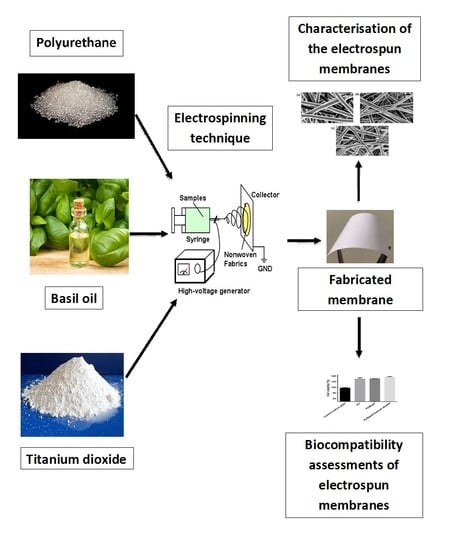
Engineered Electrospun Polyurethane Composite Patch Combined with Bi-functional Components Rendering High Strength for Cardiac Tissue Engineering
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PU and Composite Solutions
2.3. Electrospinning of PU and Composite Solutions
2.4. Physico-chemical Properties
2.4.1. Field Emission Scanning Electron Microscopy (FESEM)
2.4.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.4.3. Contact Angle Measurements
2.4.4. X-ray Diffraction Analysis (XRD) Analysis
2.4.5. Thermal Characterization
2.4.6. Surface Roughness Measurements
2.4.7. Mechanical Characterizations
2.5. Coagulation Assays
2.5.1. Activated Partial Thromboplastin Time (APTT) and Prothrombin Time (PT) Assay
2.5.2. Hemolysis Assay
2.6. Characterization of in Vitro Biocompatibility
