Development and Investigation of Lanthanum Sulfadiazine with Calcium Stearate and Epoxidised Soyabean Oil as Complex Thermal Stabilizers for Stabilizing Poly(vinyl chloride)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
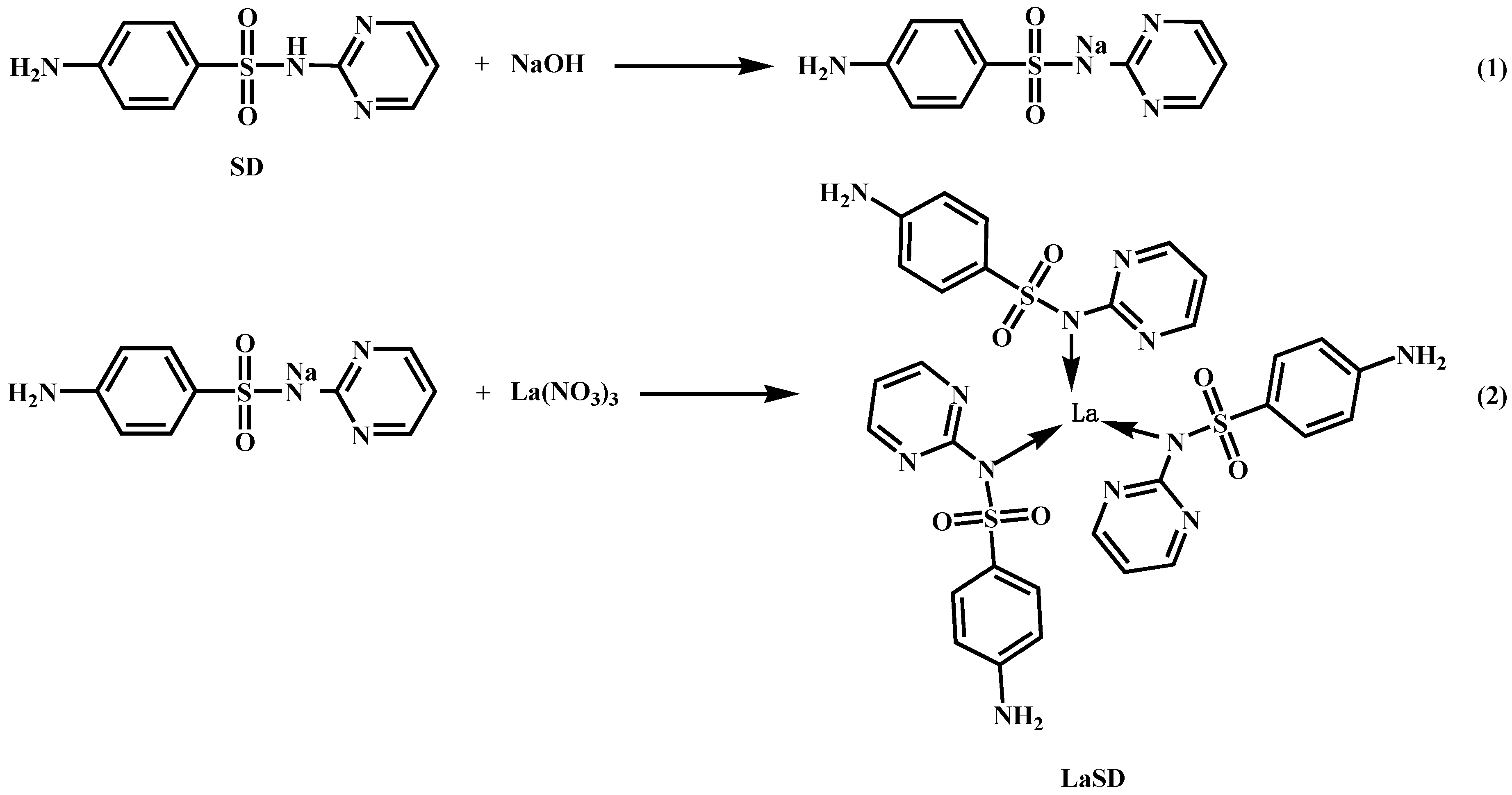
2.2. Synthesis and Characterization of LaSD
2.3. Preparation of PVC Samples
2.4. Evaluation of Stabilizing Efficiency
2.4.1. Congo Red Test
