Active Role of ZnO Nanorods in Thermomechanical and Barrier Performance of Poly(vinyl alcohol-co-ethylene) Formulations for Flexible Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zinc Oxide Nanorods Synthesis and Characterization
2.3. Production of EVOH-Based Systems
2.4. Characterization of EVOH_Nrods Formulations
2.4.1. Morphological, Spectroscopic, and Color Analysis
2.4.2. Thermal Characterization
2.4.3. Mechanical Characterization
2.4.4. Moisture Content and Oxygen Barrier Properties
2.4.5. Weathering Test
3. Results
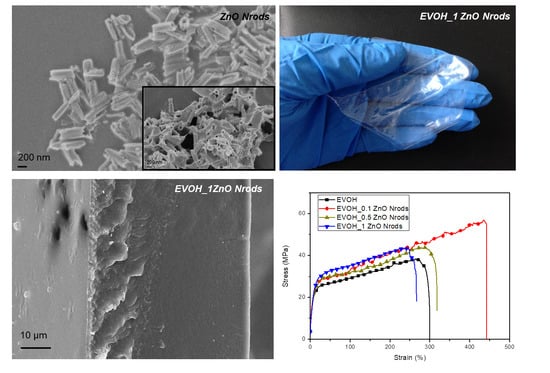
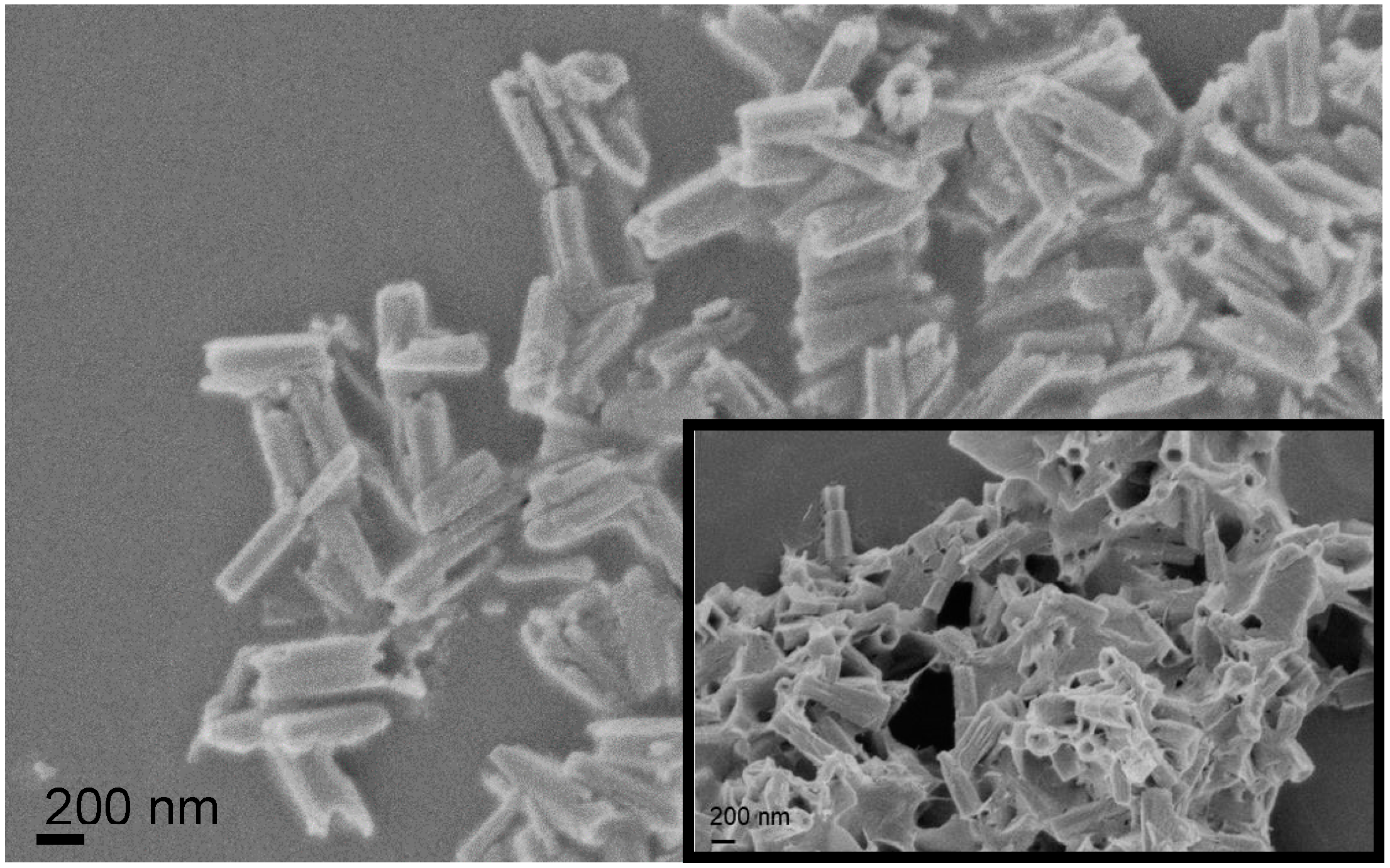
3.1. Nrods Characterization
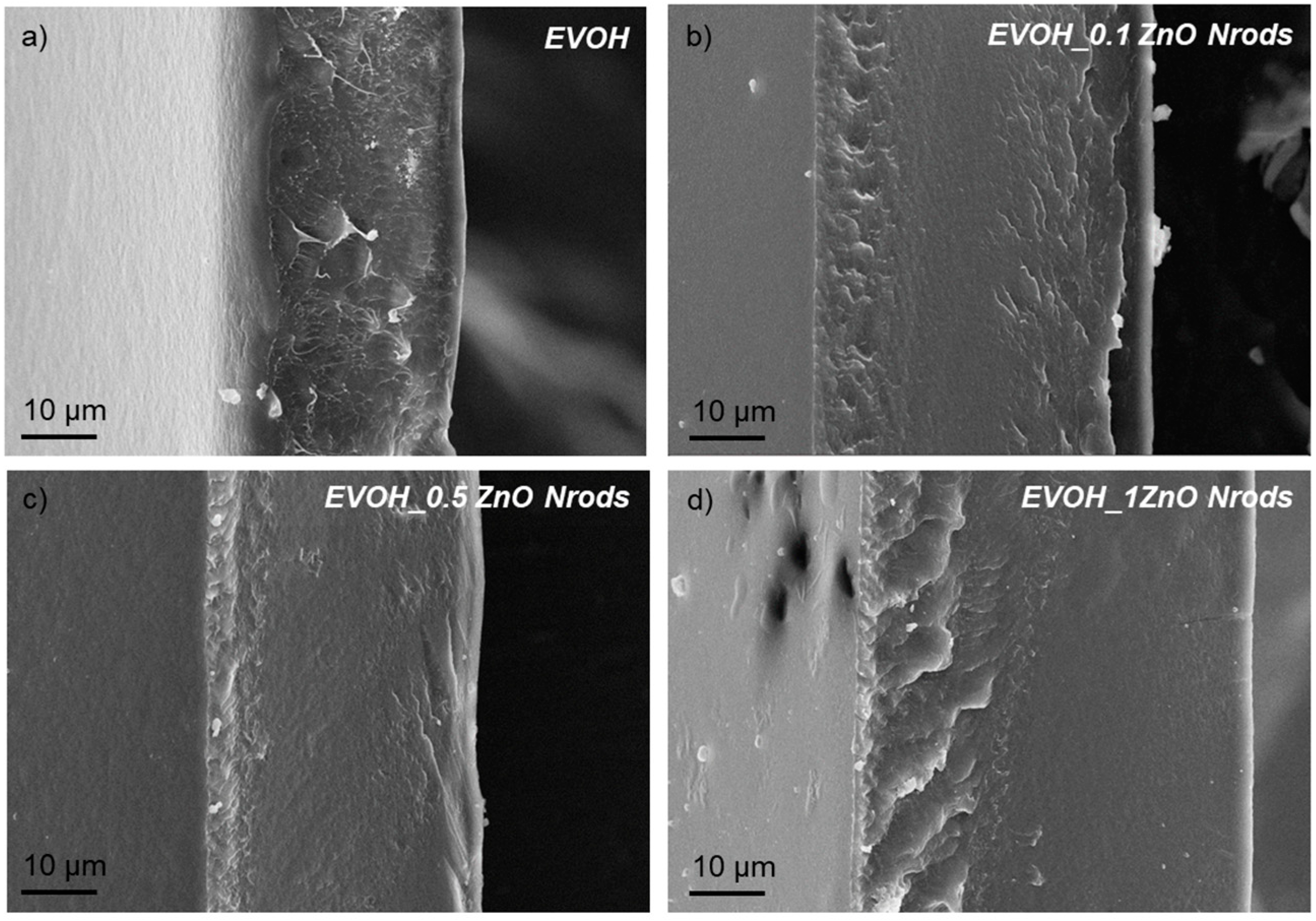
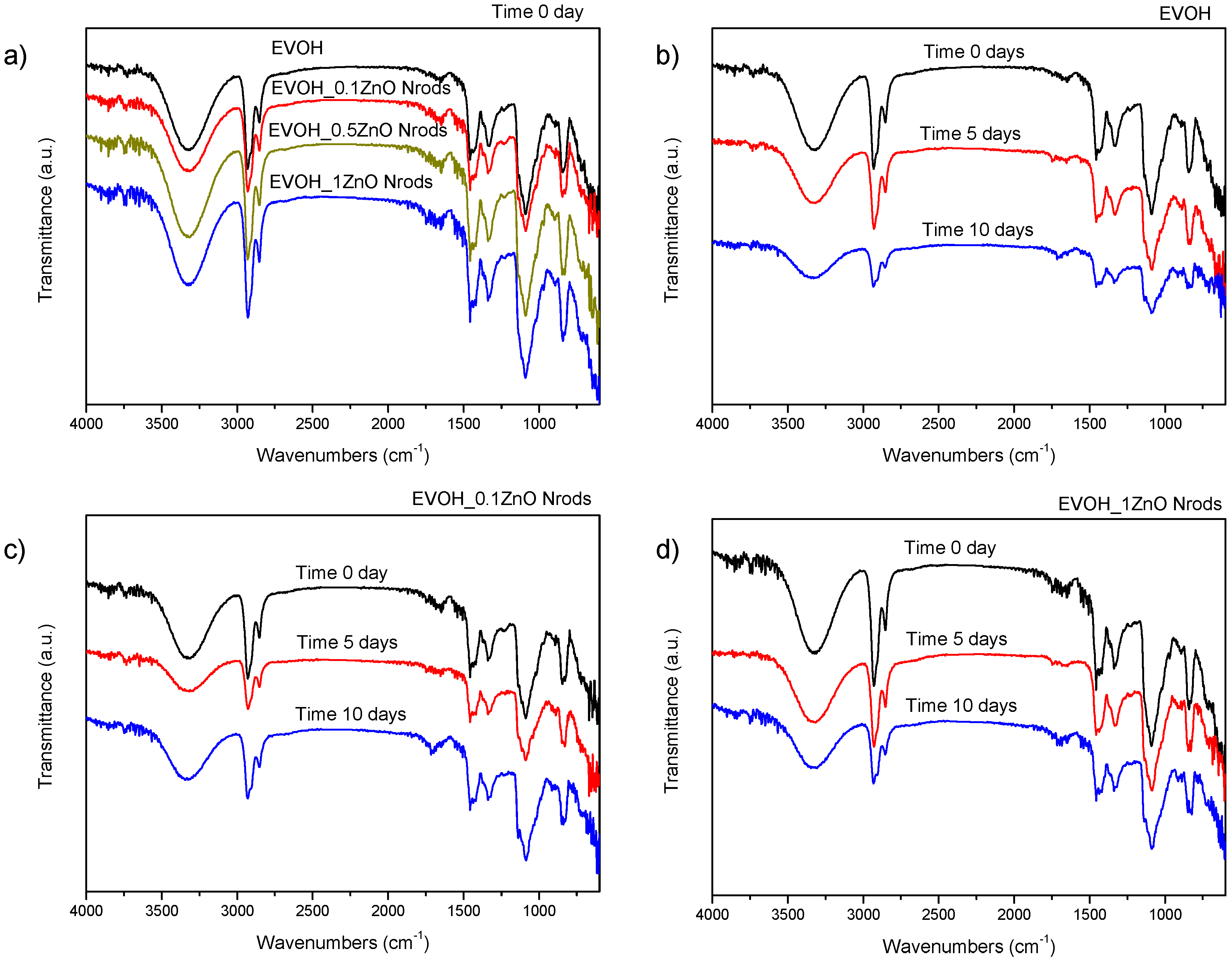
3.2. Morphological, Spectroscopic, and Color Analysis of EVOH and EVOH_Nrods Films
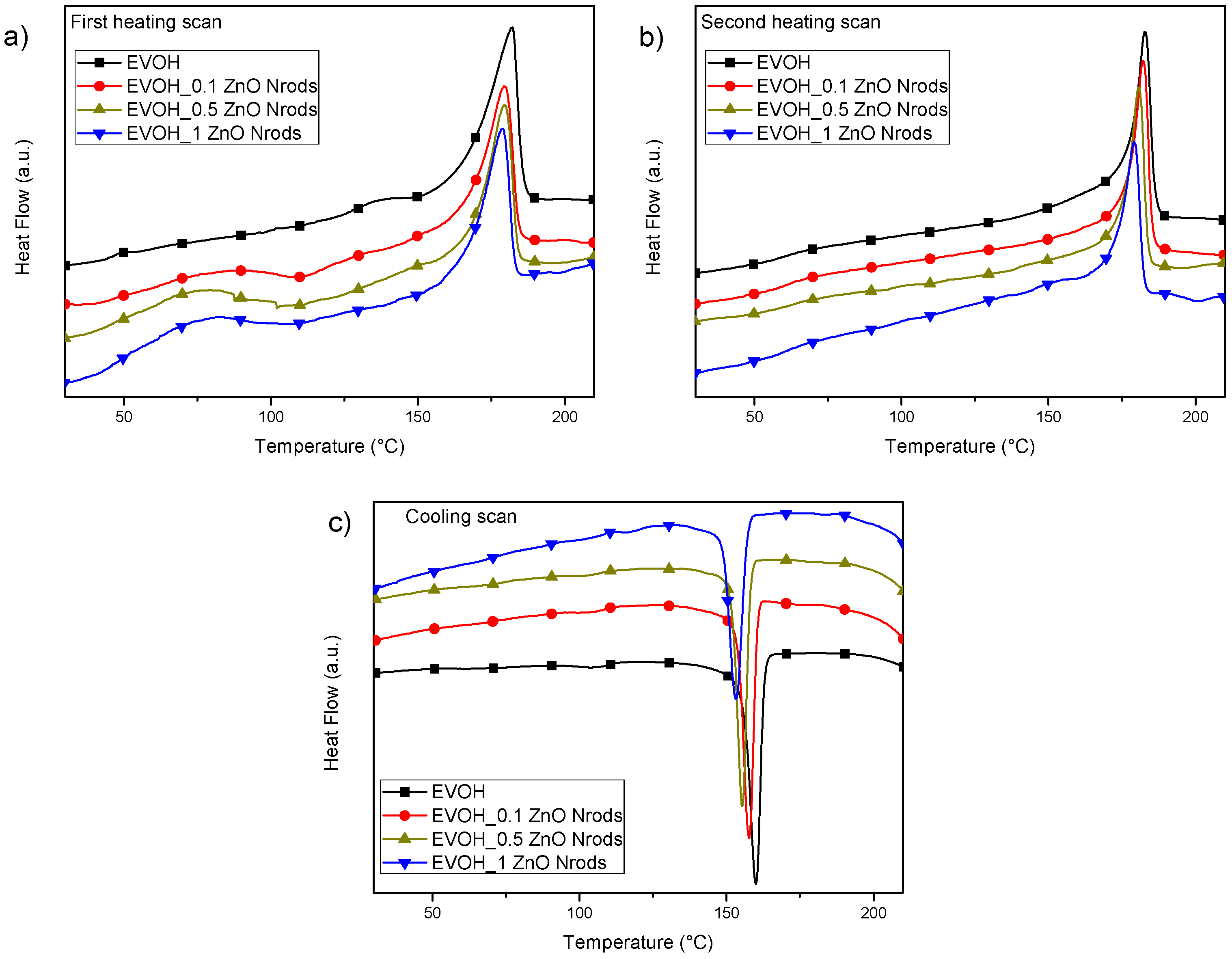
3.3. Thermal Characterizations of EVOH and EVOH_Nrods-Based Systems
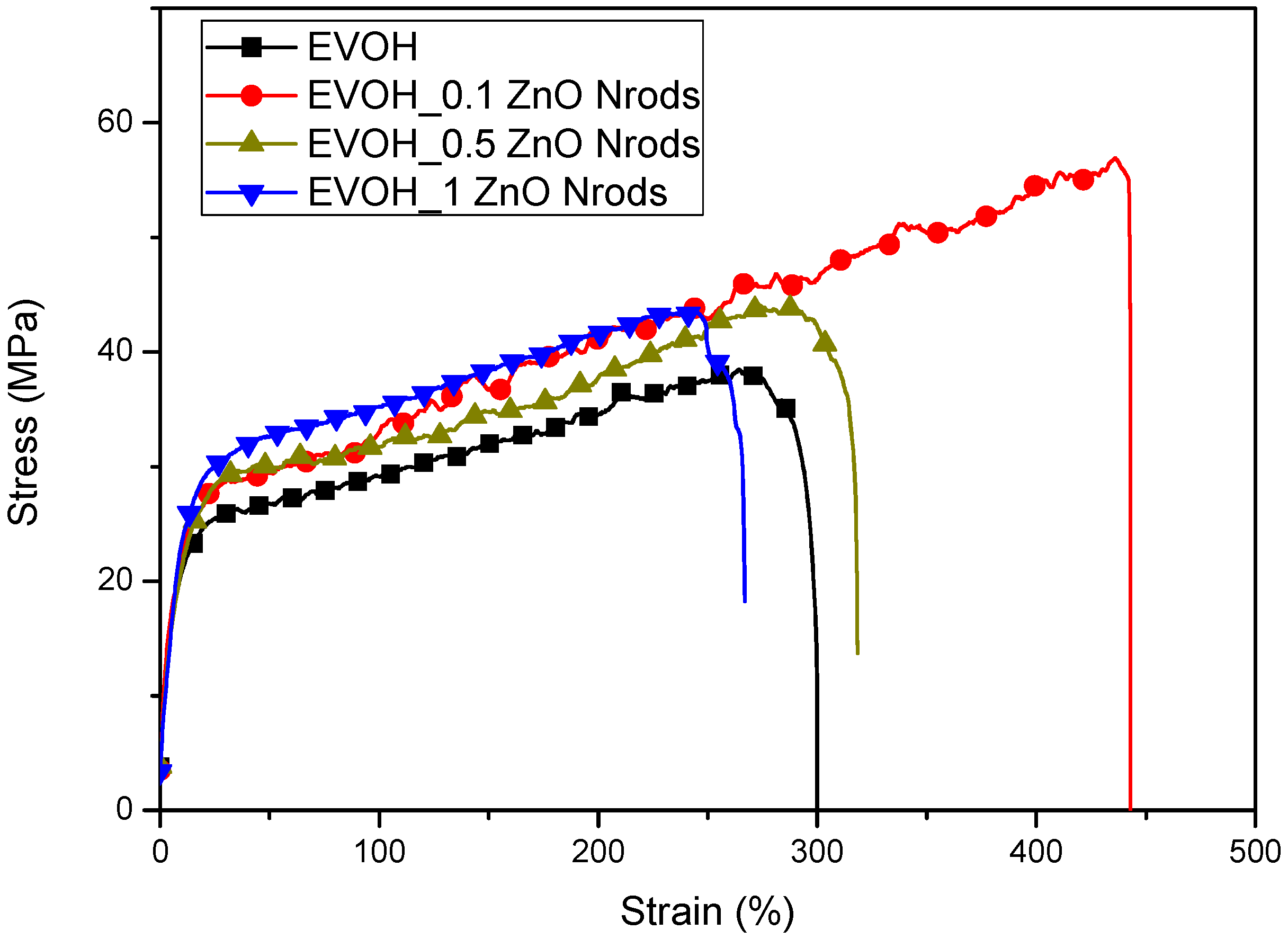
3.4. Mechanical Characterizations of EVOH and EVOH_Nrods Systems
3.5. Moisture Content and Oxygen Transmission Rate of EVOH_Nrods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ebina, T.; Mizukami, F. Flexible Transparent Clay Films with Heat-Resistant and High Gas-Barrier Properties. Adv. Mater. 2007, 19, 2450–2453. [Google Scholar] [CrossRef]
- Chaiko, D.J.; Leyva, A.A. Thermal transitions and barrier properties of olefinic nanocomposites. Chem. Mater. 2005, 17, 13–19. [Google Scholar] [CrossRef]
- Samyn, F.; Bourbigot, S.; Jama, C.; Bellayer, S.; Nazare, S.; Hull, R.; Fina, A.; Castrovinci, A.; Camino, G. Characterisation of the dispersion in polymer flame retarded nanocomposites. Eur. Polym. J. 2008, 44, 1631–1641. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. in Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Liu, X.-Q.; Wang, J.-S.; Wang, Y.-Z.; Stec, A.A.; Hull, T.R. Preparation and characterisation of a novel fire retardant PET/α-zirconium phosphate nanocomposite. Polym. Degrad. Stab. 2009, 94, 544–549. [Google Scholar] [CrossRef]
- Chandrakala, H.N.; Ramaraj, B.; Lee, J.H. Polyvinyl alcohol/carbon coated zinc oxide nanocomposites: Electrical, optical, structural and morphological characteristics. J. Alloys Compd. 2013, 580, 392–400. [Google Scholar] [CrossRef]
