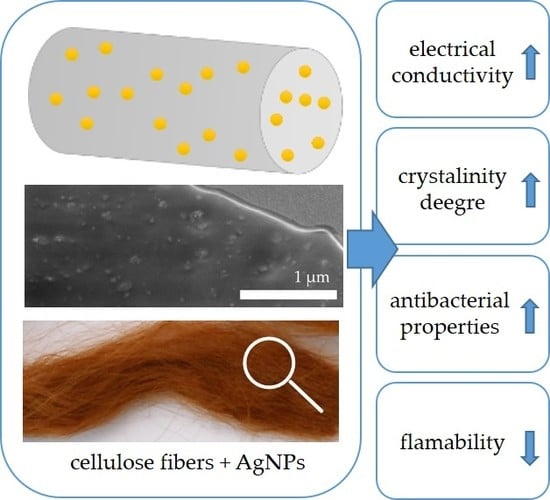
Multifunctional Nanocomposite Cellulose Fibers Doped in Situ with Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sellulose Fibers
2.3. Structure and Morphology Analysis
2.4. Thermal Properties
2.5. Determination of Antimicrobial Activity
2.6. Electrical Conductivity
2.7. Fastness Test to Washing
3. Results and Discussion
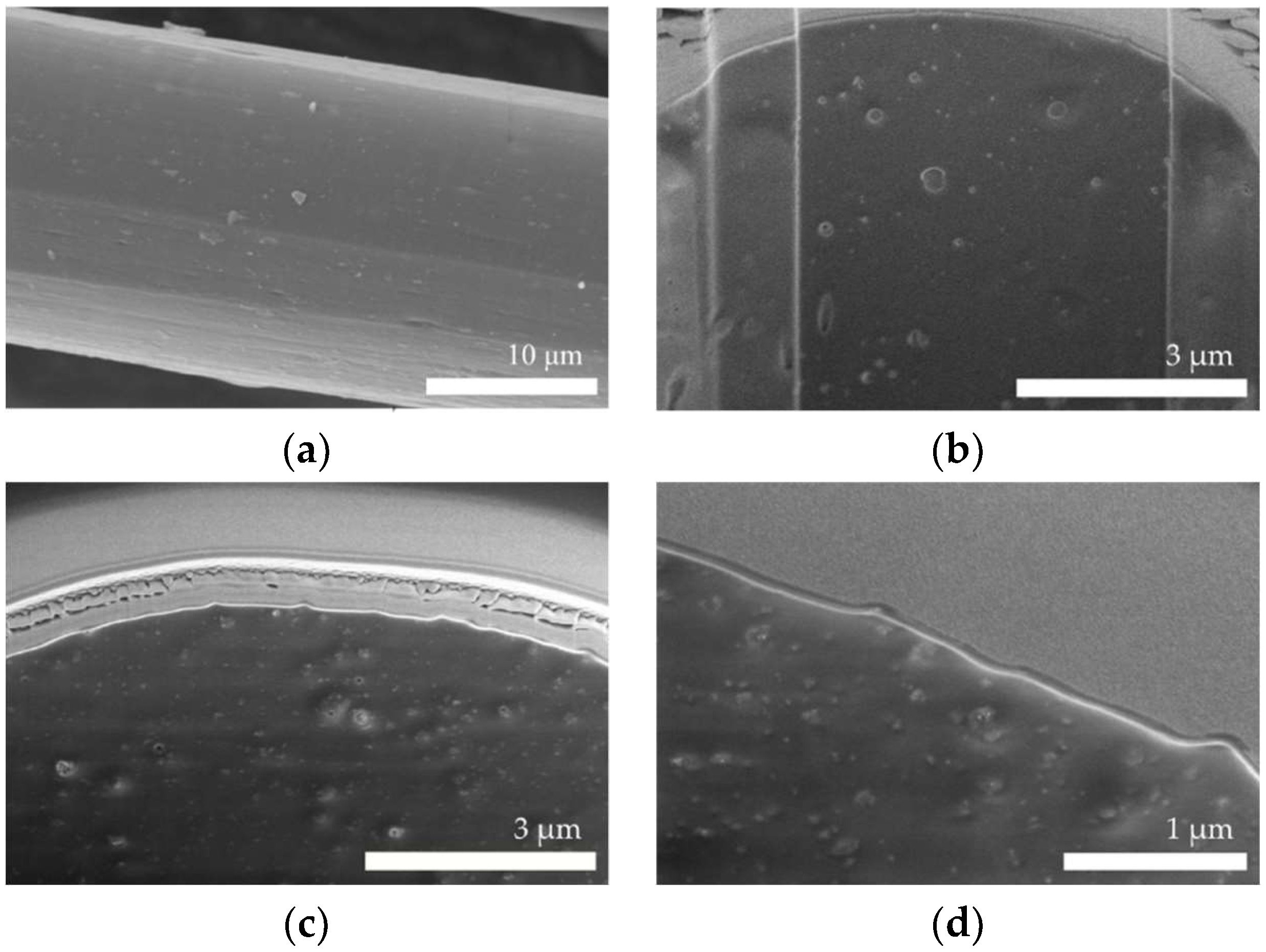
3.1. Microstructure of Fibers
3.2. Analysis of the Structural Properties (XRD)
3.3. FTIR of the Fibers
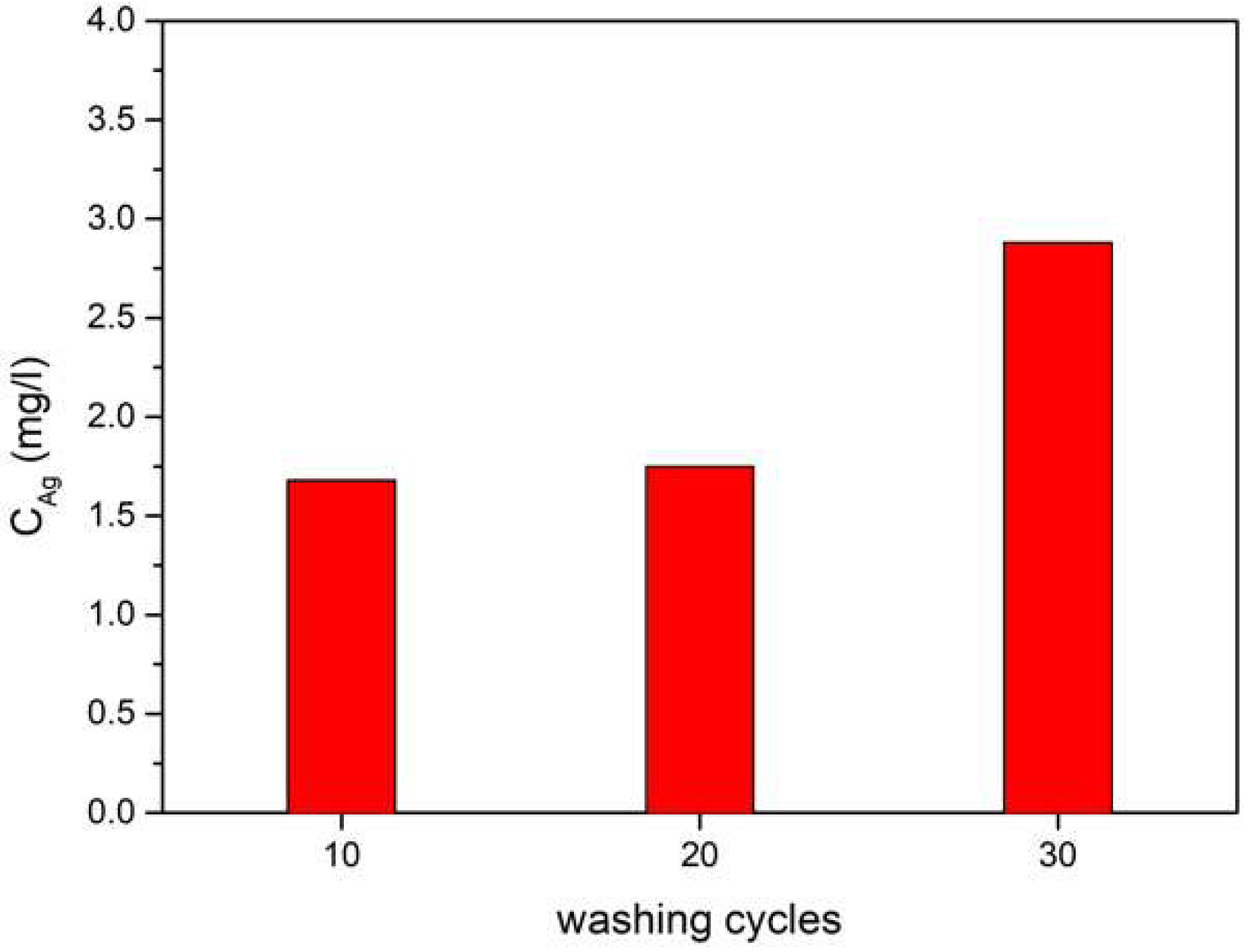
3.4. Release of Silver Nanoparticles
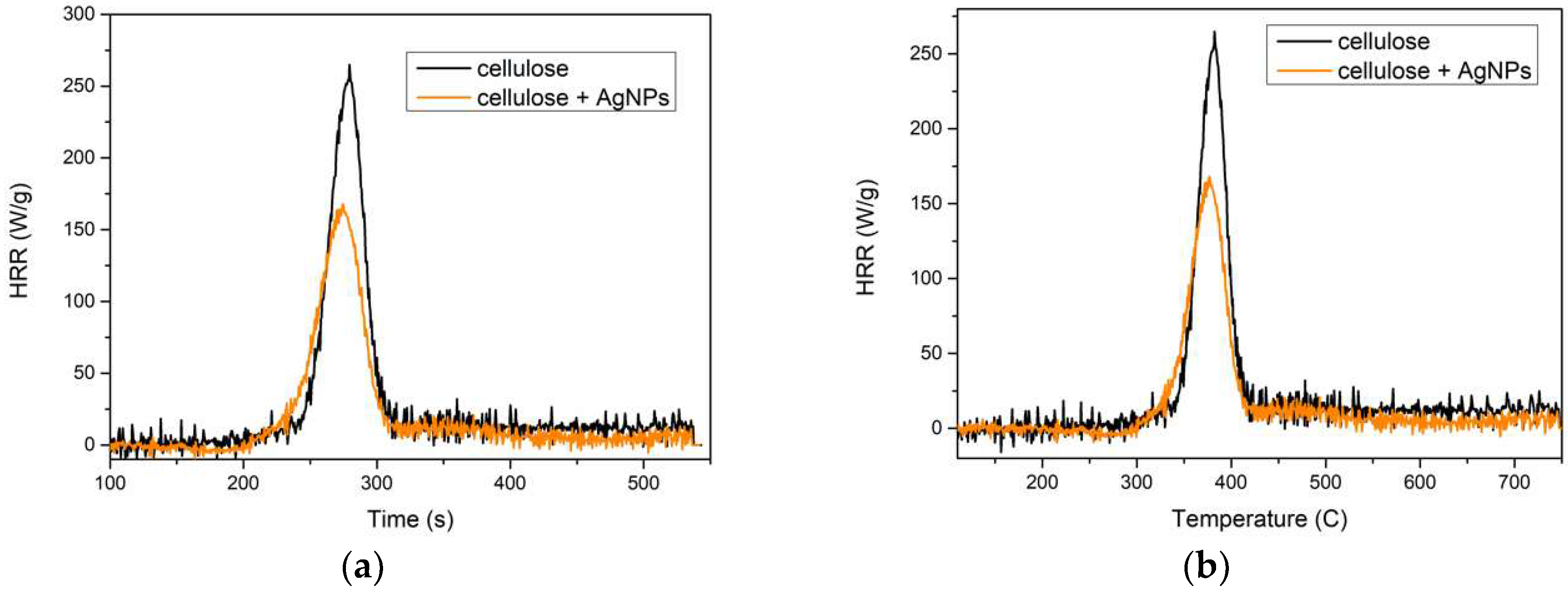
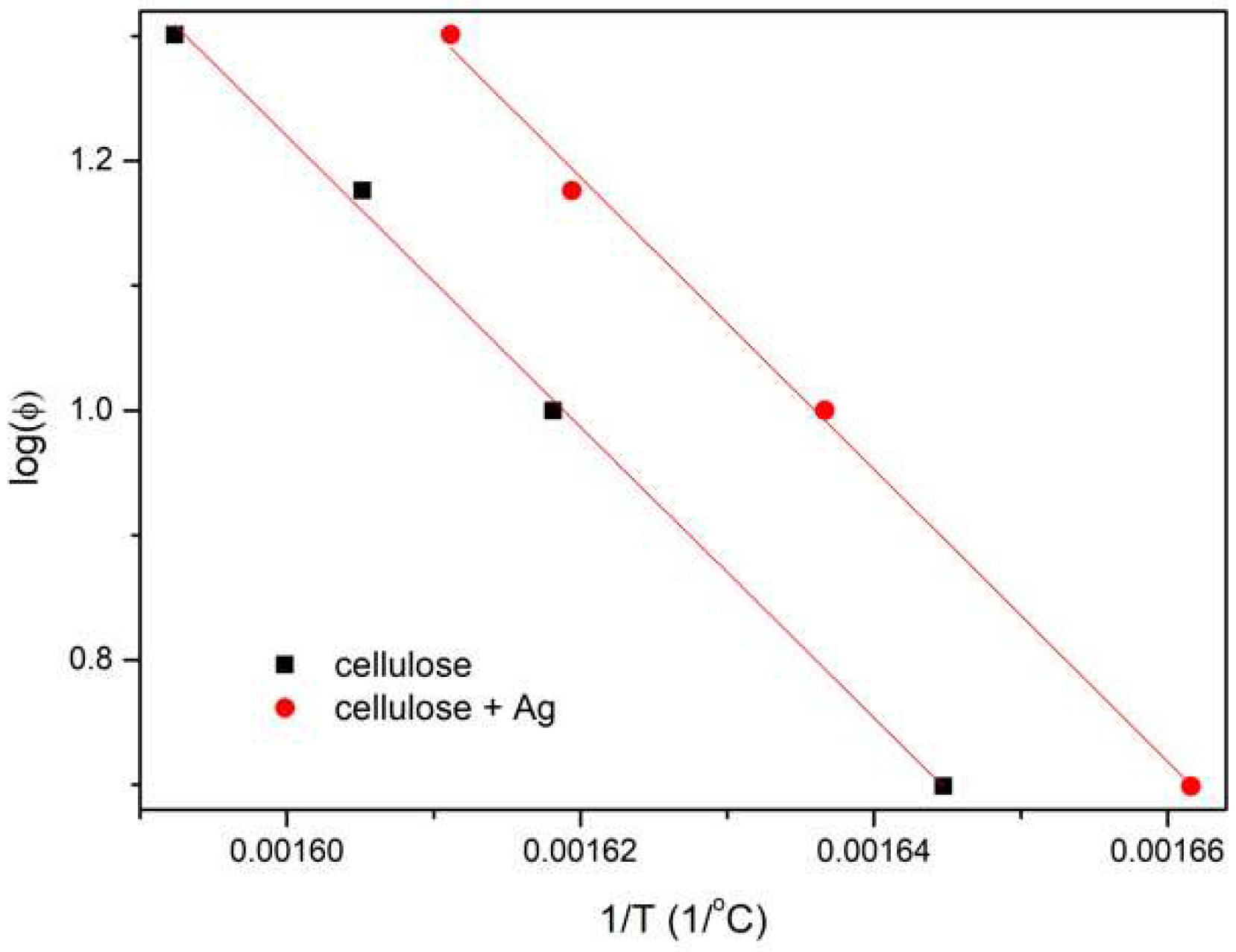
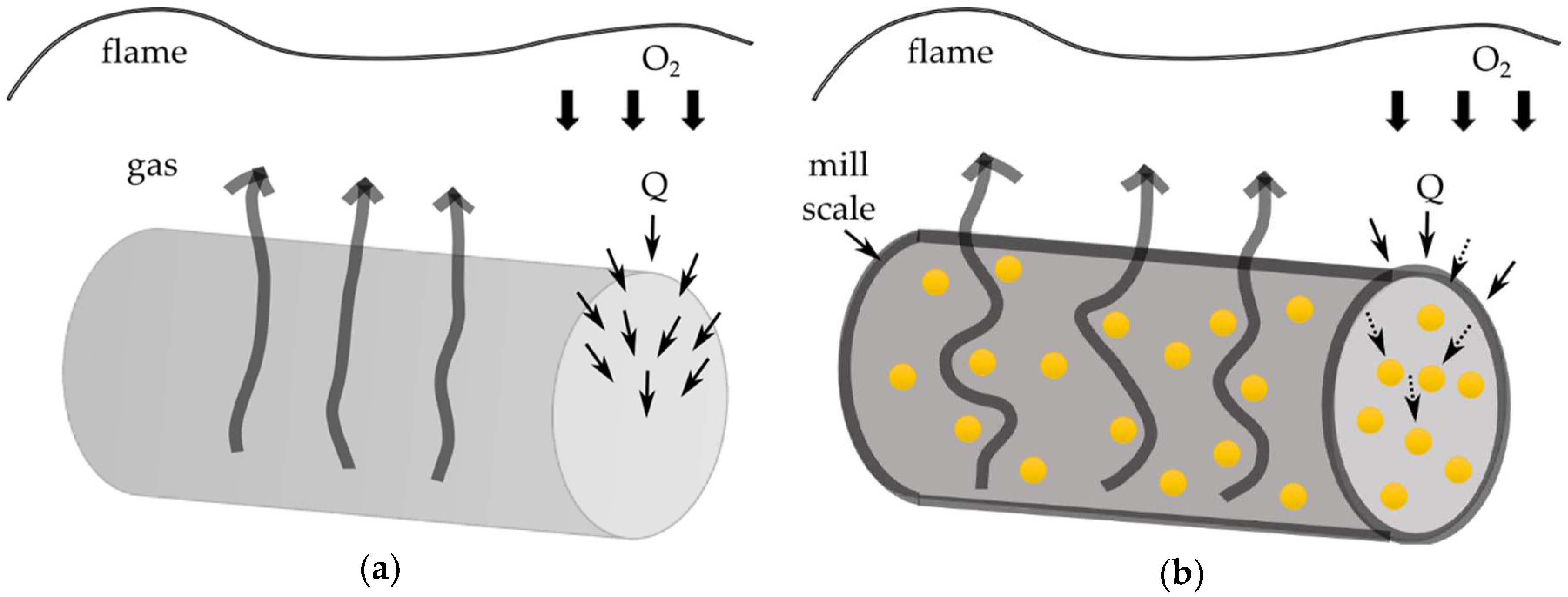
3.5. Flammability
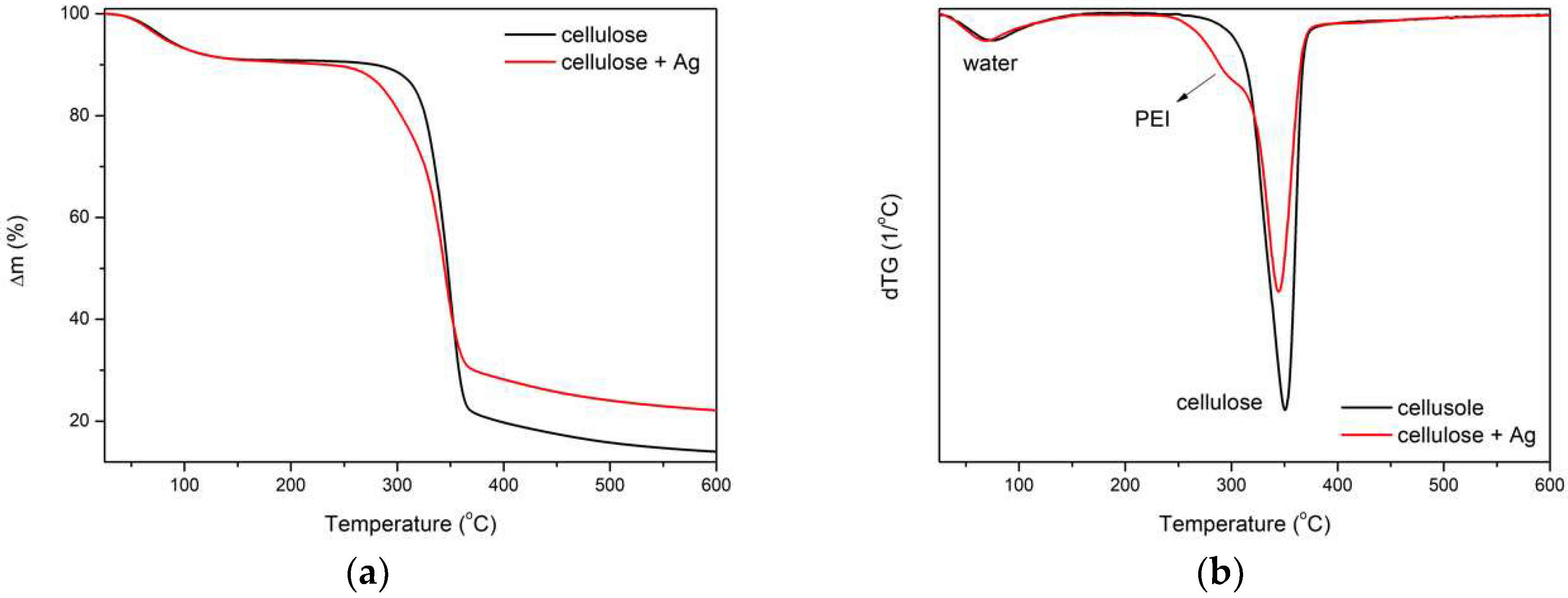
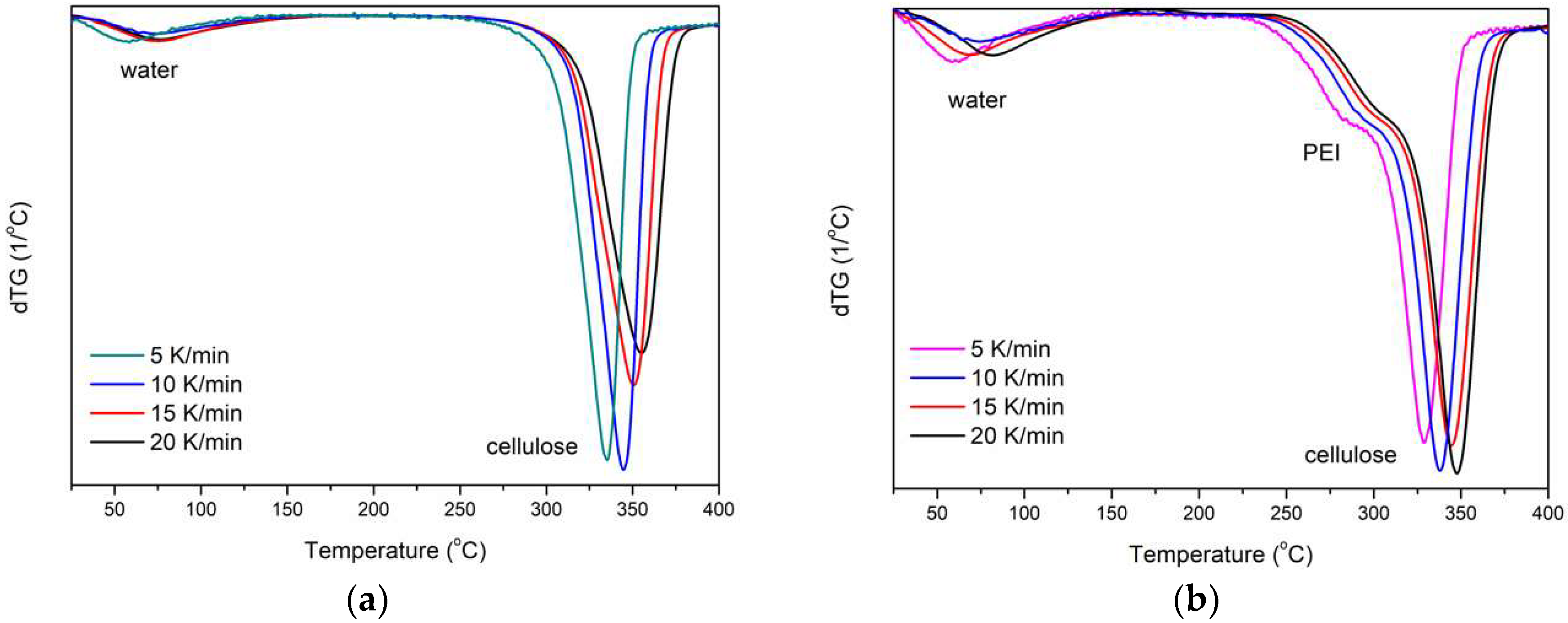
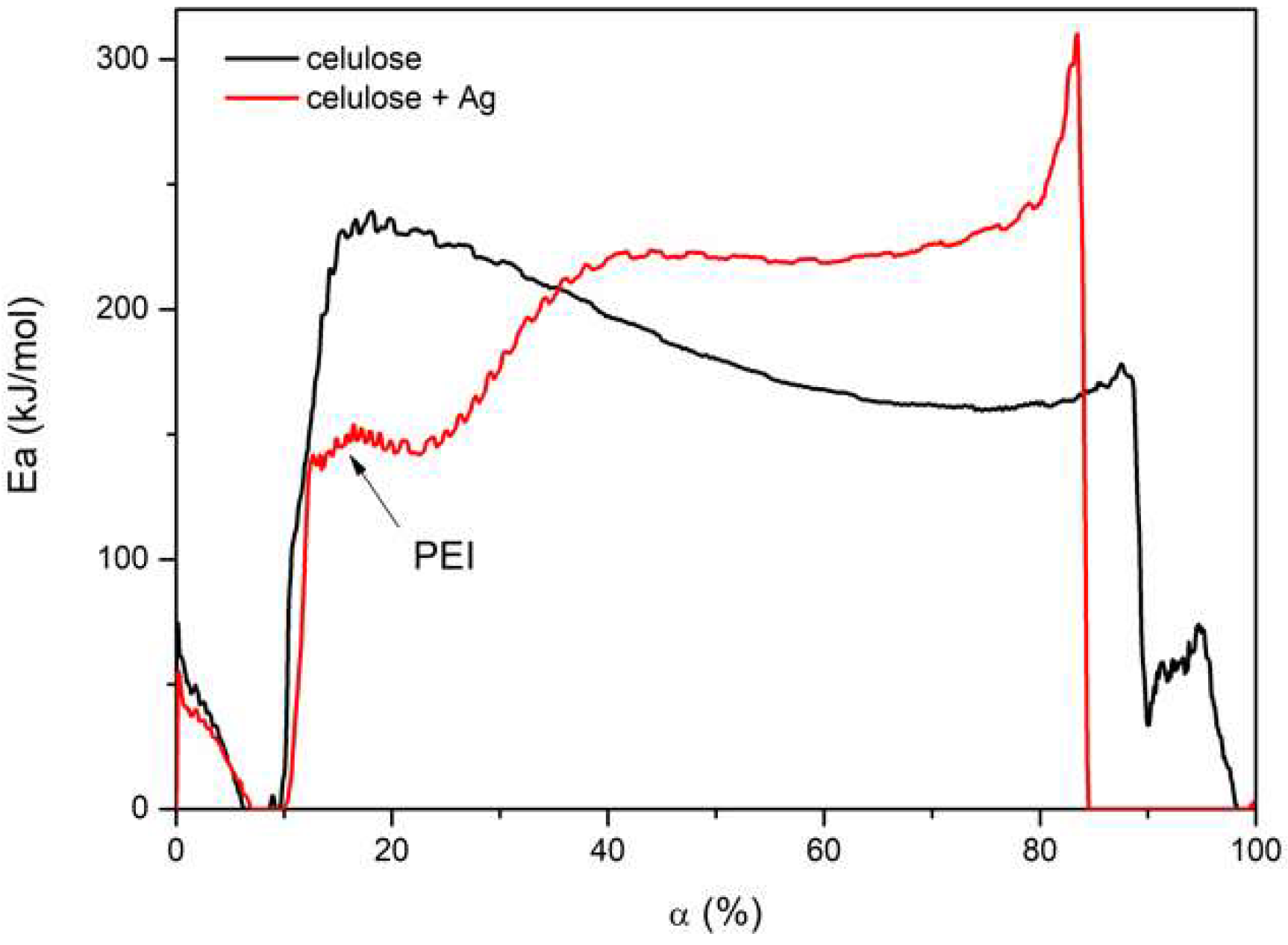
3.6. TG of Cellulose Fibers
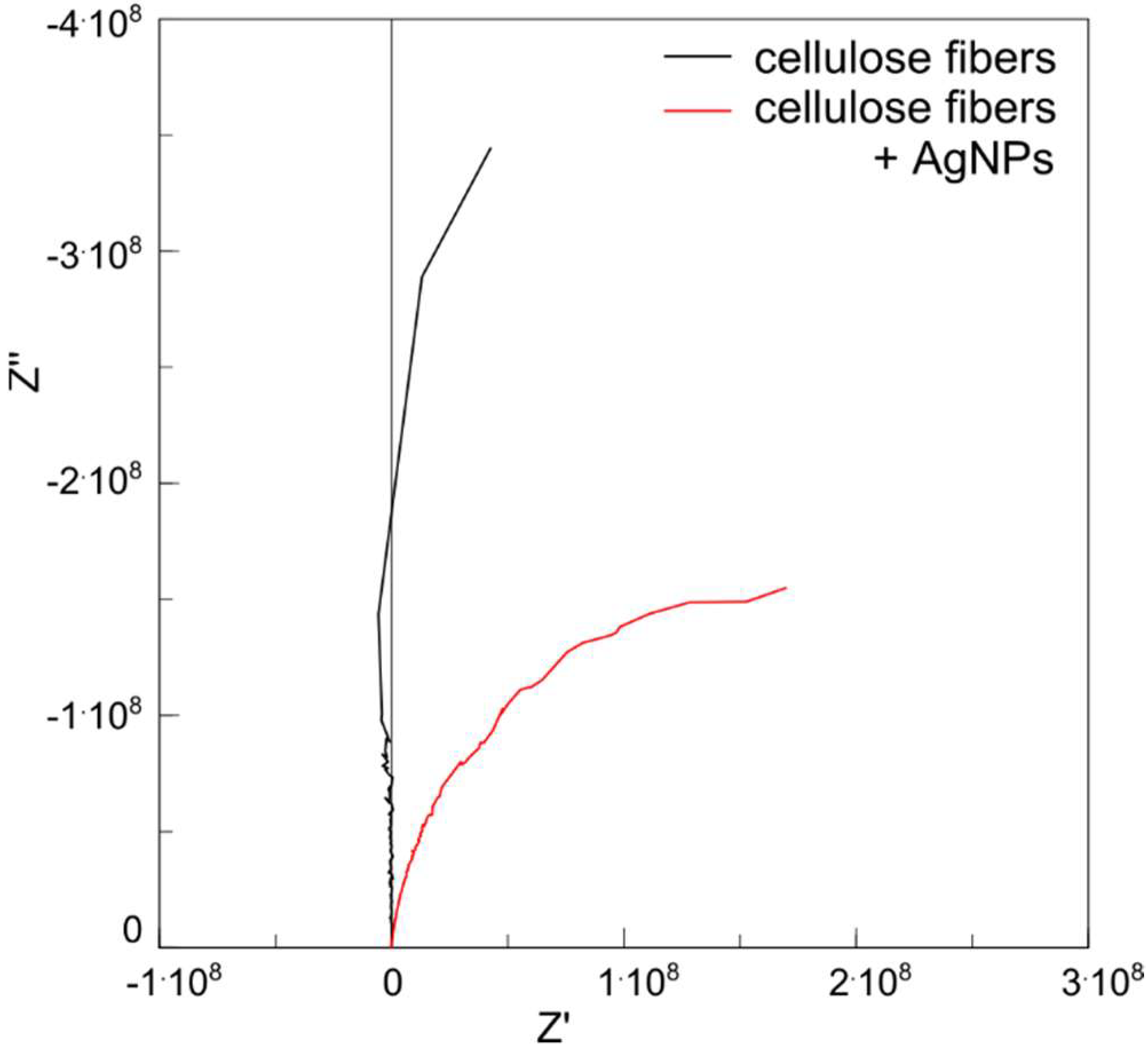
3.7. Electric Characterization of Fibers
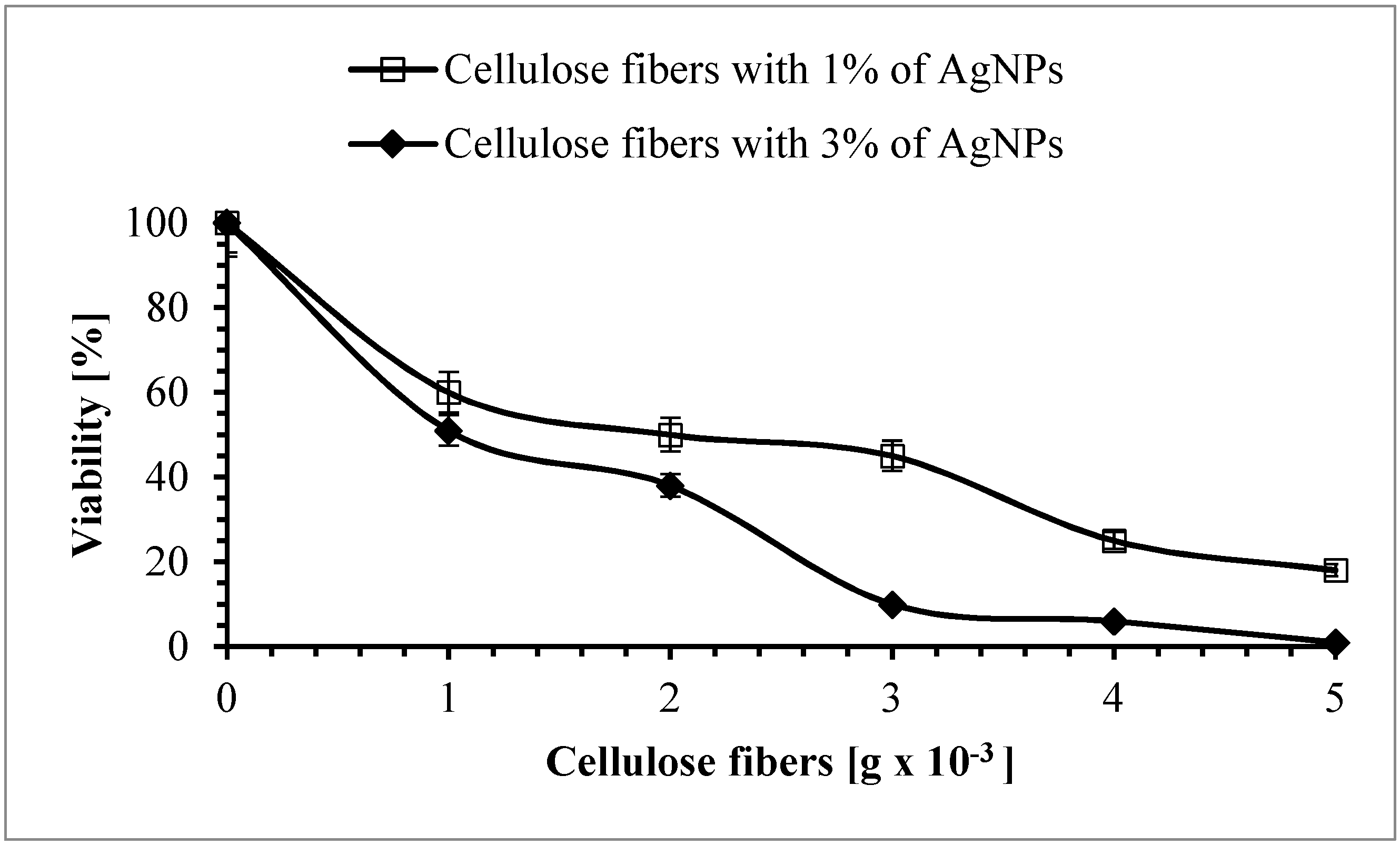
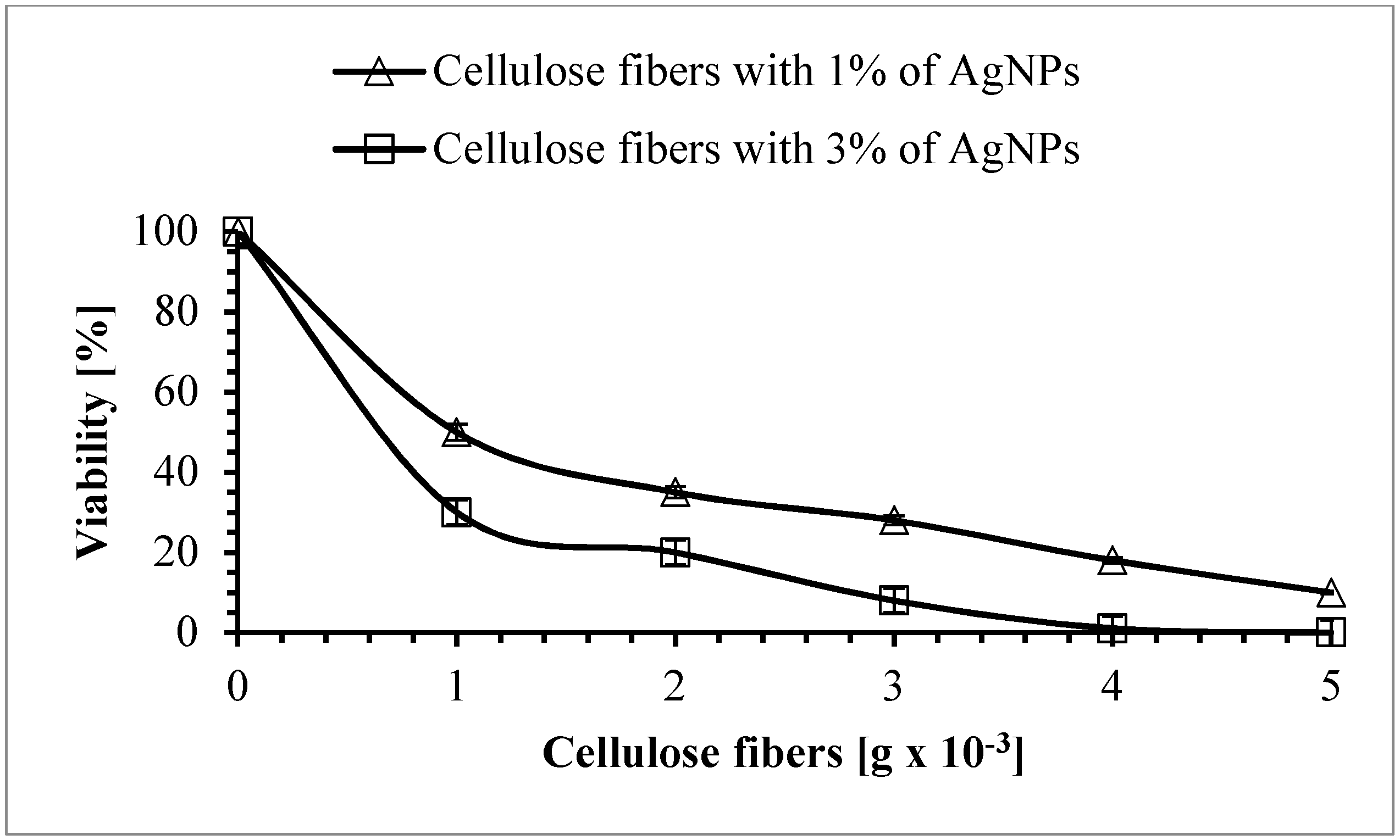
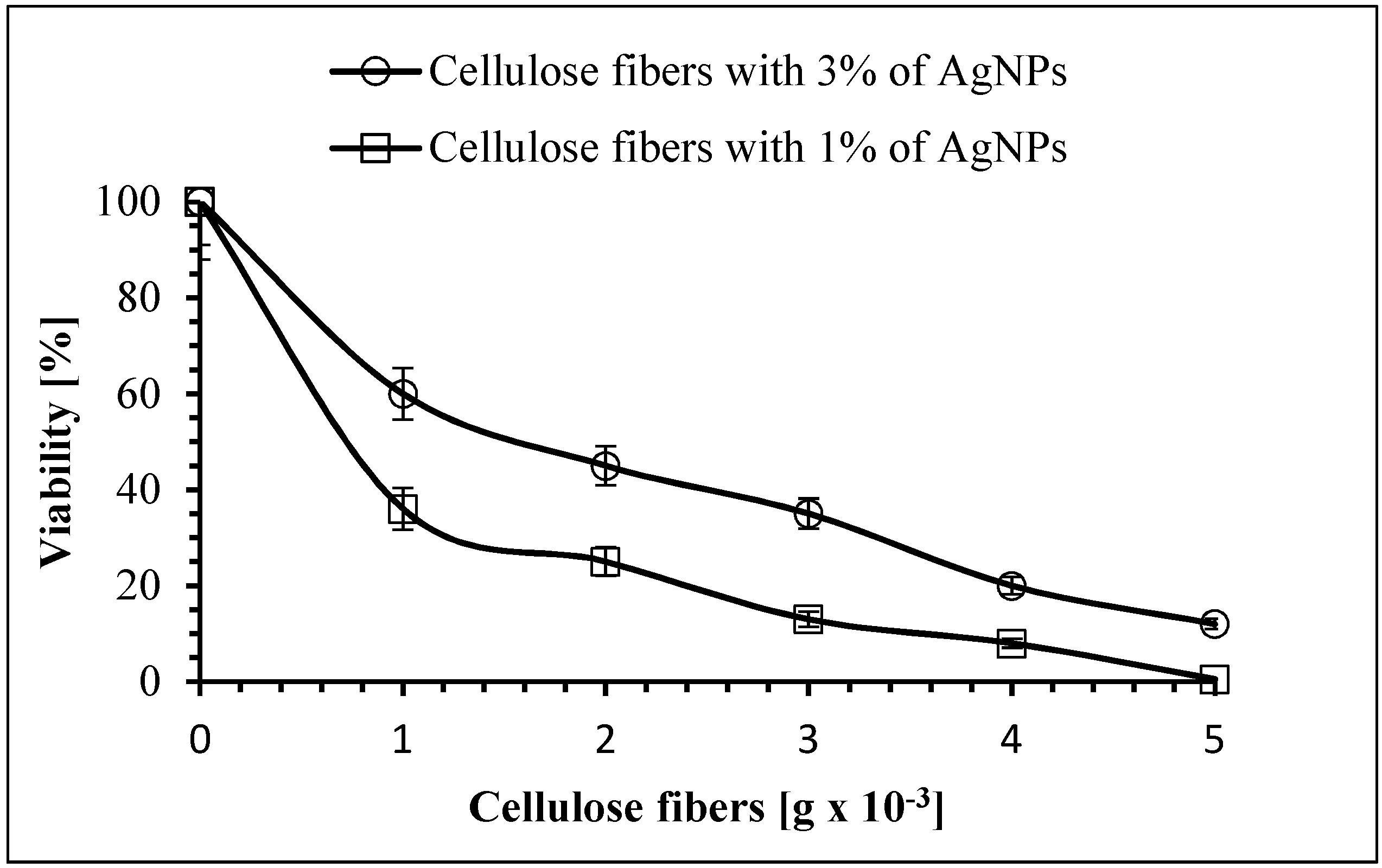
3.8. Antimicrobial Properties of the Cellulose Fibers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Peng, X. Silver nanoparticle decorated cellulose nanofibrous membrane with good antibacterial ability and high water permeability. Appl. Mater. Today 2017, 9, 130–135. [Google Scholar] [CrossRef]
- Fiedot, M.; Karbownik, I.; Maliszewska, I.H.; Rac, O.; Suchorska-Woźniak, P.; Teterycz, H. Deposition of one-dimensional zinc oxide structures on polypropylene fabrics and their antibacterial properties. Text. Res. 2015, 85, 1340–1354. [Google Scholar] [CrossRef]