2.7. Statistical Analysis
3. Result and Discussion
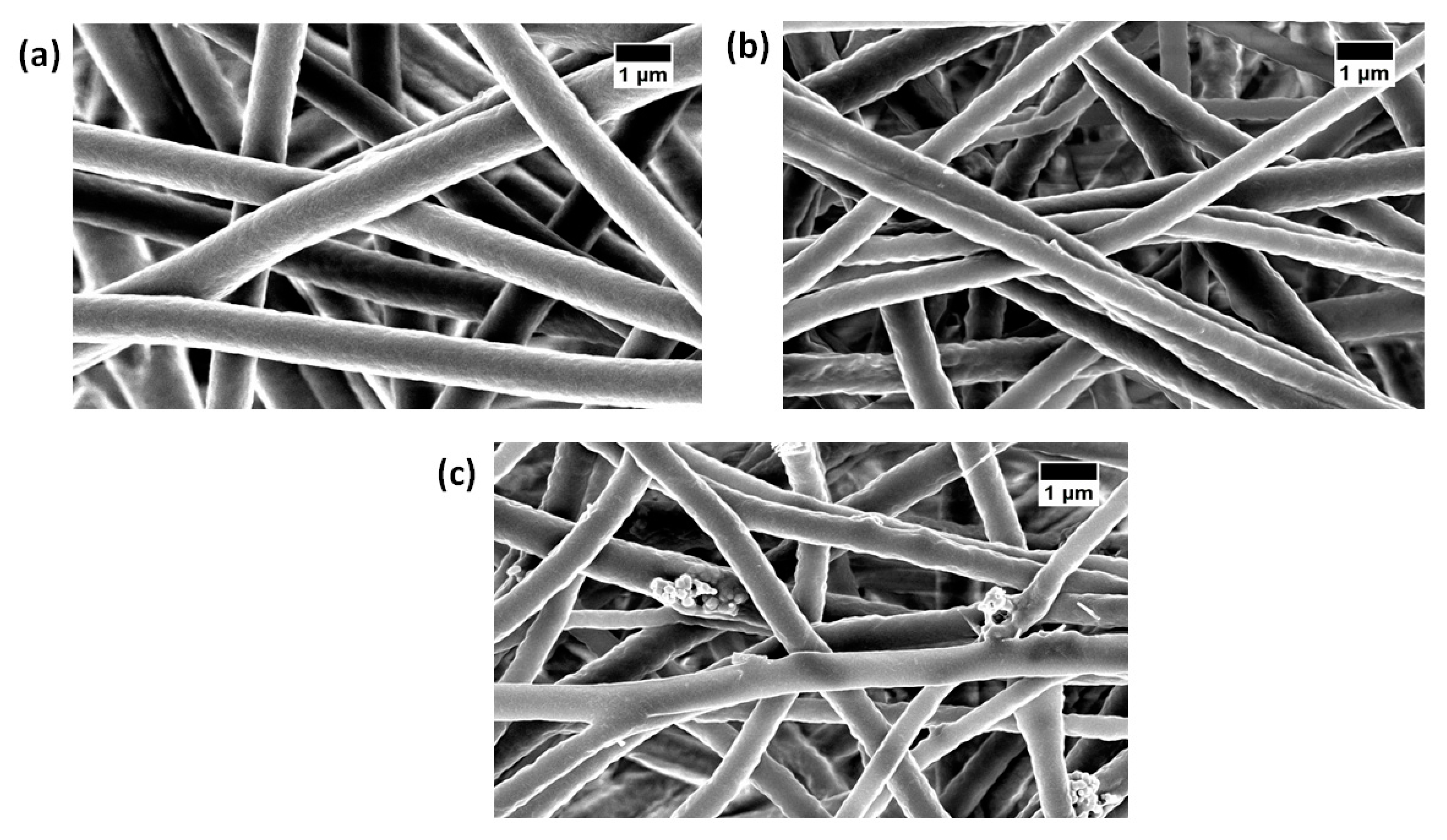
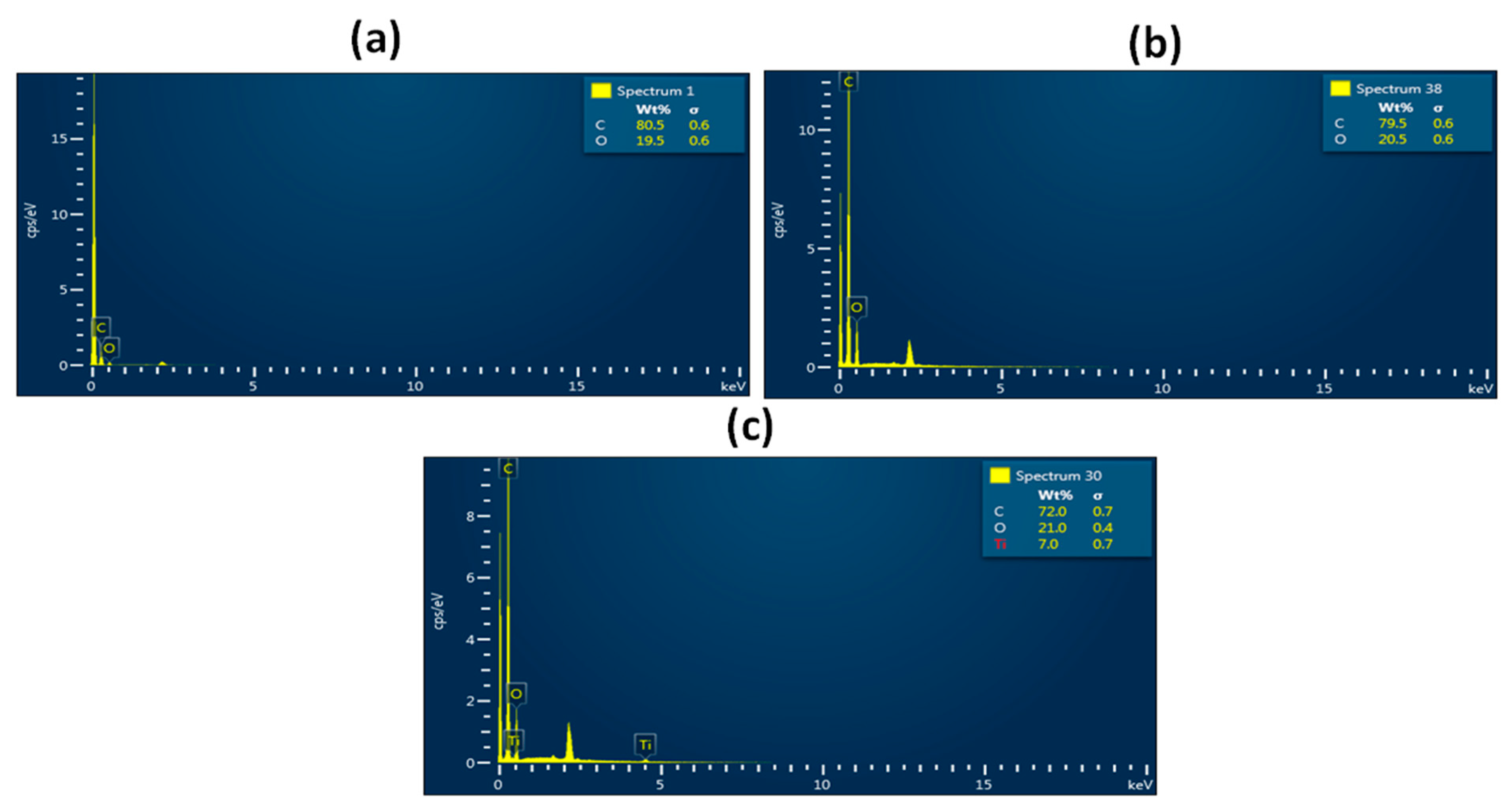
3.1. SEM Investigation
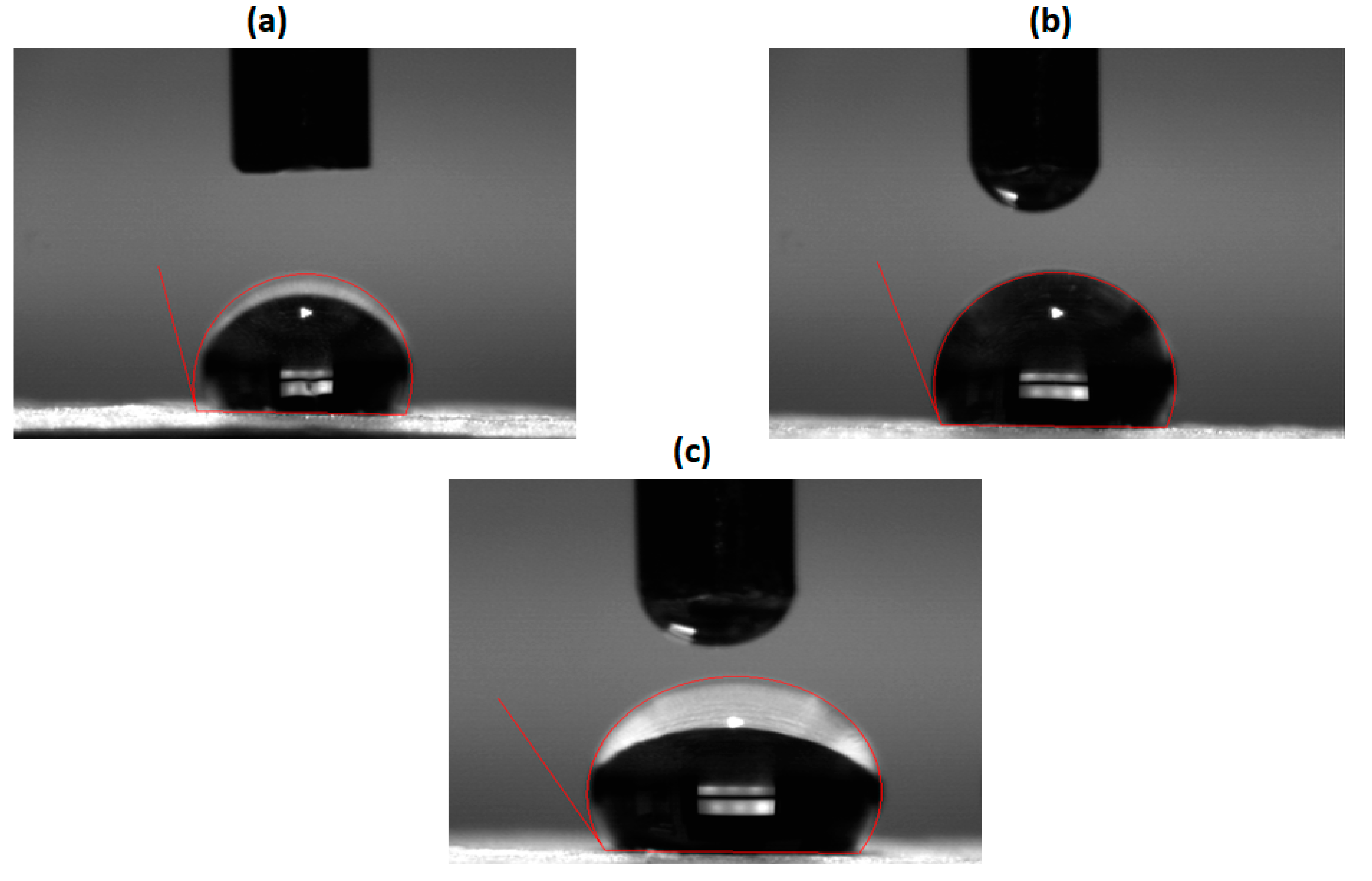
3.2. Contact Angle Measurements
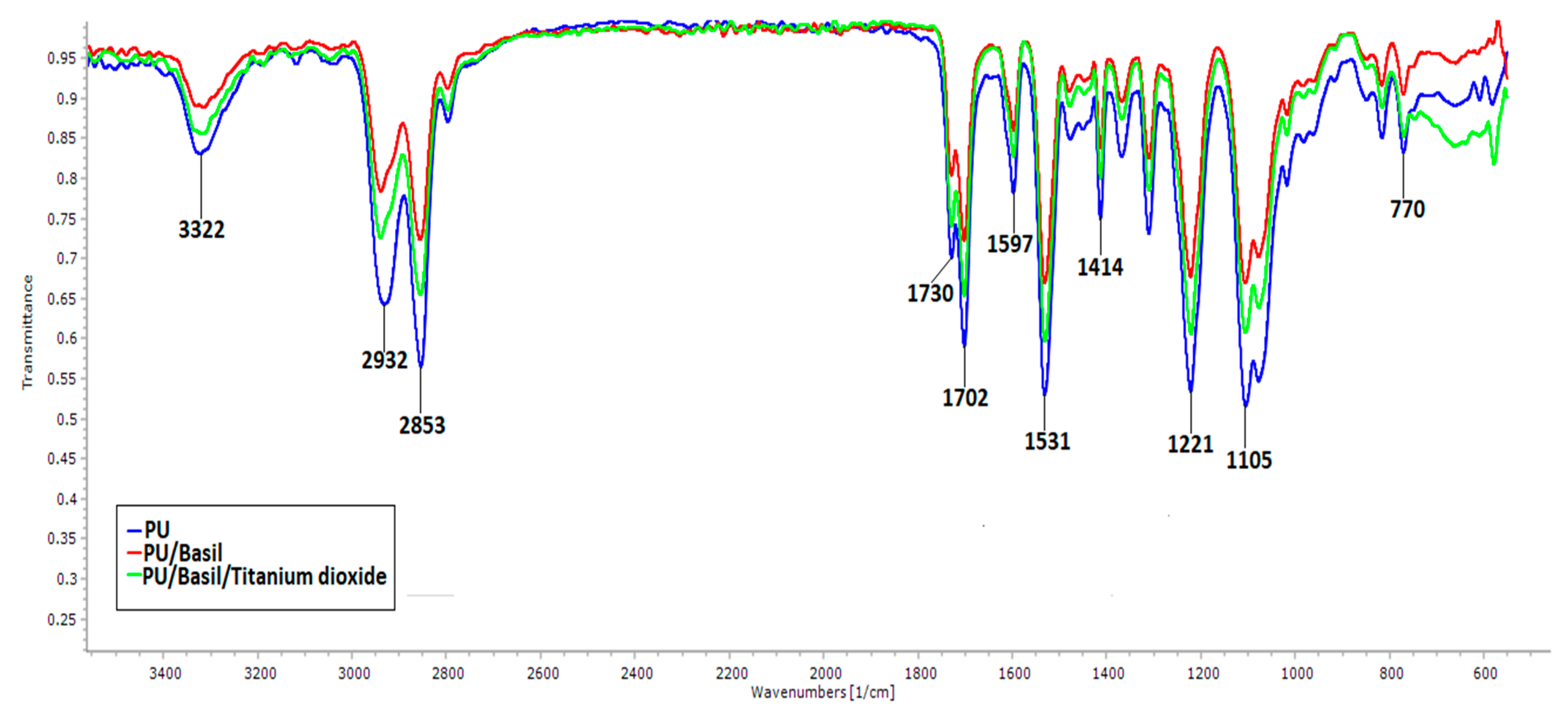
3.3. IR Analysis
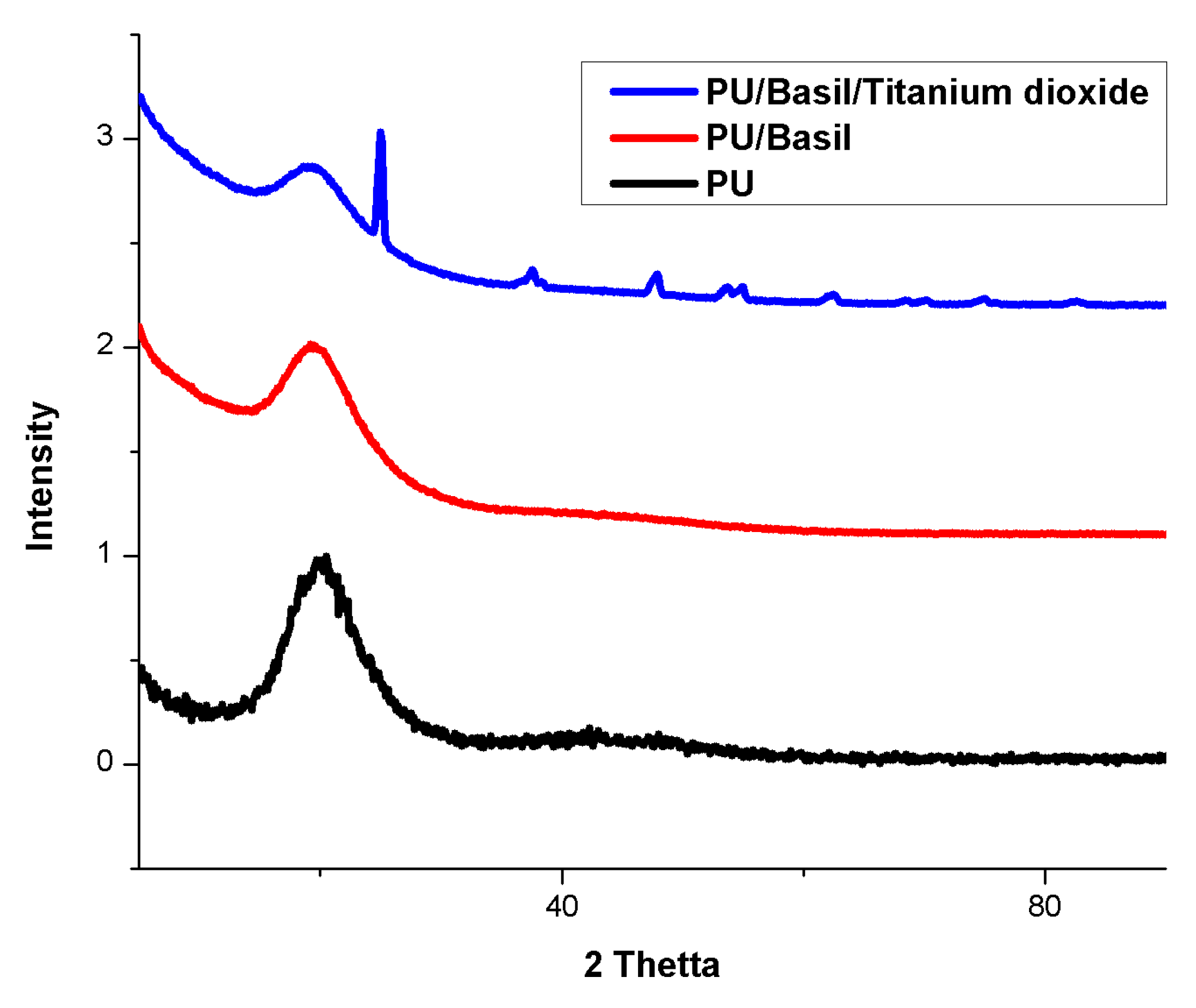
3.4. XRD Analysis
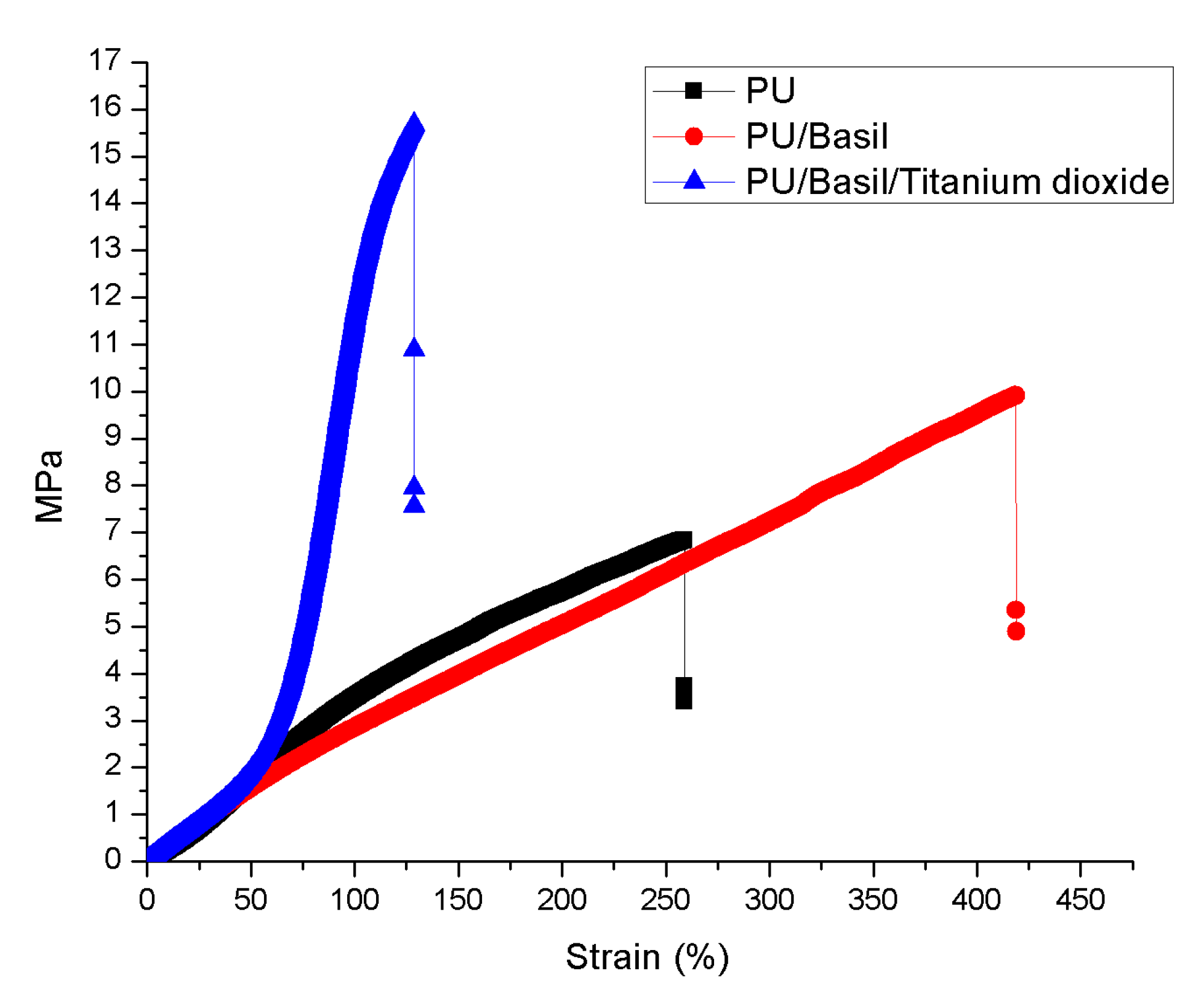
3.5. Tensile Properties
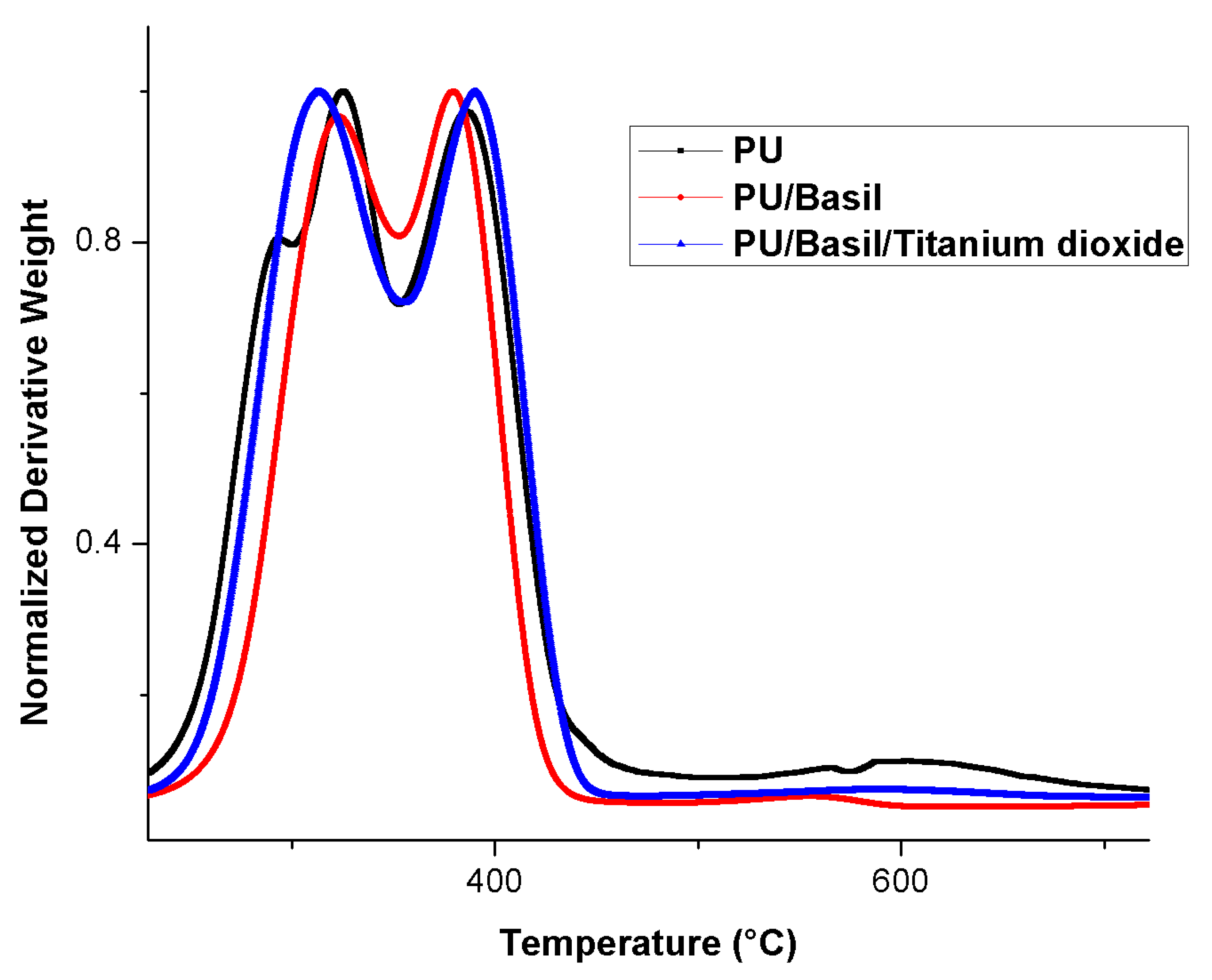
3.6. TGA Analysis
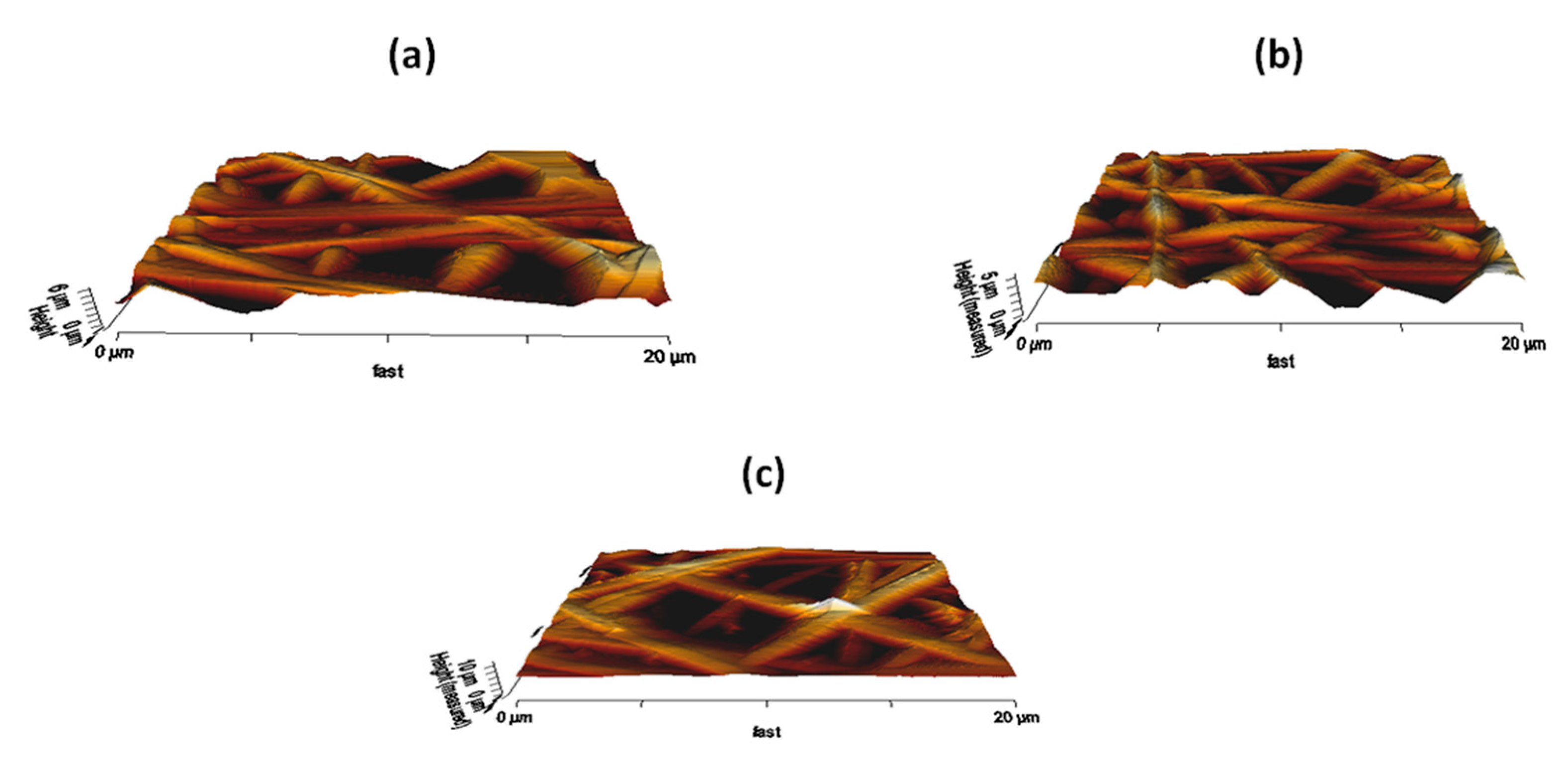
3.7. AFM Analysis
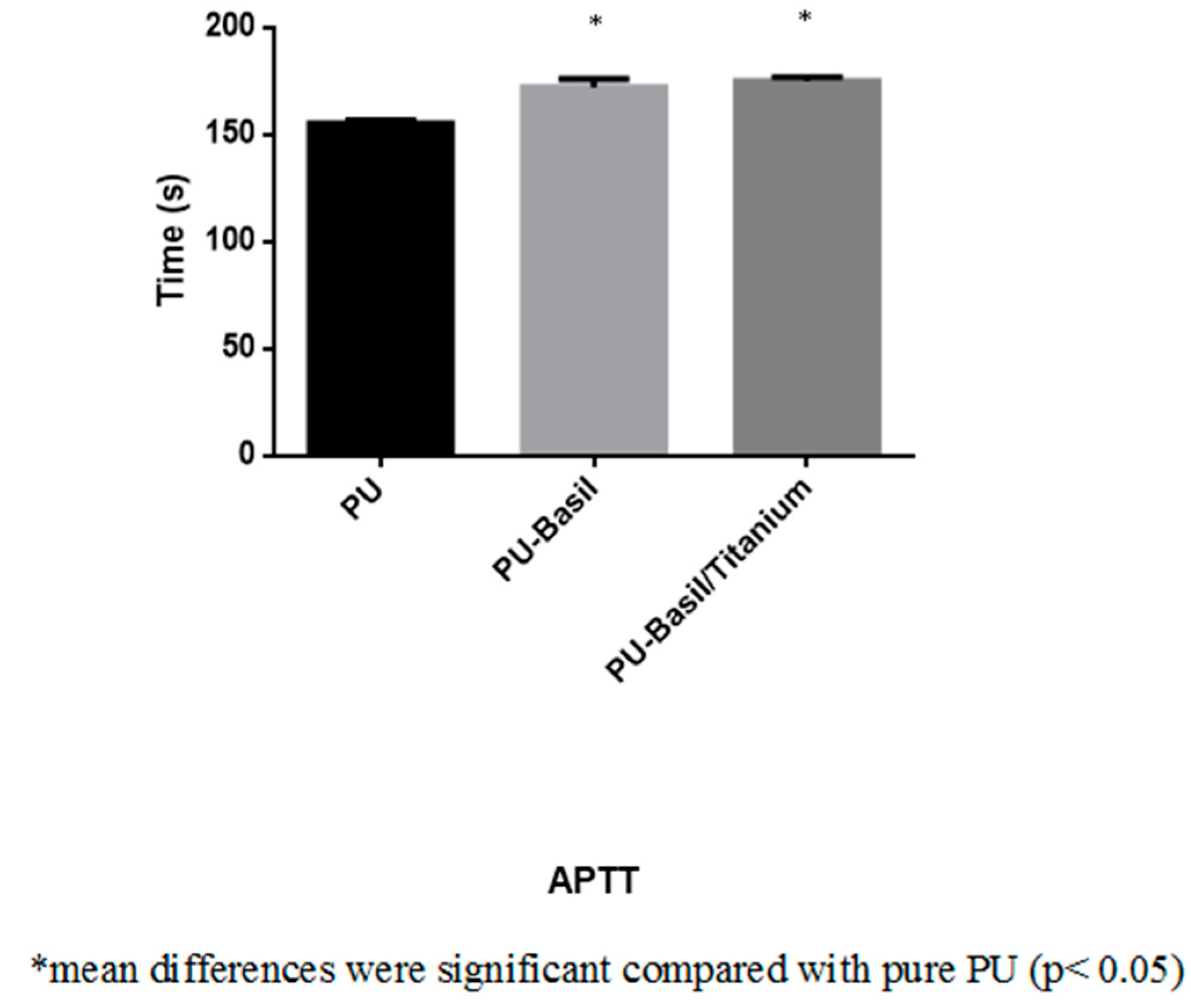
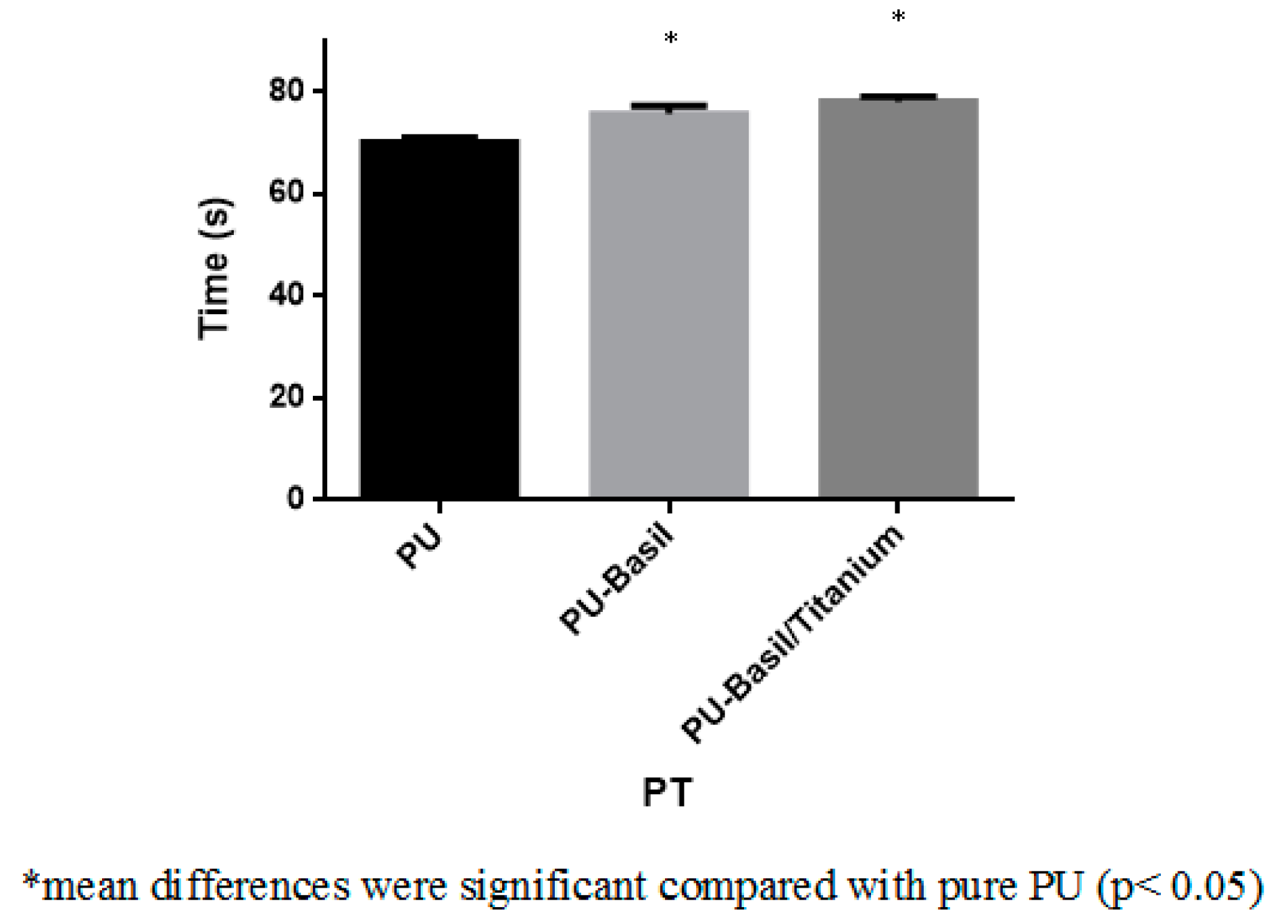
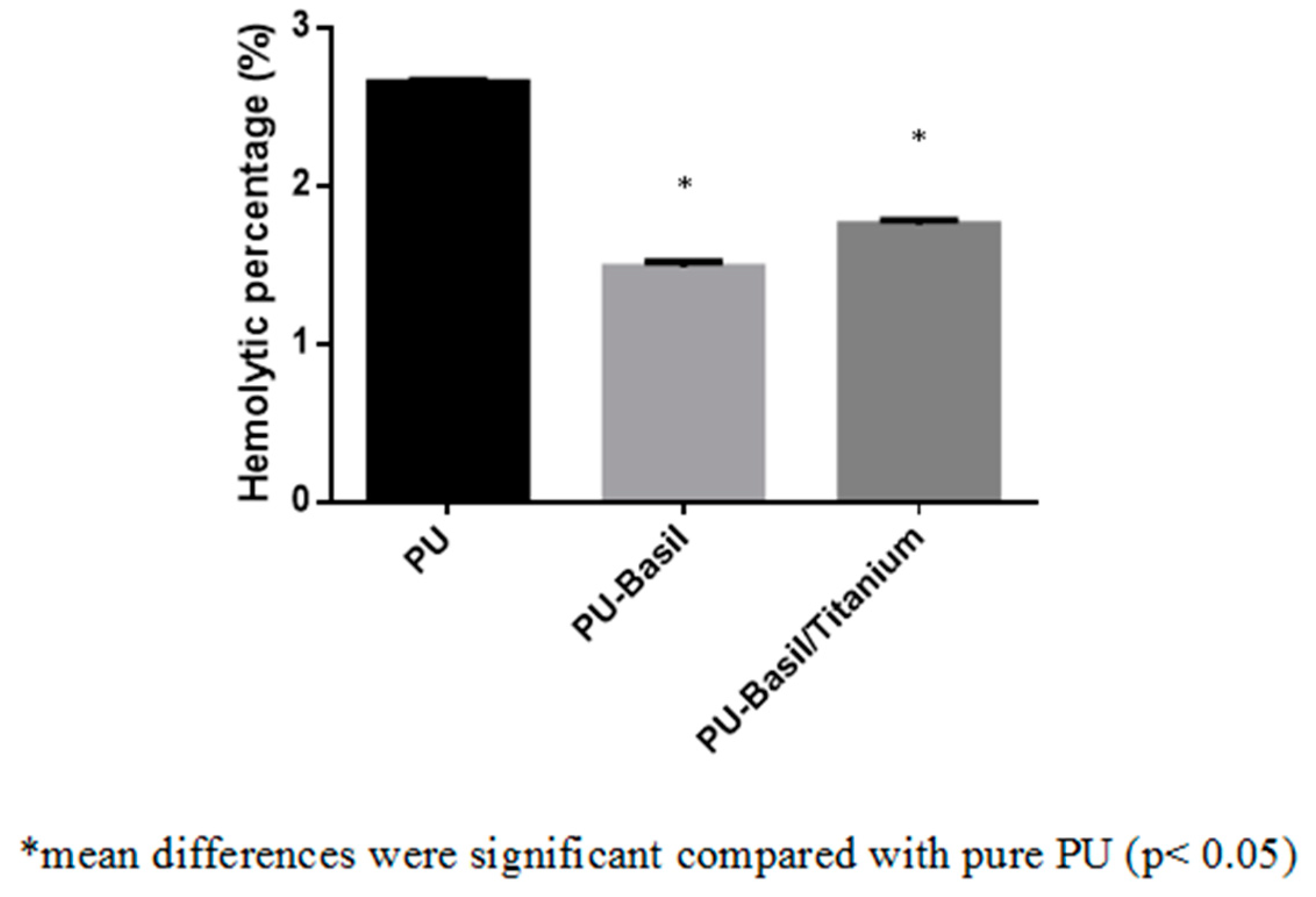
3.8. Blood Compatibility Assessments
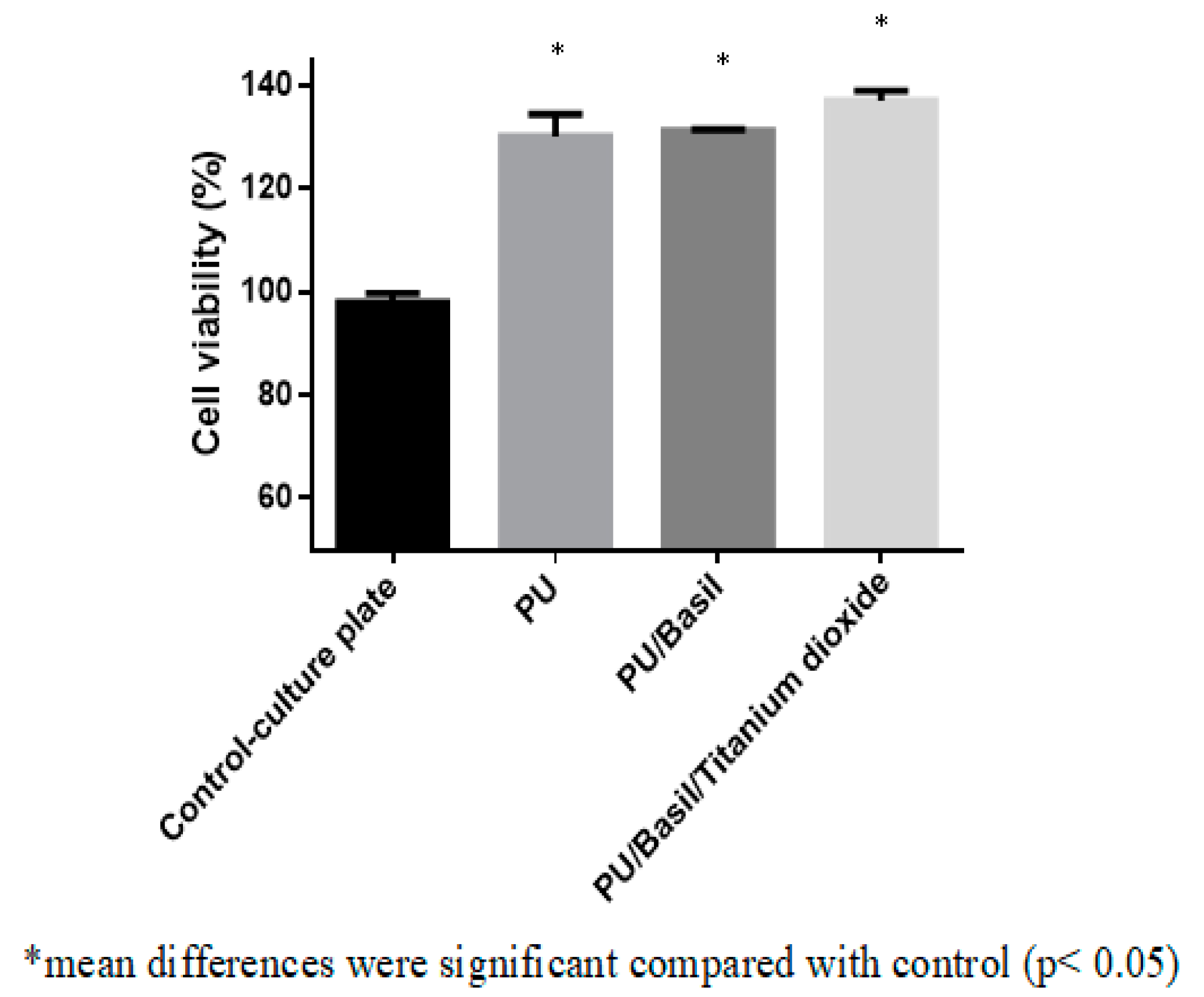
