3.1. Graft and Crosslink Structure
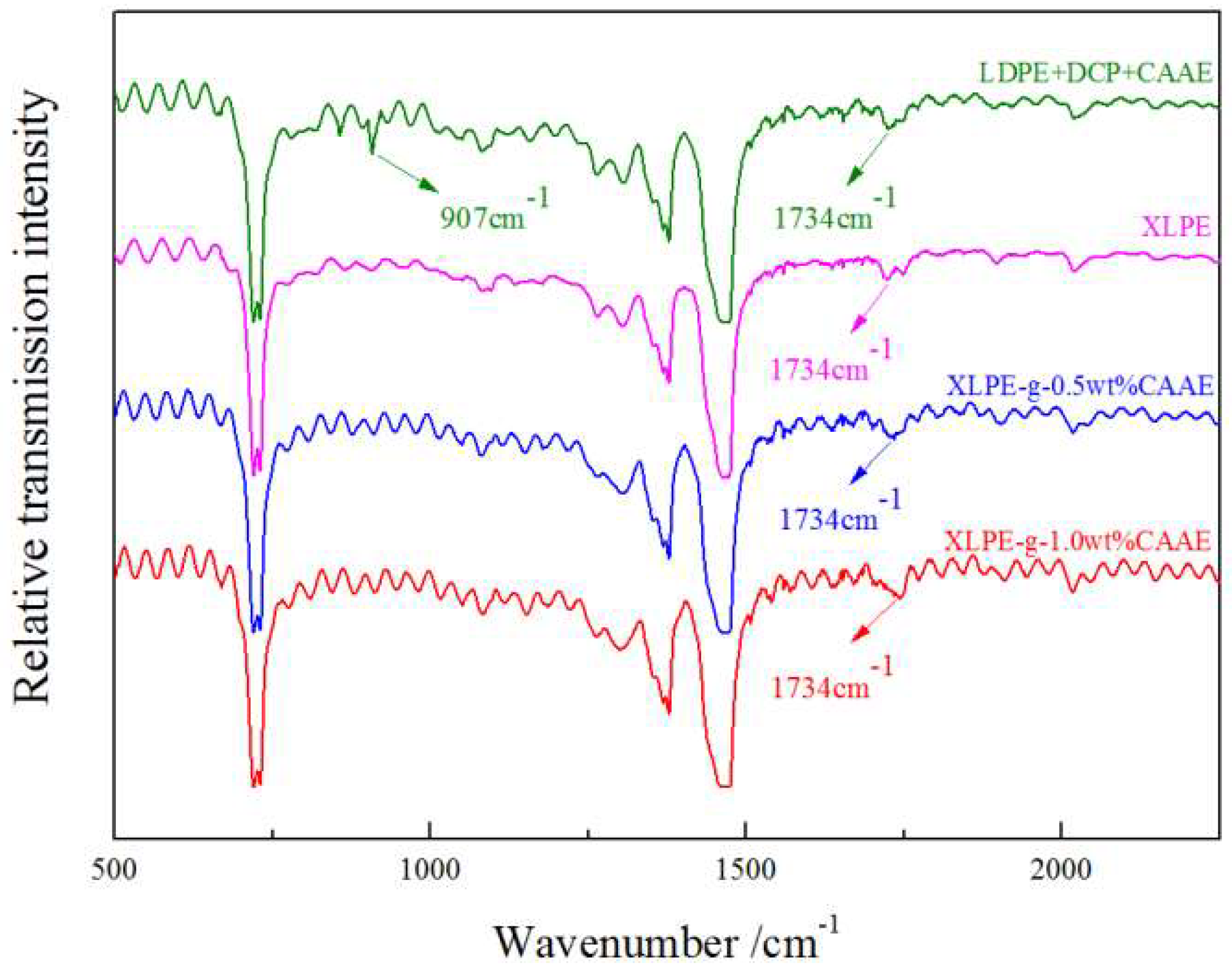
The molecular presence on chemically grafted XLPE can be verified with esters C=O vibration from IR spectroscopy, especially for grafted CAAE with characteristic stretching vibration peaks at 1734 cm
−1, as shown in
Figure 1. The newly appearing C=O peak intensity of XLPE-
g-CAAE increases with the CAAE grafting concentration, to the highest value for 1.0 wt% XLPE-
g-CAAE. Moreover, the characteristic C=C infrared absorption peak at 907 cm
−1 of CAAE monomer arises in the mixtures of LDPE, DCP, and CAAE without crosslinking and grafting reactions. However, the peak does not arise in XLPE-
g-CAAE, demonstrating that CAAE has been grafted on the molecular chain of XLPE. The FTIR results indicate explicitly that the CAAE has been substantially grafted to XLPE for each grafting concentration.
