Controlled ATRP Synthesis of Novel Linear-Dendritic Block Copolymers and Their Directed Self-Assembly in Breath Figure Arrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
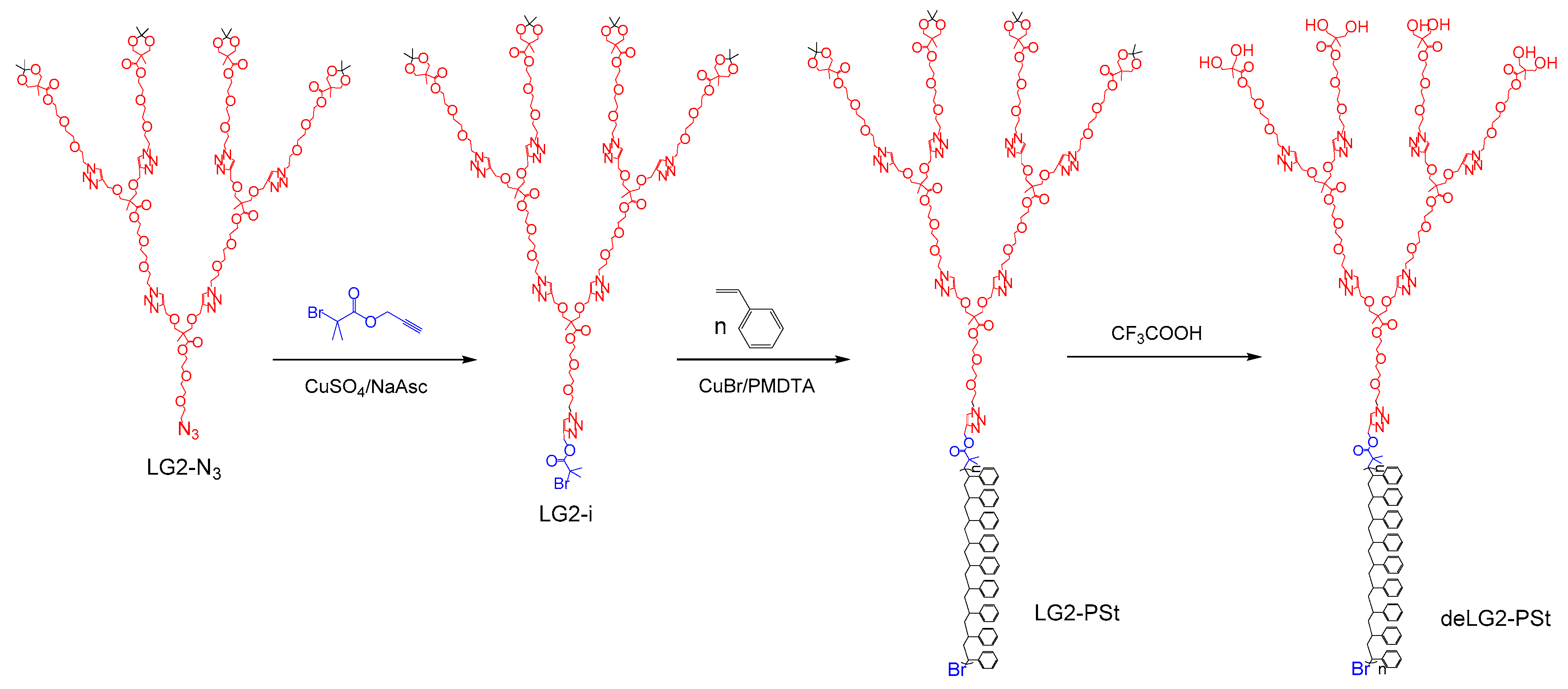
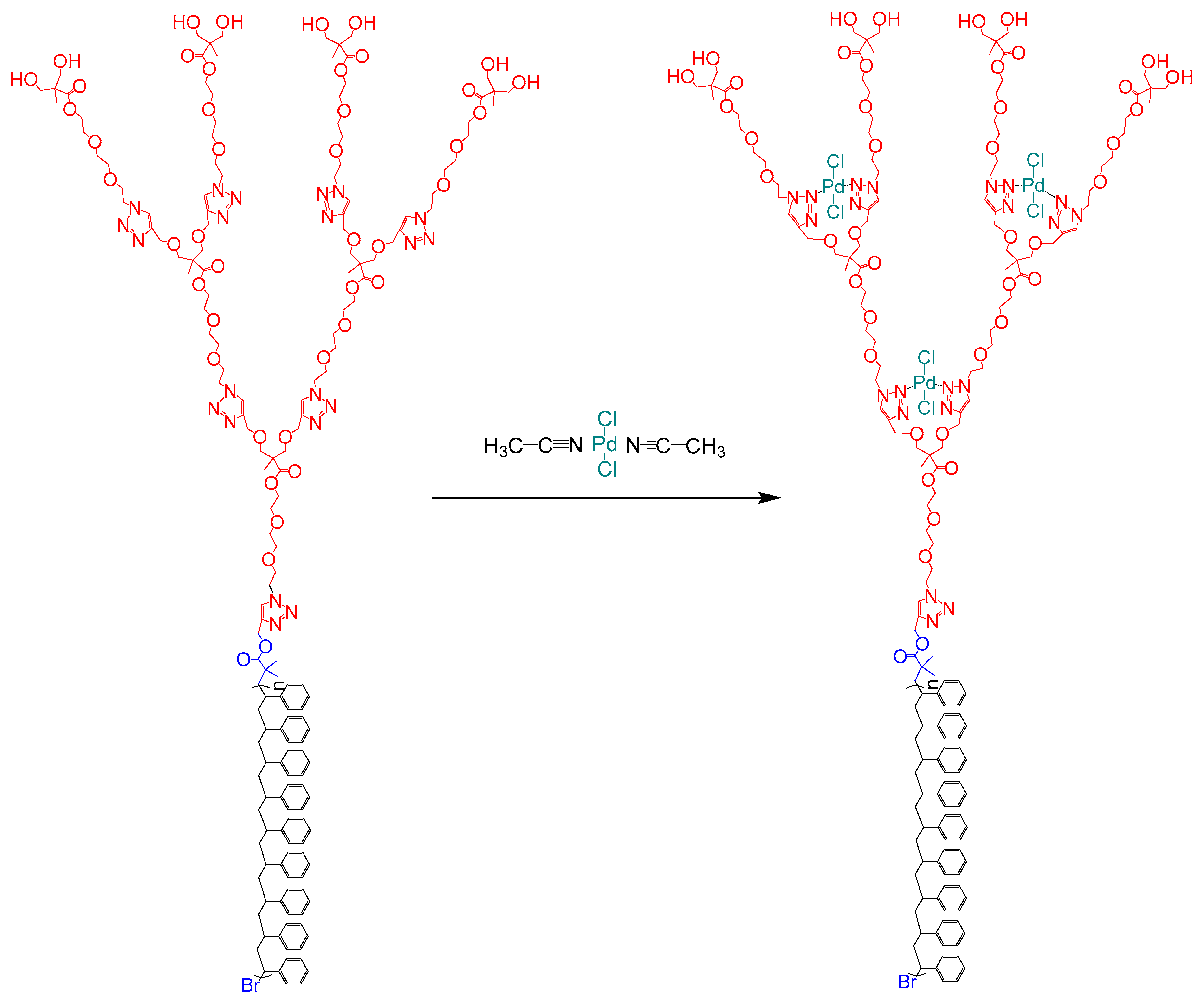
2.3. Synthesis of Dendritic Macroinitiators by “Click” Chemistry
2.3.1. First Generation PEE Dendron Initiator LG1-i
2.3.2. Second Generation PEE Dendron Initiator LG2-i
2.3.3. Third Generation PEE Dendron Initiator LG3-i
2.4. Synthesis of Linear-Dendritic block Copolymers by Dendron Initiated ATRP
2.5. Breath Figure Film Preparation
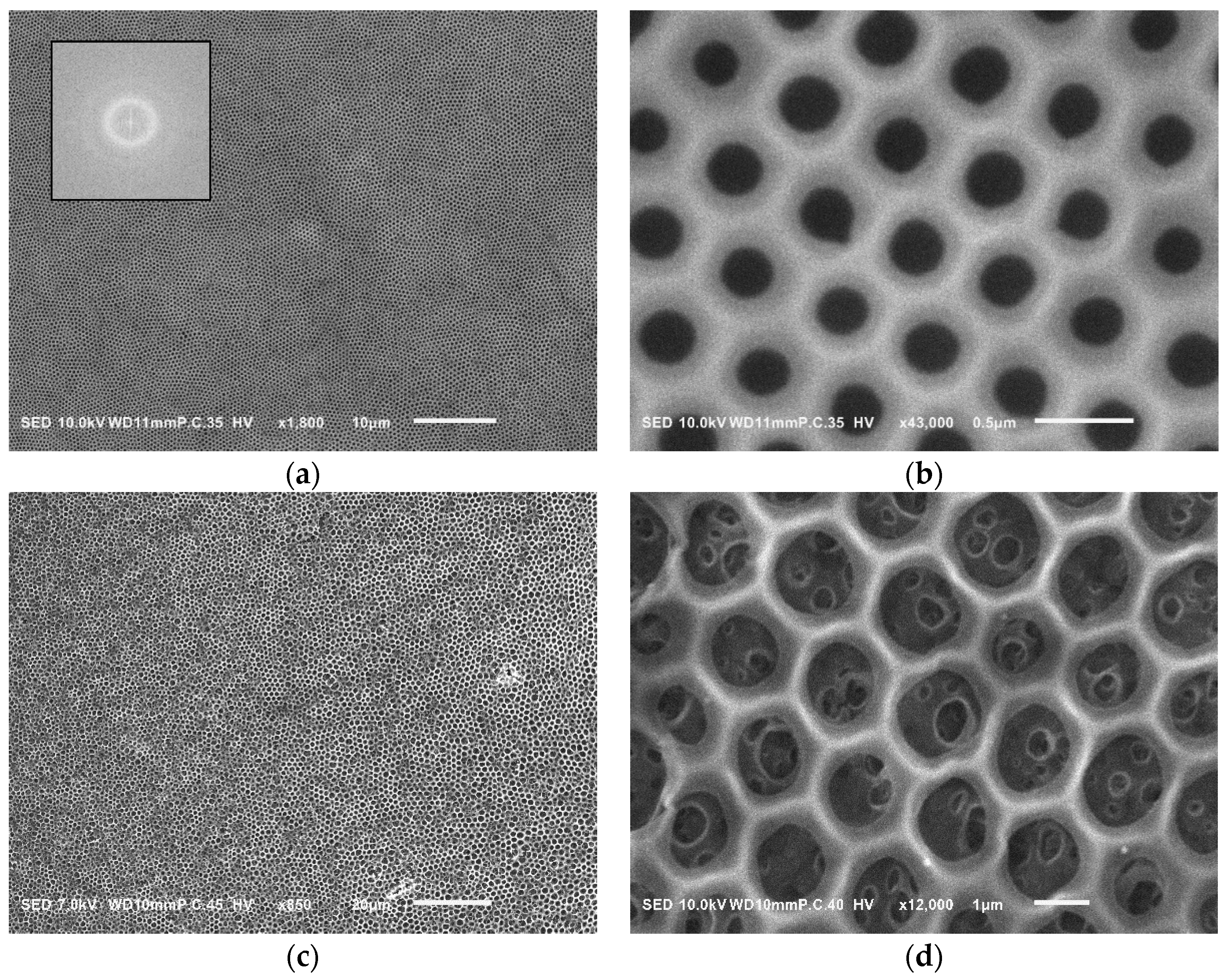
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Täuber, K.; Zimathies, A.; Yuan, J. Porous Membranes Built Up from Hydrophilic Poly(ionic liquid)s. Macromol. Rapid Commun. 2015, 36, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Cong, H.; Li, Z.; Yuan, H.; Peng, Q.; Chi, M.; Yang, S.; Yang, R.; Wickramasinghe, S.R.; Tang, J. Fabrication of highly ordered porous membranes of cellulose triacetate on ice substrates using breath figure method. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 552–558. [Google Scholar] [CrossRef]
- Du, C.; Zhang, A.; Bai, H.; Li, L. Robust Microsieves with Excellent Solvent Resistance: Cross-Linkage of Perforated Polymer Films with Honeycomb Structure. Acs Macro Lett. 2013, 2, 27–30. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Sun, H.; Xu, Y.; Wang, X.; Luo, H.; Liu, Z.; Zhang, T. Porous Ionic Membrane Based Flexible Humidity Sensor and its Multifunctional Applications. Adv. Sci. 2017, 4, 1600404. [Google Scholar] [CrossRef]
