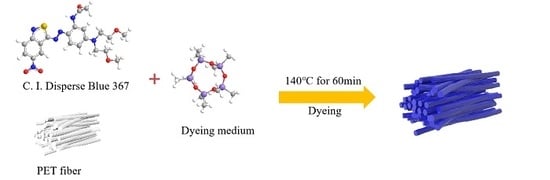
Mechanism of Accelerant on Disperse Dyeing for PET Fiber in the Silicone Solvent Dyeing System
Abstract
:1. Introduction
2. Experimental Procedure
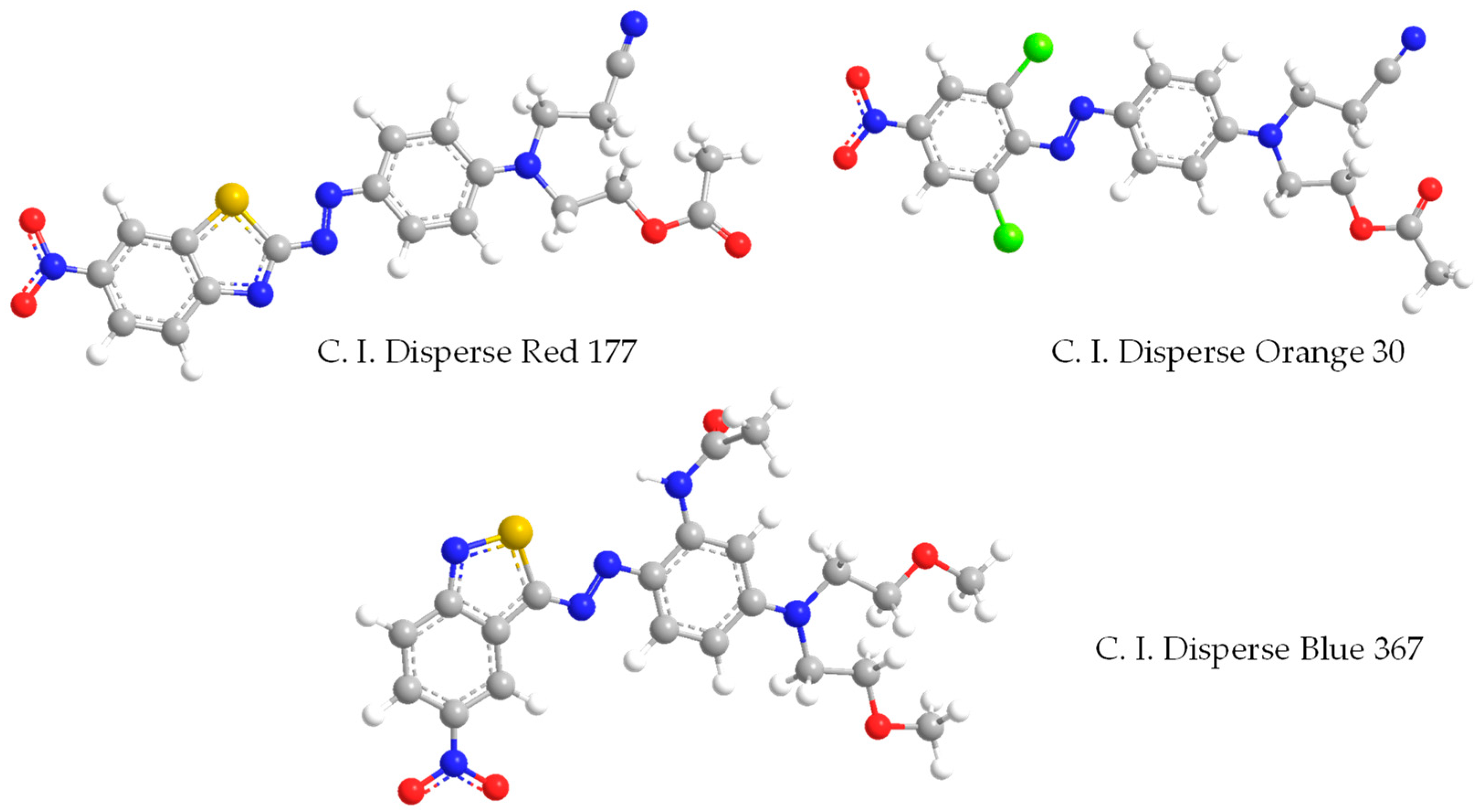
2.1. Materials
2.2. Dyeing Method
2.3. Dyeing Evaluations
2.4. The Solubility of Dye
2.5. Fiber Swelling
3. Results and Discussion
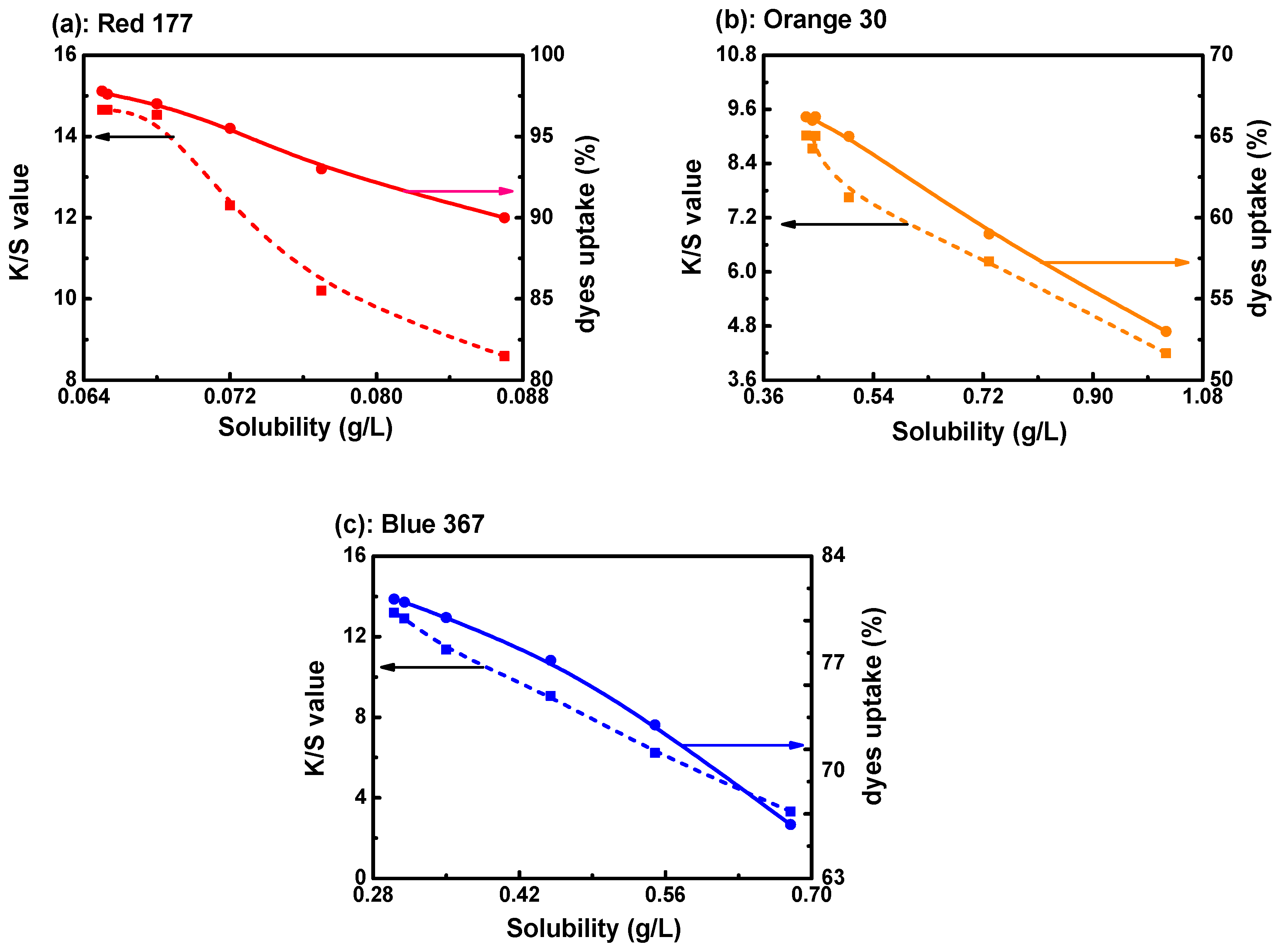
3.1. K/S Value and Dye Uptake of Disperse Dye in the D5 Solvent Dyeing System
3.2. The Solubility of Disperse Dye in D5 Solvent
3.3. The Effect of Accelerant Content on Fiber Swelling in the D5 Solvent
3.4. The Distribution of D5 in the Interior of PET Fibers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yi, Z.; Jihong, F.; Shuilin, C. Dyeing of polyester using micro-encapsulated disperse dyes in the absence of auxiliaries. Color. Technol. 2005, 121, 76–80. [Google Scholar] [CrossRef]
- Saligram, A.N.; Shukla, S.R.; Mathur, M. Dyeing of polyester fibres using ultrasound. J. Soc. Dyers Colour. 1993, 109, 263–266. [Google Scholar] [CrossRef]
- Yan, L.; Yi, Z.; Ben, Z.; Juan, D.; Shuilin, C. Effect of microencapsulation on dyeing behaviors of disperse dyes without auxiliary solubilization. J. Appl. Polym. Sci. 2011, 120, 484–491. [Google Scholar] [CrossRef]
- Urbanik, A. Effects of Solubility Parameters on Disperse Dyeing of Polyester. Text. Chem. Color. 1983, 15, 13–18. [Google Scholar]
- Pasquet, V.; Perwuelz, A.; Behary, N.; Isaad, J. Vanillin, a potential carrier for low temperature dyeing of polyester fabrics. J. Clean. Prod. 2013, 43, 20–26. [Google Scholar] [CrossRef]
- Shili, X.; Pengjun, X.; Qingyan, P.; Jiali, C.; Jiankang, H.; Faming, W.; Noor, N. Layer-by-layer assembly of polyelectrolyte multilayer onto pet fabric for highly tunable dyeing with water soluble dyestuffs. Polymers 2017, 9, 735. [Google Scholar]
