Ball Milling to Produce Composites Based of Natural Clinoptilolite as a Carrier of Salicylate in Bio-Based PA11
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zeolite-Salicylate Hybrid Filler
2.3. Incorporation of Zeolite-Salicylate into the PA11 Matrix
2.4. Characterization
2.5. Gas Transport Modeling
3. Results
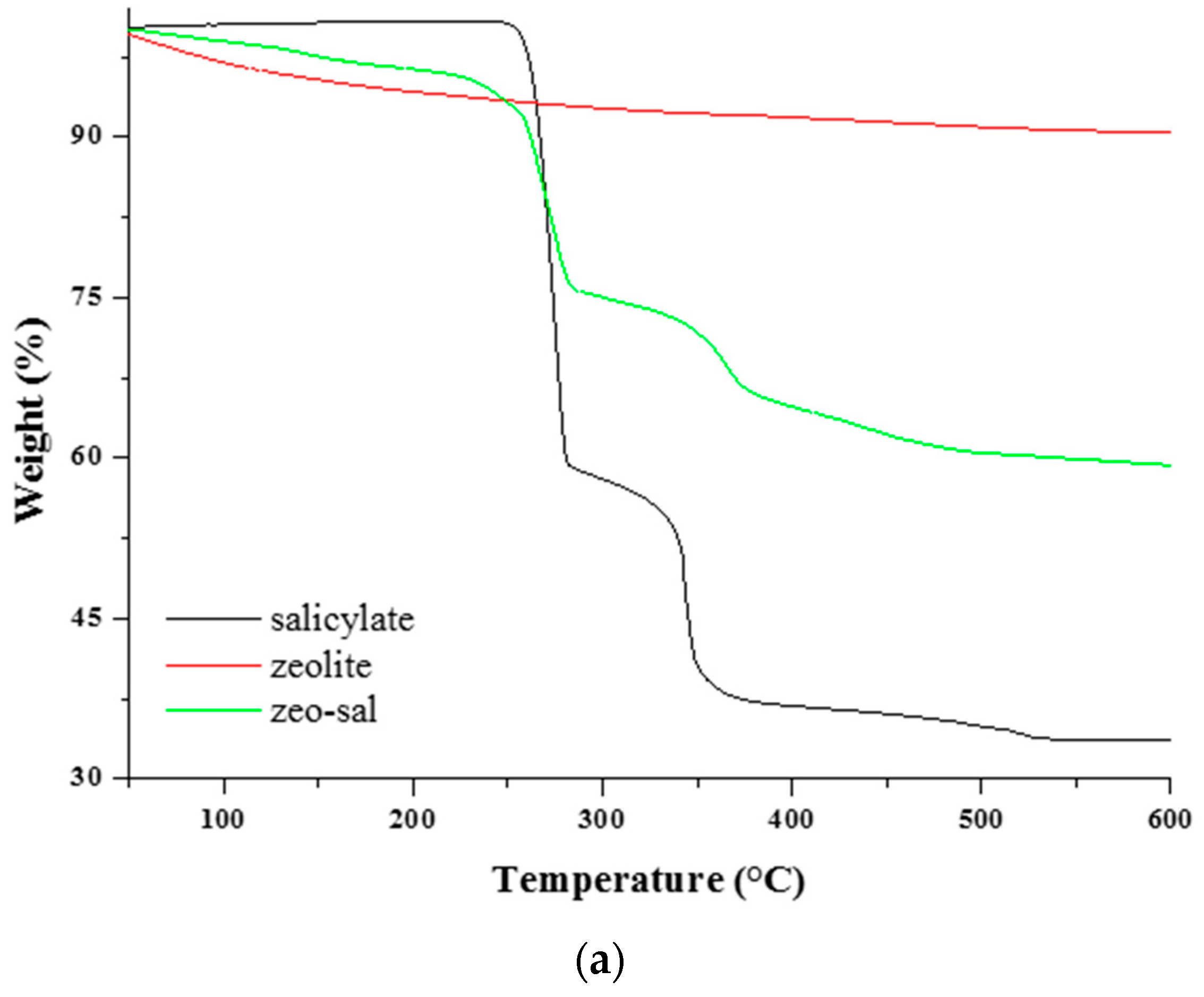
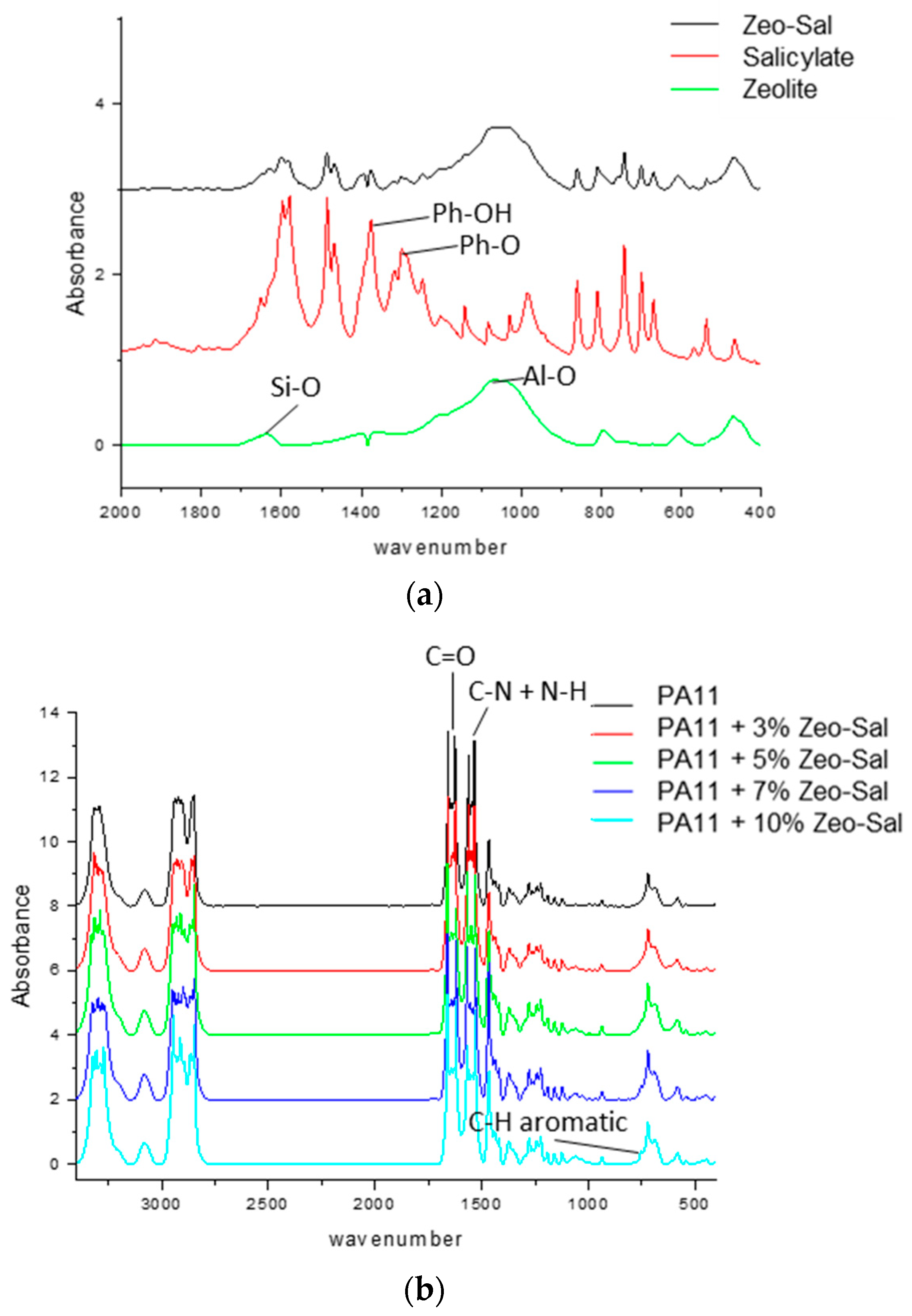
3.1. Characterization of Zeolite and Zeolite-Salicylate Hybrids
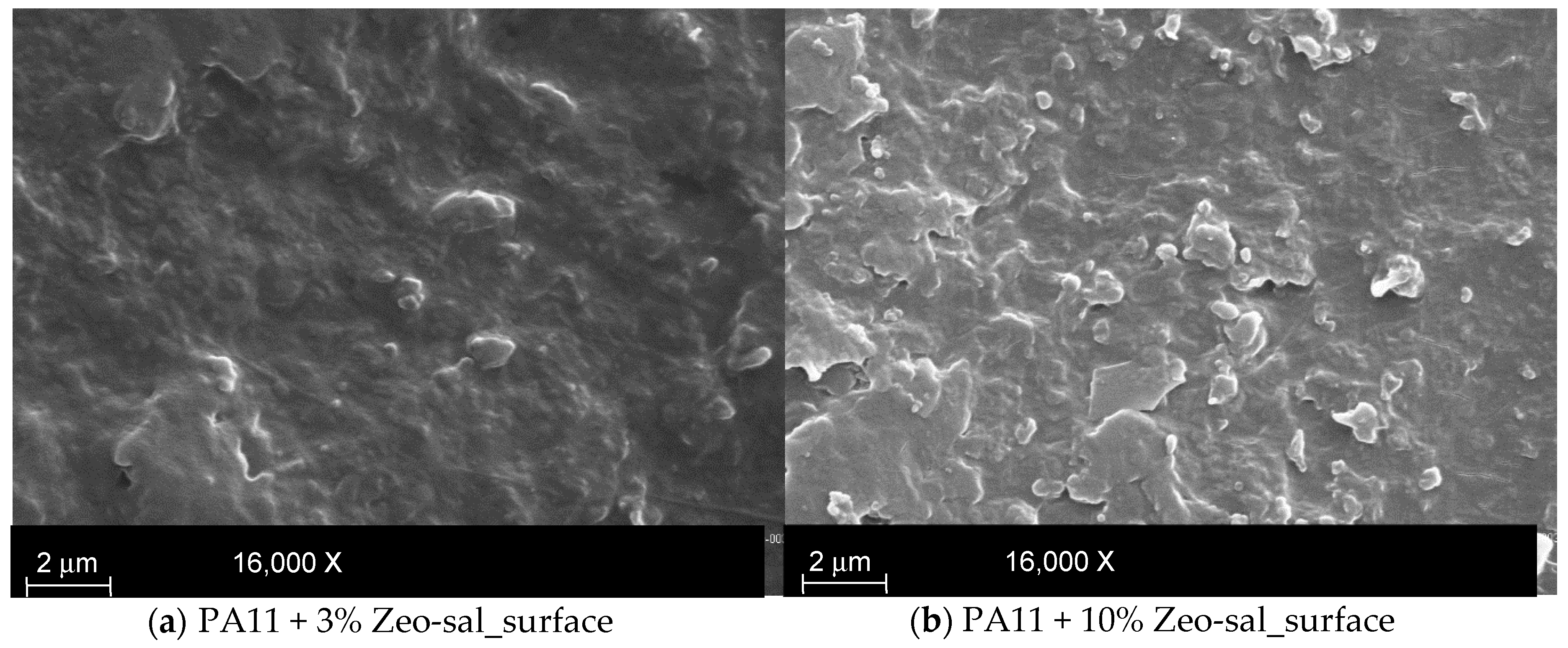
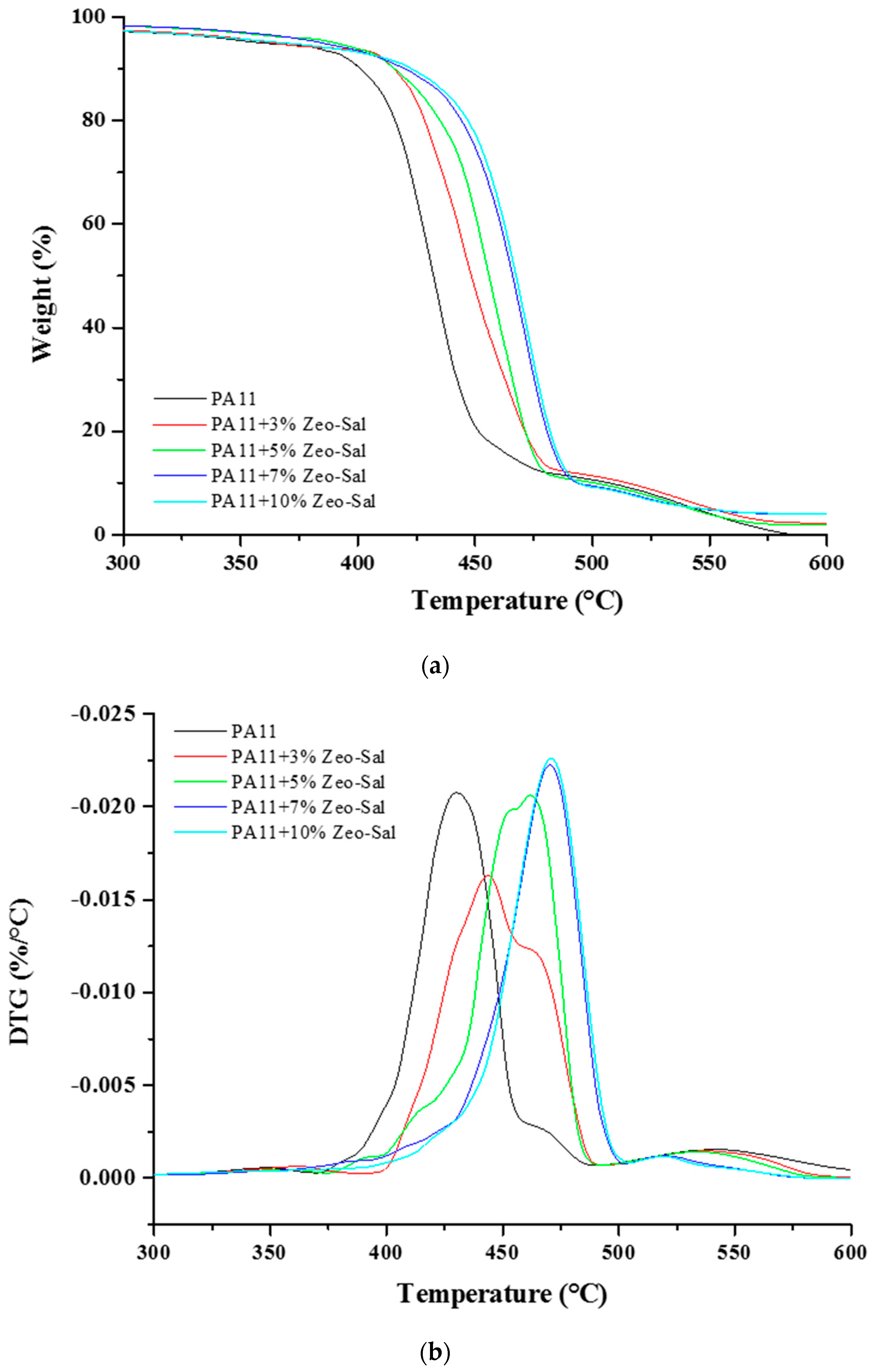
3.2. Characterization of PA11 and PA11 Composites
3.3. Gas Permeation on Films Based on PA11 and PA11 Composites
3.4. Release Kinetics of Salicylate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.; Rahman, W.; Tan, A.C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Antimicrobial poly (lactic acid)/cellulose bionanocomposite for food packaging application: A review. Food Packag. Shelf Life 2018, 17, 150–161. [Google Scholar] [CrossRef]