- Böker, A.; Lin, Y.; Chiapperini, K.; Horowitz, R.; Thompson, M.; Carreon, V.; Xu, T.; Abetz, C.; Skaff, H.; Dinsmore, A.D.; et al. Hierarchical nanoparticle assemblies formed by decorating breath figures. Nat. Mater. 2004, 3, 302–306. [Google Scholar] [CrossRef]
- Chen, P.-C.; Wan, L.-S.; Ke, B.-B.; Xu, Z.-K. Honeycomb-Patterned Film Segregated with Phenylboronic Acid for Glucose Sensing. Langmuir 2011, 27, 12597–12605. [Google Scholar] [CrossRef]
- Yin, H.; Bulteau, A.; Feng, Y.; Billon, L. CO2-Induced Tunable and Reversible Surface Wettability of Honeycomb Structured Porous Films for Cell Adhesion. Adv. Mater. Interfaces 2016, 3, 1500623. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.-F.; Shen, H.-X.; Chen, S. Quantum-dot-embedded ionomer-derived films with ordered honeycomb structures via breath figures. Chem. Commun. 2010, 46, 7376. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, Y.; Kong, J.; Zou, C.; Li, C.M.; Lu, X.; Ma, J.; Boey, F.Y.C.; Chen, X. Assembly of Graphene Sheets into Hierarchical Structures for High-Performance Energy Storage. ACS Nano 2011, 5, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- De Boer, B.; Stalmach, U.; Nijland, H.; Hadziioannou, G. Microporous Honeycomb-Structured Films of Semiconducting Block Copolymers and Their Use as Patterned Templates. Adv. Mater. 2000, 12, 1581–1583. [Google Scholar] [CrossRef]
