The Role of Surfaces in Gas Transport Through Polymer Membranes
Abstract
:1. Introduction
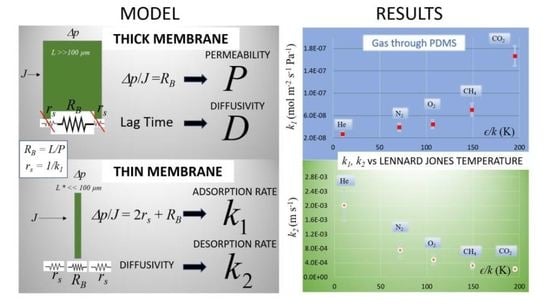
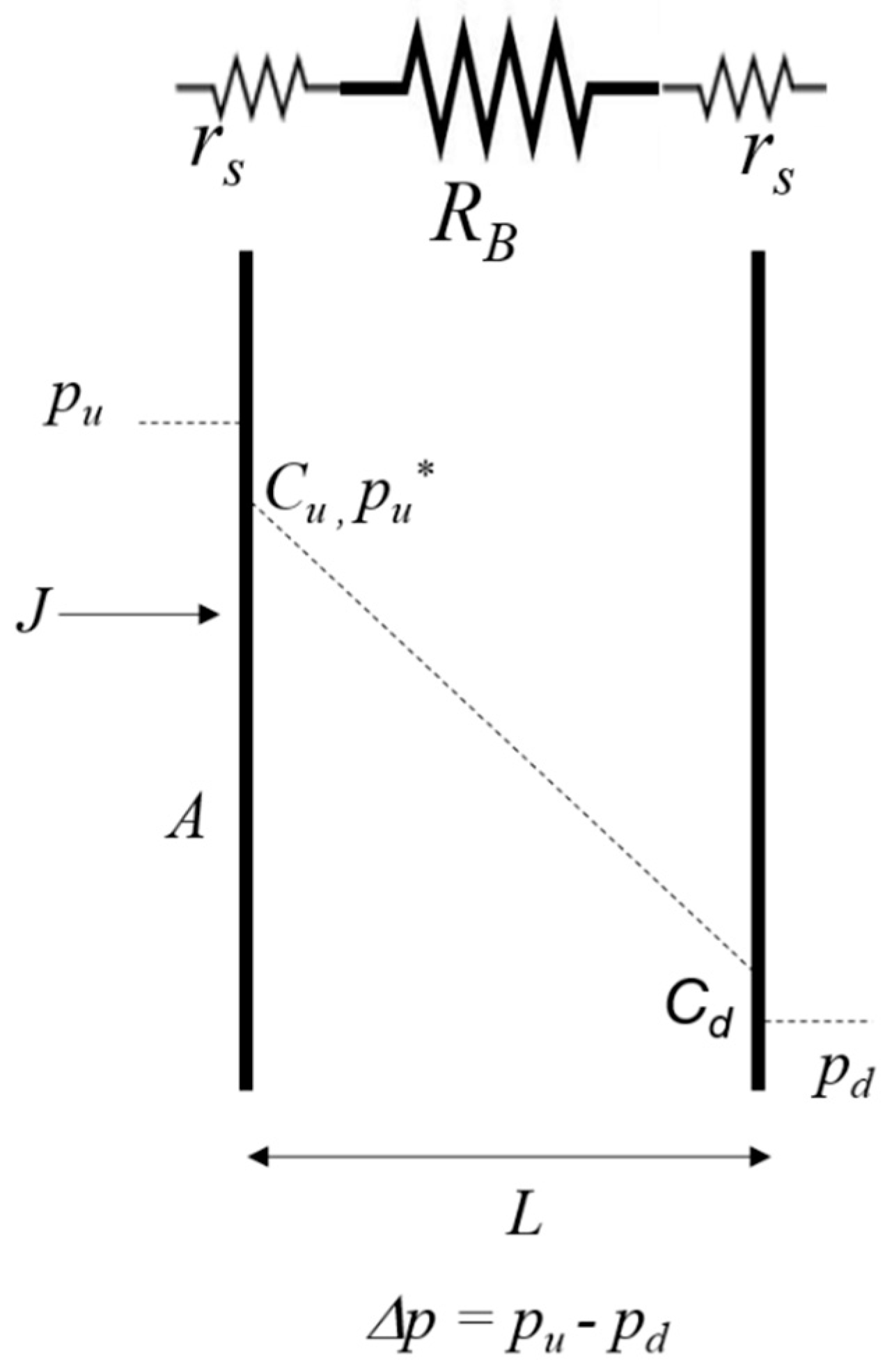
1.1. Gas Transport Model
1.2. Experimental Method
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandey, P.; Chauhan, R.S. Membranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The Solution–Diffusion Model: A Unified Approach to Membrane. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Pinnau, I., Freeman, B.D., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 159–189. ISBN 0-470-85345-X. [Google Scholar]
- Nagy, E. Basic Equation of Mass Transport through a Membrane Layer, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-12-813722-2. [Google Scholar]
- Lundstrom, J.E. Sorption, desorption and diffusion processes in membrane permeation. J. Membr. Sci. 2015, 486, 138–150. [Google Scholar] [CrossRef]
- ASTM International, Designation: D1434-82. Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Firpo, G.; Angeli, E.; Repetto, L.; Valbusa, U. Permeability thickness dependence of polydimethylsiloxane (PDMS) membranes. J. Membr. Sci. 2015, 481, 1–8. [Google Scholar] [CrossRef]