- Zhu, A.; Cai, A.; Zhang, J.; Jia, H.; Wang, J. PMMA-grafted-silica/PVC nanocomposites: Mechanical performance and barrier properties. J. Appl. Polym. Sci. 2008, 108, 2189–2196. [Google Scholar] [CrossRef]
- Topolniak, I.; Gardette, J.-L.; Therias, S. Influence of zeolite nanoparticles on photostability of ethylene vinyl alcohol copolymer (EVOH). Polym. Degrad. Stab. 2015, 121, 137–148. [Google Scholar] [CrossRef]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging: A review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innovative Food Sci. Emerging Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.M.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly (vinyl alcohol-co-ethylene) films. Polym. Degrad. Stab. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Powell, A.K.; Bonner, G. Permeation of water, methanol, fuel and alcohol-containing fuels in high-barrier ethylene–vinyl alcohol copolymer. Polym. Test. 2001, 20, 569–577. [Google Scholar] [CrossRef]
- Cabedo, L.; Lagarón, J.M.; Cava, D.; Saura, J.J.; Giménez, E. The effect of ethylene content on the interaction between ethylene-vinyl alcohol copolymers and water—II: Influence of water sorption on the mechanical properties of EVOH copolymers. Polym. Test. 2006, 25, 860–867. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Antimicrobial food packaging film based on the release of LAE from EVOH. Int. J. Food Microbiol. 2012, 157, 239–244. [Google Scholar] [CrossRef] [PubMed]
- López-Rubio, A.; Lagaron, J.M.; Giménez, E.; Cava, D.; Hernandez-Muñoz, P.; Yamamoto, T.; Gavara, R. Morphological Alterations Induced by Temperature and Humidity in Ethylene−Vinyl Alcohol Copolymers. Macromolecules 2003, 36, 9467–9476. [Google Scholar] [CrossRef]
- Lagarón, J.M.; Giménez, E.; Gavara, R.; Saura, J.J. Study of the influence of water sorption in pure components and binary blends of high barrier ethylene–vinyl alcohol copolymer and amorphous polyamide and nylon-containing ionomer. Polymer 2001, 42, 9531–9540. [Google Scholar] [CrossRef]
- Blanchard, A.; Gouanvé, F.; Espuche, E. Effect of humidity on mechanical, thermal and barrier properties of EVOH films. J. Membr. Sci. 2017, 540, 1–9. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, S.; Zhang, C.; Olah, A.; Baer, E.; Schiraldi, D.A. HDPE/EVOH Multilayered, High Barrier Films for Flexible Organic Photovoltaic Device Packaging. ACS Appl. Polym. Mater. 2019, 1, 259–266. [Google Scholar] [CrossRef]