- Cao, T.; Wei, F.; Jiao, X.; Chen, J.; Liao, W.; Zhao, X.; Cao, W. Micropatterns of Protein and Conducting Polymer Molecules Fabricated by Layer-by-Layer Self-Assembly and Photolithography Techniques. Langmuir 2003, 19, 8127–8129. [Google Scholar] [CrossRef]
- Hernández-Guerrero, M.; Stenzel, M.H. Stenzel, Honeycomb structured polymer films via breath figures. Polym. Chem. 2012, 3, 563–577. [Google Scholar] [CrossRef]
- Zhang, A.; Bai, H.; Li, L. Breath Figure: A Nature-Inspired Preparation Method for Ordered Porous Films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef] [PubMed]
- Widawski, G.; Rawiso, M.; François, B. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature 1994, 369, 387. [Google Scholar] [CrossRef]
- Stenzel, M.H.; Barner-Kowollik, C.; Davis, T.P.; Barner-Kowollik, C. Formation of honeycomb-structured, porous films via breath figures with different polymer architectures. J. Polym. Sci. A Polym. Chem. 2006, 44, 2363–2375. [Google Scholar] [CrossRef]
- Li, L.; Zhong, Y.; Li, J.; Chen, C.; Zhang, A.; Xu, J.; Ma, Z. Thermally stable and solvent resistant honeycomb structured polystyrene films via photochemical cross-linking. J. Mater. Chem. 2009, 19, 7222–7227. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Zhang, A.; Liu, X.; Cui, K.; Huang, J.; Ma, Z.; Han, Z. Fabrication of robust honeycomb polymer films: A facile photochemical cross-linking process. J. Colloid Interface Sci. 2009, 331, 446–452. [Google Scholar] [CrossRef]