- McGregor, R.; Mahajan, I.Y. Permeation of dyes through polymer films. Part 1—Acid dyes through cellulose. Trans. Faraday Soc. 1962, 58, 2484–2492. [Google Scholar] [CrossRef]
- Jadhav, J.S.; McGregor, R.; Mahajan, I.Y. Permeation of dyes through polymer films. Part 2—Chlorazol Sky Blue FF through cellulose. Trans. Faraday Soc. 1965, 61, 2569–2586. [Google Scholar] [CrossRef]
- Islam, M.A.; Buschatz, H.; Paul, D. Non-equilibrium surface reactions—A factor in determining steady state diffusion flux. J. Membr. Sci. 2002, 204, 379–384. [Google Scholar] [CrossRef]
- Fialova, K.; Petrychkovych, R.; Sharma, M.; Uchytil, P. Steady state sorption measurement and the transport mechanism in polymeric membrane during vapor permeation. J. Membr. Sci. 2006, 275, 166–174. [Google Scholar] [CrossRef]
- Firpo, G.; Angeli, E.; Repetto, L.; Guida, P.; Lo Savio, R.; Valbusa, U. The dependence on thickness of permeability and its influence on gas separations of flat and corrugated polymer membranes. J. Chromatogr. Sep. Tech. 2017, 8, 23. [Google Scholar]
- Firpo, G.; Angeli, E.; Guida, P.; Lo Savio, R.; Repetto, L.; Valbusa, U. Gas permeation through rubbery polymer nano-corrugated membranes. Sci. Rep. 2018, 8, 6345. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Cranck, J. The mathematics of Diffusions; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J. Polym. Sci. B 2000, 38, 415–434. [Google Scholar] [CrossRef]