The elongation values indicate inversely the crosslinking probability of XLPE atomic chains, representing the crosslinking reduction resulting from grafting CAAE, as listed in
Table 2 presenting thermal elongations of XLPE and XLPE-
g-CAAE. As graft active centers, the DCP are consumed by grafting CAAE but the crosslinking reactions between the macromolecular chains are not inhibited in the crosslinking process. Grafting CAAE has not decreased the thermal mechanical property and the prepared XLPE-
g-CAAE are qualified to heat resistance in electrical insulation.
3.2. Space Charge Characteristics
The space charge distributions of XLPE and XLPE-
g-CAAE at ambient temperature are measured with PEA method [
13], as exhibited in
Figure 2 presenting space charge distribution after polarization under applied DC electric field of 40 kV/mm for 130 min (left) and then with electrode short connection (right). It is noted from right panel of
Figure 2a that homocharges have been evidently accumulated near both cathode and anode in pure XLPE, with the space charge injection depth increasing with polarization time. In comparison as explicitly shown in left panels of
Figure 2b,c for XLPE-
g-CAAE, the 1.0 wt% CAAE-grafting significantly reduces space charge accumulation so that only small quantity of heterocharge accumulate at cathode with remarkably decreased homocharges accumulation at anode, while the heterocharge accumulation at cathode for 0.5 wt% CAAE-grafting is evidently aggravated so as to exacerbate internal space charge accumulation. Especially for the XLPE-
g-1.0wt%CAAE, the minimum total quantity of space charges which are mainly distributed near electrodes, has been obtained.
The space charge distributions with electrode short connection as shown in right panels of
Figure 2 illustrate that heterocharge injection has been considerably inhibited in XLPE-
g-CAAE when the CAAE-grafting concentration increases to 1.0 wt%, representing as the accumulated space charge quantity is observably abated to lower than 0.46 C/m
3 near anode. It is noted for pure XLPE that an appreciable amount of homocharges appear near both cathode and anode with peak values of 2.59 C/m
3 and 3.08 C/m
3 respectively, which decrease to 1.31 C/m
3 and 1.83 C/m
3 after 7800 s. In comparison, XLPE-
g-0.5wt% CAAE sample shows higher density of heterocharges and homocharges at cathode and anode respectively with peak values of 3.00 C/m
3 and 3.22 C/m
3, which decrease in higher speed to 2.34 C/m
3 and 2.07 c/m
3 respectively after 7800 s. The space charge accumulation inside XLPE-
g-0.5wt%CAAE is not suppressed due to that the density of grafted CAAE is not sufficient to introduce compactly distributed deep traps which can form an effective shielding layer to impede charge injection near electrode. Even, the deep traps presented by grafted-CAAE inside material can capture the injected charge to increase space charge accumulation of internal region. Therefore, the low grafting specimen of 0.5 wt% XLPE-
g-CAAE however represent deteriorated space charge distribution. Nevertheless, for XLPE-
g-1.0wt%CAAE sample, only a small amount of heterocharge and homocharge are found near cathode and anode with peak values of 1.26 C/m
3 and 0.46 C/m
3 respectively, almost retaining unchanged heterocharge until 7800 s. The space charge measurement results demonstrate that significant space charge suppression can be achieved in XLPE-
g-CAAE with substantial grafting concentration and suggest a reasonable insulation application for high-voltage DC cable.
