Influence of Al2O3 Nanoparticle Addition on a UV Cured Polyacrylate for 3D Inkjet Printing
Abstract
:1. Introduction
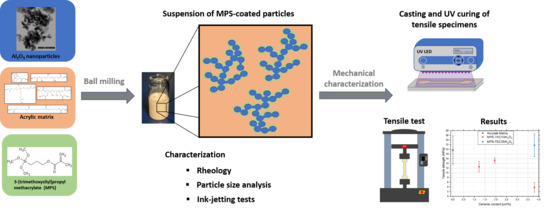
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Primary Particles Size Distribution
2.2.2. Grafting MPS onto Al2O3 Nanoparticles
2.2.3. Preparation and Characterization of Ceramic Inks
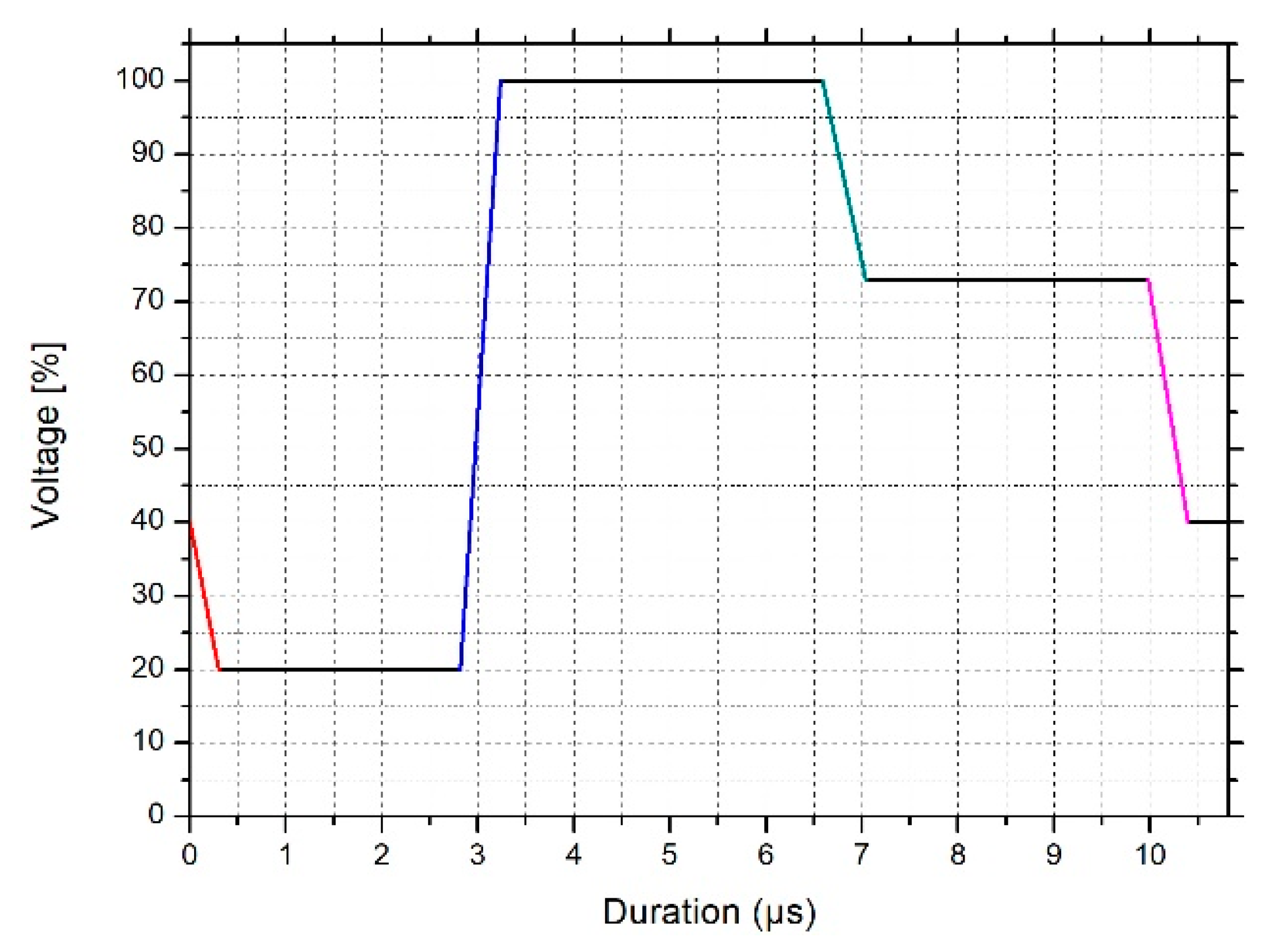
2.2.4. Tensile Testing
3. Results
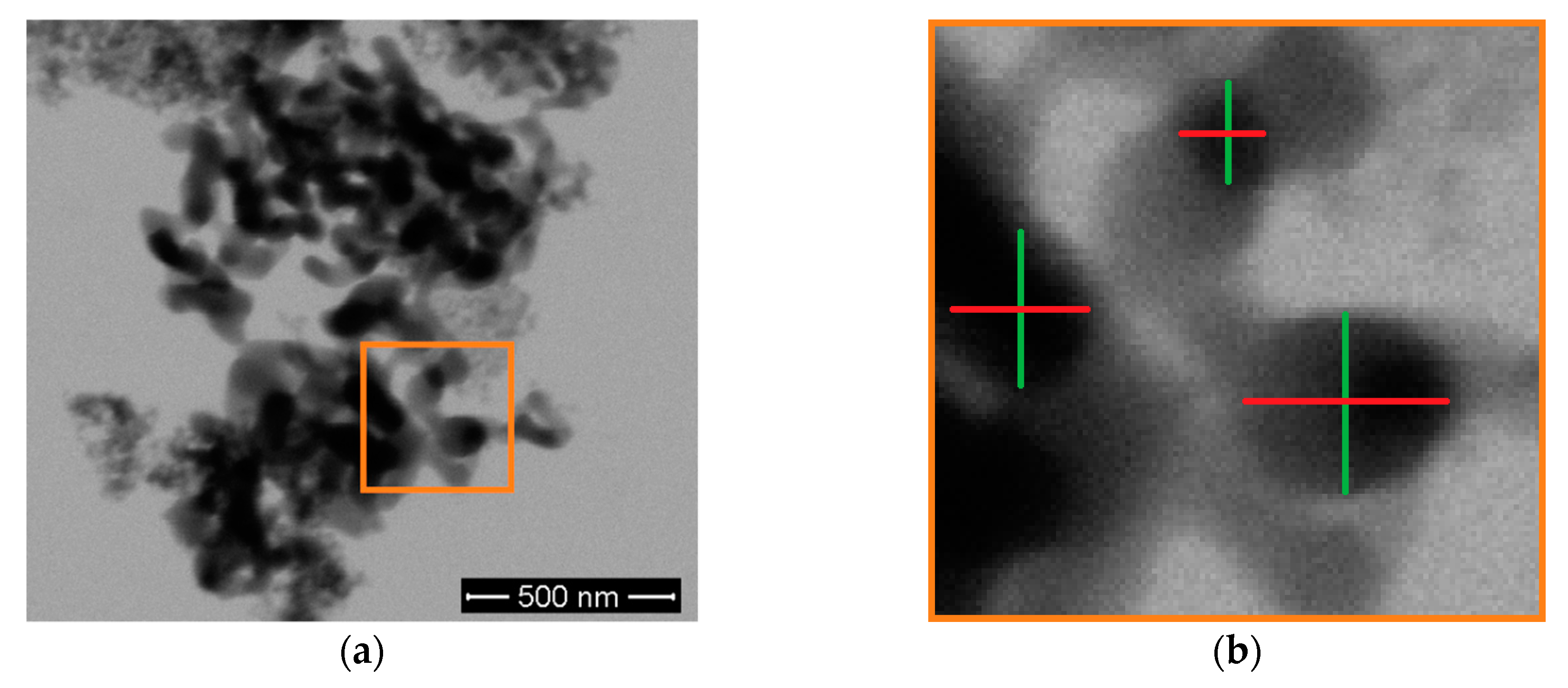
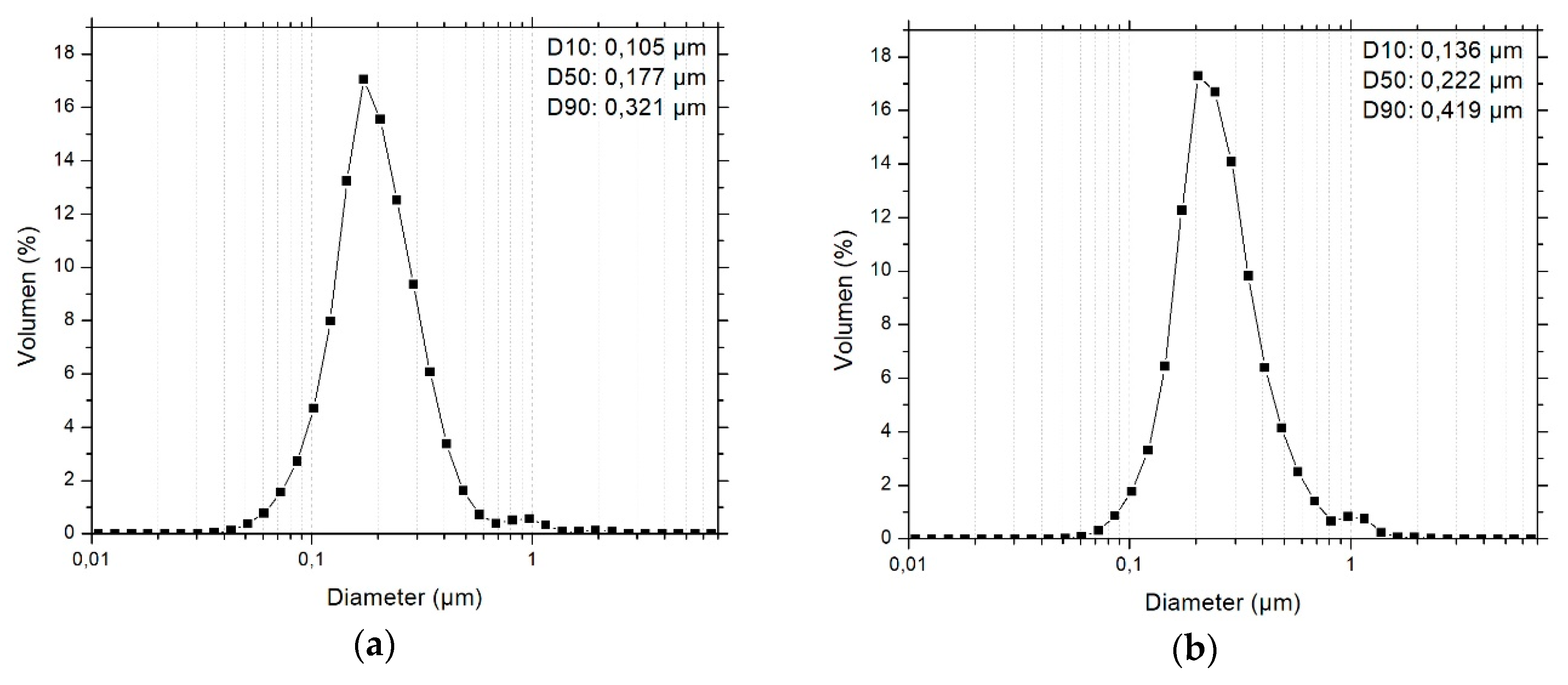
3.1. Primary Particle Size Distribution
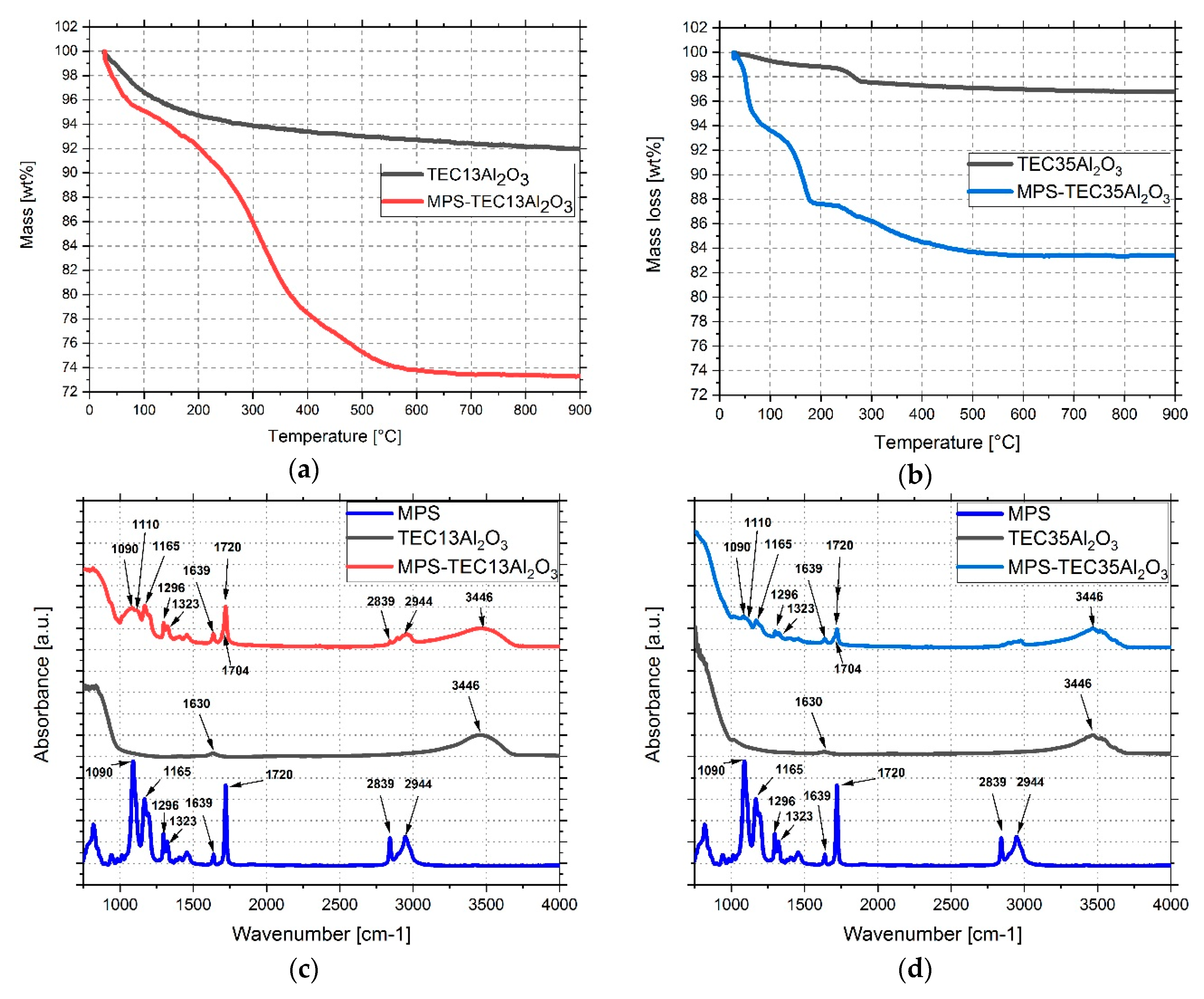
3.2. Grafting MPS on Al2O3 Nanoparticles
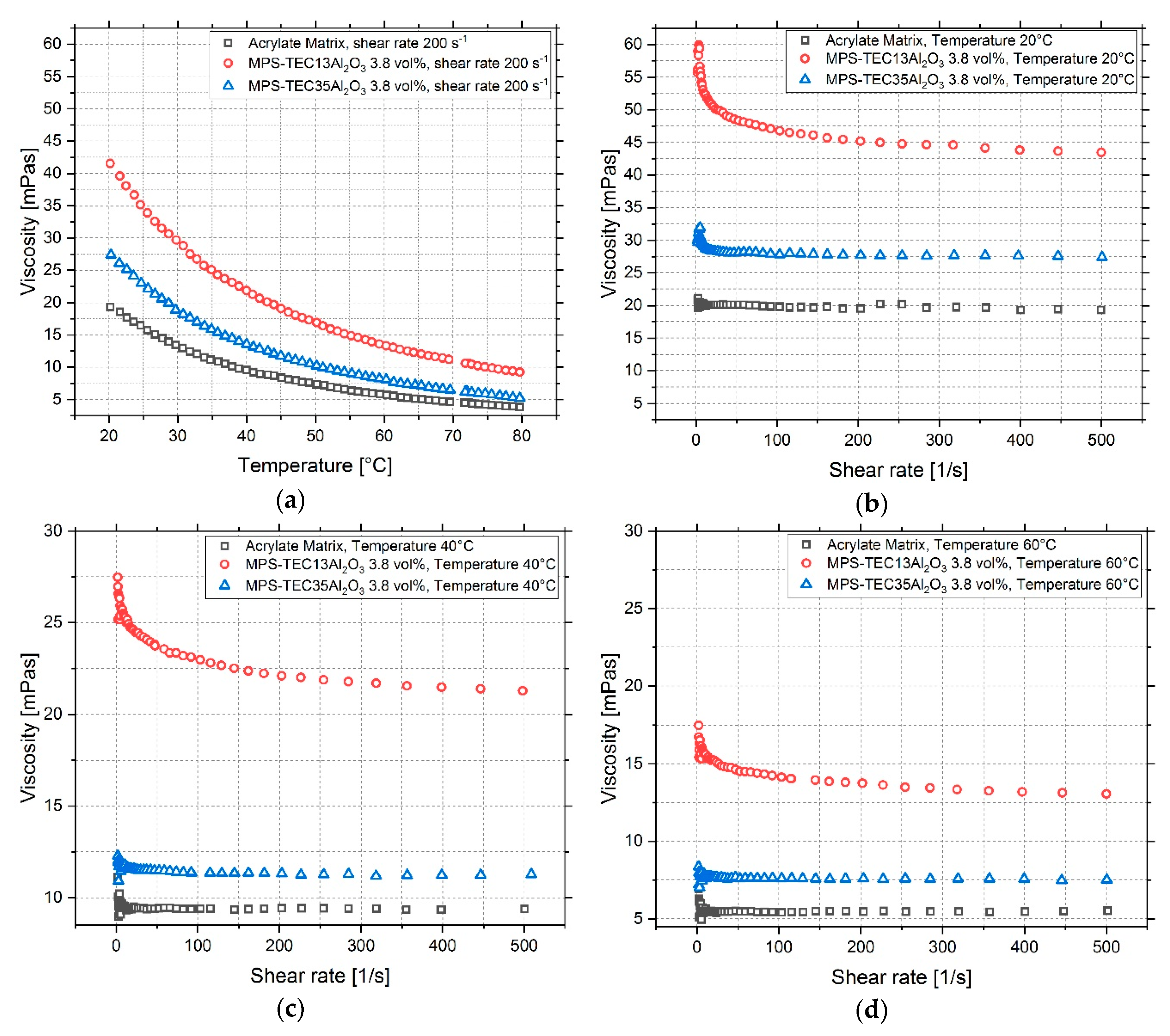
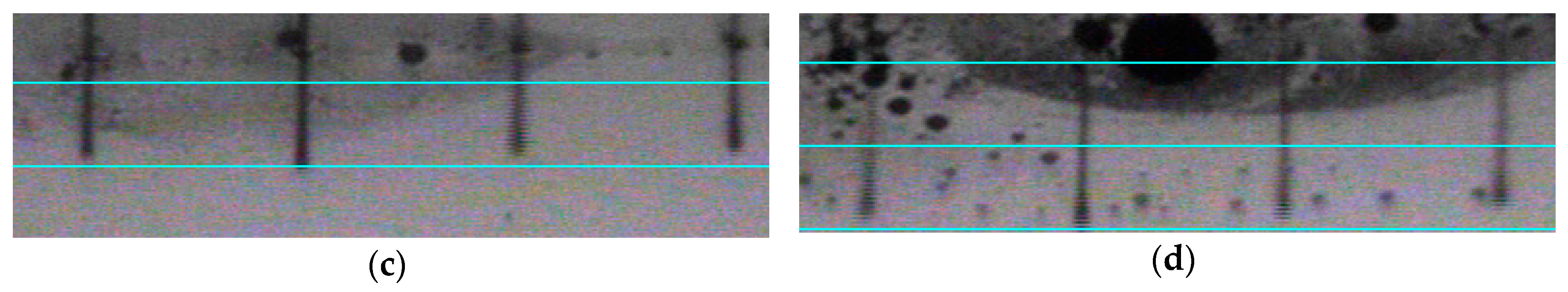
3.3. Characterization of Ceramic Inks
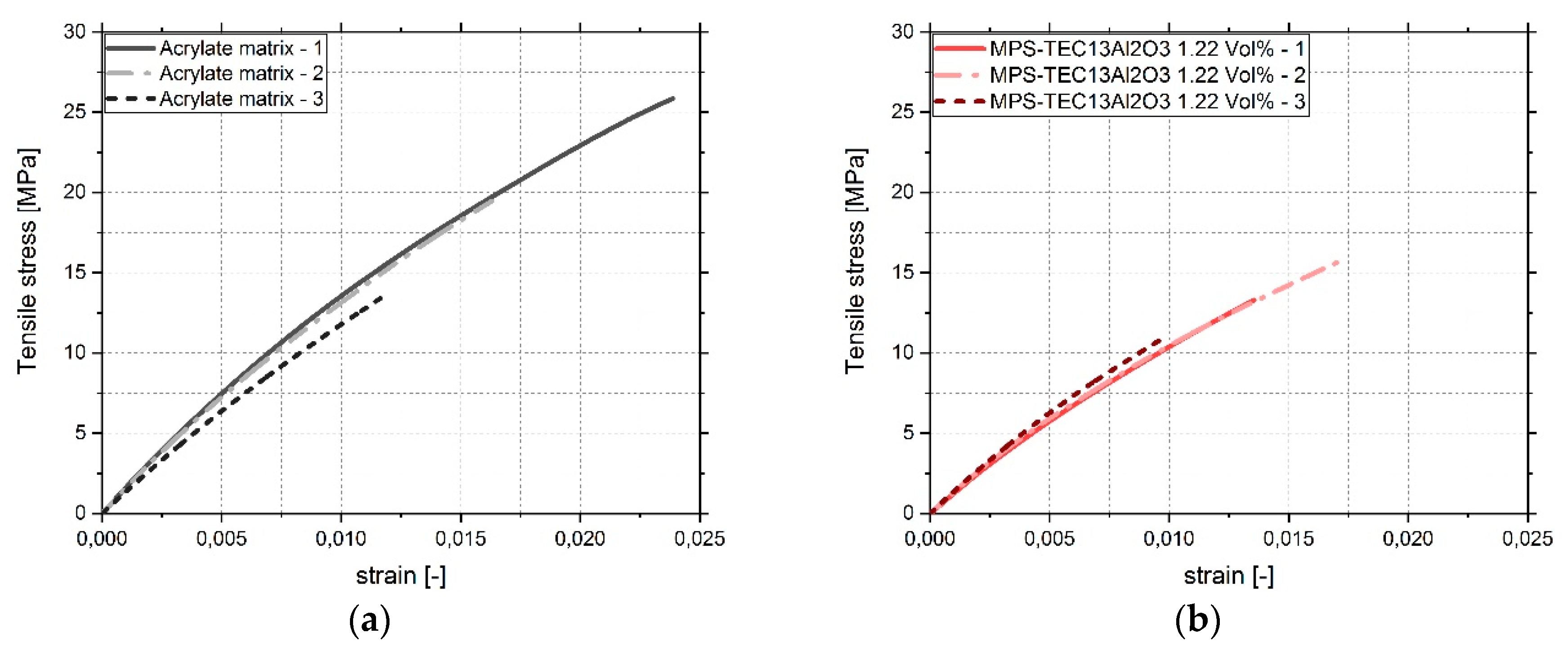
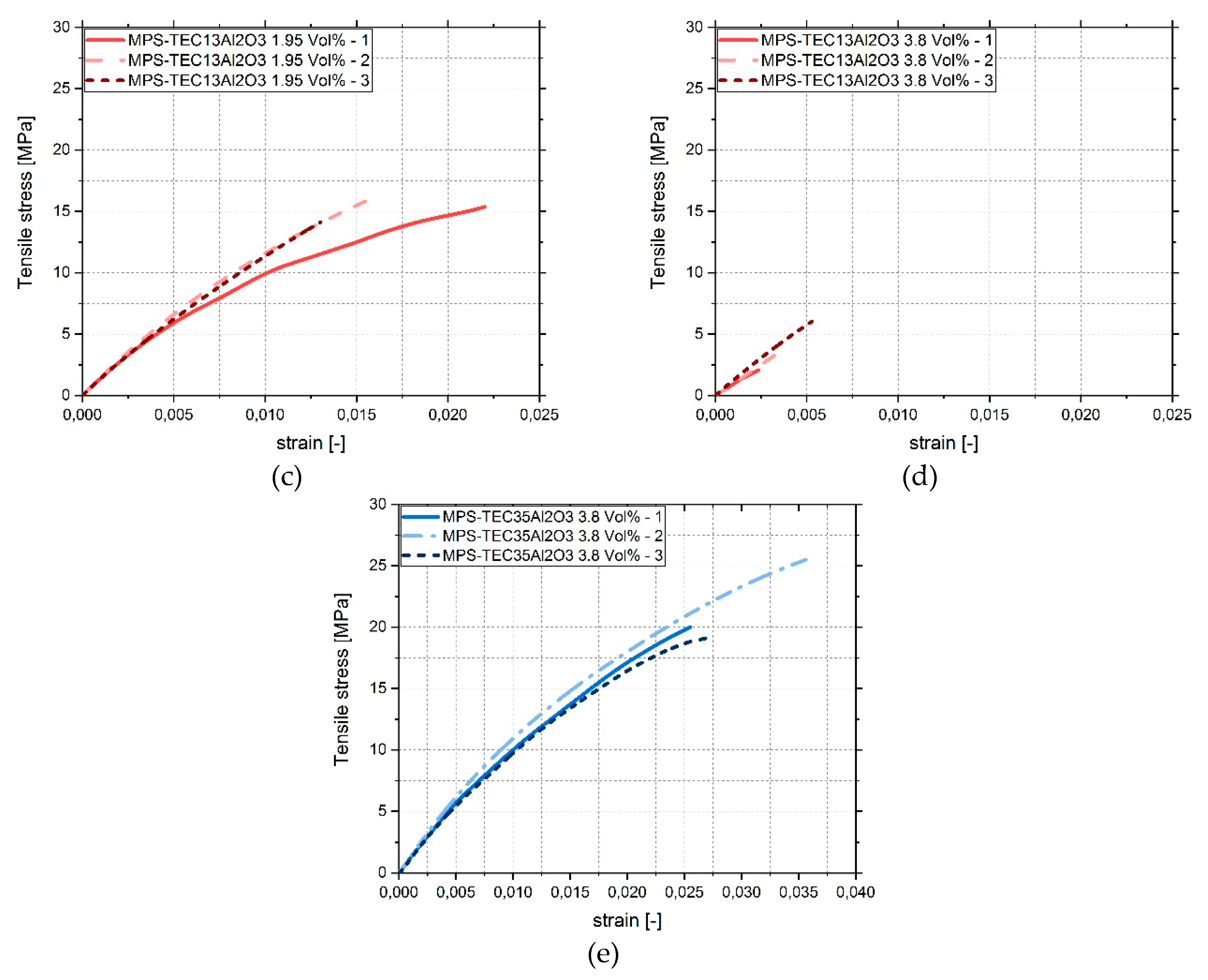
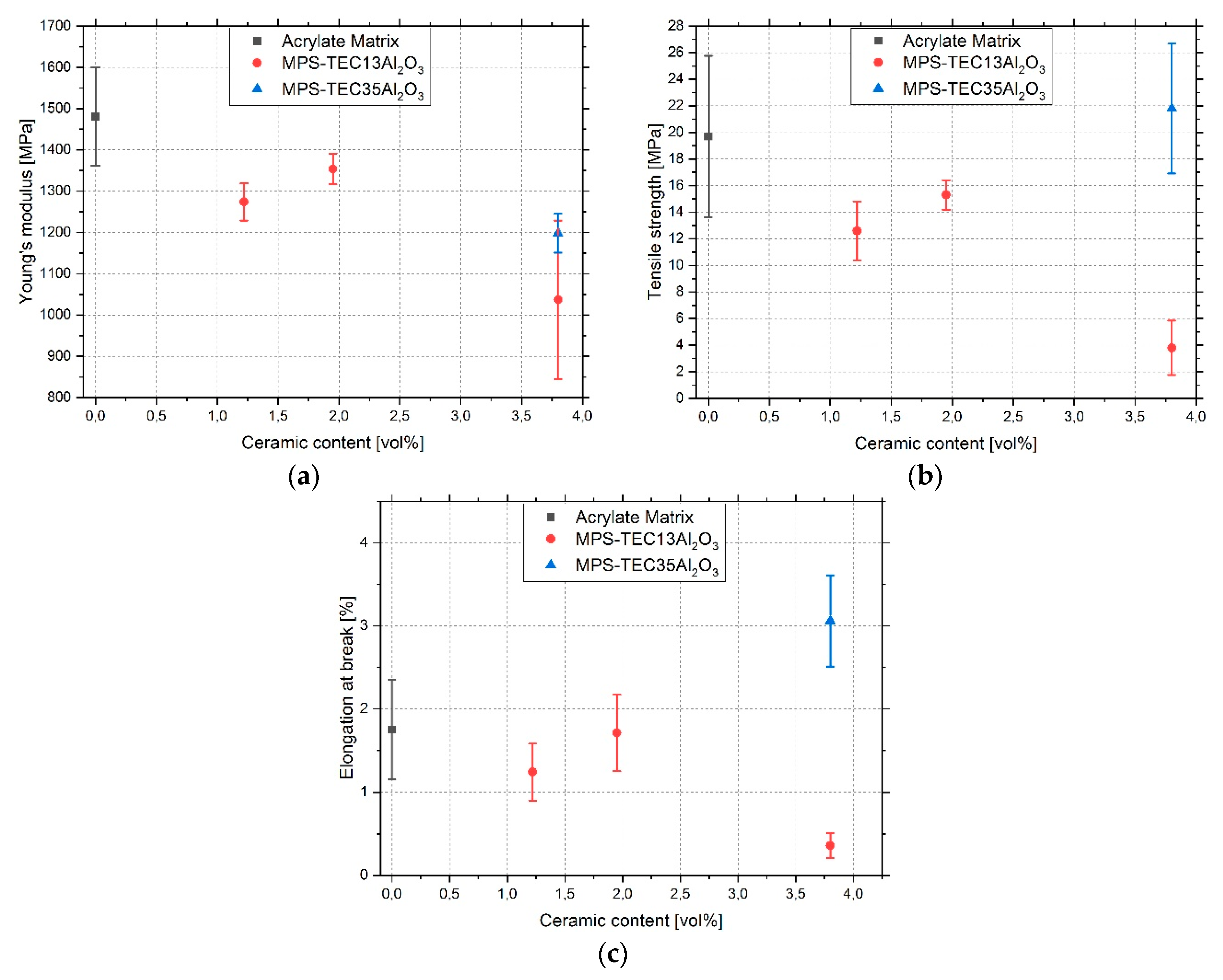
3.4. Tensile Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Magdassi, S. The Chemistry of Inkjet Inks; World Scientific Publishing: Singapore, 2010. [Google Scholar]