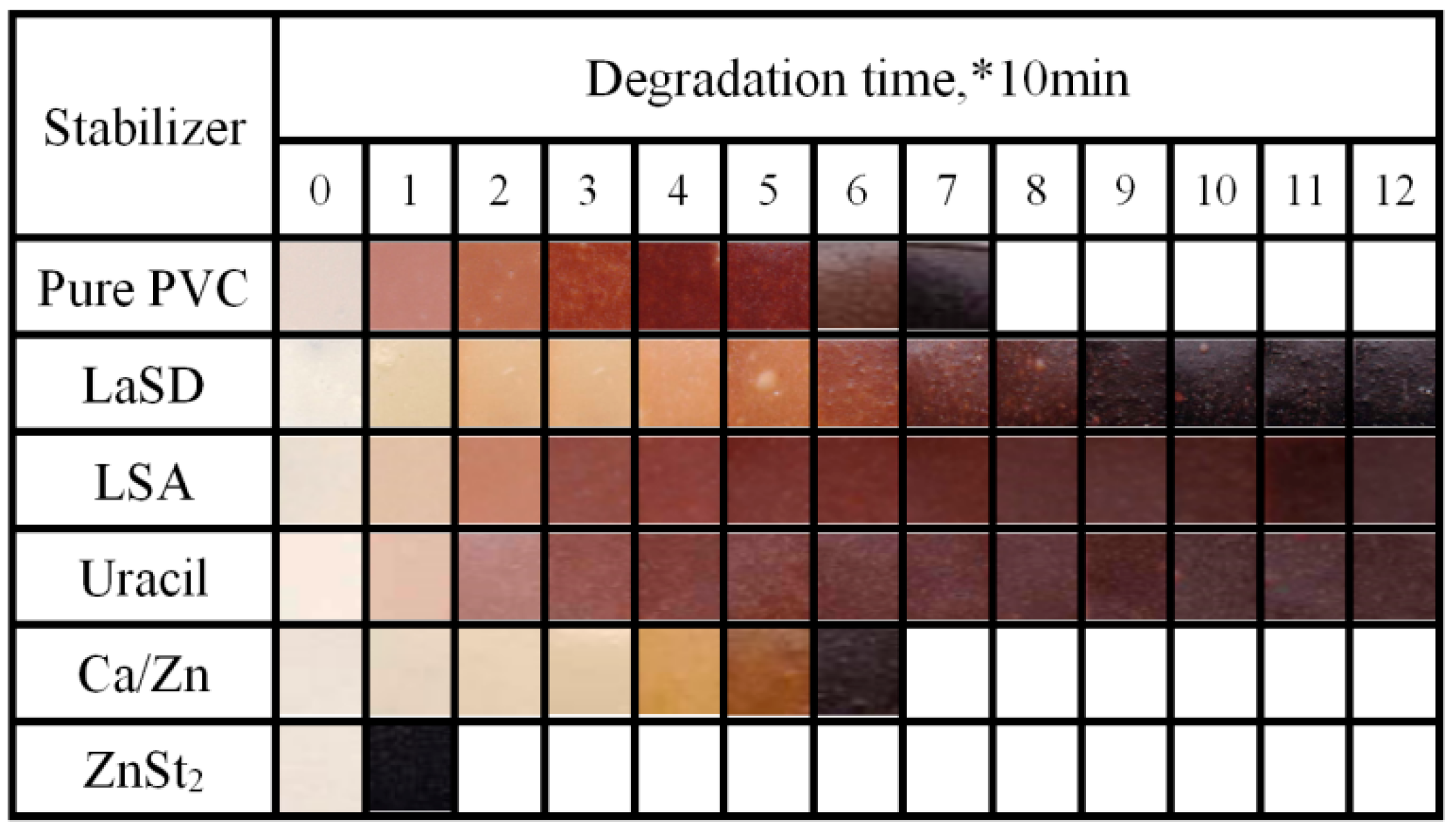
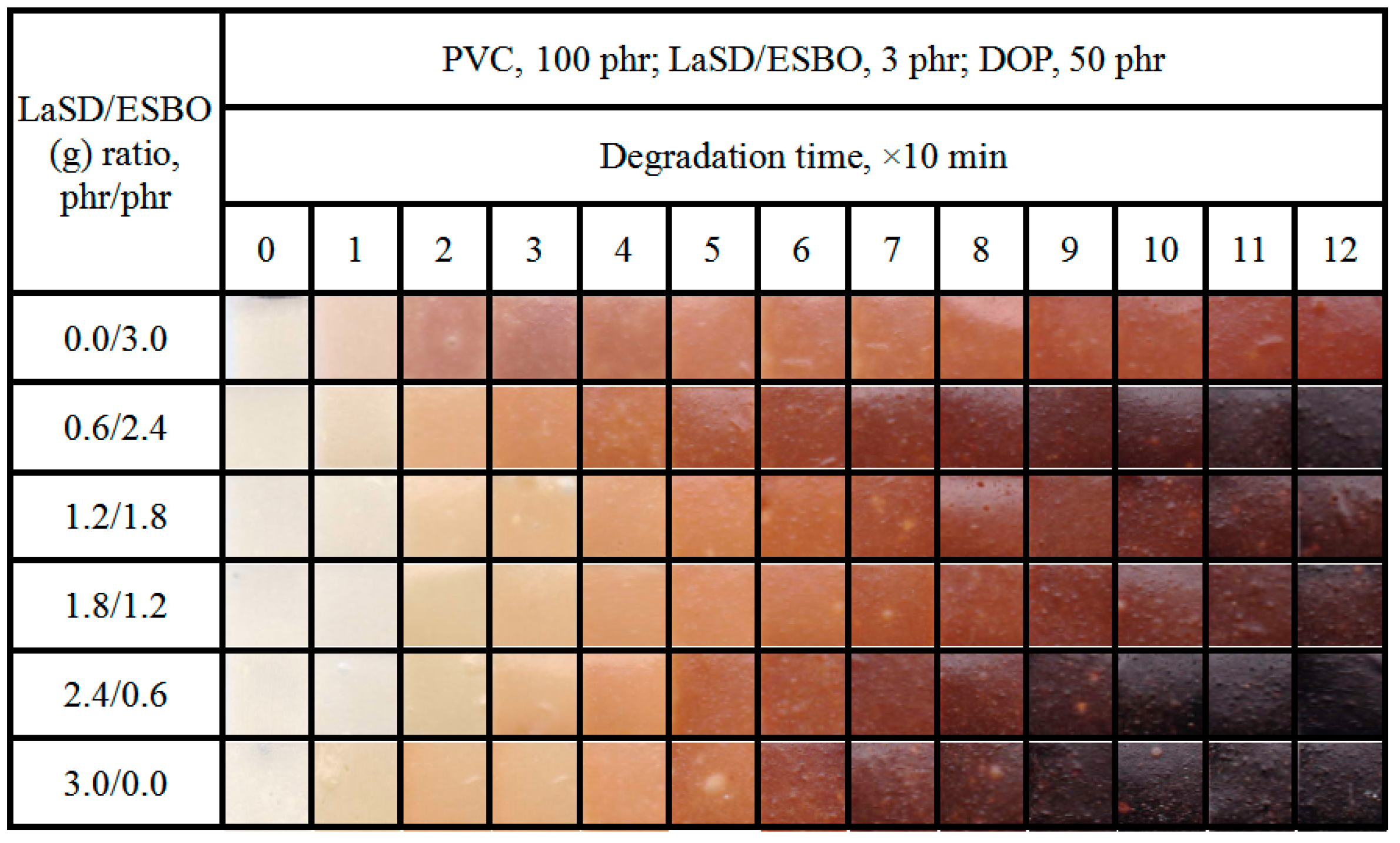
2.4.2. Discoloration Test
2.4.3. Analysis of Thermal Degradation
2.4.4. Study of the Mechanism of LaSD as PVC Thermal Stabilizer
2.5. UV-Vis Spectrum
3. Results
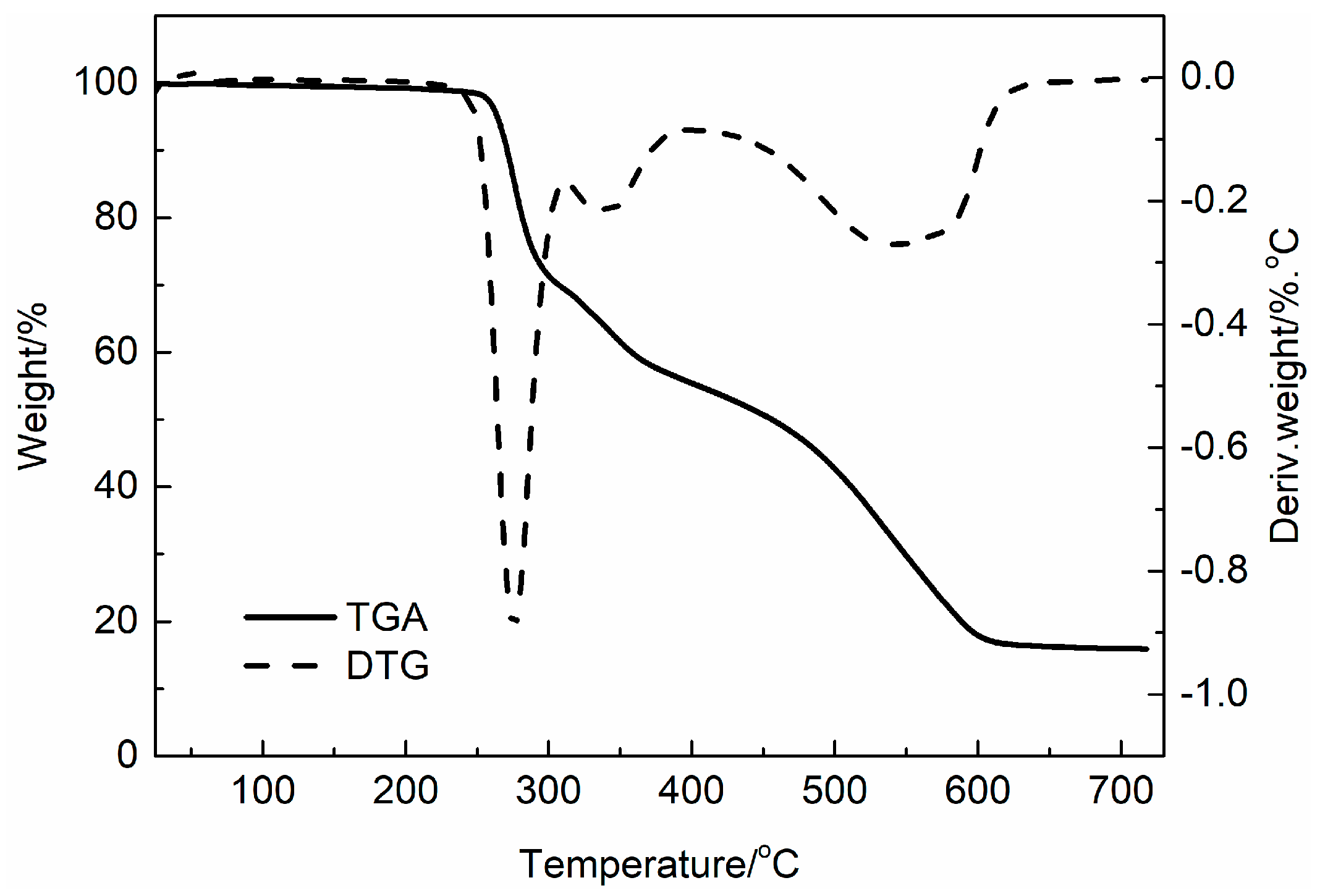
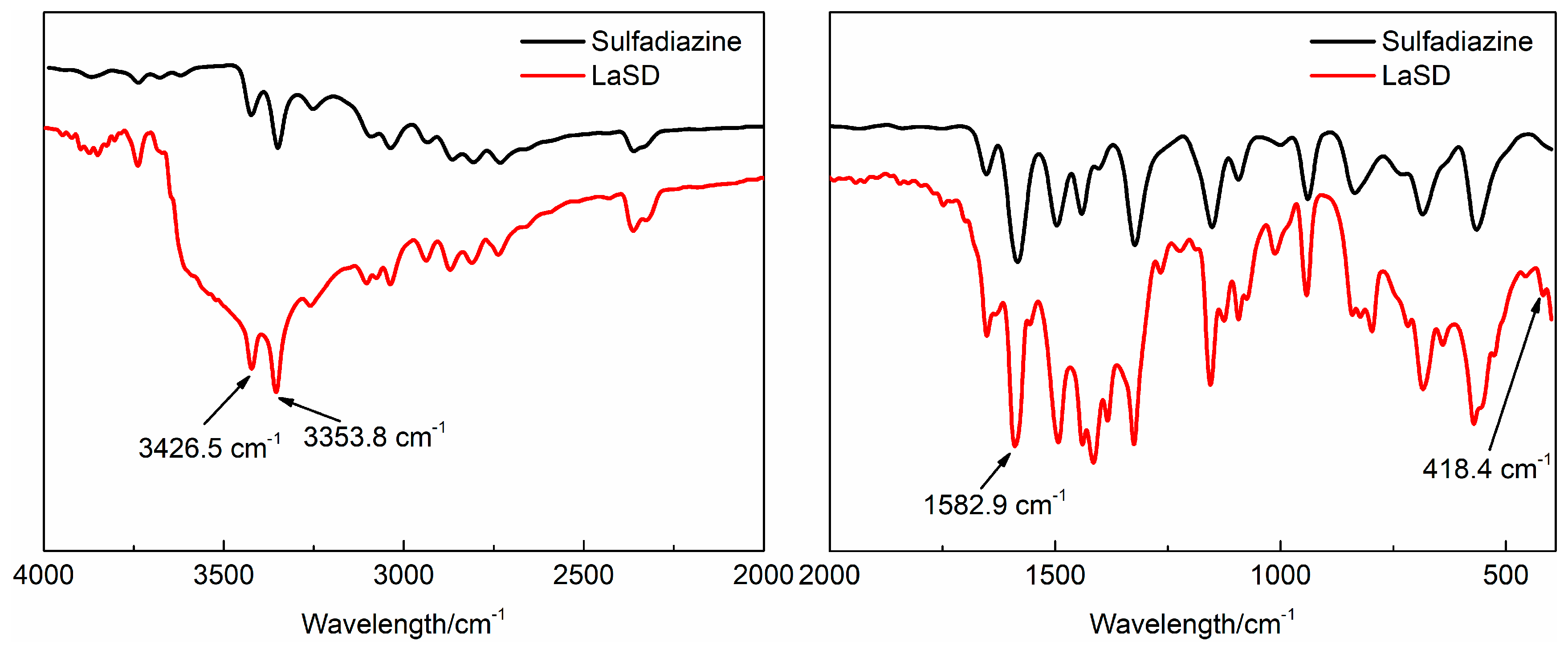
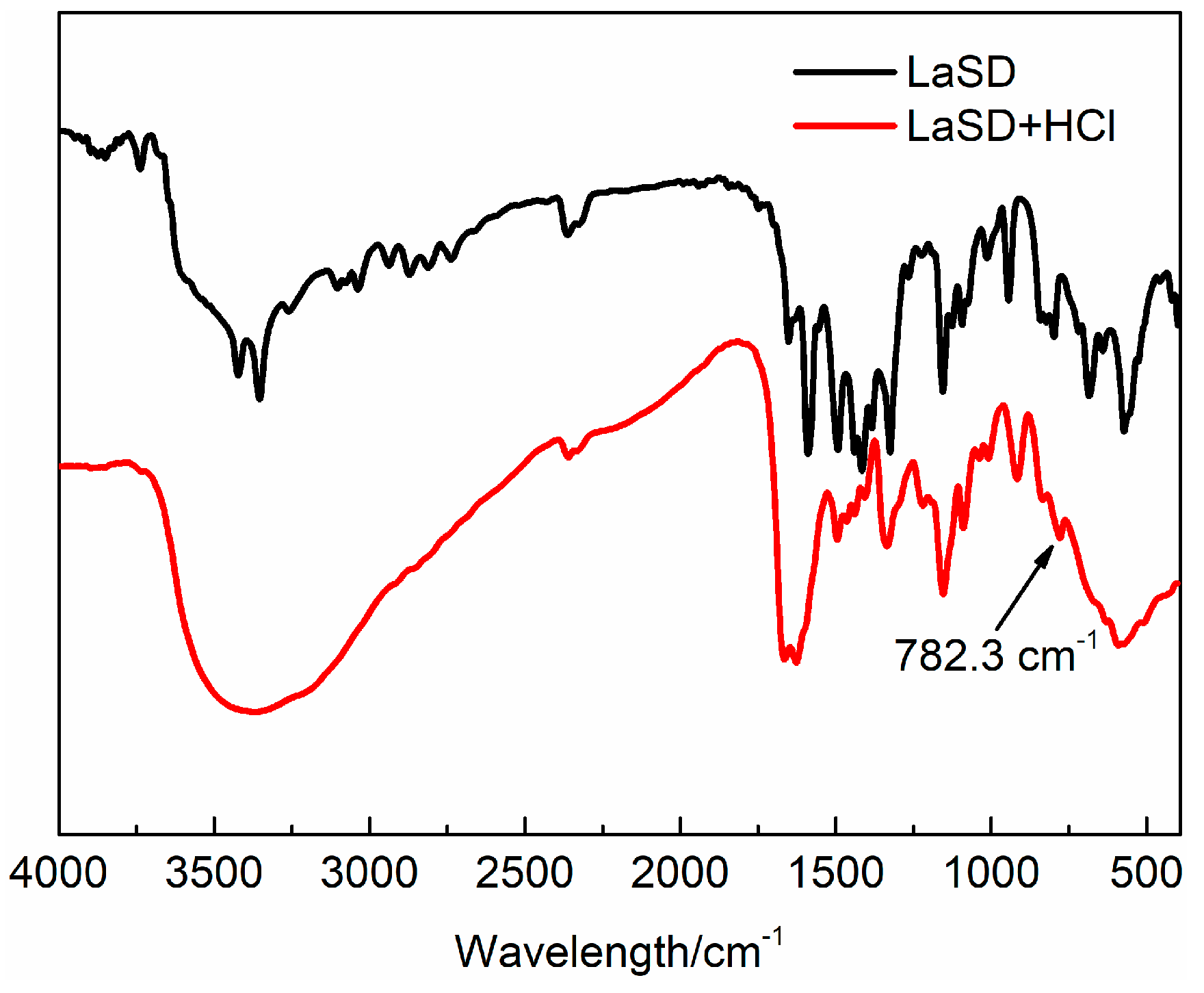
3.1. Characterization of LaSD
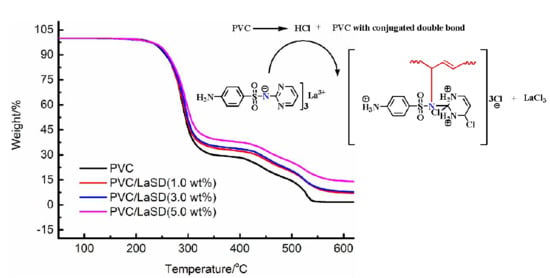
