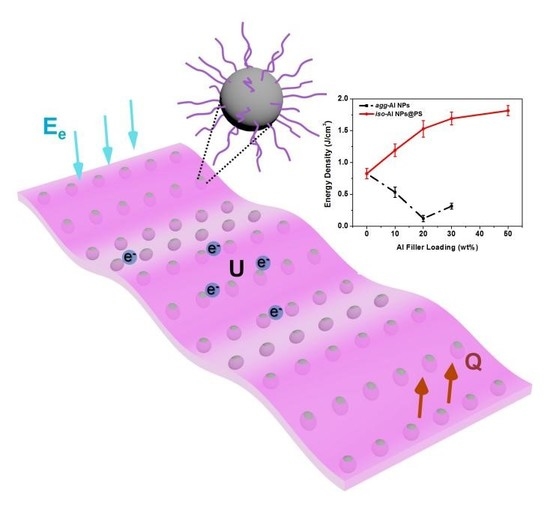
Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility
Abstract
Share and Cite
Yang, C.; Marian, C.; Liu, J.; Di, Q.; Xu, M.; Zhang, Y.; Han, W.; Liu, K. Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility. Polymers 2019, 11, 638. https://doi.org/10.3390/polym11040638
Yang C, Marian C, Liu J, Di Q, Xu M, Zhang Y, Han W, Liu K. Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility. Polymers. 2019; 11(4):638. https://doi.org/10.3390/polym11040638
Chicago/Turabian StyleYang, Chenggong, Chufarov Marian, Jie Liu, Qi Di, Mingze Xu, Yunhe Zhang, Wei Han, and Kun Liu. 2019. "Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility" Polymers 11, no. 4: 638. https://doi.org/10.3390/polym11040638
APA StyleYang, C., Marian, C., Liu, J., Di, Q., Xu, M., Zhang, Y., Han, W., & Liu, K. (2019). Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility. Polymers, 11(4), 638. https://doi.org/10.3390/polym11040638