- Hou, A.; Li, M.; Gao, F.; Xie, K.; Yu, X. One-step dyeing of polyethylene terephthalate fabric, combining pretreatment and dyeing using alkali-stable disperse dyes. Color. Technol. 2013, 129, 438–442. [Google Scholar] [CrossRef]
- Xu, S.X.; Chen, J.G.; Wang, B.J.; Yang, Y.Q. An environmentally responsible polyester dyeing technology using liquid paraffin. J. Clean. Prod. 2016, 112, 987–994. [Google Scholar] [CrossRef]
- Alkaya, E.; Demirer, G.N. Sustainable textile production: A case study from a woven fabric manufacturing mill in Turkey. J. Clean. Prod. 2014, 65, 595–603. [Google Scholar] [CrossRef]
- Cardozo, L.; Mazzer, H.R.; Santos, J.C.; Andreaus, J.; Feihrmann, A.C.; Beninca, C.; Cabral, V.F.; Zanoelo, E.F. Dyeing of polyethylene terephthalate fibers with a disperse dye in supercritical carbon dioxide. Text. Res. J. 2014, 84, 1279–1287. [Google Scholar] [CrossRef]
- Huang, G.; Dai, J.J.; Dong, F.C.; Wang, J.H.; Jia, Y.T. Compatibility of a disperse dye mixture in supercritical carbon dioxide dyeing. Color. Technol. 2013, 129, 305–311. [Google Scholar] [CrossRef]
- Hou, A.Q.; Chen, B.; Dai, J.J.; Zhang, K. Using supercritical carbon dioxide as solvent to replace water in polyethylene terephthalate (PET) fabric dyeing procedures. J. Clean. Prod. 2010, 18, 1009–1014. [Google Scholar] [CrossRef]
- Love, R.B. The Use of Non-aqueous Solvents in Dyeing I-Dyeing Polyester Fibre by the Dacsol Process. J. Soc. Dyers. Colour. 1978, 94, 440–447. [Google Scholar] [CrossRef]
- Gebert, K. The dyeing of polyester textile fabric in perchloroethylene by the exhaust process. J. Soc. Dyers. Colour. 1971, 87, 509–513. [Google Scholar] [CrossRef]