3.2. Effects of Different Thermal Stabilizers on Stabilizing PVC
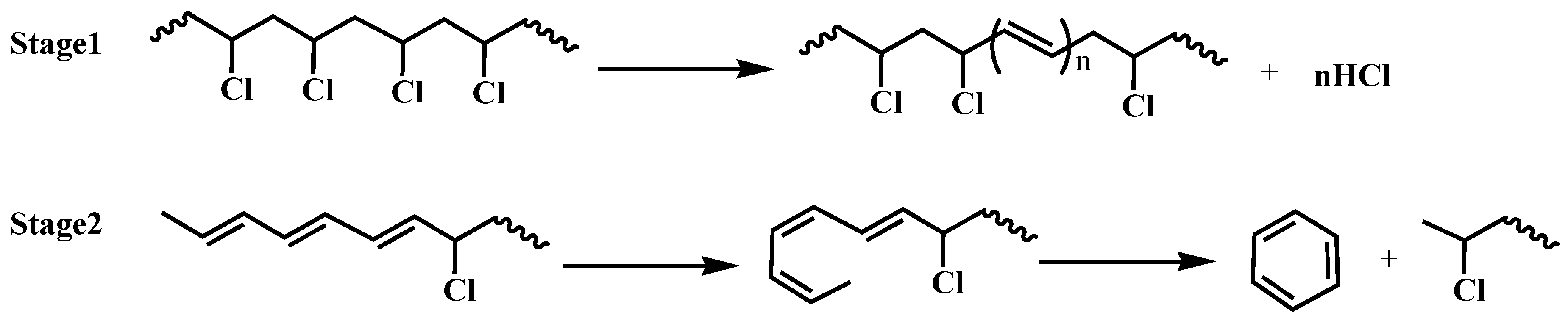
3.3. Study of the Thermal Stability Mechanism of LaSD in PVC
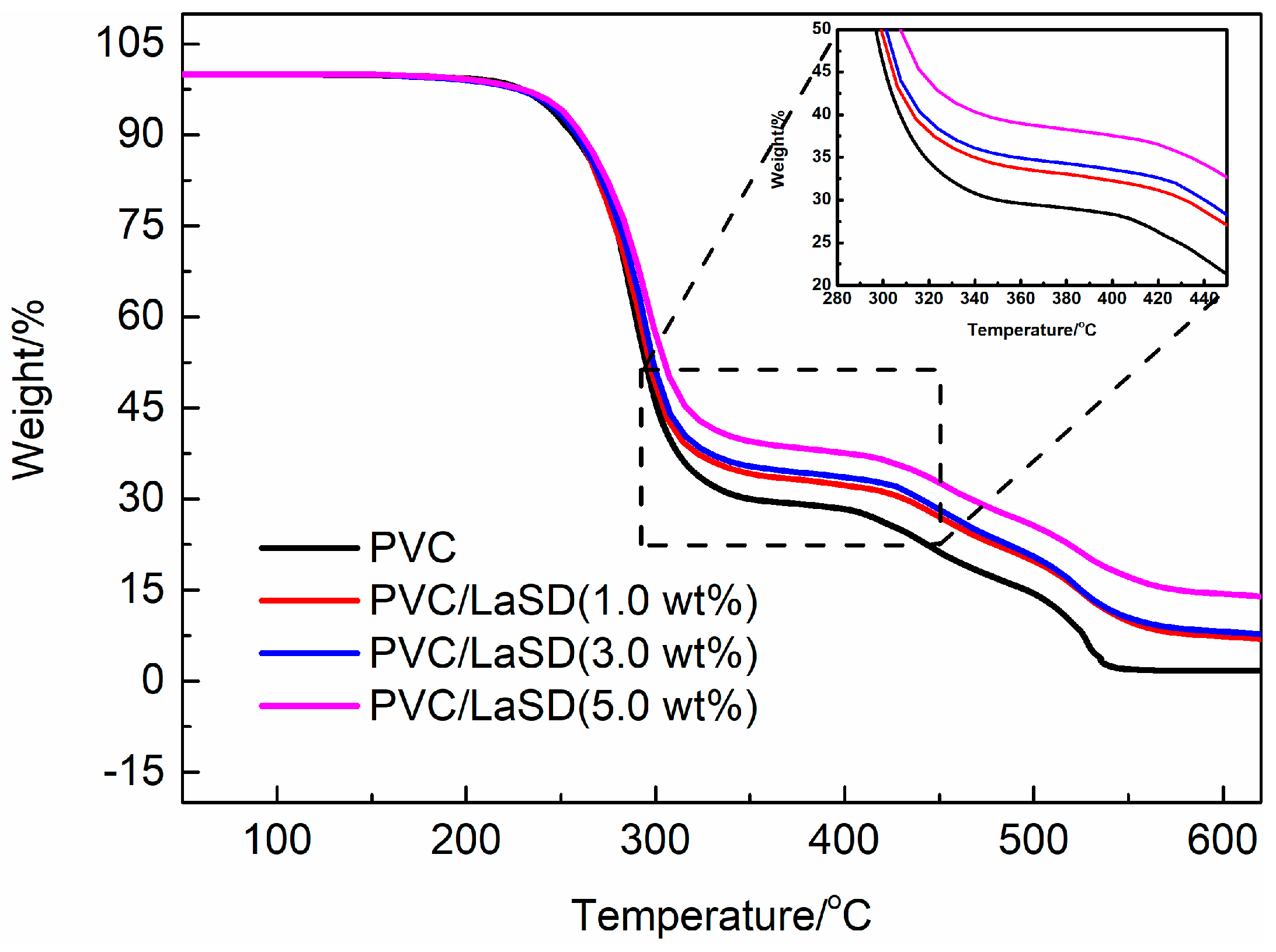
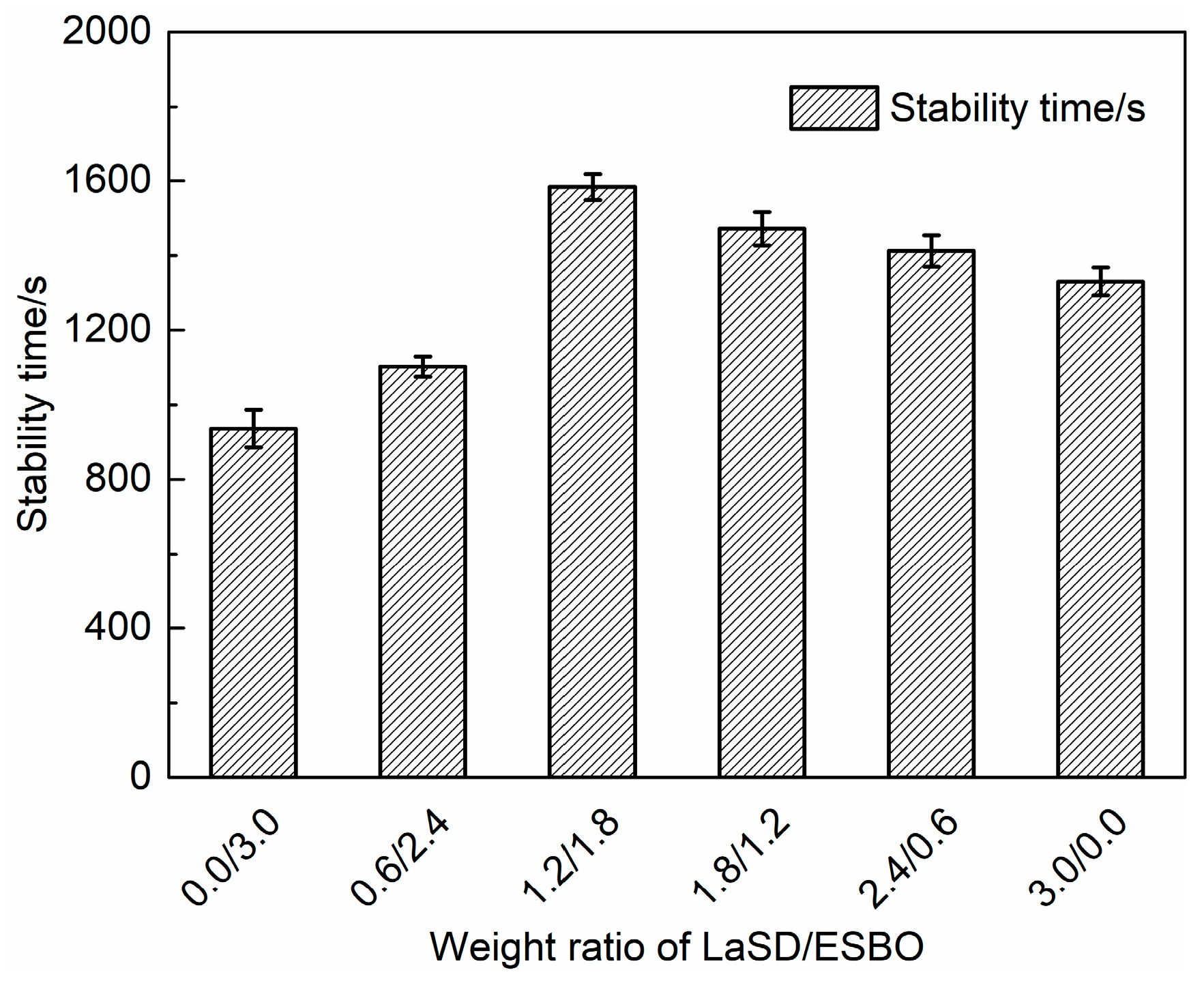
3.4. Influence of LaSD/ESBO on Stabilizing PVC
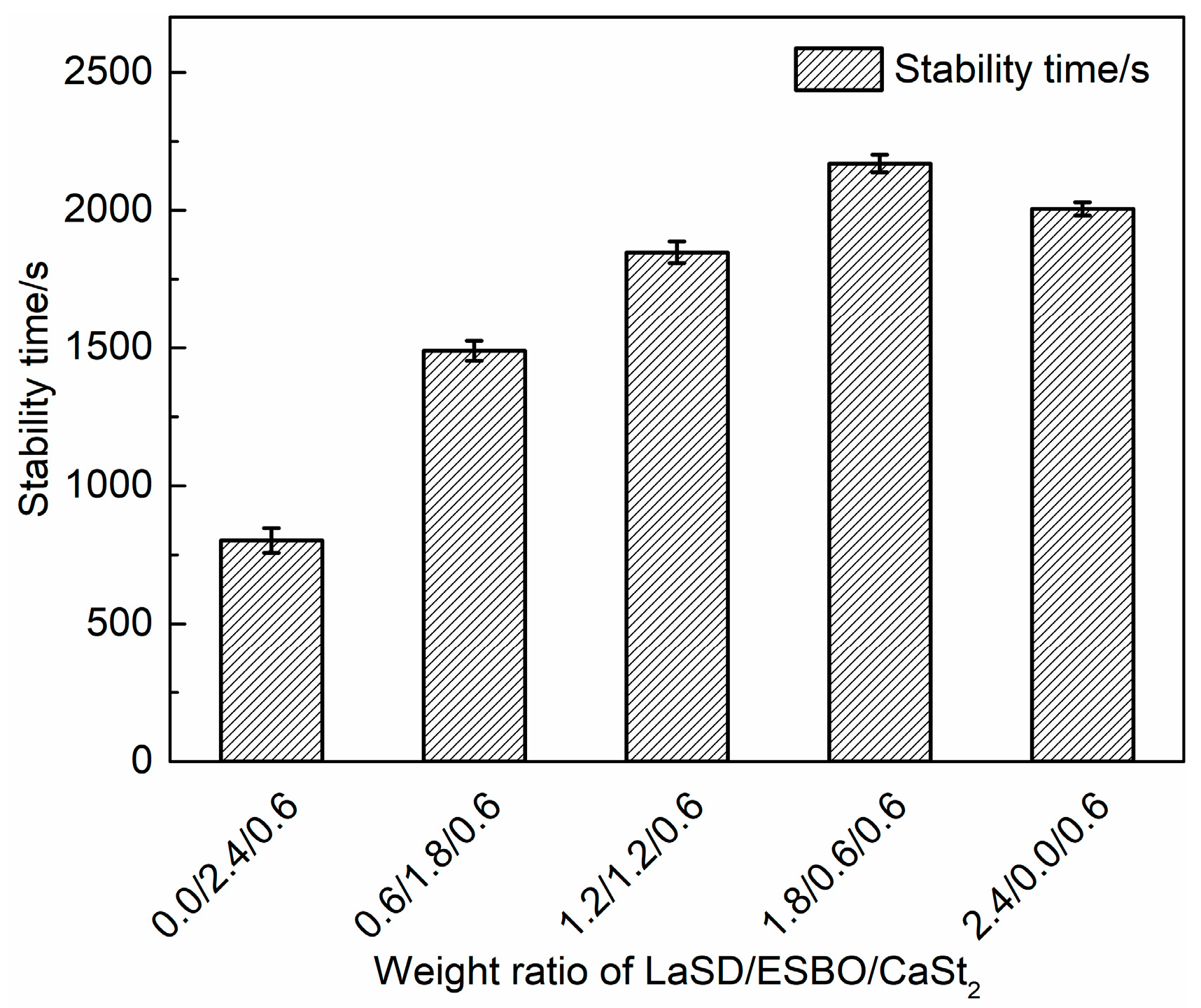
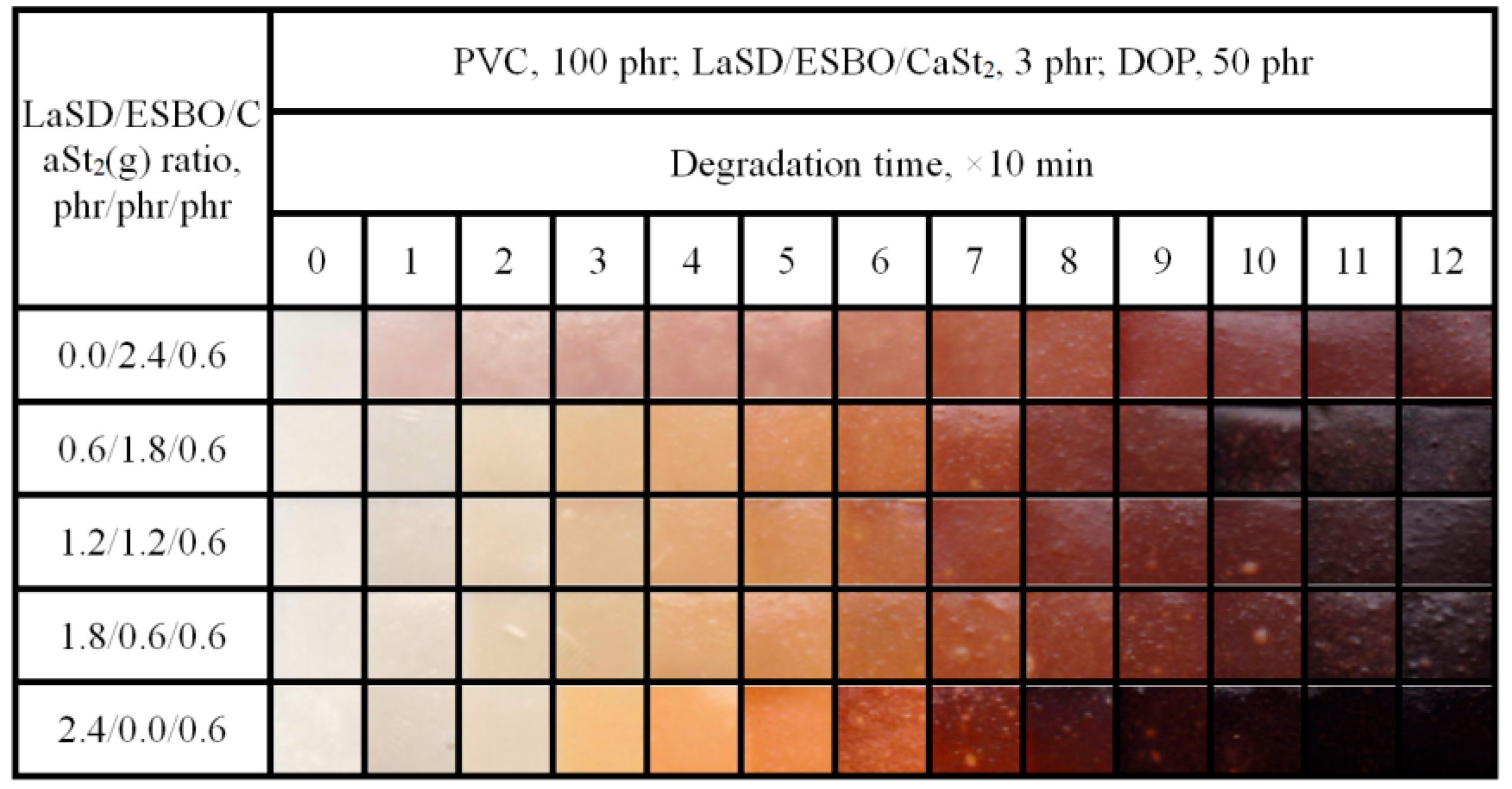