- Li, T.; Dai, Y.; Li, J.; Guo, S.; Xie, G. A high-barrier PP/EVOH membrane prepared through the multistage biaxial-stretching extrusion. J. Appl. Polym. Sci. 2017, 134, 45016. [Google Scholar] [CrossRef]
- Kim, N.; Potscavage, W.J.; Sundaramoothi, A.; Henderson, C.; Kippelen, B.; Graham, S. A correlation study between barrier film performance and shelf lifetime of encapsulated organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 101, 140–146. [Google Scholar] [CrossRef]
- Topolniak, I.; Vincze, A.; Gardette, J.-L.; Hasko, D.; Satka, A.; Therias, S.; Uherek, F. Surface analysis of EVOH and its nanocomposite photoaging: Particles effect. Vacuum 2017, 138, 125–133. [Google Scholar] [CrossRef]
- Pakravan, H.R.; Yari, H. The influence of nanostructured UV-blockers on mechanical properties of carbon fiber epoxy composites during accelerated weathering condition. Polym. Adv. Technol. 2018, 29, 970–981. [Google Scholar] [CrossRef]
- Ognibene, G.; Cristaldi, D.A.; Fiorenza, R.; Blanco, I.; Cicala, G.; Scirè, S.; Fragalà, M.E. Photoactivity of hierarchically nanostructured ZnO-PES fibre mats for water treatments. RSC Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.-J. Chitosan-Zinc Oxide hybrid composite for enhanced dye degradation and antibacterial activity. Compos. Interfaces 2013, 20, 365–377. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Bradley, E.L.; Castle, L.; Chaudhry, Q. Applications of nanomaterials in food packaging with a consideration of opportunities for developing countries. Trends in Food Science & Technology 2011, 22, 604–610. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Zheng, W.; Zhao, Y.-F.; Jin, Y.-F.; Tang, Z.-X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. Part A 2014, 31, 173–186. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Yetiskin, O.; Dadashi-Silab, S.; Khan, S.B.; Asiri, A.M.; Yagci, Y. Visible-Light-Induced Copper(I)-Catalyzed Azide-Alkyne Cycloaddition Initiated by Zinc Oxide Semiconductor Nanoparticles. Asian J. Org. Chem. 2015, 4, 442–444. [Google Scholar] [CrossRef]
- Fortunati, E.; Benincasa, P.; Balestra, G.M.; Luzi, F.; Mazzaglia, A.; Del Buono, D.; Puglia, D.; Torre, L. Revalorization of barley straw and husk as precursors for cellulose nanocrystals extraction and their effect on PVA_CH nanocomposites. Ind. Crops Prod. 2016, 92, 201–217. [Google Scholar] [CrossRef]