- Vaezi, M.; Chianrabutra, S.; Mellor, B.; Yang, S. Multiple material additive manufacturing—Part 1: A review. Virtual Phys. Prototyp. 2013, 8, 19–50. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Dorfinger, P.; Stampfl, J.; Liska, R. Toughening of Photopolymers for Stereolithography (SL). Mater. Sci. Forum 2015, 825–826, 53–59. [Google Scholar] [CrossRef]
- Ligon-Auer, S.C.; Schwentenwein, M.; Gorsche, C.; Stampfl, J.; Liska, R. Toughening of photo-curable polymer networks: A review. Polym. Chem. 2016, 7, 257–286. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, C.; Chou, T. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Johnsen, B.B.; Kinloch, A.J.; Mohammed, R.D.; Taylor, A.C.; Sprenger, S. Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 2007, 48, 530–541. [Google Scholar] [CrossRef]
- Crosby, A.J.; Lee, J. Polymer Nanocomposites: The ‘Nano’ Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Yu, A.B.; Lu, G.Q. Multiscale modeling and simulation of polymer nanocomposites. Prog. Polym. Sci. 2008, 33, 191–269. [Google Scholar] [CrossRef]
- Jancar, J.; Douglas, J.F.; Starr, F.W.; Kumar, S.K.; Cassagnau, P.; Lesser, A.J.; Sternstein, S.S.; Buehler, M.J. Current issues in research on structure–property relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Zhao, S.; Schadler, L.S.; Duncan, R.; Hillborg, H.; Auletta, T. Mechanisms leading to improved mechanical performance in nanoscale alumina filled epoxy. Compos. Sci. Technol. 2008, 68, 2965–2975. [Google Scholar] [CrossRef]
- Williams, J.G. Particle toughening of polymers by plastic void growth. Compos. Sci. Technol. 2010, 70, 885–891. [Google Scholar] [CrossRef]
- Ash, B.J.; Siegel, R.W.; Schadler, L.S. Mechanical Behavior of Alumina/Poly(methyl methacrylate) Nanocomposites. Macromolecules 2004, 37, 1358–1369. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Justice, R.S. How Nano Are Nanocomposites? Macromolecules 2007, 40, 8501–8517. [Google Scholar] [CrossRef]
- Camenzind, A.; Caseri, W.R.; Pratsinis, S.E. Flame-made nanoparticles for nanocomposites. Nano Today 2010, 5, 48–65. [Google Scholar] [CrossRef]
- Inkyo, M.; Tokunaga, Y.; Tahara, T.; Iwaki, T.; Iskandar, F.; Hogan, C.J.; Okuyama, K. Beads Mill-Assisted Synthesis of Poly Methyl Methacrylate (PMMA)-TiO2 Nanoparticle Composites. Ind. Eng. Chem. Res. 2008, 47, 2597–2604. [Google Scholar] [CrossRef]
