2.1. Sample Material and Chemicals
A commercially available scoured wool fabric (ISO 105-F01; plain woven 125 g/m2) was prepared (Testfabrics Inc. West Pittston, PA, USA). Spent coffee grounds (Coffea arabica L.) were supplied from a local coffee house in Gongju, Korea. Folin-Ciocalteu reagent, gallic acid (≥97%), vanillin (99%), (+)-catechin hydrate (≥98%), potassium iodate, sodium bicarbonate, formic acid, acetonitrile, trigonelline, protocatechuic acid, tannic acid (ACS reagent, Mw: 1701.20), chlorogenic acid, and caffeine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Free radical DPPH (1,1-diphenyl-2-picrylhydrazyl) was purchased from Calbiochem (San Diego, CA, USA). All reagents were used as received without further purification.
2.3. Dyeing Process
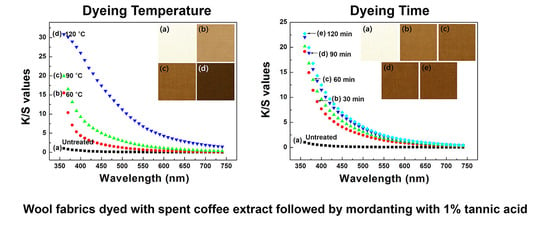
Wool fabrics were cut into 30 cm × 30 cm pieces, and each piece was immersed in a vessel containing the stock solution of the spent coffee extract (bath ratio = 1:30). A laboratory infrared (IR) dyeing machine (Daelim Starlet Co., Ltd; Gyeonggi-do, Korea) was used for the dyeing process. The temperature of the dyeing bath was gradually increased (ca. 3 °C/min) up to the designated temperature (60, 90, and 120 °C), and then that temperature was maintained while the vessels were rotated at 45 rpm for the designated dyeing time (30, 60, 90, and 120 min). The dyed wool fabrics were thoroughly rinsed with deionized water and squeezed using a padder to obtain 100 wt % of a specified wet pick-up rate.
2.5. Analysis of the Spent Coffee Extract
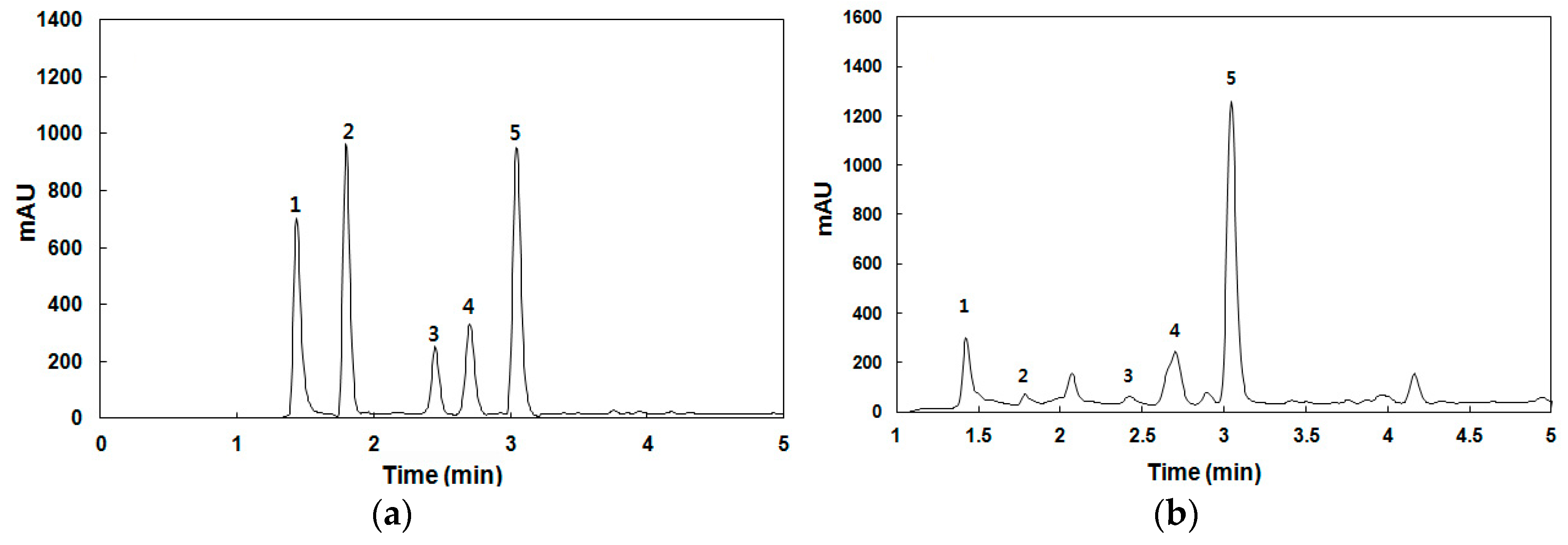
The antioxidant compounds in the spent coffee extract were analyzed by high-performance liquid chromatography (HPLC) using a diode array detector (Agilent Technologies, 1260 Infinity, Waldbronn, Germany). A Kinetex 5-μm C18 column (150 mm × 4.6 mm i.d., Phenomenex, Torrance, CA, USA) was employed at 40 °C. The antioxidant compounds were separated by a