- Shaban, M.; Abdallah, S.; Khalek, A.A. Characterization and photocatalytic properties of cotton fibers modified with ZnO nanoparticles using sol–gel spin coating technique. Beni-Seuf Univ. J. Appl. Sci. 2016, 5, 277–283. [Google Scholar] [CrossRef]
- Wang, L.H.; Ren, Y.L.; Wang, X.L.; Zhao, J.Y.; Zhang, Y.; Zeng, Q.; Gu, Y.T. Fire retardant viscose fiber fabric produced by graft polymerization of phosphorus and nitrogen-containing monomer. Cellulose 2016, 23, 2689–2700. [Google Scholar] [CrossRef]
- Fiedot, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Lewińska, A.; Teterycz, H. The relationship between the mechanism of zinc oxide crystallization and its antimicrobial properties for the surface modification of surgical meshes. Materials 2017, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; Eid, B.M.; El-Aziz, E.A.; Elmaaty, T.M.A.; Ramadan, S.M. Multifunctional cellulose-containing fabrics using modified finishing formulations. RSC Adv. 2017, 7, 33219–33230. [Google Scholar] [CrossRef]
- Cioffi, N.; Rai, M. Nano-Antimicrobials: Progress and Prospects; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Damm, C.; Münstedt, H. Kinetic aspects of the silver ion release from antimicrobial polyamide/silver nanocomposites. Appl. Phys. A 2008, 91, 479–486. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Raju, K.M.; Sambasivudu, K.; Singh, S.; Sreedhar, B. Preparation of acacia-stabilized silver nanoparticles: A green approach. J. Appl. Polym. Sci. 2007, 106, 3375–3381. [Google Scholar] [CrossRef]