- Yun, J.S.; Park, T.; Jeong, Y.H.; Cho, J.H. Development of ceramic-reinforced photopolymers for SLA 3D printing technology. Appl. Phys. A 2016, 122, 629. [Google Scholar] [CrossRef]
- Al-Turaif, H.A. Effect of nano TiO2 particle size on mechanical properties of cured epoxy resin. Prog. Org. Coat. 2010, 69, 241–246. [Google Scholar] [CrossRef]
- Lalevée, J.; Dirani, A.; El-Roz, M.; Allonas, X.; Fouassier, J.P. Silanes as New Highly Efficient Co-initiators for Radical Polymerization in Aerated Media. Macromolecules 2008, 41, 2003–2010. [Google Scholar] [CrossRef]
- Musanje, L.; Ferracane, J.L.; Sakaguchi, R.L. Determination of the optimal photoinitiator concentration in dental composites based on essential material properties. Dent. Mater. 2009, 25, 994–1000. [Google Scholar] [CrossRef]
- Gurr, M.; Hofmann, D.; Ehm, M.; Thomann, Y.; Kübler, R.; Mülhaupt, R. Acrylic Nanocomposite Resins for Use in Stereolithography and Structural Light Modulation Based Rapid Prototyping and Rapid Manufacturing Technologies. Adv. Funct. Mater. 2008, 18, 2390–2397. [Google Scholar] [CrossRef]