- Escalé, P.; Rubatat, L.; Derail, C.; Save, M.; Billon, L. pH Sensitive Hierarchically Self-Organized Bioinspired Films. Macromol. Rapid Commun. 2011, 32, 1072–1076. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, S.H.; Han, T.H.; Kim, S.O.; Lee, J. Hierarchically Ordered Polymer Films by Templated Organization of Aqueous Droplets. Adv. Funct. Mater. 2007, 17, 2315–2320. [Google Scholar] [CrossRef]
- Wu, B.-H.; Zhu, L.-W.; Ou, Y.; Tang, W.; Wan, L.-S.; Xu, Z.-K. Systematic Investigation on the Formation of Honeycomb-Patterned Porous Films from Amphiphilic Block Copolymers. J. Phys. Chem. C 2015, 119, 1971–1979. [Google Scholar] [CrossRef]
- Chen, S.; Alves, M.-H.; Save, M.; Billon, L. Synthesis of amphiphilic diblock copolymers derived from renewable dextran by nitroxide mediated polymerization: Towards hierarchically structured honeycomb porous films. Polym. Chem. 2014, 5, 5310–5319. [Google Scholar] [CrossRef]
- Connal, L.A.; Vestberg, R.; Hawker, C.J.; Qiao, G.G. Dramatic Morphology Control in the Fabrication of Porous Polymer Films. Adv. Funct. Mater. 2008, 18, 3706–3714. [Google Scholar] [CrossRef]
- Gitsov, I. Hybrid linear dendritic macromolecules: From synthesis to applications. J. Polym. Sci. A Polym. Chem. 2008, 46, 5295–5314. [Google Scholar] [CrossRef]
- Wurm, F.; Frey, H. Linear–dendritic block copolymers: The state of the art and exciting perspectives. Prog. Polym. Sci. 2011, 36, 1–52. [Google Scholar] [CrossRef]
- Andrén, O.C.J.; Walter, M.V.; Yang, T.; Hult, A.; Malkoch, M. Multifunctional Poly(ethylene glycol): Synthesis, Characterization, and Potential Applications of Dendritic–Linear–Dendritic Block Copolymer Hybrids. Macromolecules 2013, 46, 3726–3736. [Google Scholar] [CrossRef]