- Madkour, T.M.; Abdelazeem, E.A.; Tayel, A.; Mustafa, G.; Siam, R. In situ polymerization of polyurethane-silver nanocomposite foams with intact thermal stability, improved mechanical performance, and induced antimicrobial properties. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, J.; Yang, C.; Sakai, E. Preparation and application of conducting polymer/Ag/clay composite nanoparticles formed by in situ UV-induced dispersion polymerization. Sci. Rep. 2016, 6, 20470. [Google Scholar] [CrossRef] [PubMed]
- Faraji, N.; Younus, W.M.M.; Kharazmi, A.; Saion, E.; Shahmiri, M.; Tamchek, N. Synthesis, characterization and nonlinear optical properties of silver/PVA nanocomposites. J. Eur. Opt. Soc. Rapid Publ. 2012, 7. [Google Scholar] [CrossRef]
- Tang, B.; Kaur, J.; Sun, L.; Wang, X. Multifunctionalization of cotton through in situ green synthesis of silver nanoparticles. Cellulose 2013, 20, 3053–3065. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Chakraborty, S. Coating of silver nanoparticles on jute fibre by in situ synthesis. Cellulose 2017, 24, 1563–1577. [Google Scholar] [CrossRef]
- Zheng, J.; Song, F.; Wang, X.L.; Wang, Y.Z. In-situ synthesis, characterization and antimicrobial activity of viscose fiber loaded with silver nanoparticles. Cellulose 2014, 21, 3097–3105. [Google Scholar] [CrossRef]
