temkin | aT | 2.94 | 0.81 |
| bT | 1.09 | 0.733 | |
| R2 | 0.965 | 0.844 |
| Polymers | T (K) | ΔG°(kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/K·mol) |
|---|---|---|---|---|
| Chitosan-NaOH | 298 | 2.50 | −47.85 | −0.17 |
| 326 | 8.50 | - | - | |
| 341 | 9.40 | - | - | |
| β-CDS-EPI | 298 | −1.22 | −20.52 | −0.06 |
| 327 | −0.21 | - | - | |
| 344 | 1.97 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murcia-Salvador, A.; Pellicer, J.A.; Fortea, M.I.; Gómez-López, V.M.; Rodríguez-López, M.I.; Núñez-Delicado, E.; Gabaldón, J.A. Adsorption of Direct Blue 78 Using Chitosan and Cyclodextrins as Adsorbents. Polymers 2019, 11, 1003. https://doi.org/10.3390/polym11061003
Murcia-Salvador A, Pellicer JA, Fortea MI, Gómez-López VM, Rodríguez-López MI, Núñez-Delicado E, Gabaldón JA. Adsorption of Direct Blue 78 Using Chitosan and Cyclodextrins as Adsorbents. Polymers. 2019; 11(6):1003. https://doi.org/10.3390/polym11061003
Chicago/Turabian StyleMurcia-Salvador, Ainoa, José A. Pellicer, María I. Fortea, Vicente M. Gómez-López, María I. Rodríguez-López, Estrella Núñez-Delicado, and José A. Gabaldón. 2019. "Adsorption of Direct Blue 78 Using Chitosan and Cyclodextrins as Adsorbents" Polymers 11, no. 6: 1003. https://doi.org/10.3390/polym11061003
APA StyleMurcia-Salvador, A., Pellicer, J. A., Fortea, M. I., Gómez-López, V. M., Rodríguez-López, M. I., Núñez-Delicado, E., & Gabaldón, J. A. (2019). Adsorption of Direct Blue 78 Using Chitosan and Cyclodextrins as Adsorbents. Polymers, 11(6), 1003. https://doi.org/10.3390/polym11061003