gradient mobile phase consisting of (A) 0.1% formic acid and (B) acetonitrile at a flow rate of 1 mL/min. The gradient was programmed as follows: 0–10 min, 15%–37% B; 5–10 min, 37%–80% B; 10–12 min, 80%–100% B; and 12–13 min, 100%–15% B. The major antioxidant compounds were identified based on the similarities between their experimental retention times and ultraviolet-visible (UV-Vis) spectra and those of pure authentic standards (trigonelline, gallic acid, chlorogenic acid, and caffeine). The total phenolic content in the spent coffee extract was measured by the colorimetric method described by Singleton and Rossi [
10]. Folin–Ciocalteu reagent (2.5 mL, previously diluted with water 1:10, v/v) and 2 mL of 75 g/L aqueous sodium carbonate were added to 0.5 mL of an aqueous solution of the extract. The mixture was kept at 50 °C for 5 min, and after cooling, the absorbance was measured at 760 nm (Biomate5 spectrophotometer, Thermo, Waltham, MA, USA). The total phenolic content was calculated in terms of gallic acid equivalents (GAE) from the calibration curve of gallic acid standard solutions (2–40 μg/mL) and is expressed as mg gallic acid equivalent (GAE)/mg of extract (on a dry basis). The total tannin content in the spent coffee extract was determined by the vanillin/HCl method described by Broadhust and Jones [
11] with some modifications. An aliquot of the extracts (1 mL) was added to the vanillin reagent (2 mL), which had been prepared by dissolving vanillin in methanol (0.5%, w/v). Aqueous HCl (2 mL, 4%, v/v) was added to the mixture, and then the mixture was incubated in the dark. The absorbance was measured at 500 nm after 20 min of incubation. Different concentrations (500–3000 μg/mL) of (+)-catechin standards were used to calculate the condensed tannin content. The total tannin content was expressed as mg of tannic acid equivalents (TAE)/mL. All samples were analyzed in triplicate, and the mean value was calculated.