- Ashayer-Soltani, R.; Hunt, C.; Thomas, O. Fabrication of highly conductive stretchable textile with silver nanoparticles. Text. Res. J. 2016, 86, 1041–1049. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Justice, R.S. How nano are nanocomposites? Macromolecules 2007, 40, 8501–8517. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Fakirov, S. Nano-Size Polymers: Preparation, Properties, Applications; Springer: Berlin, Germany, 2016. [Google Scholar]
- Rac-Rumijowska, O.; Fiedot, M.; Karbownik, I.; Suchorska-Woźniak, P.; Teterycz, H. Synthesis of silver nanoparticles in NMMO and their in situ doping into cellulose fibers. Cellulose 2017, 24, 1355–1370. [Google Scholar] [CrossRef]
- ASTM:D 7309-07a. Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry; ASTM International: West Conshohocken, PA, USA, 2007; Available online: www.astm.org (accessed on 24 March 2019).
- Aneja, R.K. Experiments in Microbiology, Plant Pathology and Biotechnology; New Age Publishers: New Delhi, India, 2005; Volume 69, ISBN 81-224-1494-X. [Google Scholar]
- American Association of Textile Chemists and Colorists (AATCC). Standardization of Home Laundry Test Conditions. AATCC Technol. Man. 2010, 85, 401–402. [Google Scholar]
- Hinrichsen, G. Structural changes of drawn polyacrylonitrile during annealing. J. Polym. Sci. C 1971, 38, 303–319. [Google Scholar] [CrossRef]
- Rosenbrock, H.H. Some general implicit processes for the numerical solution of differential equations. Comput. J. 1963, 5, 329–330. [Google Scholar] [CrossRef]