- Ribeiro-Santosa, R.; Andrade, M.; Ramos de Melo, N.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef] [PubMed]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Nanostructures: Current uses and future applications in food science. J. Food Drug Anal. 2017, 25, 245–253. [Google Scholar] [CrossRef]
- Han, J.H. Antimicrobial food packaging. Food Technol. 2000, 54, 56–65. [Google Scholar]
- Khaneghah, A.M.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- LaCoste, A.; Schaich, K.M.; Zumbrunnen, D.; Yam, K.L. Advancing controlled release packaging through smart blending. Packag. Technol. Sci. 2005, 18, 77–87. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Advances in controlled release devices for food packaging applications. Trends Food Sci. Technol. 2010, 21, 591–598. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Corrigan, O.I. Mechanistic aspects of the release of levamisole hydrochloride from biodegradable polymers. J. Control. Release 2000, 69, 261–272. [Google Scholar] [CrossRef]
- Dunne, M.M.; Ramtoola, Z.; Corrigan, O.I. Fluphenazine release from biodegradable microparticles: Characterization and modelling of release. J. Microencapsul. 2009, 26, 403–410. [Google Scholar] [CrossRef]
- Corrigan, O.I.; Li, X. Quantifying drug release from PLGA nanoparticulates. Eur. J. Pharm. Sci. 2009, 37, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ness, J.E.; Xie, W.; Zhang, X.; Liu, F.; Cai, J.; Minshull, J.; Gross, R.A. Biosynthesis of monomers for plastics from renewable oils. J. Am. Chem. Soc. 2010, 132, 15451–15455. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Bugatti, V.; Ussia, M.; Mendichi, R.; Zampino, D.; Puglisi, C.; Carroccio, S.C. Halloysite nanotubes and thymol as photo protectors of biobased polyamide 11. Polym. Degrad. Stab. 2018, 152, 43–51. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A. Mechanical milling as a technology to produce structural and functional bio-nanocomposites. Green Chem. 2015, 17, 2610–2625. [Google Scholar] [CrossRef]
- Delogu, F.; Gorrasi, G.; Sorrentino, A. Fabrication of polymer nanocomposites via ball milling: Present status and future perspectives. Prog. Mater. Sci. 2017, 86, 75–126. [Google Scholar] [CrossRef]