3.9. Biocompatibility Assessments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardiovascular Disease. Available online: https://www.who.int/cardiovascular_diseases/en/ (accessed on 5 April 2019).
- Cardiovascular Repair And Reconstruction Devices Market Worth $4,481.5 Million By 2025. Available online: https://www.grandviewresearch.com/Press-release/global-cardiovascular-repair-reconstruction-devices-market (accessed on 5 April 2019).
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [PubMed]
- Mendis, S. Global Status Report on Noncommunicable Diseases 2014; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J., III; Gras, D. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [PubMed]
- Jaganathan, S.K.; Mani, M.P.; Ayyar, M.; Supriyanto, E. Engineered electrospun polyurethane and castor oil nanocomposite scaffolds for cardiovascular applications. J. Mater. Sci. 2017, 52, 10673–10685. [Google Scholar]
- Dacron Vascular Prosthesis. Available online: https://www.sciencedirect.com/topics/nursing-and-health-professions/dacron-vascular-prosthesis (accessed on 5 April 2019).
- Fleischer, S.; Feiner, R.; Shapira, A.; Ji, J.; Sui, X.; Daniel Wagner, H.; Dvir, T. Springlike fibers for cardiac tissue engineering. Biomaterials 2013, 34, 8599–8606. [Google Scholar]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; ARBoccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar]
- Chien, K.R.; Domian, I.J.; Parker, K.K. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science 2008, 322, 1494–1497. [Google Scholar] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar]
- Bhattarai, R.; Bachu, R.; Boddu, S.; Bhaduri, S. Biomedical applications of electrospun nanofibers: Drug and nanoparticle delivery. Pharmaceutics 2019, 11, 5. [Google Scholar]