- De Bo, F.R.; Van Langenhove, H.; Pruuost, P.; De Neve, J. Investigation of the permeability and selectivity of gases and volatile organic compounds for polydimethylsiloxane membranes. J. Membr. Sci. 2003, 215, 303–319. [Google Scholar] [CrossRef]
- Teplyakov, V.; Meares, P. Correlation aspects of selective gas permeabilities. Gas Sep Purif. 1990, 4, 66. [Google Scholar] [CrossRef]
- Petropoulos, J.H. Mechanisms and Theories for Sorption and Diffusion of Gases in Polymers. In Polymeric Gas Separation Membranes; Paul, D.R., Yampol’skii, Y.P., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 17–81. [Google Scholar]
- Van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]

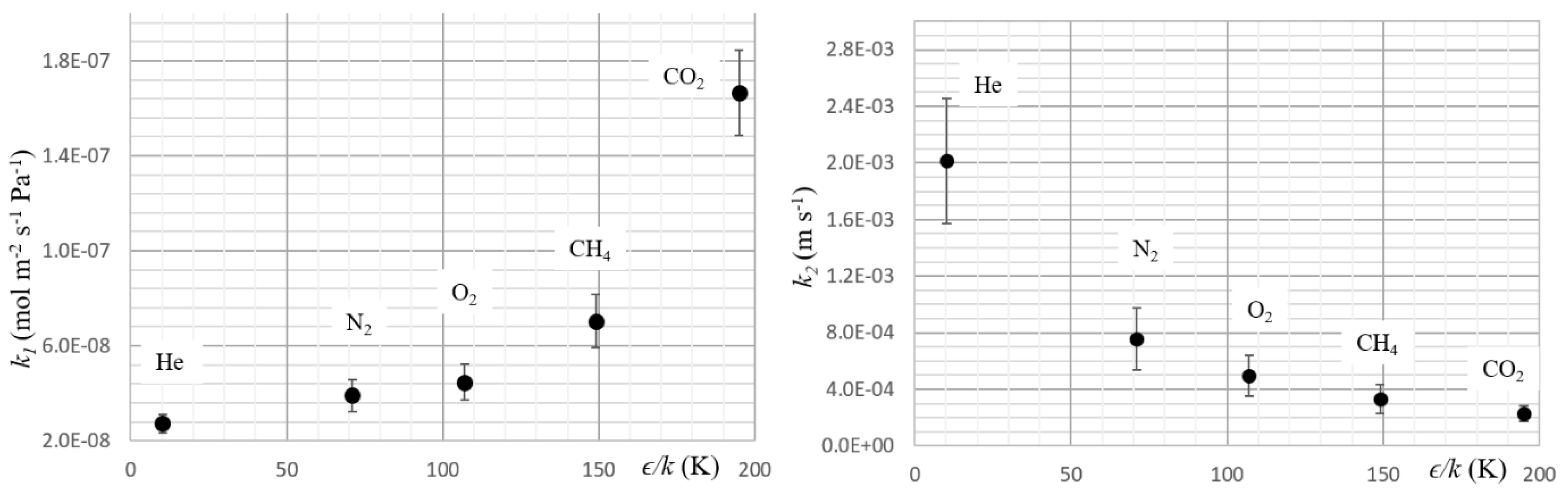
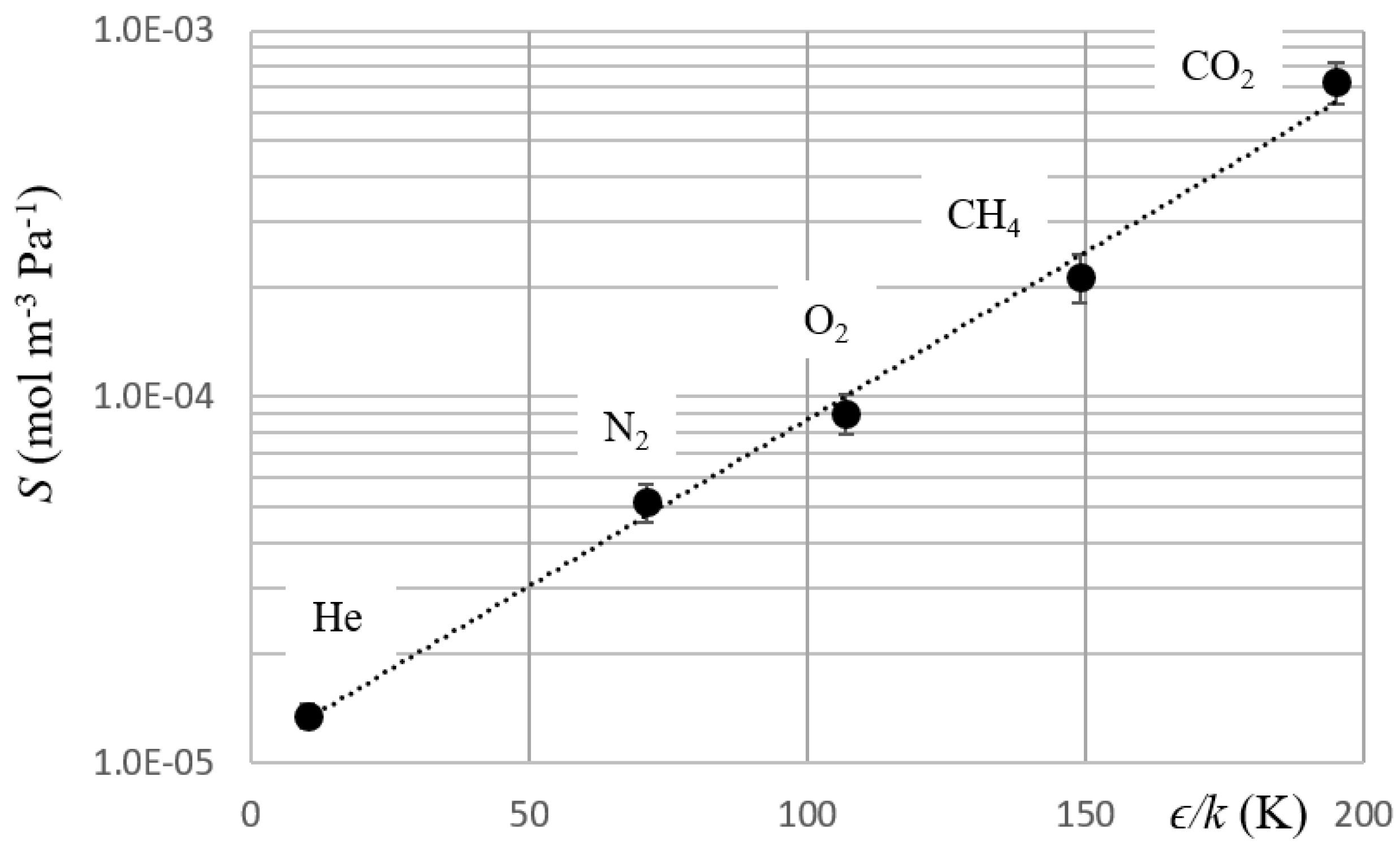
| Gas | P × 1013 (mol m−1 s−1 Pa−1) | k1 × 108 (mol m−2 s−1 Pa−1) | D × 109 (m2 s−1) | S × 104 (mol m−3 Pa) | k2 × 104 (m s−1) | ϵ/k* (K) |
|---|---|---|---|---|---|---|
| He | 2.3 ± 0.1 | 2.7 ± 0.4 | 16.8 ± 0.3 | 0.14 ± 0.01 | 20 ± 4 | 10.2 |