3.3. Electric Charge Trapping
Electric charge carrier trapping mechanism of polymer materials has essential correlation to the space charge accumulation and charge transport [
14]. Therefore, trap energy levels need to be evaluated so as to understand the space charge suppression mechanism in XLPE-
g-CAAE, which can be precisely calculated by modified TSDC theory from the TSDC measurement [
15,
16]. The TSDC testing temperature range of 0–100 °C is adopted pertaining to thermal excitation of trapped charges for both PE intrinsic traps and grafting-introduced deep traps, in which the practical ambient temperatures of HVDC cable in operation are also included. The TSDC spectra and derived trap level distributions of XLPE and XLPE-
g-CAAE are presented in
Figure 3, indicating grafting-introduced deeper traps of 1.01–1.03 eV in XLPE-
g-CAAE which are nicely confirmed in accordance with our theoretical results from first-principles calculations as illustrated by density of state (DOS) in
Figure 4a. N. Quirke et al. reported theoretically by molecular simulation that the chemical carbonyl functions will introduce deep trap levels of 0.90eV [
10], while Y. Zhou reported the higher results of 1.0eV in experiment which are well consistent with our experimental results [
11]. The energy depth and density of trap levels in XLPE-
g-CAAE are listed in
Table 3. The 1.0 wt% CAAE grafted XLPE represents the maximum intrinsic trap density and the deeper introduced trap level. The deepest trap level peaks of XLPE, 0.5 wt% and 1.0 wt% XLPE-
g-CAAE are 0.90, 1.01, and 1.03 eV with trap level densities of 7.75 × 10
20, 1.01 × 10
20 and 1.99 × 10
20 (eV
−1·m
−3) respectively, indicating that significantly numerous deeper traps have been introduced by grafting CAAE with the trapping depth slightly rising with increasing concentration of grafted radical.
The considerably reduced space charge accumulation in XLPE-g-CAAE can be attributed to the uniformly distributed deep traps introduced by grafting CAAE. The deep traps with evidently uniform density in XLPE-g-CAAE will effectively capture and fix the injected heterocharges and forms homogeneous electrostatic charge shielding layer under applied DC electric field. Consequently, the electrostatic shielding area near the electrodes will remarkably inhibit further charge injection, where the trapped heterocharges represent as blocking effect to charge injection and impede charge carrier transport. Therefore, the space charge accumulation in XLPE-g-CAAE is significantly suppressed due to the deep traps introduced by grafted polar groups of CAAE. Furthermore, grafting introduced traps 0.11eV deeper than intrinsic traps can retain the highly-efficient suppression mechanism of space accumulation at the temperature approaching to 85 °C which is significantly higher than the 55 °C for pure XLPE without grafting. These results suggest that XLPE-g-CAAE can persist in enhanced insulation performance at the experimental temperature high to 85 °C.
TSDC test detect the current produced from thermally excited charges which have been trapped by bound states of electron or holes, in which the peak value at a temperature pertains to the whole quantity of charges trapped throughout the material for a certain trapping depth. The higher density space charges deriving from charge capture by grafting-introduced deep traps only accumulate in a very thin layer region near to electrode. Accordingly, the a new peak in XLPE-g-CAAE TSDC spectra arises at a higher temperature signifying larger tapping depth with the peak intensity increasing as grafting density increases, while the lower temperature peak corresponding intrinsic traps from PE defects is reduced, which confirms the space charge suppression by chemically grafting CAAE.
As shown in
Figure 4a, our first-principles DOS results predict that the CAAE-grafting can introduce one shallow hole trap state (occupied bound state with intrinsic energy level close to VB band-edge) and two electron trap states (unoccupied bound states with intrinsic energy levels near to CB band-edge in band-gap) in the PE-
g-CAAE system. The energy levels of these bound states in band-gap described as trap level depths are listed in far right column of
Table 3, in which 1.0 eV electron trap level is well comparable with our experimental TSDC results. In order to investigate which polar bonding atoms in the grafted CAAE account for the produced deep trap states, the first-principles calculations on allyl acetate-grafted-PE (PE-
g-AA) with absent chlorine as deleted from PE-
g-CAAE are also performed for electronic structure in ground state, as the DOS results shown in
Figure 4b. It is indicated from comparison between
Figure 4a,b that the second electron trap state completely vanishes for PE-
g-AA without polar-bonding chlorine atom, while the hole trap and first electron trap states retain unchanged in both energy level and density, demonstrating the deeper 1.8 eV trap presentation by chlorine of grafted CAAE which needs experimental confirmation with more higher temperature TSDC. Further, it can be verified from the projected density of states (PDOS) on the specific atoms of polar-bonding carbonyl (C=O) that the shallow hole trap sate and the first electron trap state origin primarily from oxygen and carbon in polar C=O segment, respectively. The molecular modeling of PE-
g-CAAE after being relaxed by geometry optimization is present in
Figure 4d.