- Walter, M.V.; Lundberg, P.; Hult, D.; Hult, A.; Malkoch, M. A one component methodology for the fabrication of honeycomb films from biocompatible amphiphilic block copolymer hybrids: A linear–dendritic–linear twist. Polym. Chem. 2013, 4, 2680. [Google Scholar] [CrossRef]
- Mongkhontreerat, S.; Walter, M.V.; Cai, Y.; Brismar, H.; Hult, A.; Malkoch, M. Functional porous membranes from amorphous linear dendritic polyester hybrids. Polym. Chem. 2015, 6, 2390–2395. [Google Scholar] [CrossRef]
- Liu, X.; Gitsov, I. Thermosensitive Amphiphilic Janus Dendrimers with Embedded Metal Binding Sites. Synthesis and Self-Assembly. Macromolecules 2018, 51, 5085–5100. [Google Scholar] [CrossRef]
- Gitsov, I. Synthesis and Self-Assembly of Linear-Dendritic Hybrid Polymers. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2436–2446. [Google Scholar]
- Angell, Y.; Burgess, K. Base Dependence in Copper-Catalyzed Huisgen Reactions: Efficient Formation of Bistriazoles. Angew. Chem. 2007, 119, 3723–3725. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Elias, H.G. An Introduction to Polymer Science, 1st ed.; VCH: Weinheim, Germany, 1997; p. 69. [Google Scholar]
- Connal, L.A.; Vestberg, R.; Hawker, C.J.; Qiao, G.G. Synthesis of Dendron Functionalized Core Cross-linked Star Polymers. Macromolecules 2007, 40, 7855–7863. [Google Scholar] [CrossRef]