| N2 | 1.8 ± 0.1 | 3.9 ± 0.7 | 3.5 ± 0.2 | 0.52 ± 0.06 | 8 ± 2 | 71 |
| O2 | 2.8 ± 0.2 | 4.5 ± 0.7 | 3.1 ± 0.2 | 0.9 ± 0.1 | 5 ± 1 | 107 |
| CH4 | 4.6 ± 0.3 | 7 ± 1 | 2.2 ± 0.2 | 2.1 ± 0.3 | 3 ± 1 | 149 |
| CO2 | 17 ± 1 | 17 ± 2 | 2.4 ± 0.2 | 7.3 ± 0.9 | 2.3 ± 0.6 | 195 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firpo, G.; Angeli, E.; Guida, P.; Pezzuoli, D.; Repetto, D.; Repetto, L.; Valbusa, U. The Role of Surfaces in Gas Transport Through Polymer Membranes. Polymers 2019, 11, 910. https://doi.org/10.3390/polym11050910
Firpo G, Angeli E, Guida P, Pezzuoli D, Repetto D, Repetto L, Valbusa U. The Role of Surfaces in Gas Transport Through Polymer Membranes. Polymers. 2019; 11(5):910. https://doi.org/10.3390/polym11050910
Chicago/Turabian StyleFirpo, Giuseppe, Elena Angeli, Patrizia Guida, Denise Pezzuoli, Diego Repetto, Luca Repetto, and Ugo Valbusa. 2019. "The Role of Surfaces in Gas Transport Through Polymer Membranes" Polymers 11, no. 5: 910. https://doi.org/10.3390/polym11050910
APA StyleFirpo, G., Angeli, E., Guida, P., Pezzuoli, D., Repetto, D., Repetto, L., & Valbusa, U. (2019). The Role of Surfaces in Gas Transport Through Polymer Membranes. Polymers, 11(5), 910. https://doi.org/10.3390/polym11050910