- Harris, F.; Guion, T. A new approach to solvent dyeing with non-ionic dyes. Text. Res. J. 1972, 42, 626–627. [Google Scholar] [CrossRef]
- Drews, M.J.; Jordan, C. The effect of supercritical CO2 dyeing conditions on the morphology of polyester fibers. Text. Chem. Color. 1998, 30, 13–20. [Google Scholar]
- Beltrame, P.L.; Castelli, A.; Selli, E.; Mossa, A.; Testa, G.; Bonfatti, A.M.; Seves, A. Dyeing of cotton in supercritical carbon dioxide. Dyes Pigments 1998, 39, 335–340. [Google Scholar] [CrossRef]
- Long, J.J.; Cui, C.L.; Zhang, Y.Q.; Yuan, G.H. Clean fixation of dye on cotton in supercritical carbon dioxide with a heterogeneous and phase transfer catalytic reaction. Dyes Pigments 2015, 115, 88–95. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Gao, S.H.; Zheng, L.J.; Zhang, J.; Du, B.; Wei, J. The research of cleaning process for supercritical carbon dioxide dyeing equipment. Adv. Mater. Res. 2014, 1048, 515–518. [Google Scholar] [CrossRef]
- Li, S.Z.; Liu, J.Q.; Li, Y.Q.; Li, L.M. Study on the dyeing of PET fiber with disperse dyes in D5 media. Adv. Mater. Res. 2011, 331, 334–337. [Google Scholar] [CrossRef]
- Li, S.Z.; Liu, J.Q.; Li, Y.Q.; Li, L.M. Dyeing behavior of polyester with disperse dyes in decamethylcyclopentasiloxane(d5) media. Dyeing Finish. 2012, 2, 1–4. [Google Scholar]
- Wu, H.; Liu, J.Q. Study on Waterless Dyeing Process of Polyester with Disperse Dyes in D5 under Normal Pressure and High Temperature. J. Zhejiang Sci. Univ. B 2015, 33, 584–590. [Google Scholar]
- Burns-Naas, L.A.; Mast, R.W.; Meeks, R.G.; Mann, P.C.; Thevenaz, P. Inhalation toxicology ofdecamethylcyclopentasiloxane (D5) following a 3-monthnose-only exposure inFischer 344 rats. Toxicol. Sci. 1998, 43, 230–240. [Google Scholar] [CrossRef] [PubMed]