- Rabiej, M.; Rabiej, S. Analiza Rentgenowskich Krzywych Dyfrakcyjnych Polimerów za Pomocą Programu Komputerowego WAXSFIT; Wydawnictwo Akademii Techniczno-Humanistycznej: Bielsko-Biała, Poland, 2006. [Google Scholar]
- Patterson, A. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D. Determination of crystal structure of polyaniline and substituted polyanilines through powder X-ray diffraction analysis. Polym. Test. 2008, 27, 851–857. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Dai, D.; Huang, B. Fourier transform infrared spectroscopy for natural fibres. In Fourier Transform-Materials Analysis; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Azari, A.; Kalantary, R.R.; Ghanizadeh, G.; Kakavandi, B.; Farzadkia, M.; Ahmadi, E. Iron–silver oxide nanoadsorbent synthesized by co-precipitation process for fluoride removal from aqueous solution and its adsorption mechanism. RSC Adv. 2015, 5, 87377–87391. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Jovic, M.; Ragaisiene, A.; Rukuiziene, Z.; Milasius, R.; Mikucioniene, D.; Gaan, S. Flammability of cellulose-based fibers and the effect of structure of phosphorus compounds on their flame retardancy. Polymers 2016, 8, 293. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Song, X.; Gu, X.; Sun, H.; Fu, C.; Meng, F. Synthesis of superparamagnetic iron oxide nanoparticles modified with MPEG-PEI via photochemistry as new MRI contrast agent. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G. Effect of phosphorus flame retardants on thermo-oxidative decomposition of cotton. Polym. Degrad. Stab. 2007, 92, 968–974. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Y.; Kai, Y.; Jin, R.G. Structural evolution and kinetic study of high isotacticity poly (acrylonitrile) during isothermal pre-oxidation. Carbon Lett. 2011, 12, 229–235. [Google Scholar] [CrossRef]
- Coats, A.V.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285. [Google Scholar] [CrossRef]
- Vyazovkin, S. Model-free kinetics. J. Therm. Anal. Calorim. 2006, 83, 45–51. [Google Scholar] [CrossRef]
- Gniotek, K.; Zięba, J.; Frydrysiak, M.; Tokarska, M. Pomiary rezystancji przejścia między dwiema nitkami elektroprzewodzącymi. Pomiary Automatyka Kontrola 2010, 56, 1020–1023. [Google Scholar]
- Dakal, T.C.H.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microb. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Aniket Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 2, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2008, 52, 662–668. [Google Scholar] [CrossRef]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2010, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Hwang, I.S.; Lee, J.; Hwang, J.H.; Kim, K.J.; Lee, D.G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Antibacterial characterization of silver nanoparticles against E. coil ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Ghosh, M.J.M.; Sinha, S.; Chakraborty, A.; Mallick, S.K.; Bandyopadhyay, M.; Mukherjee, A. In vitro and in vivo genotoxicity of silver nanoparticles. Mutat. Res. 2012, 12, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, W.J.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar]