- Clarizia, G.; Bernardo, P.; Gorrasi, G.; Zampino, D.; Carroccio, S.C. Influence of the preparation method and photo-oxidation treatment on the thermal and gas transport properties of dense films based on a poly (ether-block-amide) copolymer. Materials 2018, 11, 1326. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Cambridge University Press: Cambridge, UK, 2010; Volume 1. [Google Scholar]
- Bouma, R.H.B.; Checchetti, A.; Chidichimo, G.; Drioli, E. Permeation through a heterogeneous membrane: The effect of the dispersed phase. J. Membr. Sci. 1997, 128, 141–149. [Google Scholar] [CrossRef]
- Spielberg, E.T.; Campbell, P.S.; Szeto, K.C.; Mallick, B.; Schaumann, J.; Mudring, A.-V. Sodium salicylate: An in-depth thermal and photophysical study. Chem. Eur. J. 2018, 24, 15638–15648. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, Z.; Zhang, H.; Liu, S.; Cheng, S.Z.D. Crystal transitions of nylon 11 under drawing and annealing. Polymer 2001, 42, 5543–5547. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. (Eds.) Collection of Simulated XRD POWDER Patterns for Zeolites; Elsevier: Amsterdam, The Netherlands, 2001; pp. 184–187. [Google Scholar]
- Herrera, M.; Matuschek, G.; Kettrup, A. Main products and kinetics of the thermal degradation of polyamides. Chemosphere 2001, 42, 601–607. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D.; Lewin, M. Thermal decomposition of aliphatic nylons. Polym. Int. 1999, 48, 532–557. [Google Scholar] [CrossRef]
- Jin, X.; Sun, J.; Shiqing Zhang, J.; Gu, X.; Bourbigot, S.; Li, H.; Tang, W.; Zhang, S. Preparation of a novel intumescent flame retardant based on supramolecular interactions and its application in polyamide 11. ACS Appl. Mater. Interfaces 2017, 9, 24964–24975. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ortega, H.; Méndez, J.A.; Mutjé, P.; Tarrés, Q.; Espinach, F.X.; Ardanuy, M. Evaluation of thermal and thermomechanical behaviour of bio-based polyamide 11 based composites reinforced with lignocellulosic fibres. Polymers 2017, 9, 522. [Google Scholar] [CrossRef]