3.4. Electric Conductivity and Breakdown Strength
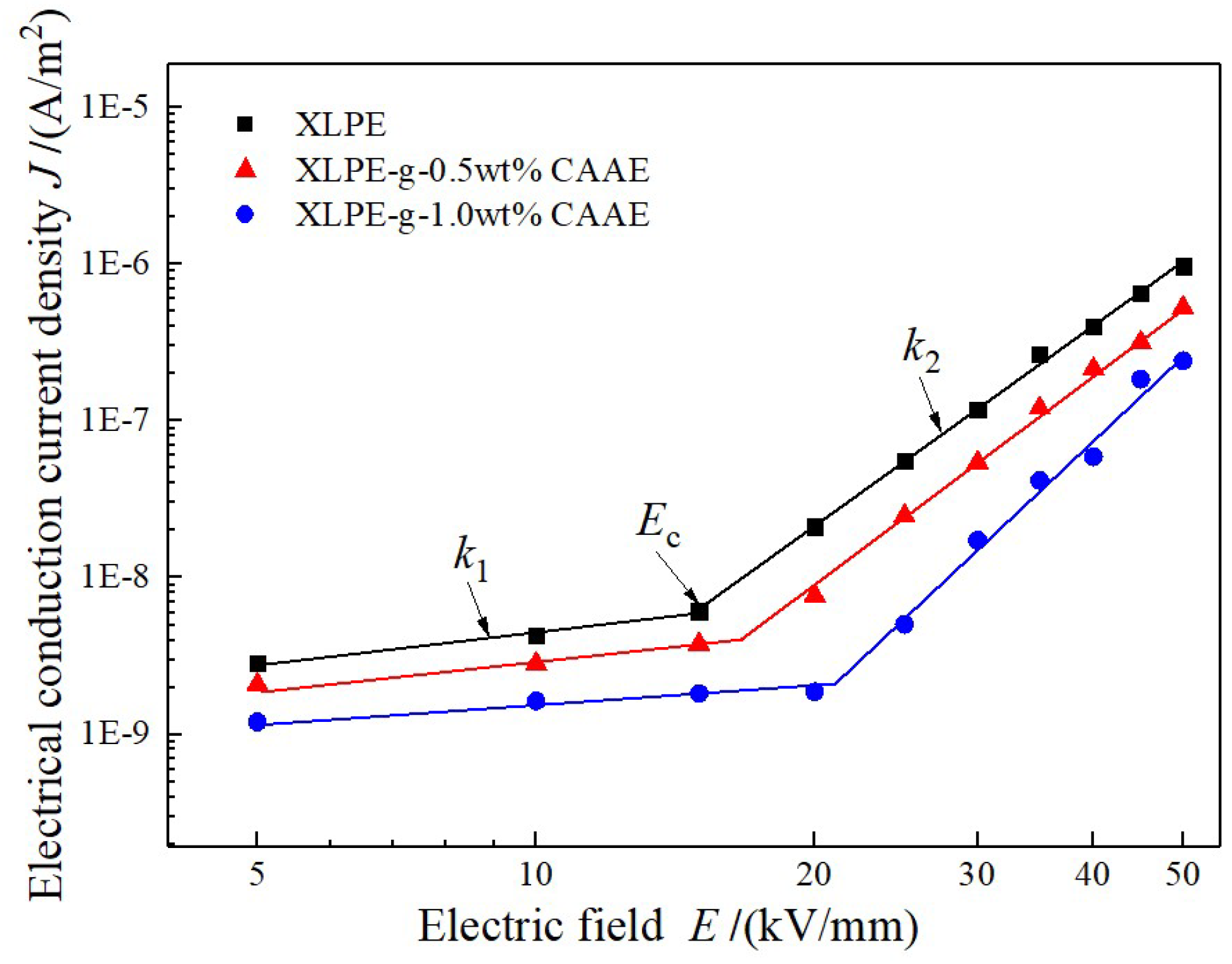
The conduction current density
J depending on varying applied DC electric field E are measured to investigate the effect of CAAE-grafting-introduced deep traps on the charge carrier transport characteristics in XLPE-
g-CAAE. The
E–
J curves of both XLPE and XLPE-
g-CAAE represent as two distinct rising stages, as shown in
Figure 5. The dependence of conduction current density
J varying with electric field strength
E can be represented as:
and the logarithm with 10 as base (lg) be formularized by:
where the A denotes the factor attributed to sample properties and size and
k represents the nonlinear coefficient. The lg(
J) is proportional to lg(
E) with the gradient
k describing the nonlinearity of material electrical conductivity [
17,
18]. In bi-logarithm coordinates, the pure XLPE and XLPE-
g-CAAE all exhibit two stages of almost perfect linear relationship of the conduction current density increasing with applied electric field in relatively smaller and larger gradients at lower and higher applied electric field respectively, rendering a critical turning point at a demarcated electric field. The slopes at lower and higher electric field
k1,
k2, and the critical electric field
Ec as described nonlinear parameters are listed in
Table 4. The electric resistivity and critical electric field of XLPE-
g-CAAE increases apparently with CAAE grafting concentration, respectively approaching to one magnitude and 45% higher of 1.0 wt% CAAE grafted XLPE than those of pure XLPE, reasonably suggesting the promising low dielectric loss and high working electric field adequate for most practical applications including HVDC cables.