- Gurr, M.; Thomann, Y.; Nedelcu, M.; Kübler, R.; Könczöl, L.; Mülhaupt, R. Novel acrylic nanocomposites containing in-situ formed calcium phosphate/layered silicate hybrid nanoparticles for photochemical rapid prototyping, rapid tooling and rapid manufacturing processes. Polymer 2010, 51, 5058–5070. [Google Scholar] [CrossRef]
- Hong, R.Y.; Qian, J.Z.; Cao, J.X. Synthesis and characterization of PMMA grafted ZnO nanoparticles. Powder Technol. 2006, 163, 160–168. [Google Scholar] [CrossRef]
- Siddiquey, I.A.; Ukaji, E.; Furusawa, T.; Sato, M.; Suzuki, N. The effects of organic surface treatment by methacryloxypropyltrimethoxysilane on the photostability of TiO2. Mater. Chem. Phys. 2007, 105, 162–168. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Liso, M.J.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the reaction of γ—methacryloxypropyltrimethoxysilane (γ—MPS) with slate surfaces. J. Mater. Sci. 1999, 34, 3867–3873. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, A.; Gan, F. Improvement of polyacrylate coating by filling modified nano-TiO2. Appl. Surf. Sci. 2006, 252, 8635–8640. [Google Scholar] [CrossRef]