- Bugatti, V.; Vertuccio, L.; Viscusi, G.; Gorrasi, G. Antimicrobial membranes of bio-based PA 11 and HNTs filled with lysozyme obtained by an electrospinning process. Nanomaterials 2018, 8, 139. [Google Scholar] [CrossRef]
- Philip, D.; John, A.; Panicker, C.Y.; Varghese, H.T. FT-Raman, FT-IR and surface enhanced raman scattering spectra of sodium salicylate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 1561–1566. [Google Scholar] [CrossRef]
- Pucciariello, R.; Villani, V.; Belviso, S.; Gorrasi, G.; Tortora, M.; Vittoria, V. Phase Behavior of Modified Montmorillonite-Poly(ε-caprolactone) Nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2004, 43, 1321–1332. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nada, A.; Kamal, D.E. Density functional theory and FTIR spectroscopic study of carboxyl group. Indian J. Pure Appl. Phys. 2005, 43, 911–917. [Google Scholar]
- Paul, D.R.; Kemp, D.R. The diffusion time lag in polymer membranes containing adsorptive fillers. J. Polym. Sci. Polym. Symp. 1973, 41, 79–93. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57–74. [Google Scholar] [CrossRef]
- Milallos, R.G.; Alexander, K.; Riga, A. Investigation of the interaction between acidic, basic, neutral, and zwitterionic drugs with poly-l-lactic acid by thermal and analytical methods. J. Anal. Calorim. 2008, 93, 289–294. [Google Scholar] [CrossRef]
- He, J.; Zhong, C.; Mi, J. Modeling of drug release from bioerodible polymer matrices. J. Drug Deliv. 2005, 12, 251–259. [Google Scholar] [CrossRef]




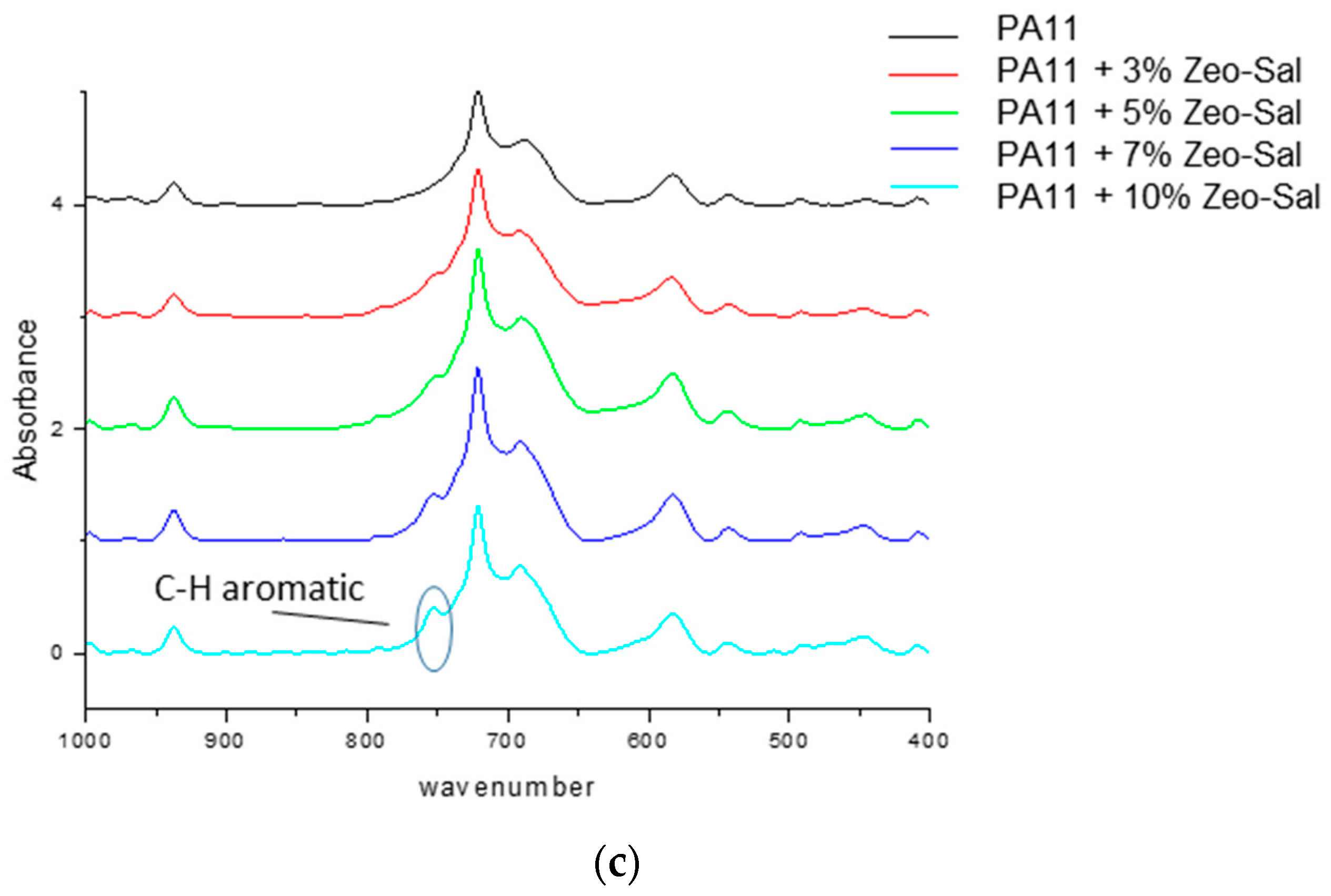
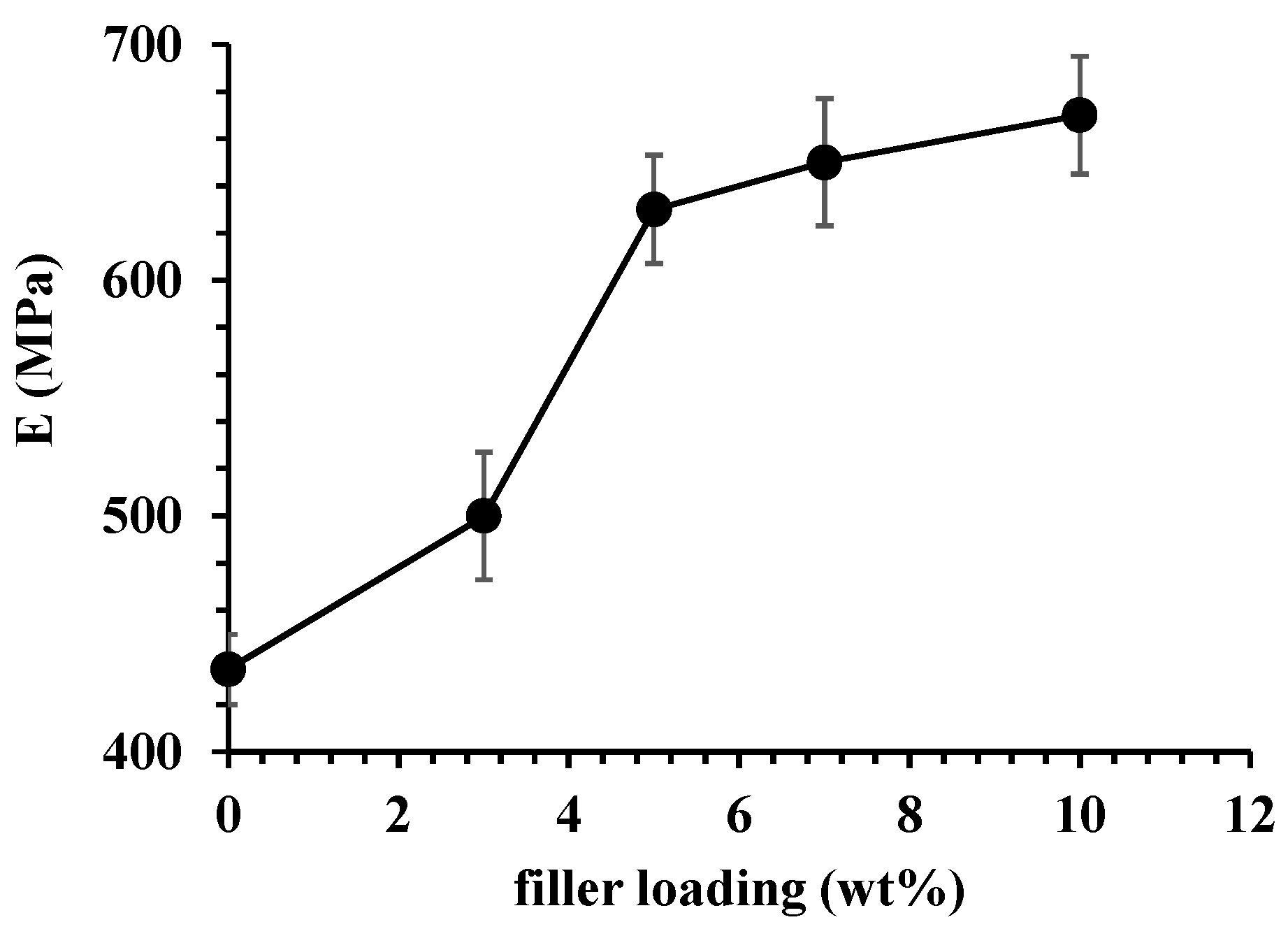
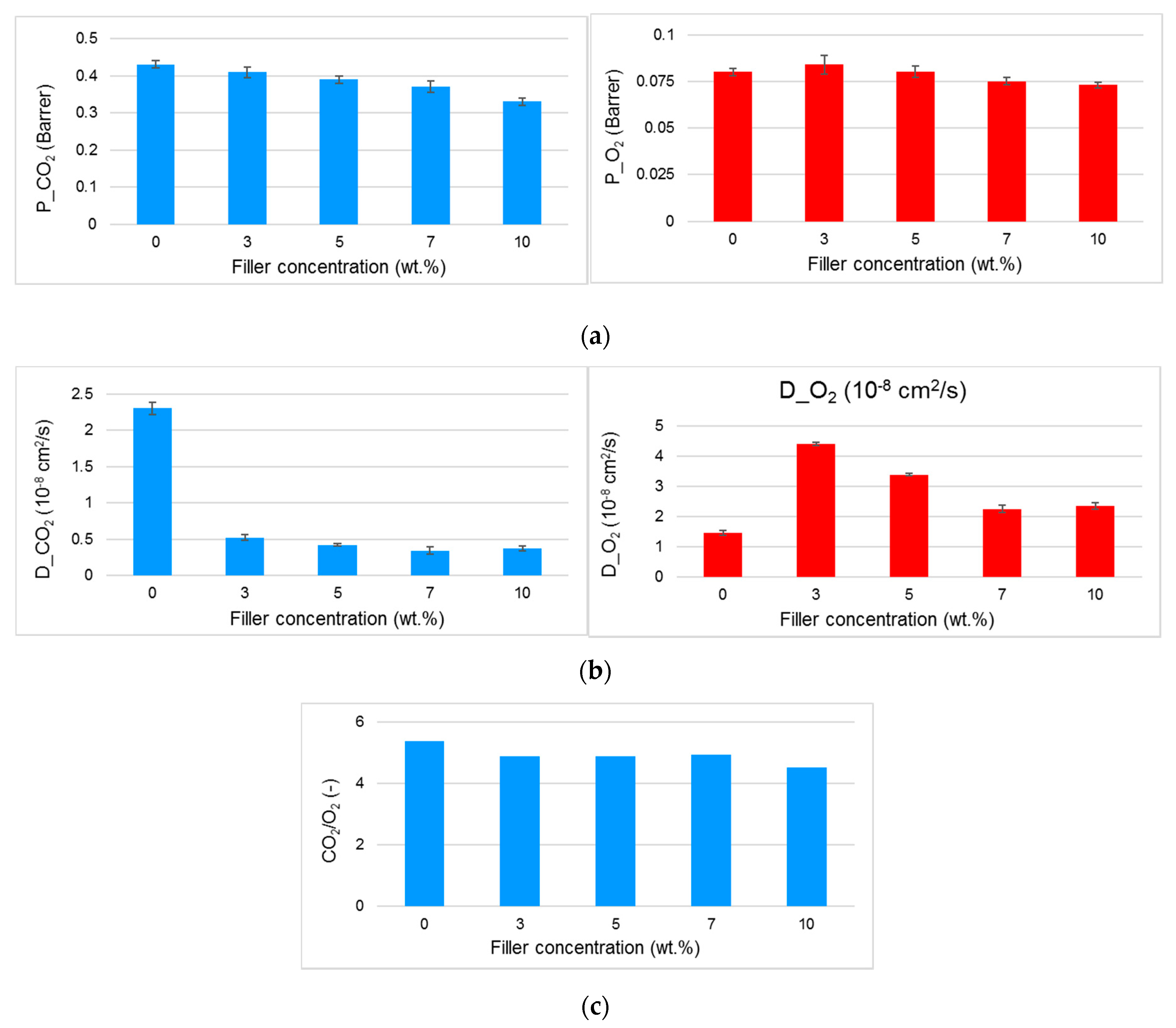
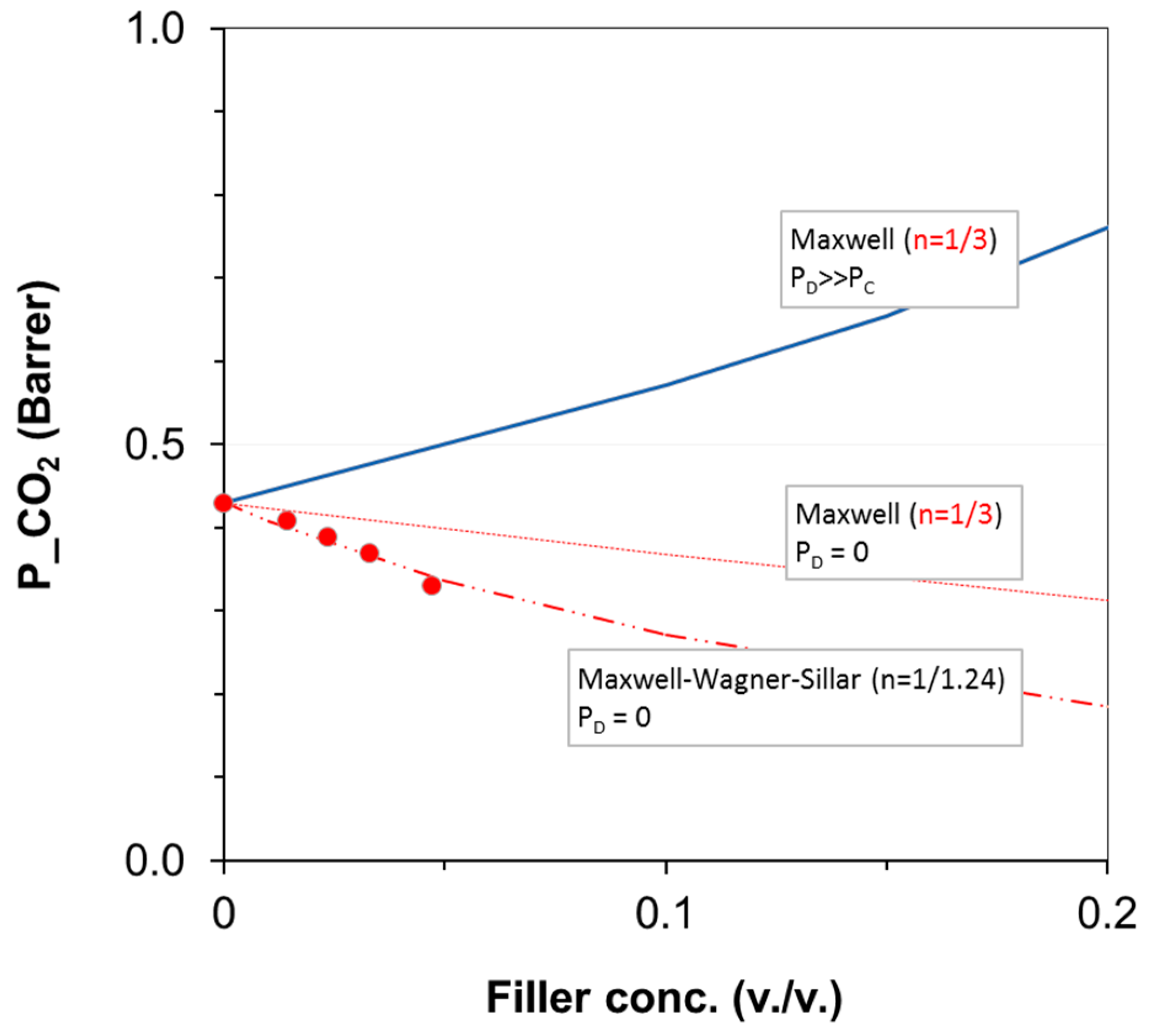

| Filler loading (wt %) | fb (%) | t2max (h) | k1 (h−1) | k2 (h−1) |
|---|---|---|---|---|
| 3.0 | 65 | 47 | 3.03E-01 | 3.89E-02 |
| 5.0 | 43 | 43 | 3.09E-01 | 4.23E-02 |
| 7.0 | 38 | 42 | 3.43E-01 | 4.09E-02 |