- Li, J.; Rong, K.; Zhao, H.; Li, F.; Lu, Z.; Chen, R. Highly selective antibacterial activities of silver nanoparticles against Bacillus subtilis. J. Nanosci. Nanotechnol. 2013, 13, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, F.; Ghassempour, A.; Aliahmadi, A.; Esmaeili, M.A. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res. Microbiol. 2011, 162, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad- spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103–225111. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 5, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rac-Rumijowska, O.; Fiedot-Toboła, M.; Suchorska-Woźniak, P.; Karbownik, I.; Stafiniak, A.; Teterycz, H. Depositing of conductive silver nanoparticles layer on cellulose fibers. In Proceedings of the 2017 21st European Microelectronics and Packaging Conference (EMPC) & Exhibition, Warsaw, Poland, 10–13 September 2017; pp. 1–4. [Google Scholar]

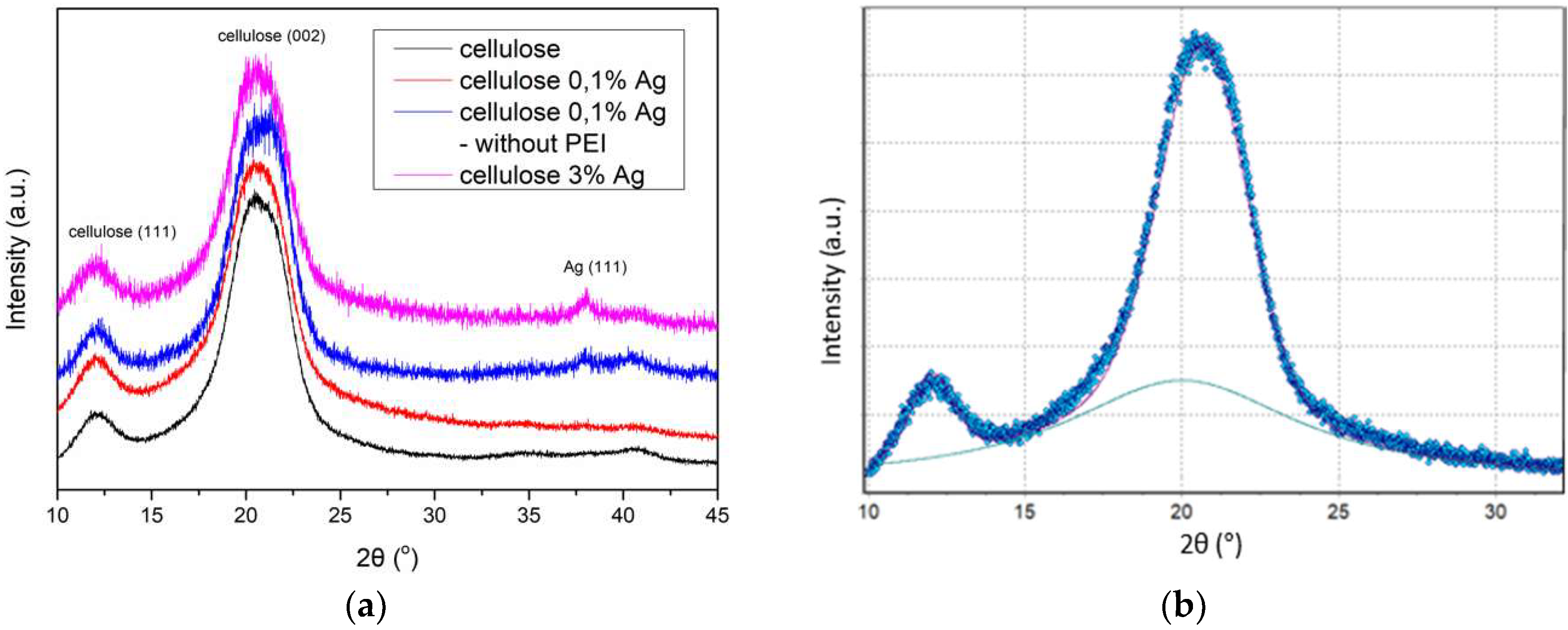

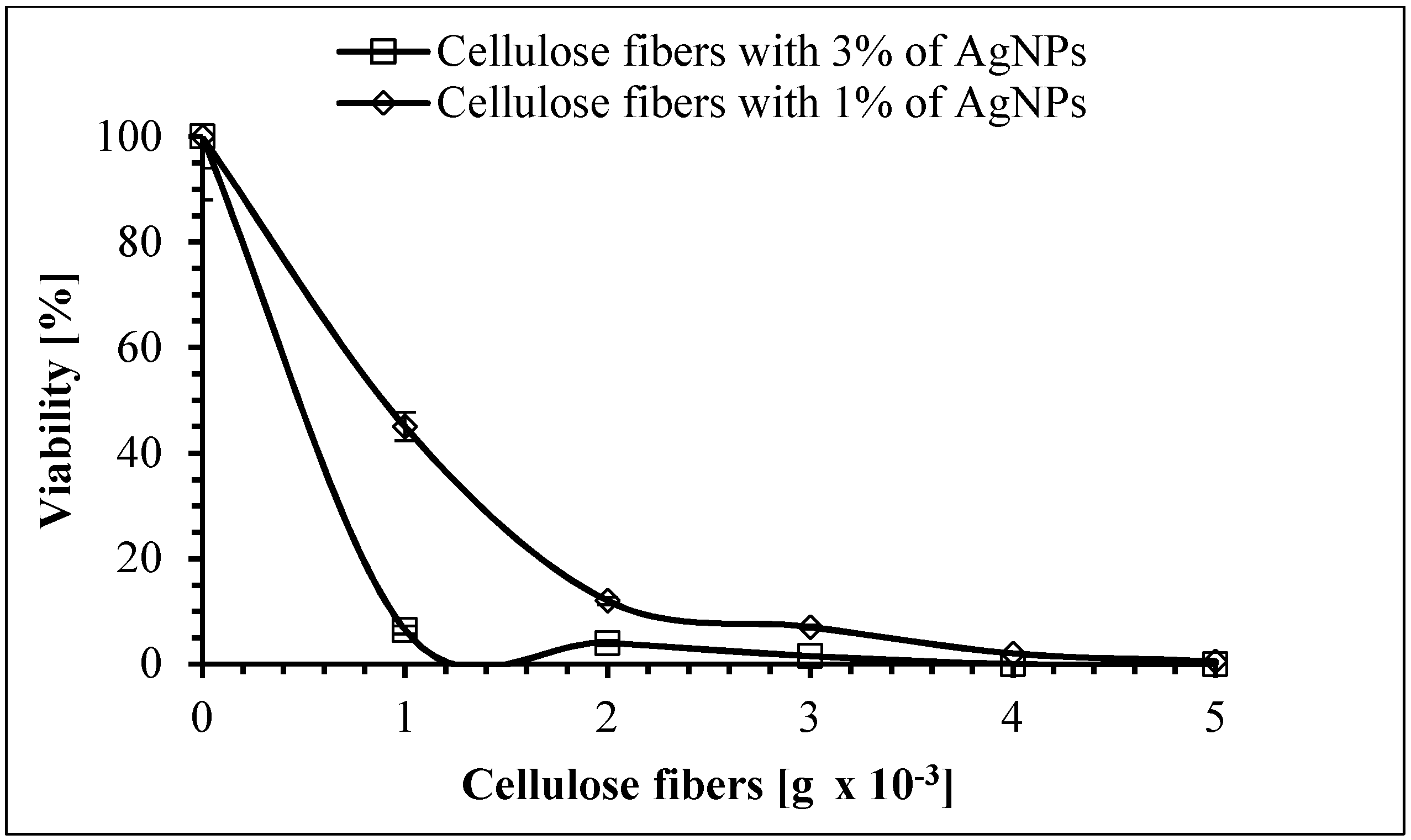
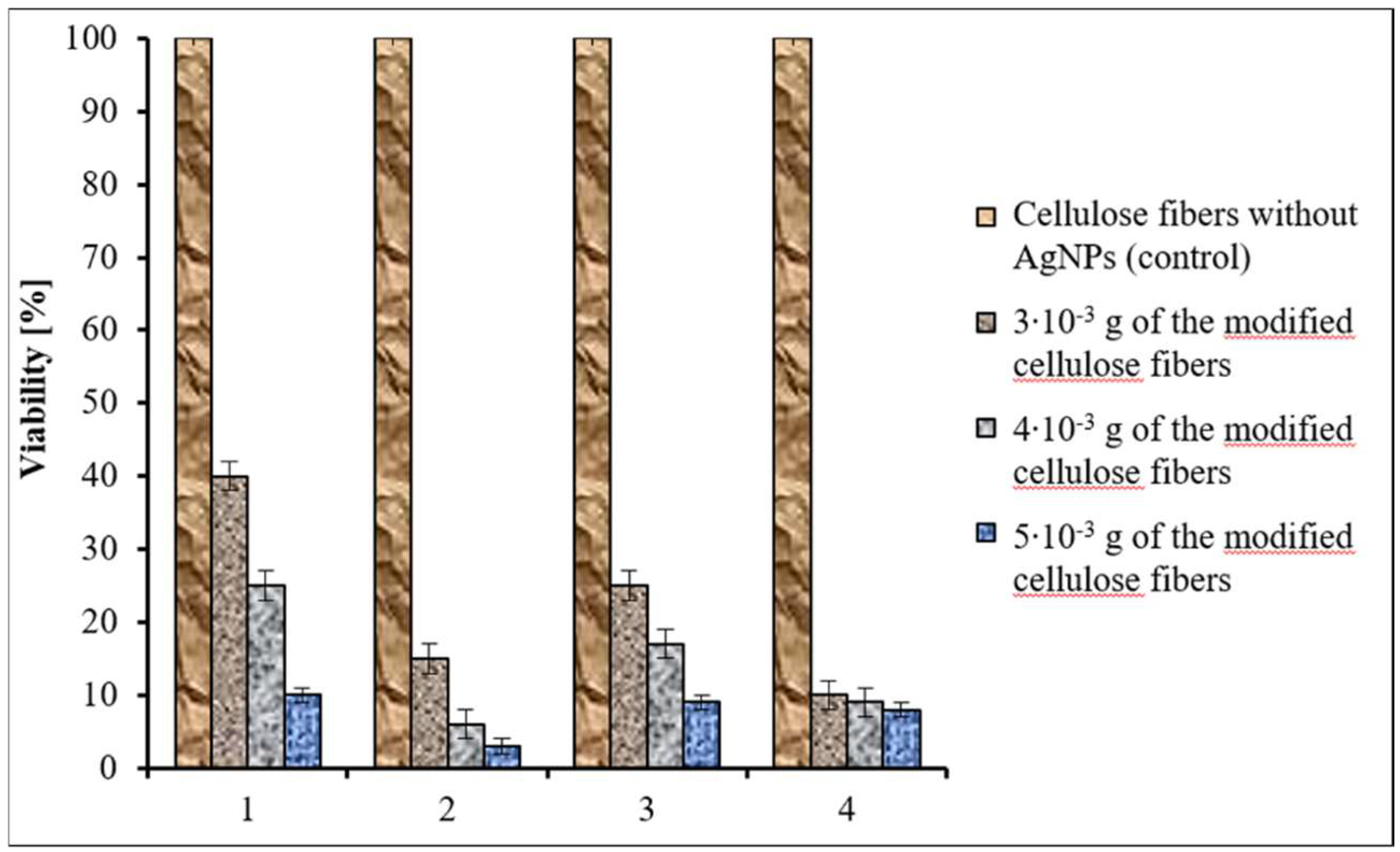
| Ag Concentration [wt %] | Crystallinity Degree | L(002) [nm] | Ag L(111) [nm] | R [Å] |
|---|---|---|---|---|
| – | 0.54 | 4 | - | 5.36 |
| 0.1 | 0.71 | 4 | 7 | 5.37 |
| 0.1 (without PEI) | 0.58 | 3 | 14 | 5.33 |
| 3 | 0.55 | 4 | 11 | 5.28 |
| Fibers | T max [s] | T max [°C] | HRR [W/g] | THR [kJ/g] |
|---|---|---|---|---|
| Cellulose | 279.5 | 382 | 274.5 | 10.56 |