- Martȷnez-Abad, A.; Lagaron, J.M.; Ocio, M.J. Development and Characterization of Silver-Based Antimicrobial Ethylene–Vinyl Alcohol Copolymer (EVOH) Films for Food-Packaging Applications. J. Agric. Food Chem. 2012, 60, 5350–5359. [Google Scholar] [CrossRef]
- Faisant, J.B.; Aït-Kadi, A.; Bousmina, M.; Descheˆnes, L. Morphology, thermomechanical and barrier properties of polypropylene-ethylene vinyl alcohol blends. Polymer 1998, 39, 533–545. [Google Scholar] [CrossRef]
- Roohani, M.; Habibi, Y.; Belgacem, N.M.; Ebrahim, G.; Karimi, A.N.; Dufresne, A. Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites. Eur. Polym. J. 2008, 44, 2489–2498. [Google Scholar] [CrossRef]
- Badran, R.I.; Umar, A.; Al-Heniti, S.; Al-Hajry, A.; Al-Harbi, T. Synthesis and characterization of zinc oxide nanorods on silicon for the fabrication of p-Si/n-ZnO heterojunction diode. J. Alloys Compd. 2010, 508, 375–379. [Google Scholar] [CrossRef]
- Sajimol Augustine, M.; Jeeju, P.P.; Sreevalsa, V.G.; Jayalekshmi, S. Excellent UV absorption in spin-coated thin films of oleic acid modified zinc oxide nanorods embedded in Polyvinyl alcohol. J. Phys. Chem. Solids 2012, 73, 396–401. [Google Scholar] [CrossRef]
- Wahab, R.; Kim, Y.-S.; Lee, K.; Shin, H.-S. Fabrication and growth mechanism of hexagonal zinc oxide nanorods via solution process. J. Mater. Sci. 2010, 45, 2967–2973. [Google Scholar] [CrossRef]
- Sadeghi, K.; Shahedi, M. Physical, mechanical, and antimicrobial properties of ethylene vinyl alcohol copolymer/chitosan/nano-ZnO (ECNZn) nanocomposite films incorporating glycerol plasticizer. J. Food Meas. Charact. 2016, 10, 137–147. [Google Scholar] [CrossRef]
- Murariu, M.; Paint, Y.; Murariu, O.; Raquez, J.M.; Bonnaud, L.; Dubois, P. Current progress in the production of PLA–ZnO nanocomposites: Beneficial effects of chain extender addition on key properties. J. Appl. Polym. Sci. 2015, 132, 42480. [Google Scholar] [CrossRef]
- Abu-Eittah, R.H.; El-Tawil, B.A.H. The electronic absorption spectra of some coumarins. A molecular orbital treatment. Can. J. Chem. 1985, 63, 1173–1179. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Giménez, E.; Saura, J.J. Degradation of high barrier ethylene–vinyl alcohol copolymer under mild thermal-oxidative conditions studied by thermal analysis and infrared spectroscopy. Polym. Int. 2001, 50, 635–642. [Google Scholar] [CrossRef]