- For a diagram and in-depth discussion of these processes see Maruyama, N.; Koito, T.; Nishida, J.; Sawadaishi, T.; Cieren, X.; Ijiro, K.; Karthaus, O.; Shimomura, M. Mesoscopic patterns of molecular aggregates on solid substrates. Thin Solid Film. 1998, 327–329, 854–856. [Google Scholar] [CrossRef]
- Chen, A.; Blakey, I.; Whittaker, A.K.; Peng, H. The influence of casting parameters on the surface morphology of PS-b -P4VP honeycomb films. J. Polym. Sci. A Polym. Chem. 2016, 54, 3721–3732. [Google Scholar] [CrossRef]
- Hrakins, W.D.; Feldman, A. Films. The Spreading of Liquids and the Spreading Coefficient. J. Am. Chem. Soc. 1921, 44, 2665–2685. [Google Scholar]
- Voronoi, G. Nouvelles applications des paramétres continus à la théorie des forms quadratiques. J. Reine Angew. Math. 1907, 133, 97–178. [Google Scholar]
- Madej, W.; Budkowski, A.; Raczkowska, J.; Rysz, J. Breath Figures in Polymer and Polymer Blend Films Spin-Coated in Dry and Humid Ambiance. Langmuir 2008, 24, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- de Leon, A.; Munos-Bonilla, A.; Fernández-Garcia, M.; Rodriguez-Hernández, J. Breath figures method to control the topography and functionality of polymeric surfaces in porous films and microspheres. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 851–859. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Wu, L. Micro-patterned polystyrene surfaces surfactant-encapsulated polyoxometalate complex via breath figures. Polymer 2009, 50, 2113–2122. [Google Scholar] [CrossRef]