The
E–
J curves characteristics will generally represent as based on space-charge limited current (SCLC) theory [
19], according to which the conduction current in solid dielectrics obeys the Ohm’s law (
k1 = 1) at lower applied electric field while will increase intensively complying with the Child’s law [
20] (
k2 = 2) when the applied electric field grows exceeding critical point. The Child’s law describes
E–
J as that no traps exist or all traps are occupied, actually without considering the trapping/detrapping process for conduction current under varying applied electric field. Therefore, the present
E–
J curves of XLPE and XLPE-
g-CAAE exhibit characteristic trap-limited SCLC performance with
k2 distinctly greater than 2 deriving from deep traps, indicating charge carrier traps existing in all samples and remarkably higher trap density of XLPE-
g-CAAE than XLPE. Furthermore, our first-principles calculated DOS illustrates that the graft-induced electronic bound states with low density energy levels very near to the PE conduction band bottom are merged into conduction band and represent as new band-edge determining the electron charge carrier transport. The new band-edge states have the intrinsic nature of bound state with the wave function substantially being localized around the correlated atoms in grafted molecules and thus render considerably reduced electronic mobility compared with neat PE under low carrier injection, in consistence with the experimental results of remarkably decreased electrical conductivity from grafting CAAE. The deeper traps in XLPE-
g-CAAE than XLPE can capture charge carriers to lower energy levels being effective at higher temperature, and decrease conduction current with higher the critical electric field of
E–
J curves, agreeing well with the TSDC results. Furthermore, compared with pure XLPE, the conductivity of XLPE-
g-CAAE, although increasing with electric field at a higher rate, is lower and decreases with grafting density in the testing range of electric field that exceeds the upper limit of HVDC cable working ambience. Therefore, it is definitely proved that the impedance to carrier transport has been achieved by chemically grafting CAAE onto the molecular chain of the XLPE applied for HVDC cables.
The dielectric breakdown strength (DBS) testing results for all the XLPE and XLPE-
g-CAAE are analyzed by Weibull statistics as provided with 2-parameter Weibull fitting in
Figure 6 [
21]. The 63.2% probability cumulative DBS together with fitting parameters of Weibull distribution for all the XLPE and XLPE-
g-CAAE are listed in
Table 5. The evident DBS enhancement can be presented by the field strength at 63.2% breakdown probability calculated from scale and location parameters of the fitted Weibull distribution. The chemically grafted XLPE exhibit remarkable DBS improvement directly compared with neat XLPE, demonstrating the evident deep-trap effect deriving from C=O group in grafted CAAE. The XLPE-
g-CAAE give 9.1% higher of characteristic 63.2% DBS compared with pure XLPE, which is attributed to the deep traps introduced by CAAE graft, capturing charge carriers and suppressing space charge accumulation under DC electric field. Furthermore, it is explicit that the CAAE grafting concentration has substantially affected the DBS. Although the XLPE-
g-CAAE samples with different concentrations of grafted CAAE have almost the same characteristic DBS, the Weibull shape factor notably increases with CAAE grafting concentration, indicating the DBS can be significantly improved by raising grafted CAAE concentration, as shown in
Table 5. The chemical modification effect of CAAE graft on DBS is still retained for the low breakdown probabilities as shown in
Figure 6. Comparing the DBS results, it is reasonable to correlate DBS improvement with the increment of trap level depth and density. Those results support the electron trapping model of the grafting polar group and demonstrate that the CAAE is mostly expected as chemically grafting molecule to improve dielectric properties of polymer materials. The polar C=O group in grafted CAAE accounts essentially for DBS improvement of XLPE-
g-CAAE. Further evidence is shown that the candidate molecules with higher polarity or multiple polar group have been grafted to polymers for chemical modification so as to further increase DBS.