- McMullin, T.S.; Yang, Y.; Campbell, J.; Clewell, H.J.; Plotzke, K.; Andersen, M.E. Development of an integrated multi-species and multi-dose route PBPK model for volatile methyl siloxanes–D4 and erse dye has a higher solubility in the solvent D5. Regul. Toxicol. Pharm. 2016, 74, S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Surita, S.C.; Tansel, B. Emergence and fate of cyclic volatile polydimethylsiloxanes (D4, D5) in municipal waste streams: Release mechanisms, partitioning and persistence in air, water, soil and sediments. Sci. Total. Environ. 2014, 468, 46–52. [Google Scholar] [CrossRef]
- Tabata, I.; Lyu, J.; Cho, S.; Tominaga, T.; Hori, T. Relationship between the solubility of disperse dyes and the equilibrium dye adsorption in supercritical fluid dyeing. Color. Technol. 2010, 117, 346–351. [Google Scholar] [CrossRef]
- Ferri, A.; Banchero, M.; Manna, L.; Sicardi, S. An experimental technique for measuring high solubilities of dyes in supercritical carbon dioxide. J. Supercrit. Fluid. 2004, 30, 41–49. [Google Scholar] [CrossRef]
- Tissera, N.D.; Wijesena, R.N.; de Silva, K.N. Ultrasound energy to accelerate dye uptake and dye–fiber interaction of reactive dye on knitted cotton fabric at low temperatures. Ultrason. Sonochem. 2016, 29, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Naebe, M.; Cookson, P.G.; Rippon, J.; Brady, R.P.; Wang, X.; Brack, N.; van Riessen, G. Effects of plasma treatment of wool on the uptake of sulfonated dyes with different hydrophobic properties. Text. Res. J. 2010, 80, 312–324. [Google Scholar] [CrossRef]
- Ibarra, L.; Chamorro, C. Short fiber–elastomer composites. Effects of matrix and fiber level on swelling and mechanical and dynamic properties. J. Appl. Polym. Sci. 1991, 43, 1805–1819. [Google Scholar] [CrossRef]