- Sakatani, Y.; Boisiérre, C.; Grosso, D.; Nicole, L.; Soler-Illia, G.J.A.A.; Sanchez, C. Coupling Nanobuilding Block and Breath Figures Approaches for the Designed Construction of Hierarchically Templated Porous Materials and Membranes. Chem. Mater. 2008, 20, 1049–1056. [Google Scholar] [CrossRef]
- Zhu, L.-W.; Ou, Y.; Wan, L.-S.; Xu, Z.-K. Polystyrenes with Hydrophilic End Groups: Synthesis, Characterization, and Effects on the Self-Assembly of Breath Figure Arrays. J. Phys. Chem. B 2014, 118, 845–854. [Google Scholar] [CrossRef]
- Ji, E.; Pellerin, V.; Ehrenfeld, F.; Laffore, A.; Bousquet, A.; Billon, L. Hierarchical honeycomb-structured films by directed self-assembly in “breath figure” templating of ionizable “clicked” PH3T-b-PMMA diblock copolymers: An ionic group/counter-ion effect on porous polymer film morphology. Chem. Commun. 2017, 53, 1876–1879. [Google Scholar] [CrossRef] [PubMed]

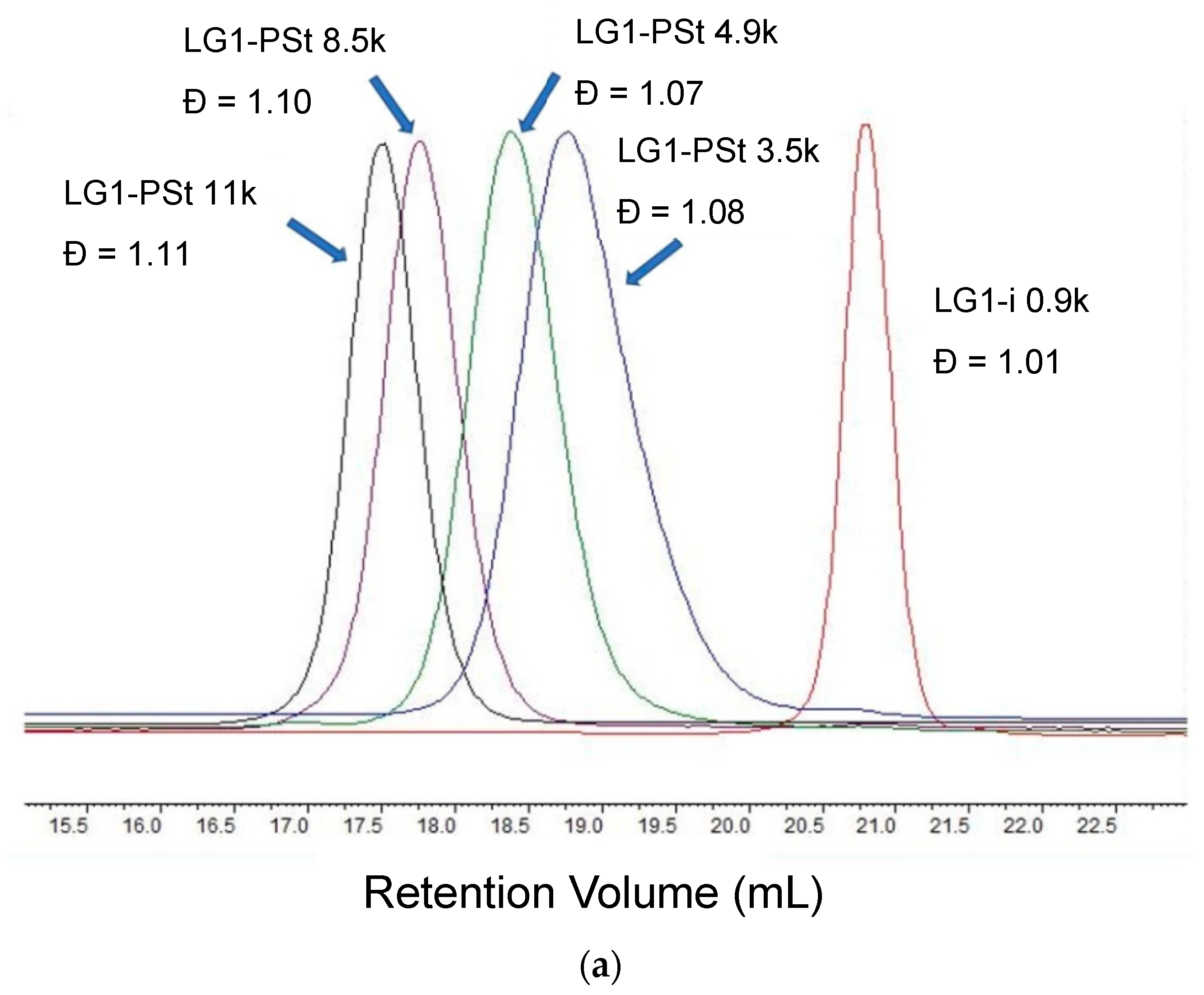
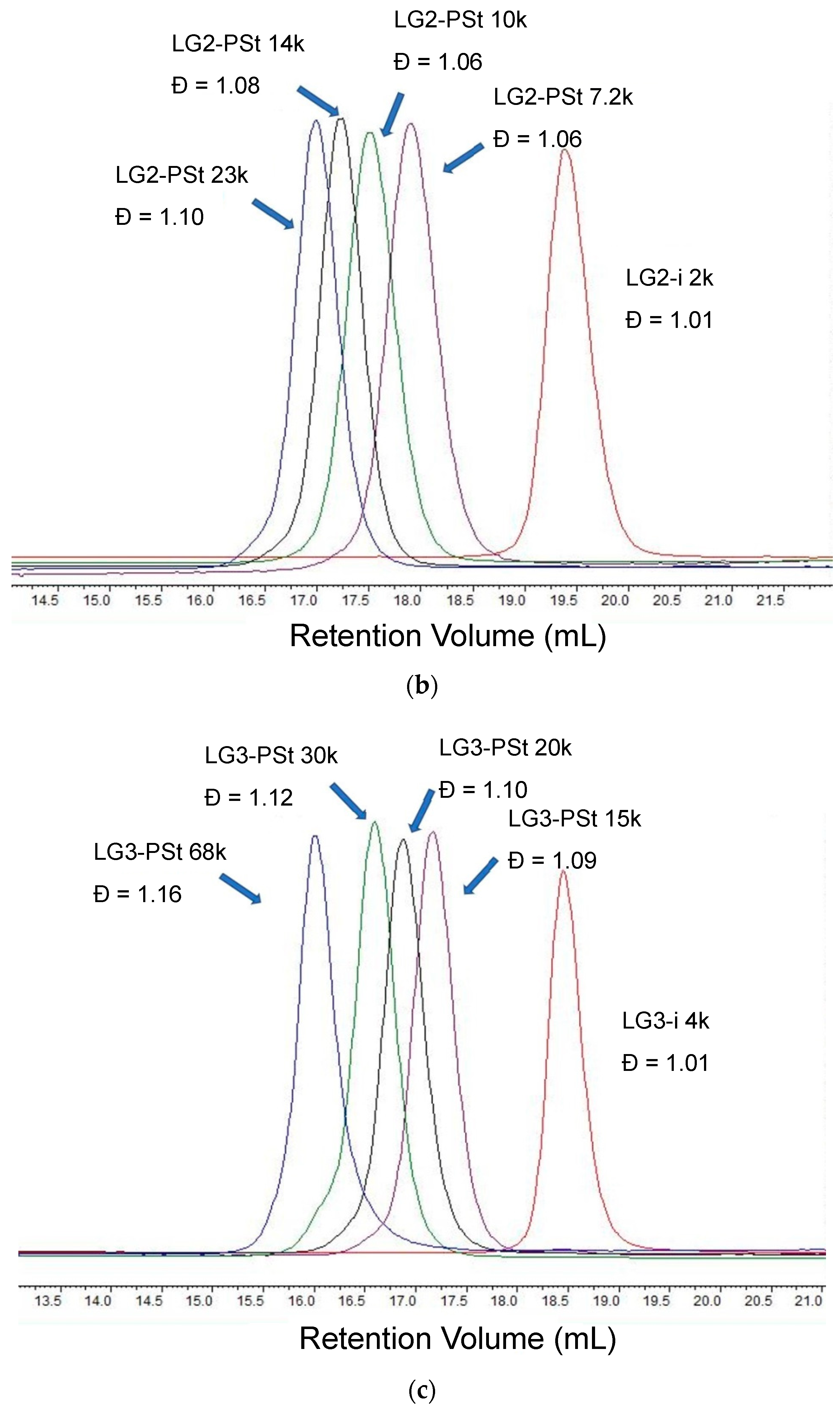
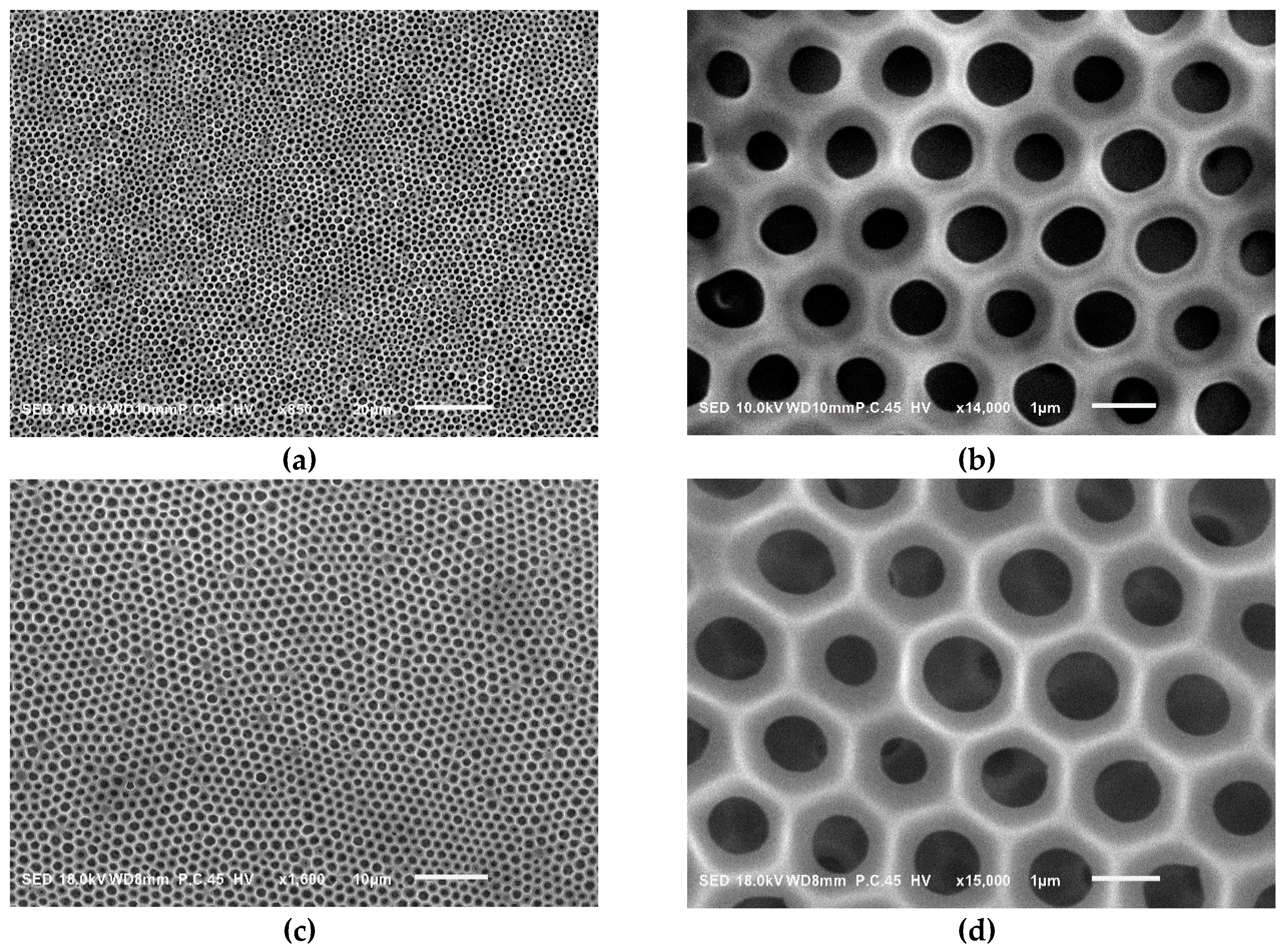
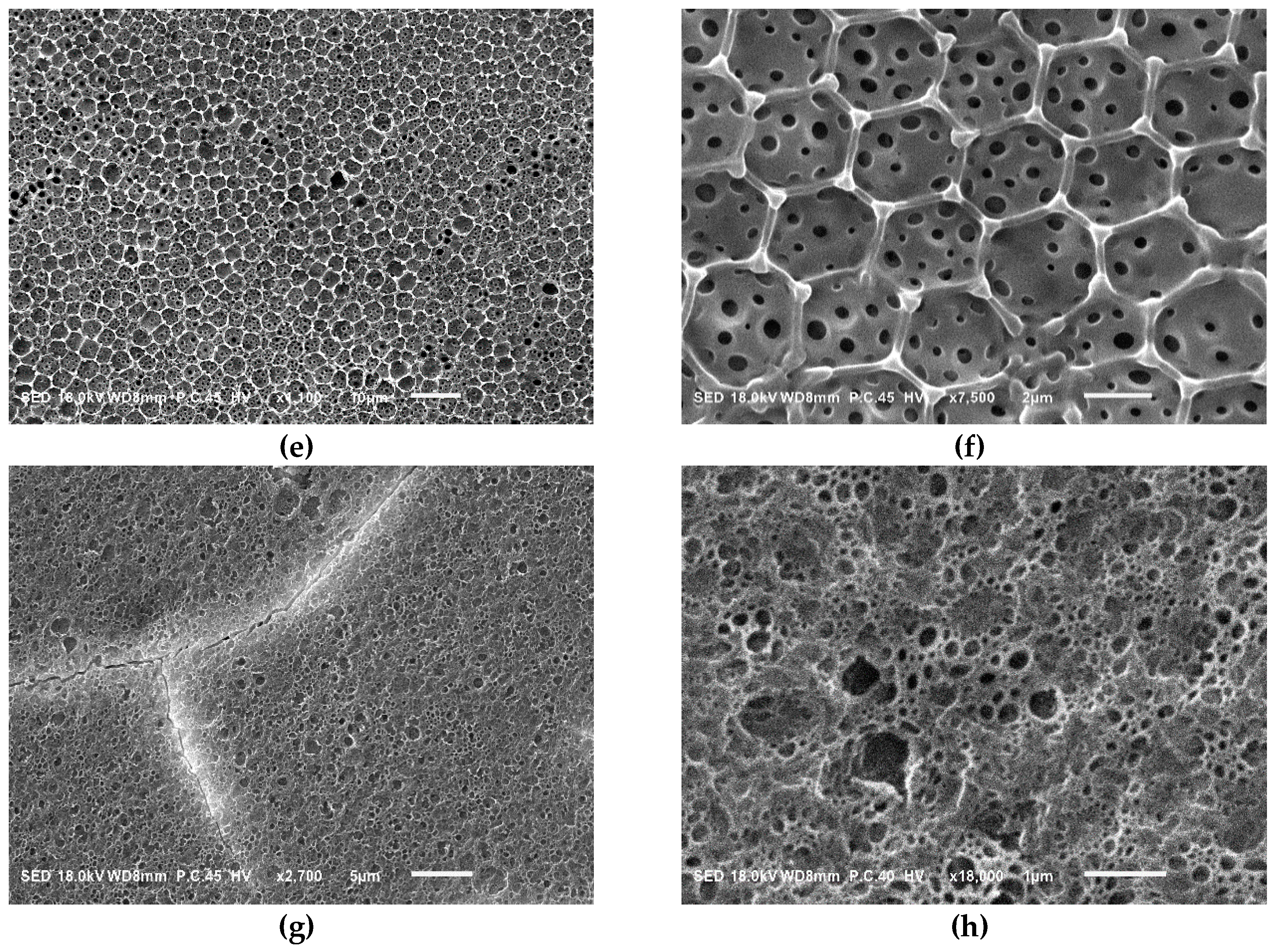
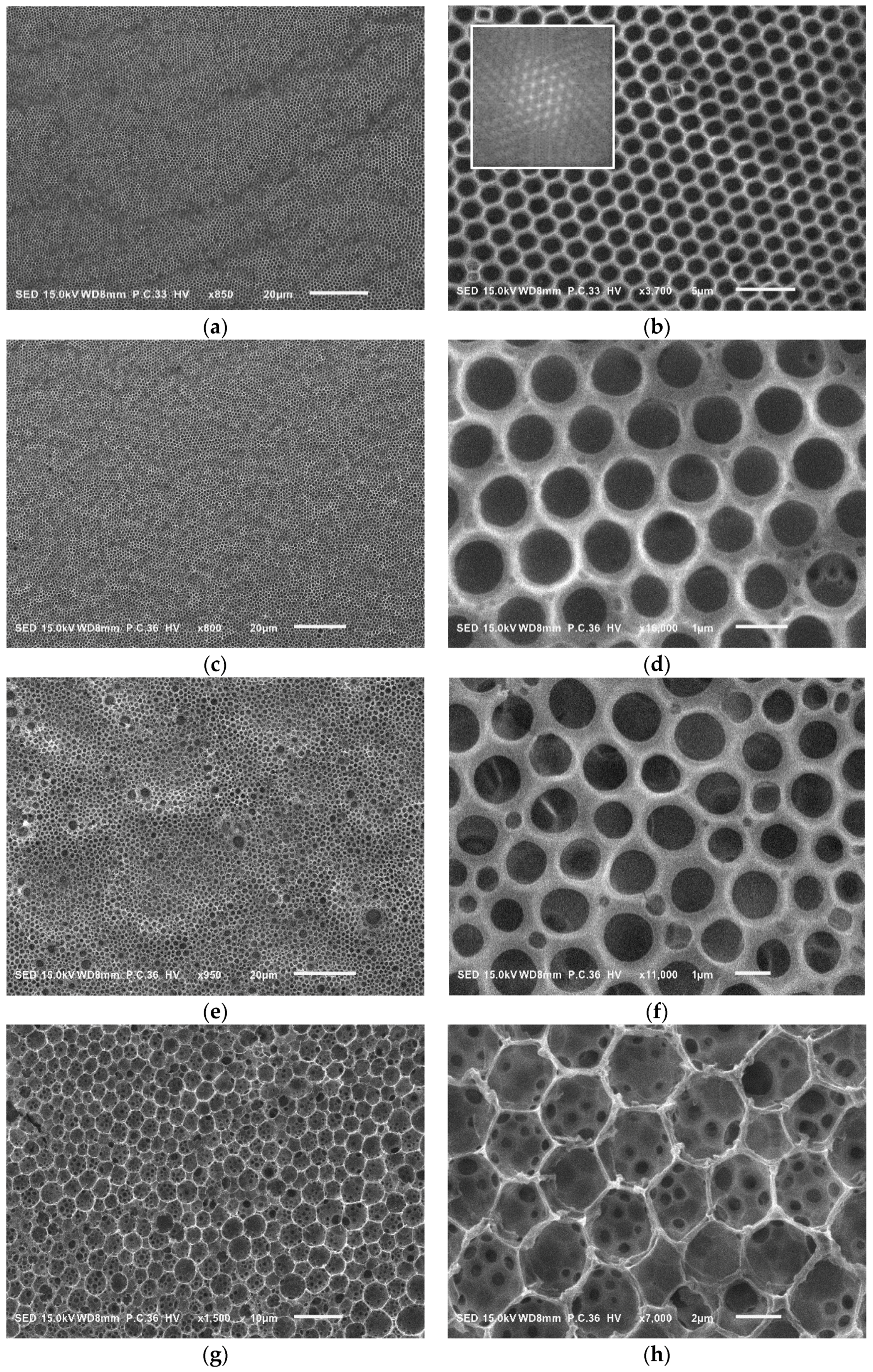
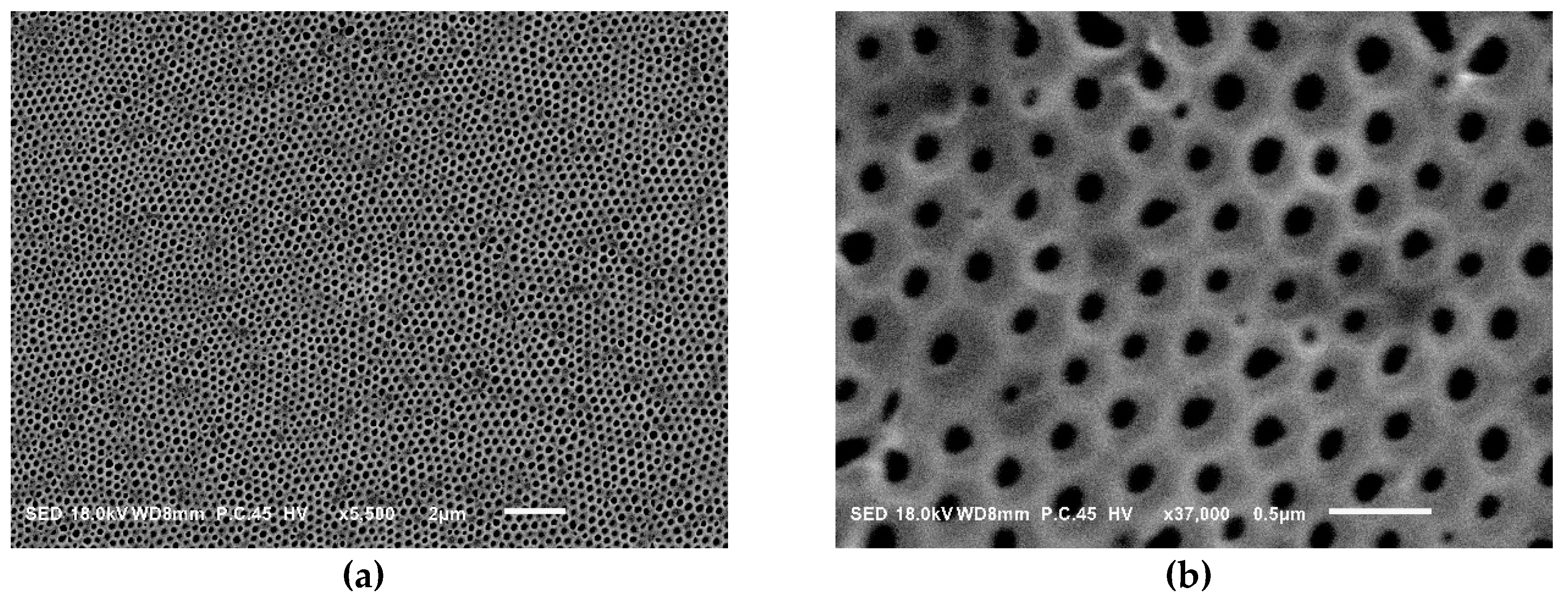

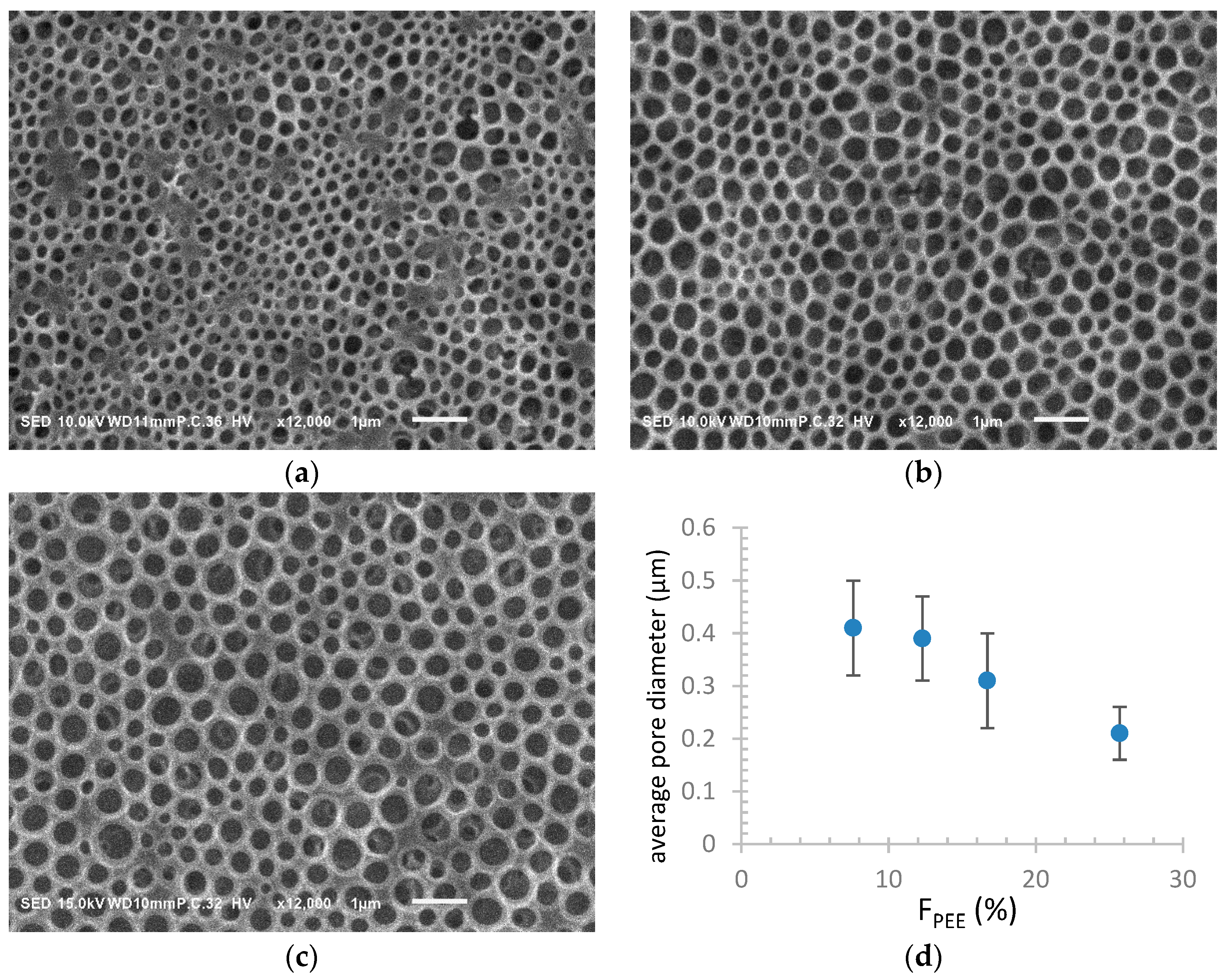
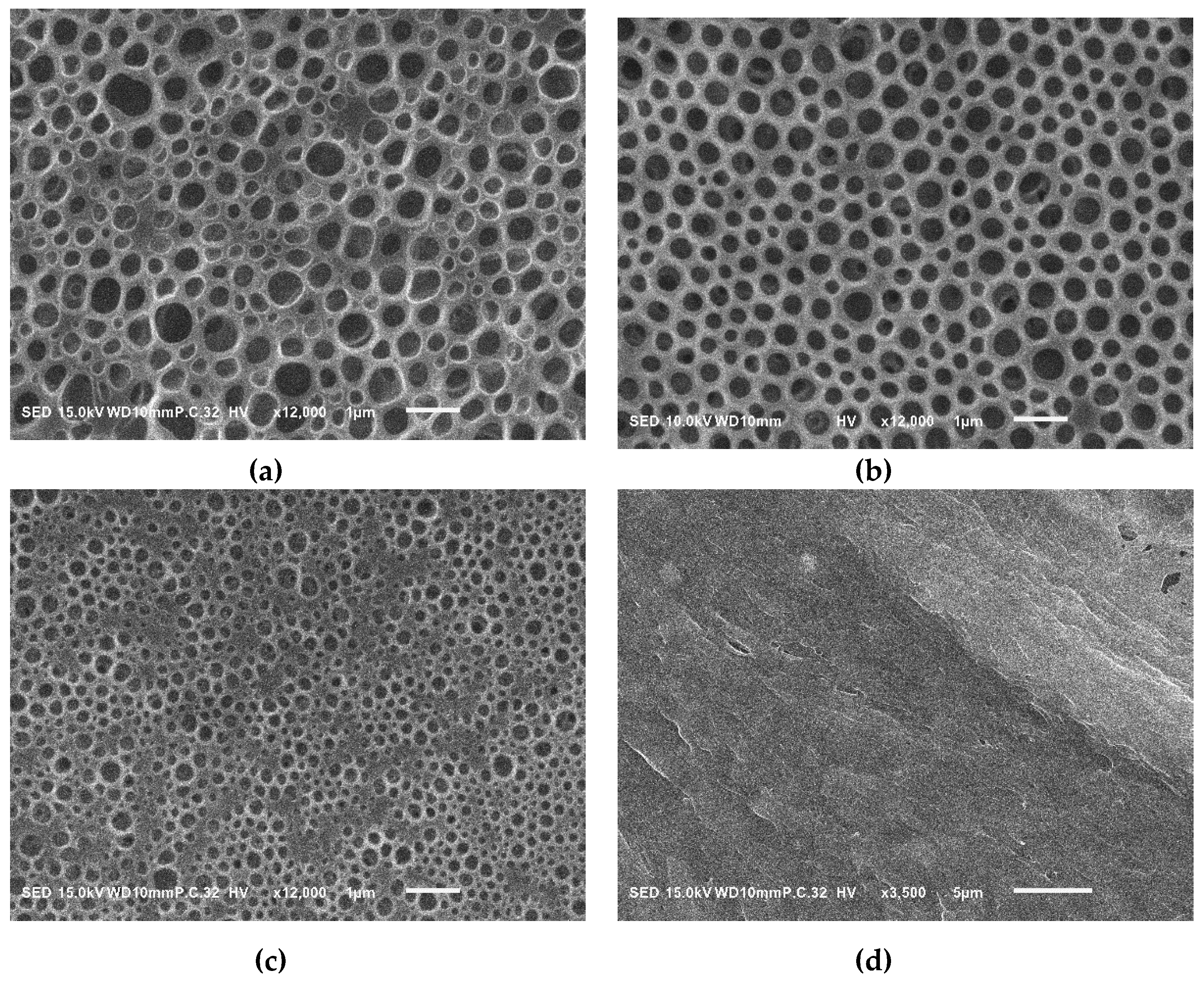
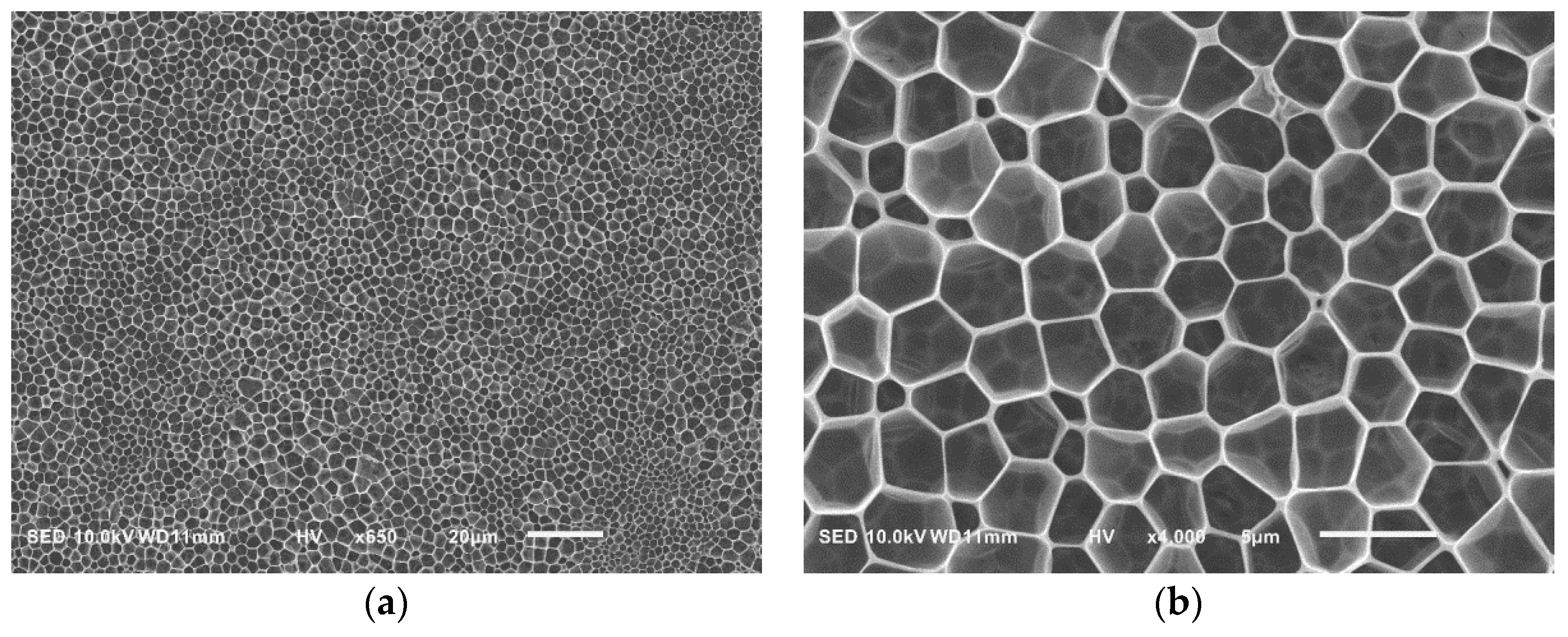
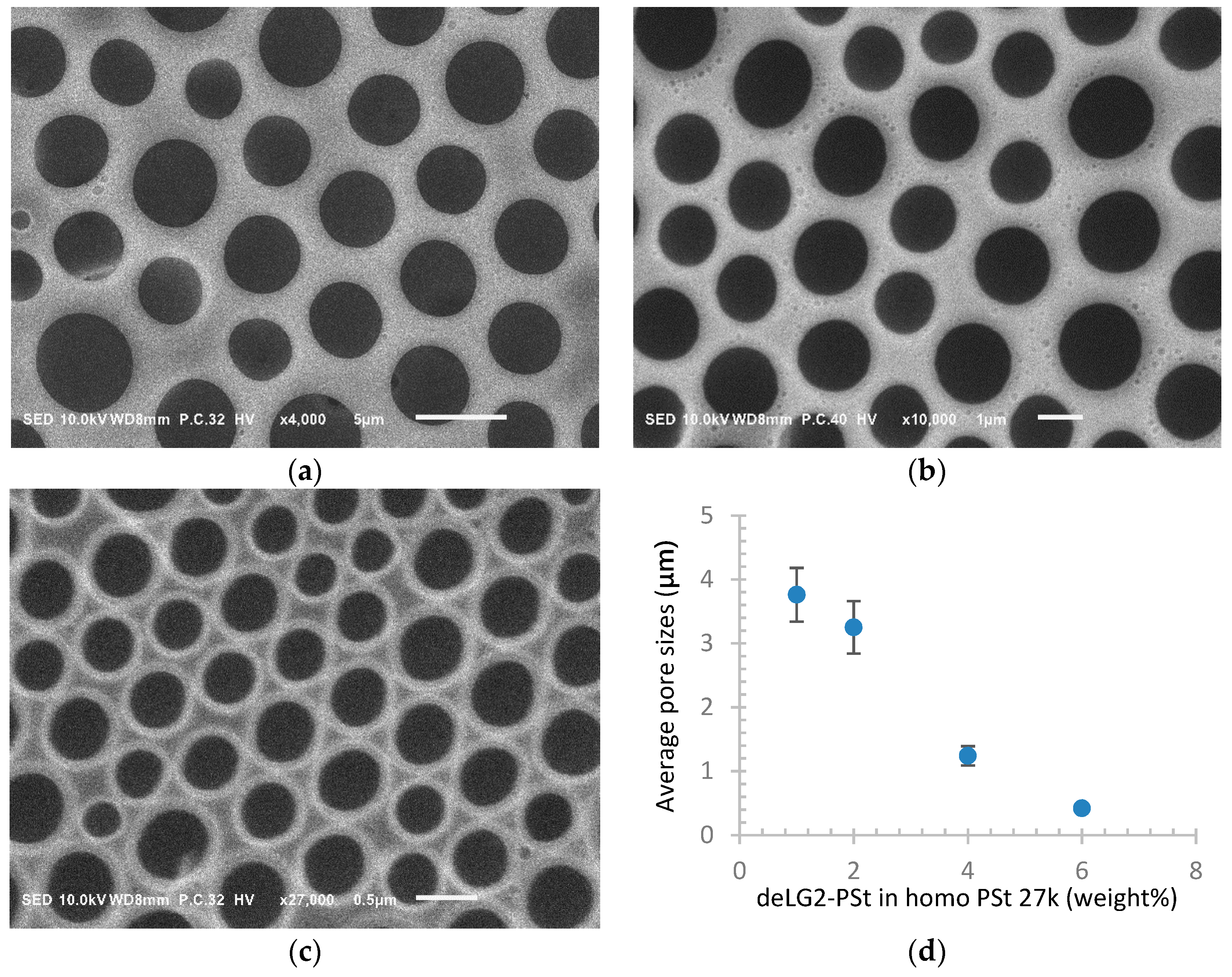
| Sample a | FPEE b (%) | γ c (mN m−1) | γ’ d (mN m−1) | Cloud Point e (%) | Pore Size (μm) | Pore Regularity |
|---|---|---|---|---|---|---|
| LG1-PSt 11k | 9.2 | 29.7 | 23.3 | 18.7 | 0.85 ± 0.15 | fair |
| LG1-PSt 8.5k | 12.6 | 29.5 | 21.4 | 20.0 | 0.90 ± 0.12 | fair |