| 10.0 | 44 | 107 | 4.02E-01 | 4.65E-02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugatti, V.; Bernardo, P.; Clarizia, G.; Viscusi, G.; Vertuccio, L.; Gorrasi, G. Ball Milling to Produce Composites Based of Natural Clinoptilolite as a Carrier of Salicylate in Bio-Based PA11. Polymers 2019, 11, 634. https://doi.org/10.3390/polym11040634
Bugatti V, Bernardo P, Clarizia G, Viscusi G, Vertuccio L, Gorrasi G. Ball Milling to Produce Composites Based of Natural Clinoptilolite as a Carrier of Salicylate in Bio-Based PA11. Polymers. 2019; 11(4):634. https://doi.org/10.3390/polym11040634
Chicago/Turabian StyleBugatti, Valeria, Paola Bernardo, Gabriele Clarizia, Gianluca Viscusi, Luigi Vertuccio, and Giuliana Gorrasi. 2019. "Ball Milling to Produce Composites Based of Natural Clinoptilolite as a Carrier of Salicylate in Bio-Based PA11" Polymers 11, no. 4: 634. https://doi.org/10.3390/polym11040634
APA StyleBugatti, V., Bernardo, P., Clarizia, G., Viscusi, G., Vertuccio, L., & Gorrasi, G. (2019). Ball Milling to Produce Composites Based of Natural Clinoptilolite as a Carrier of Salicylate in Bio-Based PA11. Polymers, 11(4), 634. https://doi.org/10.3390/polym11040634