2.6. Characteristics of the Fabrics Dyed with the Spent Coffee Extract
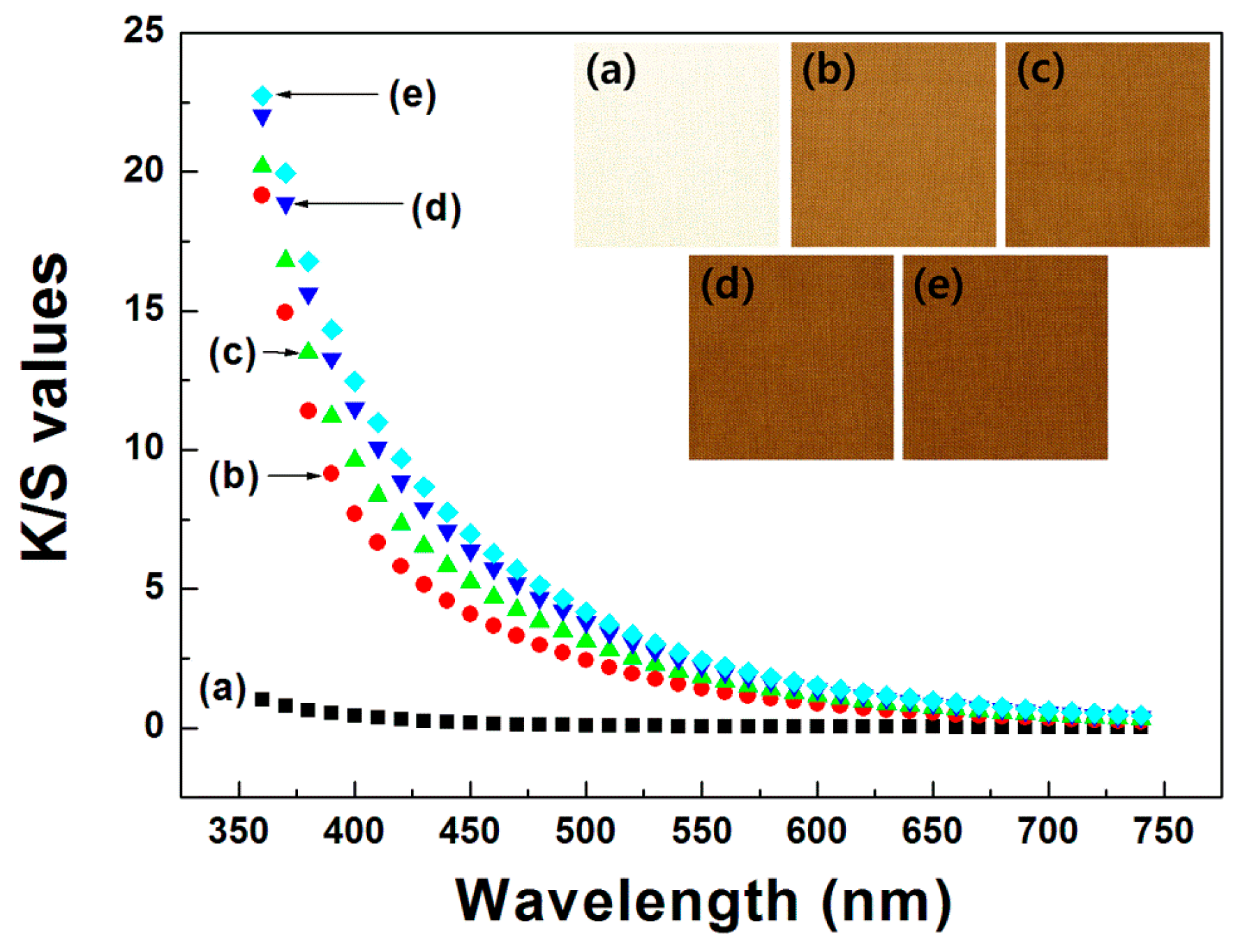
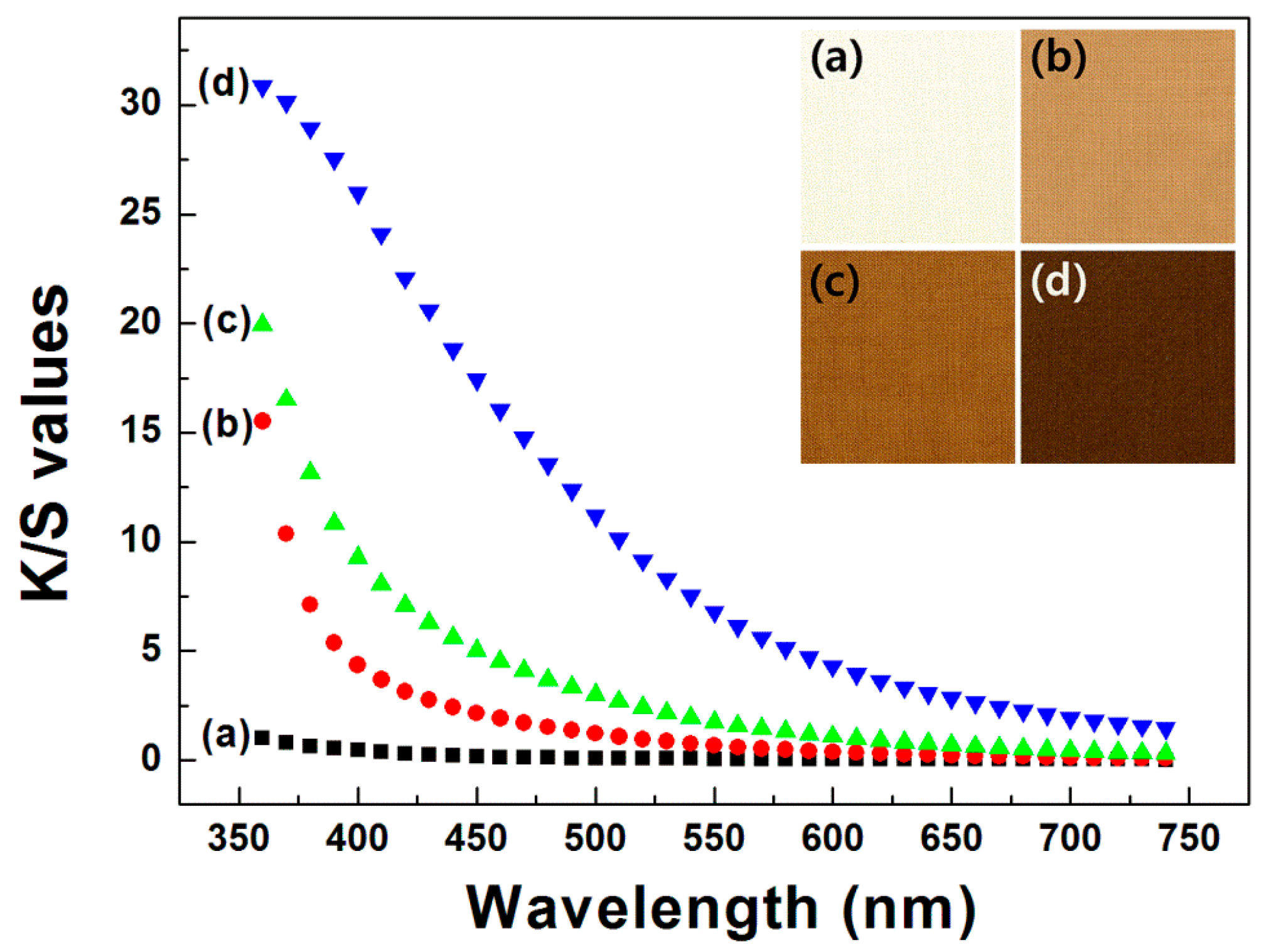
Color properties, in terms of the L*, a*, and b* values, and color differences, ΔE, of the dyed fabrics were investigated using a spectrophotometer (CM-2500d, Konica Minolta, Inc., Osaka, Japan). The color strength (K/S) values were assessed using the Kubelka–Munk Equation (1):
In the above equation, R is the decimal fraction of the reflectance of the dyed fabric.
Color fastness was investigated as follows: color fastness to washing (ISO 105 C06: 2010, A2S, 30 min mechanical wash at 40 ± 2 °C in 0.4% European Colorfastness Establishment (ECE) reference detergent and 0.1% sodium perborate tetrahydrate solution with 10 steel balls); color fastness to light (ISO 105 B02: 2014, Xenon-arc lamp, blue scale). All tests were conducted at least in triplicate for all samples.
The shrinkage rate of dyed fabrics was determined based on the fabric count values measured via fabric analyzing glass, and calculated using Equation (2):
In the above equation, D and P represent the fabric count values of dyed fabrics and pristine fabrics, respectively.
The tensile strength of the fabrics was measured by the cut strip method (modified ASTM D5035) only in the weft direction using an Instron 5543 system (Norwood, MA, USA); 300 mm/min, gauge length: 50 mm, specimen width: 25 mm.