| LG1-PSt 4.9k | 21.5 | 29.1 | 18.1 | 28.5 | 0.40 ± 0.20 | poor |
| LG1-PSt 3.5k | 27.8 | 29.2 | 18.6 | 31.0 | - | very poor |
| LG2-PSt 23k | 10.2 | 28.6 | 16.1 | 18.0 | 1.10 ± 0.11 | fair |
| LG2-PSt 14k | 16.9 | 27.9 | 15.7 | 19.4 | 1.02 ± 0.06 | fair |
| LG2-PSt 10k | 23.0 | 28.5 | 13.2 | 21.5 | 0.84 ± 0.42 | poor |
| LG3-PSt 68k | 7.6 | 26.5 | 17.9 | 11.5 | 1.1 | very good |
| LG3-PSt 30k | 16.7 | 25.2 | 16.5 | 16.6 | 0.95 ± 0.06 | good |
| LG3-PSt 20k | 25.7 | 24.4 | 12.3 | 20.0 | 1.14 ± 0.30 | poor |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Monzavi, T.; Gitsov, I. Controlled ATRP Synthesis of Novel Linear-Dendritic Block Copolymers and Their Directed Self-Assembly in Breath Figure Arrays. Polymers 2019, 11, 539. https://doi.org/10.3390/polym11030539
Liu X, Monzavi T, Gitsov I. Controlled ATRP Synthesis of Novel Linear-Dendritic Block Copolymers and Their Directed Self-Assembly in Breath Figure Arrays. Polymers. 2019; 11(3):539. https://doi.org/10.3390/polym11030539
Chicago/Turabian StyleLiu, Xin, Tina Monzavi, and Ivan Gitsov. 2019. "Controlled ATRP Synthesis of Novel Linear-Dendritic Block Copolymers and Their Directed Self-Assembly in Breath Figure Arrays" Polymers 11, no. 3: 539. https://doi.org/10.3390/polym11030539
APA StyleLiu, X., Monzavi, T., & Gitsov, I. (2019). Controlled ATRP Synthesis of Novel Linear-Dendritic Block Copolymers and Their Directed Self-Assembly in Breath Figure Arrays. Polymers, 11(3), 539. https://doi.org/10.3390/polym11030539