- Chouhan, S.; Bajpai, A.K.; Bajpai, J.; Katare, R.; Dhoble, S.J. Mechanical and UV absorption behavior of zinc oxide nanoparticles: Reinforced poly(vinyl alcohol-g-acrylonitrile) nanocomposite films. Polym. Bull. 2017, 74, 4119–4141. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Chmela, S.; Wiles, D.M. The oxidative degradation of ethylene/vinyl alcohol copolymers. Polym. Degrad. Stab. 1991, 31, 255–267. [Google Scholar] [CrossRef][Green Version]
- Skrovanek, D.J.; Howe, S.E.; Painter, P.C.; Coleman, M.M. Hydrogen bonding in polymers: Infrared temperature studies of an amorphous polyamide. Macromolecules 1985, 18, 1676–1683. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Kubacka, A.; Ferrer, M.; Cerrada, M.L.; Fernández-García, M.; Fernández-García, M. Role of TiO2 morphological characteristics in EVOH–TiO2 nanocomposite films: Self-degradation and self-cleaning properties. RSC Adv. 2013, 3, 8541–8550. [Google Scholar] [CrossRef]
- Su, Z.; Zhao, Y.; Xu, Y.; Zhang, X.; Zhu, S.; Wang, D.; Wu, J.; Han, C.C.; Xu, D. Spectroscopic Investigation of the Polymorphism and Side Group Location of Ethylene Copolymers. Macromolecules 2004, 37, 3249–3256. [Google Scholar] [CrossRef]
- Földes, E.; Pukánszky, B. Miscibility–structure–property correlation in blends of ethylene vinyl alcohol copolymer and polyamide 6/66. J. Colloid Interface Sci. 2005, 283, 79–86. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Ruseckaite, R.A.; Vázquez, A. Kinetic analysis of thermal degradation in poly(ethylene–vinyl alcohol) copolymers. J. Appl. Polym. Sci. 2003, 90, 3157–3163. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Hechenleitner, A.A.W.; Lima, S.M.; Andrade, L.H.C.; Caires, A.R.L.; Pineda, E.A.G. Preparation, characterization, and photoluminescence study of PVA/ZnO nanocomposite films. Mater. Chem. Phys. 2011, 128, 371–376. [Google Scholar] [CrossRef]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.L.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-Performance Polylactide/ZnO Nanocomposites Designed for Films and Fibers with Special End-Use Properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef]