- Cui, W.; Li, X.; Zhou, S.; Weng, J. Investigation on process parameters of electrospinning system through orthogonal experimental design. J. Appl. Polym. Sci. 2007, 103, 3105–3112. [Google Scholar]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar]
- Kharaziha, M.; Nikkhah, M.; Shin, S.R.; Annabi, N.; Masoumi, N.; Gaharwar, A.K.; Camci-Unal, G.; Khademhosseini, A. PGS: Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials 2013, 34, 6355–6366. [Google Scholar] [PubMed]
- Orlova, Y.; Magome, N.; Liu, L.; Chen, Y.; Agladze, K. Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials 2011, 32, 5615–5624. [Google Scholar] [PubMed]
- Zong, X.; Bien, H.; Chung, C.Y.; Yin, L.; Fang, D.; Hsiao, B.S.; Chu, B.; Entcheva, E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 2005, 26, 5330–5338. [Google Scholar]
- Vats, A.; Tolley, N.S.; Polak, J.M.; Gough, J.E. Scaffolds and biomaterials for tissue engineering: A review of clinical applications. Clin. Otolaryngol. Allied Sci. 2003, 28, 165–172. [Google Scholar]
- Shen, Z.; Lu, D.; Li, Q.; Zhang, Z.; Zhu, Y. Synthesis and characterization of biodegradable polyurethane for hypopharyngeal tissue engineering. BioMed Res. Int. 2015, 2015, 871202. [Google Scholar] [PubMed]
- Polymer Properties Database. Available online: https://polymerdatabase.com/polymer%20classes/Polyurethane%20type.html (accessed on 18 March 2019).
- Domenech, M.; Polo-Corrales, L.; Ramirez-Vick, J.E.; Freytes, D.O. Tissue engineering strategies for myocardial regeneration: Acellular versus cellular scaffolds? Tissue. Eng. Part B Rev. 2016, 22, 438–458. [Google Scholar]
- Jaganathan, S.K.; Mani, M.P.; Ayyar, M.; Krishnasamy, N.P.; Nageswaran, G. Blood compatibility and physicochemical assessment of novel nanocomposite comprising polyurethane and dietary carotino oil for cardiac tissue engineering applications. J. Appl. Polym. Sci. 2018, 135, 45691. [Google Scholar]
- Jaganathan, S.K.; Mani, M.P. Electrospun polyurethane nanofibrous composite impregnated with metallic copper for wound-healing application. 3 Biotech 2018, 8, 327. [Google Scholar]
- Jaganathan, S.K.; Mani, M.P. Single-stage synthesis of electrospun polyurethane scaffold impregnated with zinc nitrate nanofibers for wound healing applications. J. Appl. Polym. Sci. 2019, 136, 46942. [Google Scholar]
- Basil. Available online: https://en.wikipedia.org/wiki/Basil (accessed on 18 March 2019).