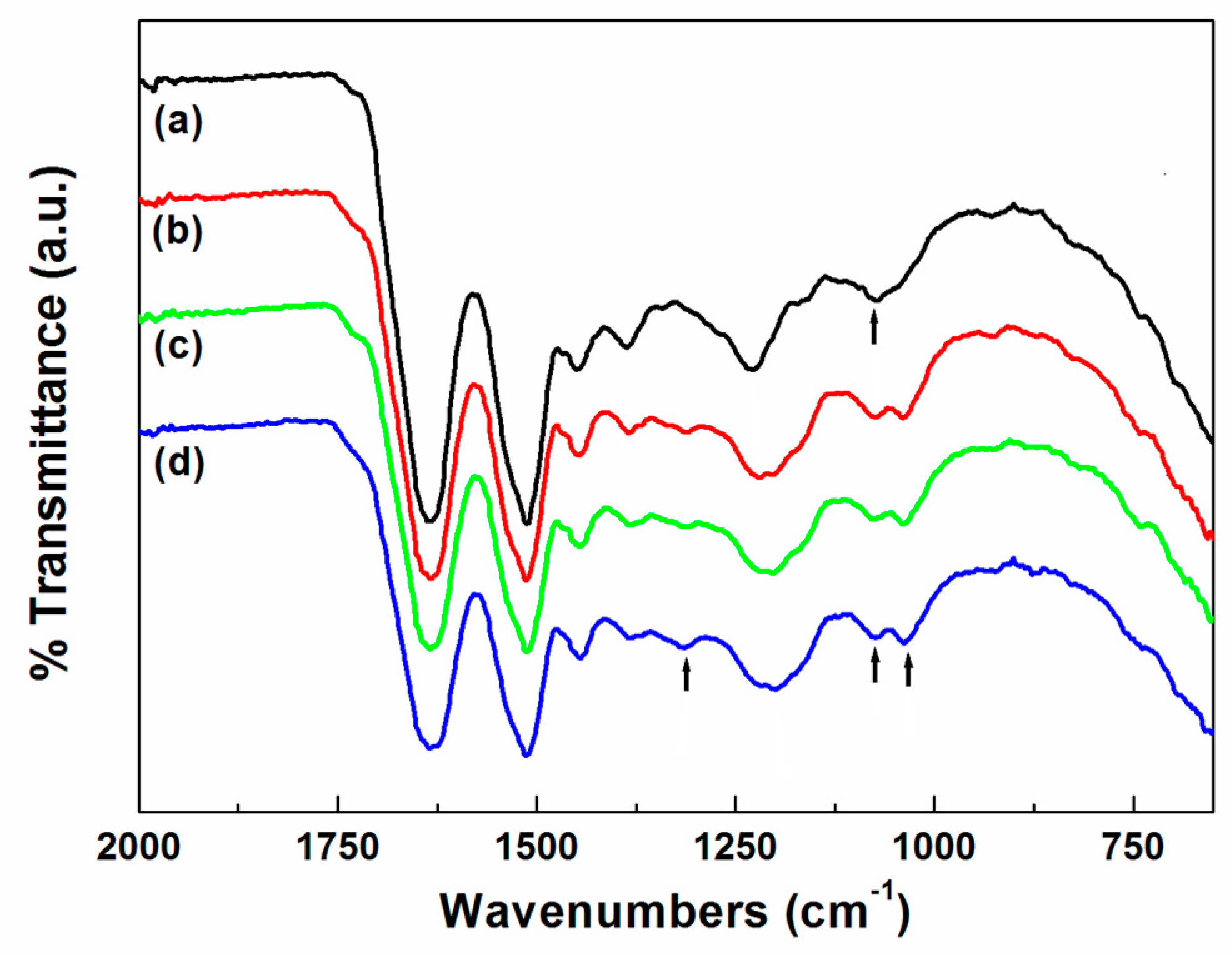
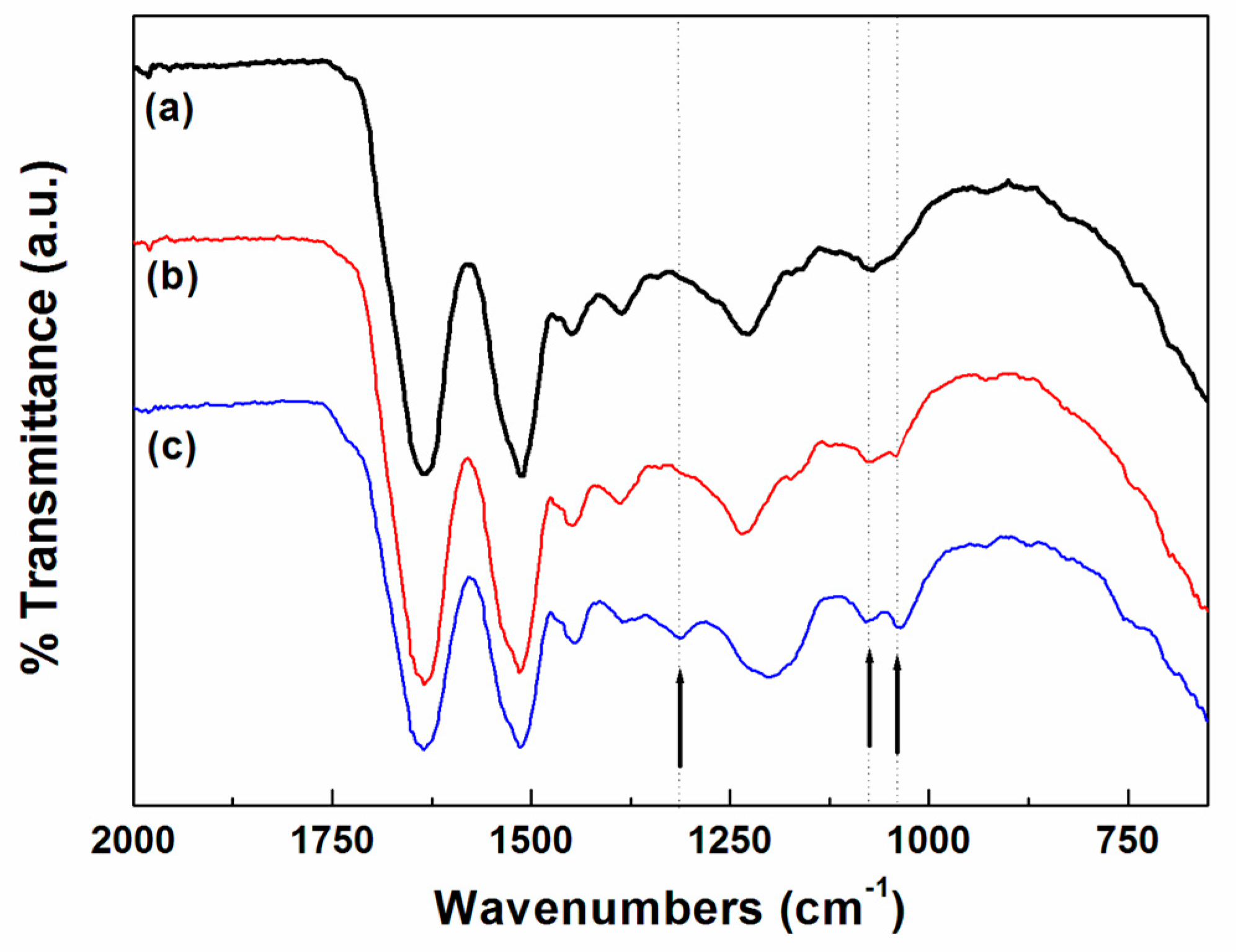
Fourier transform infrared (FT–IR) spectrometry was performed using a Spectrum 100 Optica FT–IR instrument (PerkinElmer, Waltham, MA, USA) with a resolution of 4 cm−1. The FT–IR measurements were carried out using an attenuated total reflectance (ATR) technique.
The ability of the dyed fabrics to impede microbial growth and retention was tested using
Staphylococcus aureus (ATCC 6538; a Gram-positive bacterium) and
Klebsiella pneumoniae (ATCC 4352; a Gram-negative bacterium) cultures according to an established protocol (KS K 0693).
In the above equation, A and B represent the surviving bacterial cells (colony-forming units mL-1) on the plates inoculated with the bacterial solution derived from the dyed fabric and a control solution derived from untreated fabric, respectively.
The antioxidant activity of the dyed fabrics was measured with DPPH using a previously reported method [
12]. More details are presented in our previous papers [
6,
7]. Lower absorbances of the solutions indicated higher DPPH scavenging abilities. The DPPH scavenging ability was calculated using Equation (4).
In the above equation, S and C represent the absorbance at 517 nm of the sample from the dyed fabric and that of the control from the untreated fabric, respectively.