- Abutalib, M.M. Effect of zinc oxide nanorods on the structural, thermal, dielectric and electrical properties of polyvinyl alcohol/carboxymethyle cellulose composites. Phys. B 2019, 557, 108–116. [Google Scholar] [CrossRef]
- Abd-Elrahman, M.I. Synthesis of Polyvinyl Alcohol–Zinc Oxide Composite by Mechanical Milling: Thermal and Infrared Studies. Nanoscale Microscale Thermophys. Eng. 2013, 17, 194–203. [Google Scholar] [CrossRef]
- Restrepo, I.; Benito, N.; Medinam, C.; Mangalaraja, R.V.; Flores, P.; Rodriguez-Llamazares, S. Development and characterization of polyvinyl alcohol stabilized polylactic acid/ZnO nanocomposites. Mater. Res. Express 2017, 4, 105019. [Google Scholar] [CrossRef]
- Lizundia, E.; Penayo, M.C.; Guinault, A.; Vilas, J.L.; Domenek, S. Impact of ZnO nanoparticle morphology on relaxation and transport properties of PLA nanocomposites. Polym. Test. 2019, 75, 175–184. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, H.M. Enhancement of thermal, mechanical, and barrier properties of ethylene vinyl alcohol copolymer by incorporation of graphene nanosheets: Effect of functionalization of graphene oxide. High Perform. Polym. 2014, 27, 694–704. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced Mechanical Properties of Graphene-Based Poly(vinyl alcohol) Composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Li, P.; Song, G.; Yin, L.; Wang, L.; Ma, G. New toughened polypropylene/organophilic montmorillonite nanocomposites. J. Appl. Polym. Sci. 2008, 108, 2116–2121. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Das, K.; Sonakshi, M.; Reddy, G.S.M.; Aderibigbe, B.; Sadiku, R.; Ray, S.S. Structure and properties of highly toughened biodegradable polylactide/ZnO biocomposite films. Int. J. Biol. Macromol. 2014, 64, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crops Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Díez-Pascual, M.A.; Díez-Vicente, L.A. Poly(3-hydroxybutyrate)/ZnO Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Giovanale, G.; Mazzaglia, A.; Torre, L.; Balestra, G.M. Cellulose nanocrystals from Actinidia deliciosa pruning residues combined with carvacrol in PVA_CH films with antioxidant/antimicrobial properties for packaging applications. Int. J. Biol. Macromol. 2017, 104, 43–55. [Google Scholar] [CrossRef]