- Benltoufa, S.; Fayala, F.; Nasrallah, S.B. Determination of yarn and fiber diameters after swelling using a capillary rise method. J. Text. Inst. 2012, 103, 517–522. [Google Scholar] [CrossRef]
- Liu, R.Y.F.; Hu, Y.S.; Schiraldi, D.A.; Hiltner, A.; Baer, E. Crystallinity and oxygen transport properties of PET bottle walls. J. Appl. Polym. Sci. 2004, 94, 671–677. [Google Scholar] [CrossRef]
- Lee, K.W.; Chung, Y.S.; Kim, J.P. Characteristics of ultrasonic dyeing on poly (ethylene terephthalate). Text. Res. J. 2003, 73, 751–755. [Google Scholar]



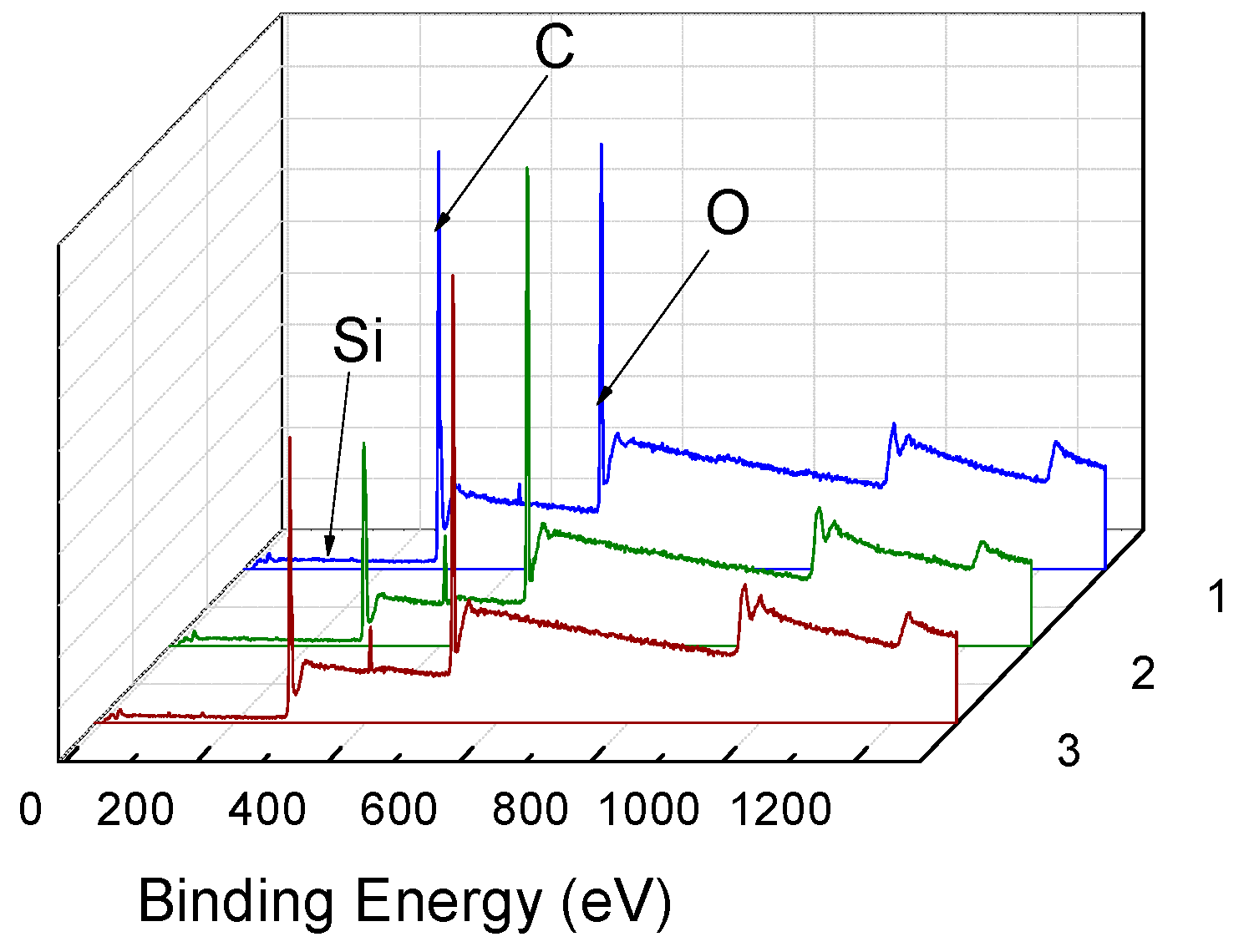

| Element | Atomic % | ||
|---|---|---|---|
| Before Dyeing | After Dyeing without Adding Accelerant | After Dyeing with 20% Accelerant | |
| C | 63.37 | 63.29 | 63.23 |
| O | 35.80 | 35.19 | 35.25 |
| Si | 0.83 | 1.52 | 1.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Cheng, W.; Gao, Y.; Zhu, L.; Pei, L. Mechanism of Accelerant on Disperse Dyeing for PET Fiber in the Silicone Solvent Dyeing System. Polymers 2019, 11, 520. https://doi.org/10.3390/polym11030520
Wang J, Cheng W, Gao Y, Zhu L, Pei L. Mechanism of Accelerant on Disperse Dyeing for PET Fiber in the Silicone Solvent Dyeing System. Polymers. 2019; 11(3):520. https://doi.org/10.3390/polym11030520
Chicago/Turabian StyleWang, Jiping, Wenqing Cheng, Yuanyuan Gao, Lei Zhu, and Liujun Pei. 2019. "Mechanism of Accelerant on Disperse Dyeing for PET Fiber in the Silicone Solvent Dyeing System" Polymers 11, no. 3: 520. https://doi.org/10.3390/polym11030520
APA StyleWang, J., Cheng, W., Gao, Y., Zhu, L., & Pei, L. (2019). Mechanism of Accelerant on Disperse Dyeing for PET Fiber in the Silicone Solvent Dyeing System. Polymers, 11(3), 520. https://doi.org/10.3390/polym11030520