- Guo, Z.; Pereira, T.; Choi, O.; Wang, Y.; Hahn, H.T. Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties. J. Mater. Chem. 2006, 16, 2800–2808. [Google Scholar] [CrossRef]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- Song, L.; Ye, Q.; Ge, X.; Misra, A.; Spencer, P. Tris(trimethylsilyl)silane as a co-initiator for dental adhesive: Photo-polymerization kinetics and dynamic mechanical property. Dent. Mater. 2016, 32, 102–113. [Google Scholar] [CrossRef]
- Megias-Alguacil, D.; Tervoort, E.; Cattin, C.; Gauckler, L.J. Contact angle and adsorption behavior of carboxylic acids on α-Al2O3 surfaces. J. Colloid Interface Sci. 2011, 353, 512–518. [Google Scholar] [CrossRef]
- Wong, J.C.H.; Tervoort, E.; Busato, S.; Ermanni, P.; Gauckler, L.J. Engineering macroporous composite materials using competitive adsorption in particle-stabilized foams. J. Colloid Interface Sci. 2012, 383, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Macroporous Ceramics from Particle-Stabilized Wet Foams. J. Am. Ceram. Soc. 2007, 90, 16–22. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B. Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv. Colloid Interface Sci. 2004, 110, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Blokhus, A.M. Adsorption of Benzoic Acid on α-Alumina and γ-Boehmite. J. Colloid Interface Sci. 1994, 166, 259–262. [Google Scholar] [CrossRef]