- López-de-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Lepot, N.; Van Bael, M.K.; Van den Rul, H.; D’Haen, J.; Peeters, R.; Franco, D.; Mullens, J. Influence of incorporation of ZnO nanoparticles and biaxial orientation on mechanical and oxygen barrier properties of polypropylene films for food packaging applications. J. Appl. Polym. Sci. 2011, 120, 1616–1623. [Google Scholar] [CrossRef]
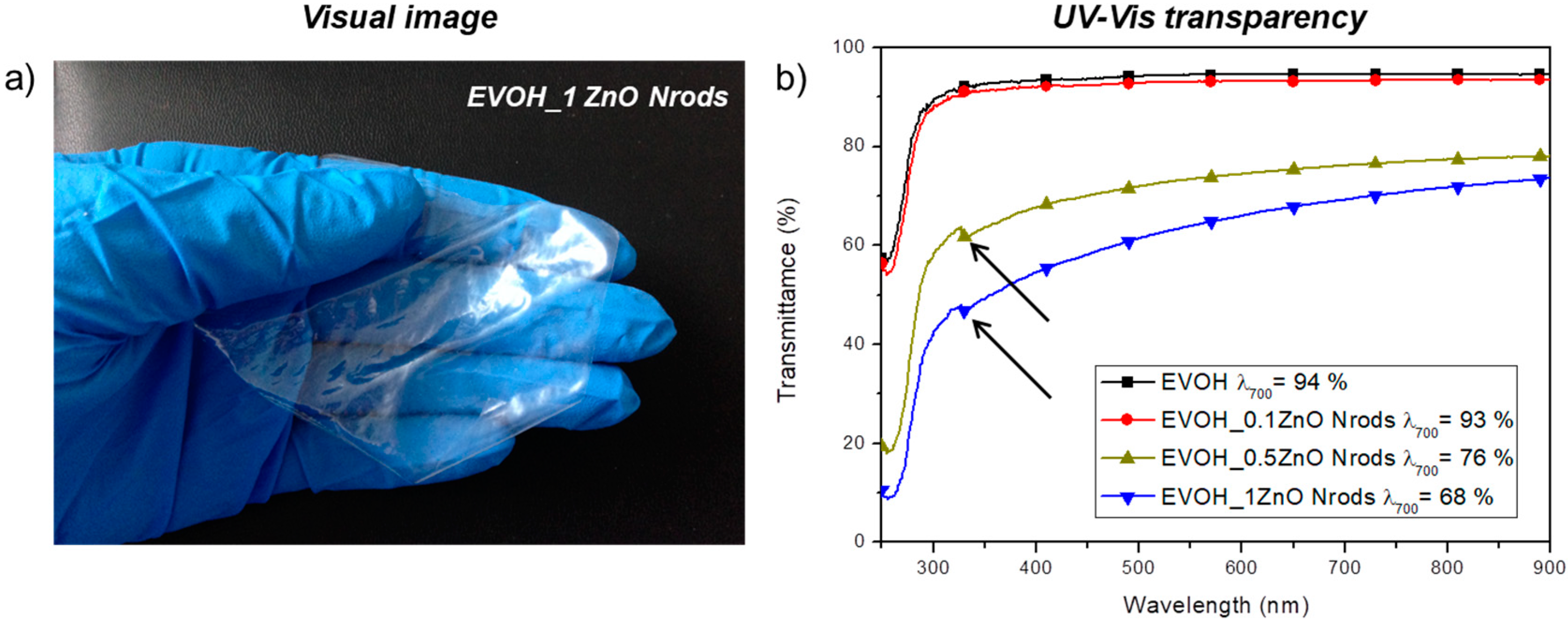

| Formulations | L* | a* | b* | ∆E* | Gloss (°) |
|---|---|---|---|---|---|
| White Control | 99.47 ± 0.00 | −0.08 ± 0.01 | −0.08 ± 0.01 | - | 121 ± 0 |
| Time 0 day | |||||
| EVOH | 98.78 ± 0.06 | −0.09 ± 0.01 | 0.14 ± 0.00 | 0.72 ± 0.06 | 157 ± 2 |
| EVOH_0.1ZnO Nrods | 98.21 ± 0.03 | −0.10 ± 0.01 | 0.14 ± 0.00 | 1.28 ± 0.03 | 154 ± 4 |
| EVOH_0.5ZnO Nrods | 98.32 ± 0.13 | −0.08 ± 0.00 | 0.20 ± 0.02 | 1.19 ± 0.14 | 157 ± 1 |
| EVOH_1ZnO Nrods | 98.78 ± 0.02 | −0.10 ± 0.00 | 0.22 ± 0.02 | 0.79 ± 0.02 | 158 ± 3 |
| Time 5 days | |||||
| EVOH | 98.12 ± 0.20 | −0.09 ± 0.01 | 0.28 ± 0.05 | 1.40 ± 0.18 | 138 ± 4 |
| EVOH_0.1ZnO Nrods | 97.40 ± 0.25 | −0.06 ± 0.02 | 0.18 ± 0.05 | 2.09 ± 0.25 | 136 ± 5 |
| EVOH_1ZnO Nrods | 97.28 ± 0.11 | −0.11 ± 0.01 | 0.45 ± 0.06 | 2.25 ± 0.09 | 132 ± 2 |
| Time 10 days | |||||
| EVOH | 98.18 ± 0.02 | −0.22 ± 0.01 | 0.86 ± 0.10 | 2.23 ± 0.25 | 137 ± 4 |
| EVOH_0.1ZnO Nrods | 97.47 ± 0.33 | −0.22 ± 0.01 | 0.86 ± 0.10 | 2.04 ± 0.10 | 137 ± 4 |
| EVOH_1ZnO Nrods | 97.71 ± 0.02 | −0.21 ± 0.02 | 0.87 ± 0.01 | 2.01 ± 0.03 | 147 ± 2 |
| Formulations | First Heating Scan | |||||
| Tg (°C) | ΔHm (J g−1) | Tm (°C) | Xm (%) | |||
| EVOH | 48.8±0.1 | 82.8 ± 0.4 | 180.7 ± 2.2 | 40.9 ± 0.2 | ||
| EVOH_0.1ZnONrods | 59.5 ± 0.1 | 67.6 ± 2.4 | 179.8 ± 0.7 | 33.4 ± 1.2 | ||
| EVOH_0.5ZnONrods | 59.6 ± 0.1 | 66.5 ± 0.1 | 179.5 ± 0.3 | 33.0 ± 0.1 | ||
| EVOH_1ZnONrods | 57.4 ± 0.3 | 61.5 ± 2.7 | 178.0 ± 1.0 | 30.7 ± 1.3 | ||
| Second Heating Scan | ||||||
| Tg (°C) | ΔHm (J g−1) | Tm (°C) | Xm (%) | |||
| EVOH | 64.6 ± 0.3 | 83.2 ± 0.1 | 182.9 ± 0.1 | 41.1 ± 0.1 | ||
| EVOH_0.1ZnONrods | 63.5 ± 0.1 | 70.3 ± 1.5 | 181.9 ± 0.3 | 34.8 ± 0.7 | ||
| EVOH_0.5ZnONrods | 66.0 ± 2.3 | 68.2 ± 0.3 | 178.7 ± 2.7 | 33.8 ± 0.1 | ||
| EVOH_1ZnONrods | 64.7 ± 0.1 | 64.7 ± 0.1 | 178.7 ± 0.7 | 30.8 ± 0.1 | ||
| Cooling Scan | ||||||