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [PubMed]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335. [Google Scholar]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [PubMed]
- Chen, S.; Hao, Y.; Cui, W.; Chang, J.; Zhou, Y. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis. J. Mater. Sci. 2013, 48, 6567–6577. [Google Scholar]
- Jaganathan, S.K.; Mani, M.P. Enriched mechanical, thermal, and blood compatibility of single stage electrospun polyurethane nickel oxide nanocomposite for cardiac tissue engineering. Polym. Compos. 2018. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Pichiah, P.T.; Gnanasekaran, G.; Seenivasan, K.; Barakat, N.A.; Cha, Y.S.; Jung, C.H.; Shanmugam, A.; Kim, H.Y. Emu oil-based electrospun nanofibrous scaffolds for wound skin tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 454–460. [Google Scholar]
- Theivasanthi, T.; Alagar, M. Titanium dioxide (TiO2) Nanoparticles XRD Analyses: An Insight. arXiv, 2013; arXiv:1307.1091. [Google Scholar]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 1–27. [Google Scholar]
- Vorp, D.A.; Schiro, B.J.; Ehrlich, M.P.; Juvonen, T.S.; Ergin, M.A.; Griffith, B.P. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann. Thorac. Surg. 2003, 75, 1210–1214. [Google Scholar] [PubMed]
- Hasan, A.; Ragaert, K.; Swieszkowski, W.; Selimovic, S.; Paul, A.; Camci-Unal, G.; Mofrad, M.R.K.; Khademhosseini, A. Biomechanical properties of native and tissue engineered heart valve constructs. J. Biomech. 2014, 7, 1949–1963. [Google Scholar]
- Jeon, H.J.; Kim, J.S.; Kim, T.G.; Kim, J.H.; Yu, W.R.; Youk, J.H. Preparation of poly (ɛ-caprolactone)-based polyurethane nanofibers containing silver nanoparticles. Appl. Surf. Sci. 2008, 254, 5886–5890. [Google Scholar]
- Parizek, M.; Kasalkova, N.; Bacakova, L.; Slepicka, P.; Lisa, V.; Blazkova, M.; Svorcik, V. Improved adhesion, growth and maturation of vascular smooth muscle cells on polyethylene grafted with bioactive molecules and carbon particles. Int. J. Mol. Sci. 2009, 10, 4352–4374. [Google Scholar]
- Ayyar, M.; Mani, M.P.; Jaganathan, S.K.; Rathanasamy, R. Preparation, characterization and blood compatibility assessment of a novel electrospun nanocomposite comprising polyurethane and ayurvedic-indhulekha oil for tissue engineering applications. Biomed. Eng. Biomed. Tech. 2018, 63, 245–253. [Google Scholar]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 66–75. [Google Scholar]
- Sharifi, F.; Irani, S.; Zandi, M.; Soleimani, M.; Atyabi, S.M. Comparative of fibroblast and osteoblast cells adhesion on surface modified nanofibrous substrates based on polycaprolactone. Prog. Biomater. 2016, 5, 213–222. [Google Scholar]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/material interfaces: Influence of surface chemistry and surface topography on cell adhesion. J. Adh. Sci. Technol. 2010, 24, 831–852. [Google Scholar]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Moses, R.L.; Jacobs, J.J. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001, 7, 55–71. [Google Scholar]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2011, 63, 209–220. [Google Scholar] [PubMed]
- Dong, S.; Sun, J.; Li, Y.; Li, J.; Cui, W.; Li, B. Electrospun nanofibrous scaffolds of poly (L-lactic acid)-dicalcium silicate composite via ultrasonic-aging technique for bone regeneration. Mater. Sci. Eng. C 2014, 35, 426–433. [Google Scholar]
- Tian, F.; Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Yokoyama, Y.; Estrada, G.G.; Kobayashi, H. Quantitative analysis of cell adhesion on aligned micro-and nanofibers. J. Biomed. Mater. Res. Part A 2008, 84, 291–299. [Google Scholar]
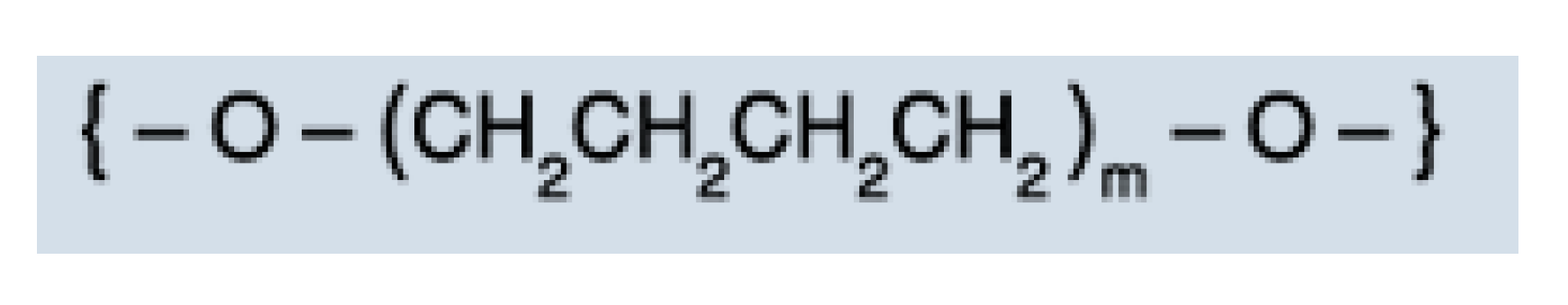

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, M.P.; Jaganathan, S.K.; Faudzi, A.A.M.; Sunar, M.S. Engineered Electrospun Polyurethane Composite Patch Combined with Bi-functional Components Rendering High Strength for Cardiac Tissue Engineering. Polymers 2019, 11, 705. https://doi.org/10.3390/polym11040705
Mani MP, Jaganathan SK, Faudzi AAM, Sunar MS. Engineered Electrospun Polyurethane Composite Patch Combined with Bi-functional Components Rendering High Strength for Cardiac Tissue Engineering. Polymers. 2019; 11(4):705. https://doi.org/10.3390/polym11040705
Chicago/Turabian StyleMani, Mohan Prasath, Saravana Kumar Jaganathan, Ahmad Athif Mohd Faudzi, and Mohd Shahrizal Sunar. 2019. "Engineered Electrospun Polyurethane Composite Patch Combined with Bi-functional Components Rendering High Strength for Cardiac Tissue Engineering" Polymers 11, no. 4: 705. https://doi.org/10.3390/polym11040705
APA StyleMani, M. P., Jaganathan, S. K., Faudzi, A. A. M., & Sunar, M. S. (2019). Engineered Electrospun Polyurethane Composite Patch Combined with Bi-functional Components Rendering High Strength for Cardiac Tissue Engineering. Polymers, 11(4), 705. https://doi.org/10.3390/polym11040705