- Woo, H.; Hong, L.; Kim, S.; Park, S.; Song, S.; Ham, H. Photopolymerization of Methyl Methacrylate with Secondary Silanes. Bull. Korean Chem. Soc. 1995, 16, 775. [Google Scholar]
- Kim, B.; Choi, J.; Yang, S.; Yu, S.; Cho, M. Influence of crosslink density on the interfacial characteristics of epoxy nanocomposites. Polymer 2015, 60, 186–197. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, P.; Dong, S. The Influence of Crosslink Density on the Failure Behavior in Amorphous Polymers by Molecular Dynamics Simulations. Materials 2016, 9, 234. [Google Scholar] [CrossRef]
- Gürgen, S.; Kuşhan, M.C.; Li, W. Shear thickening fluids in protective applications: A review. Prog. Polym. Sci. 2017, 75, 48–72. [Google Scholar] [CrossRef]
- Hanemann, T. Nanoparticle surface polarity influence on the flow behaviour of polymer, atrix composites. Polym. Compos. 2013, 34, 1425–1432. [Google Scholar] [CrossRef]

| Filler | Content [vol %] |
|---|---|
| MPS-TEC13Al2O3 | 1.22 |
| MPS-TEC13Al2O3 | 1.95 |
| MPS-TEC13Al2O3 | 3.80 |
| MPS-TEC35Al2O3 | 3.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, D.; Burchard, S.; Crespo, J.; Megnin, C.; Gutsch, S.; Zacharias, M.; Hanemann, T. Influence of Al2O3 Nanoparticle Addition on a UV Cured Polyacrylate for 3D Inkjet Printing. Polymers 2019, 11, 633. https://doi.org/10.3390/polym11040633
Graf D, Burchard S, Crespo J, Megnin C, Gutsch S, Zacharias M, Hanemann T. Influence of Al2O3 Nanoparticle Addition on a UV Cured Polyacrylate for 3D Inkjet Printing. Polymers. 2019; 11(4):633. https://doi.org/10.3390/polym11040633
Chicago/Turabian StyleGraf, Dennis, Sven Burchard, Julian Crespo, Christof Megnin, Sebastian Gutsch, Margit Zacharias, and Thomas Hanemann. 2019. "Influence of Al2O3 Nanoparticle Addition on a UV Cured Polyacrylate for 3D Inkjet Printing" Polymers 11, no. 4: 633. https://doi.org/10.3390/polym11040633
APA StyleGraf, D., Burchard, S., Crespo, J., Megnin, C., Gutsch, S., Zacharias, M., & Hanemann, T. (2019). Influence of Al2O3 Nanoparticle Addition on a UV Cured Polyacrylate for 3D Inkjet Printing. Polymers, 11(4), 633. https://doi.org/10.3390/polym11040633