| Tg (°C) | ΔHc (J g−1) | Tc (°C) | ΔHc (J g−1) | Tc (°C) | Xc (%) | |
| EVOH | 61.0 ± 0.7 | 2.2 ± 0.2 | 104.9 ± 0.4 | 71.8 ± 0.4 | 159.9 ± 0.2 | 36.6 ± 0.3 |
| EVOH_0.1ZnONrods | 60.4 ± 0.7 | 1.2 ± 0.1 | 104.1 ± 0.8 | 60.1 ± 2.5 | 157.7 ± 0.1 | 30.3 ± 1.2 |
| EVOH_0.5ZnONrods | 60.6 ± 0.7 | 1.2 ± 0.1 | 102.0 ± 0.4 | 62.8 ± 0.4 | 155.2 ± 0.3 | 31.8 ± 0.3 |
| EVOH_1ZnONrods | 65.2 ± 0.7 | 0.9 ± 0.1 | 101.6 ± 0.1 | 56.3 ± 1.1 | 152.5 ± 0.9 | 28.6 ± 0.6 |
| Formulation | Mechanical Properties | ||
|---|---|---|---|
| σb (MPa) | εb (%) | EYoung (MPa) | |
| EVOH | 45 ± 4 | 265 ± 28 | 440 ± 65 |
| EVOH_0.1ZnO Nrods | 55 ± 3 | 410 ± 30 | 415 ± 30 |
| EVOH_0.5ZnO Nrods | 50 ± 9 | 365 ± 100 | 345 ± 80 |
| EVOH_1ZnO Nrods | 40 ± 6 | 240 ± 15 | 300 ± 10 |
| Formulations | MC (%) @ 1 Week | MC (%) @ 5 Week |
|---|---|---|
| EVOH | 1.51 ± 0.08 | 1.55 ± 0.15 |
| EVOH_0.1ZnO Nrods | 1.50 ± 0.06 | 1.60 ± 0.15 |
| EVOH_0.5ZnO Nrods | 1.45 ± 0.02 | 1.55 ± 0.10 |
| EVOH_1ZnO Nrods | 1.35 ± 0.0.5 | 1.43 ± 0.07 |
| Formulations | OTR (cm3 m−2 days−1) | e (mm) |
|---|---|---|
| EVOH | 1.97 | 0.08 |
| EVOH_0.1ZnO Nrods | 2.74 | 0.06 |
| EVOH_0.5ZnO Nrods | 4.79 | 0.06 |
| EVOH_1ZnO Nrods | 6.10 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzi, F.; Di Michele, A.; Torre, L.; Puglia, D. Active Role of ZnO Nanorods in Thermomechanical and Barrier Performance of Poly(vinyl alcohol-co-ethylene) Formulations for Flexible Packaging. Polymers 2019, 11, 922. https://doi.org/10.3390/polym11050922
Luzi F, Di Michele A, Torre L, Puglia D. Active Role of ZnO Nanorods in Thermomechanical and Barrier Performance of Poly(vinyl alcohol-co-ethylene) Formulations for Flexible Packaging. Polymers. 2019; 11(5):922. https://doi.org/10.3390/polym11050922
Chicago/Turabian StyleLuzi, Francesca, Alessandro Di Michele, Luigi Torre, and Debora Puglia. 2019. "Active Role of ZnO Nanorods in Thermomechanical and Barrier Performance of Poly(vinyl alcohol-co-ethylene) Formulations for Flexible Packaging" Polymers 11, no. 5: 922. https://doi.org/10.3390/polym11050922
APA StyleLuzi, F., Di Michele, A., Torre, L., & Puglia, D. (2019). Active Role of ZnO Nanorods in Thermomechanical and Barrier Performance of Poly(vinyl alcohol-co-ethylene) Formulations for Flexible Packaging. Polymers, 11(5), 922. https://doi.org/10.3390/polym11050922