| Cellulose + 3% AgNPs | 274.5 | 376 | 167.88 | 7.21 |
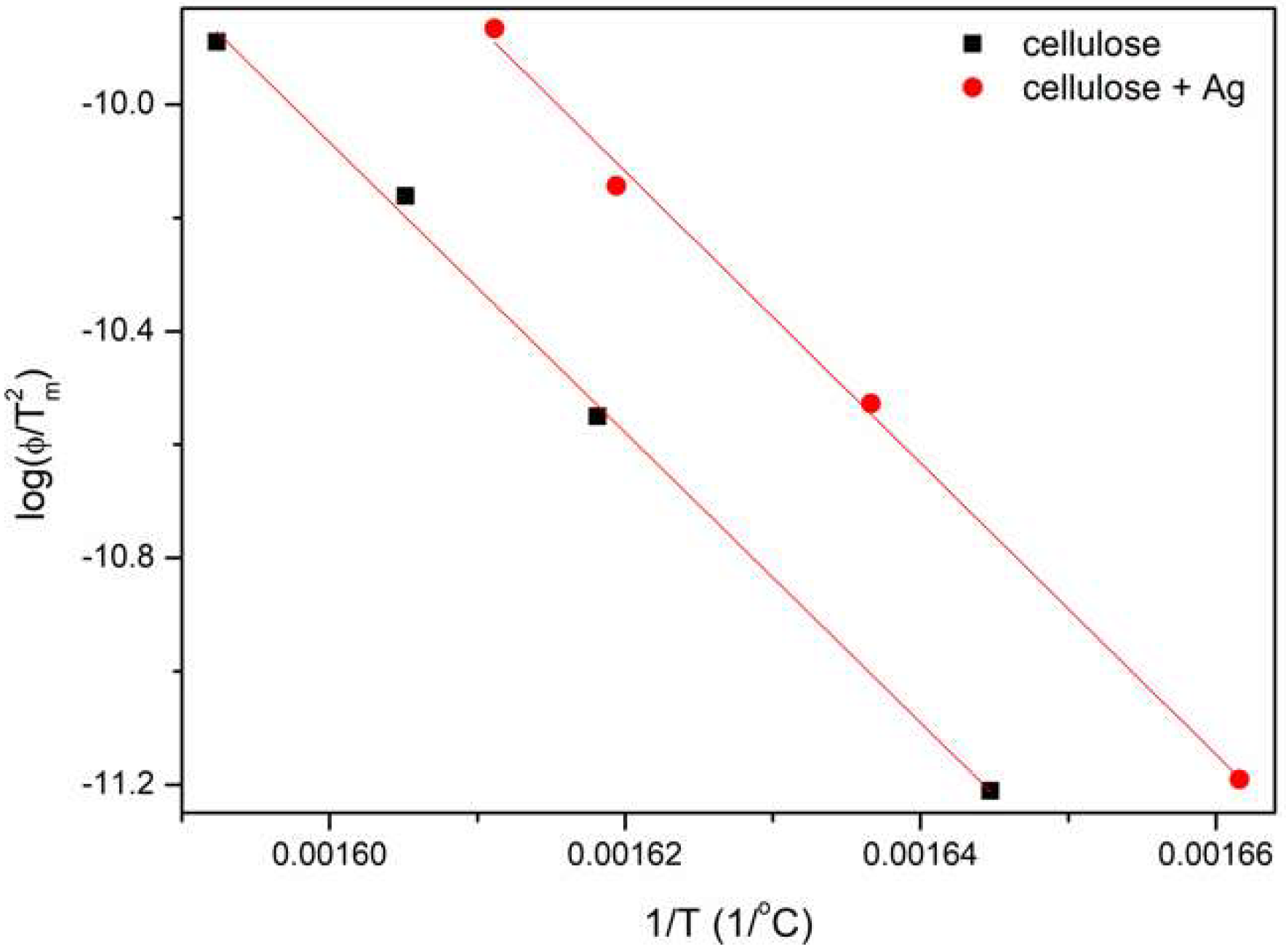
| Fibers | Ea KAS [kJ/mol] | Ea FWO [kJ/mol] | Ea śr. [kJ/mol] |
|---|---|---|---|
| Cellulose | 212.38 | 211.71 | 212.04 |
| Cellulose + 3% AgNPs | 213.56 | 212.72 | 213.14 |
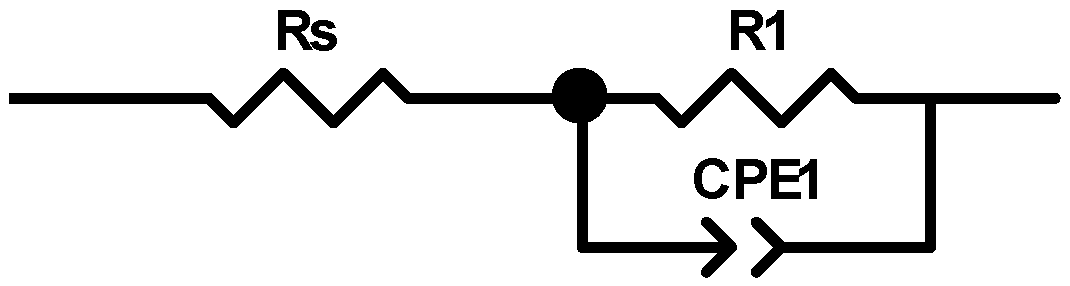
| Fibers | Rs [Ω] | R1 [Ω/cm] | CPE1-T [F] | CPE1-P |
|---|---|---|---|---|
| Cellulose | 920 | 2.18 × 1016 | 1.58 × 10−11 | 1 |
| Cellulose + 3% AgNPs | 920 | 2.98 × 108 | 4.64 × 10−11 | 0.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rac-Rumijowska, O.; Maliszewska, I.; Fiedot-Toboła, M.; Karbownik, I.; Teterycz, H. Multifunctional Nanocomposite Cellulose Fibers Doped in Situ with Silver Nanoparticles. Polymers 2019, 11, 562. https://doi.org/10.3390/polym11030562
Rac-Rumijowska O, Maliszewska I, Fiedot-Toboła M, Karbownik I, Teterycz H. Multifunctional Nanocomposite Cellulose Fibers Doped in Situ with Silver Nanoparticles. Polymers. 2019; 11(3):562. https://doi.org/10.3390/polym11030562
Chicago/Turabian StyleRac-Rumijowska, Olga, Irena Maliszewska, Marta Fiedot-Toboła, Iwona Karbownik, and Helena Teterycz. 2019. "Multifunctional Nanocomposite Cellulose Fibers Doped in Situ with Silver Nanoparticles" Polymers 11, no. 3: 562. https://doi.org/10.3390/polym11030562
APA StyleRac-Rumijowska, O., Maliszewska, I., Fiedot-Toboła, M., Karbownik, I., & Teterycz, H. (2019). Multifunctional Nanocomposite Cellulose Fibers Doped in Situ with Silver Nanoparticles. Polymers, 11(3), 562. https://doi.org/10.3390/polym11030562