3.5. Influence of LaSD/ESBO/CaSt2 on Stabilizing PVC
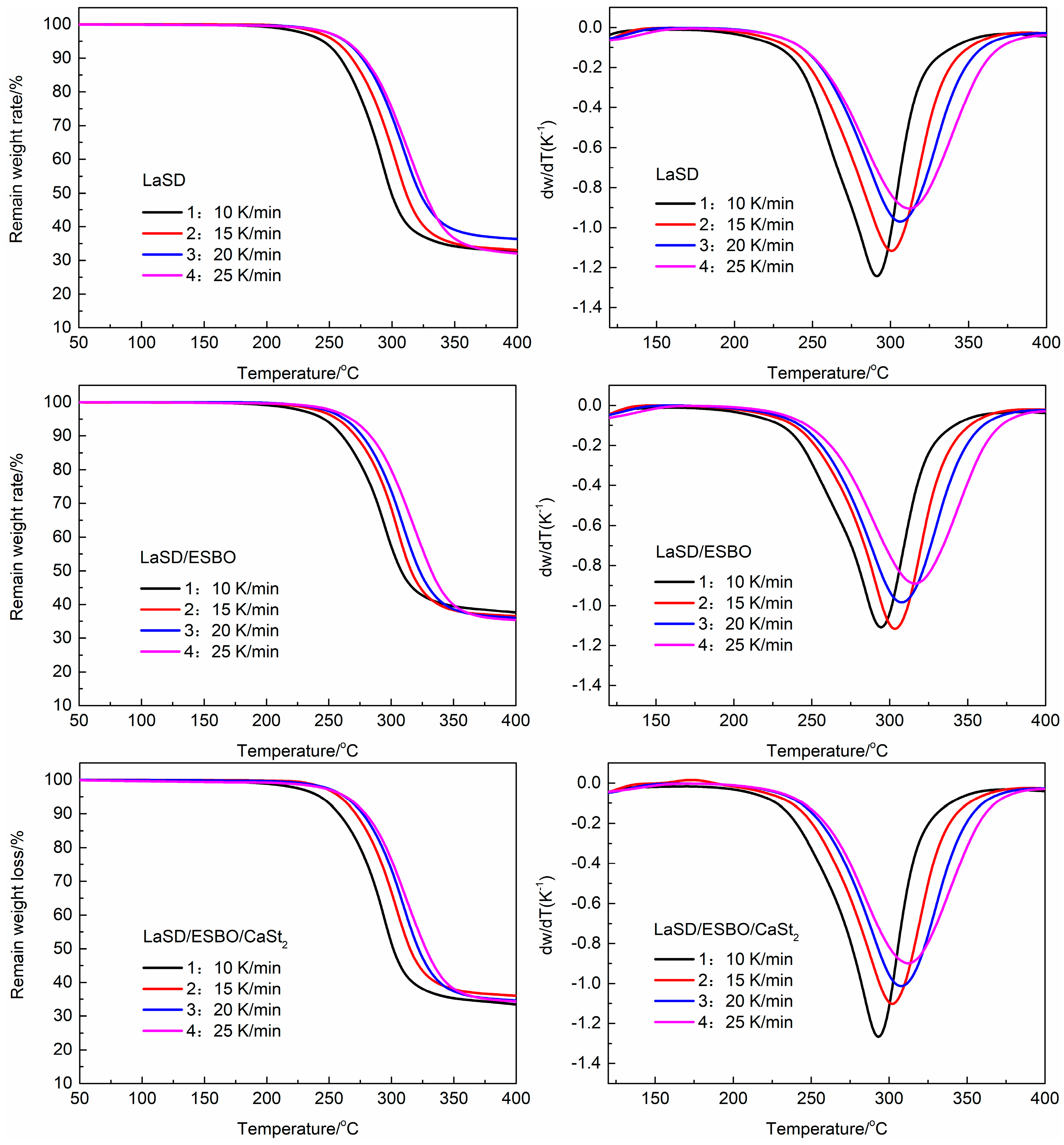
3.6. Thermal Decomposition Kinetics
3.7. UV-Vis Spectrum Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jia, P.; Zhang, M.; Hu, L.; Wang, R.; Sun, C.; Zhou, Y. Cardanol Groups Grafted on Poly(vinyl chloride)-Synthesis, Performance and Plasticization Mechanism. Polymers 2017, 9, 621. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Wang, Y.; Huang, J.; Nie, X.; Jiang, J. Synthesis and Properties of a Novel Environmental Epoxidized Glycidyl Ester of Ricinoleic Acetic Ester Plasticizer for Poly(vinyl chloride). Polymers 2017, 9, 640. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, Physical and Topography of Photochemical Process of PVC Films in the Presence of Schiff Base Metal Complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Lim, K.M.; Ching, Y.C.; Gan, S.N. Effect of Palm Oil Bio-Based Plasticizer on the Morphological, Thermal and Mechanical Properties of Poly(Vinyl Chloride). Polymers 2015, 7, 2031–2043. [Google Scholar] [CrossRef]
- Ye, F.; Ye, Q.; Zhan, H.; Ge, Y.; Ma, X.; Xu, Y.; Wang, X. Synthesis and Study of Zinc Orotate and Its Synergistic Effect with Commercial Stabilizers for Stabilizing Poly(Vinyl Chloride). Polymers 2019, 11, 194. [Google Scholar] [CrossRef]
- Ye, F.; Guo, X.J.; Zhan, H.H.; Lin, J.X.; Lou, W.C.; Ma, X.T.; Wang, X. The synergistic effect of zinc urate with calcium stearate and commercial assistant stabilizers for stabilizing poly(vinyl chloride). Polym. Degrad. Stab. 2018, 156, 193–201. [Google Scholar] [CrossRef]
- Bjorn, A.; Horsing, M.; Karlsson, A.; Mersiowsky, I.; Ejlertsson, J. Impacts of temperature on the leaching of organotin compounds from poly(vinyl chloride) plastics-A study conducted under simulated landfill conditions. J. Vinyl. Addit. Technol. 2007, 13, 176–188. [Google Scholar] [CrossRef]
- Kalouskova, R.; Novotna, M.; Vymazal, Z. Investigation of thermal stabilization of poly(vinyl chloride) by lead stearate and its combination with synthetic hydrotalcite. Polym. Degrad. Stab. 2004, 85, 903–909. [Google Scholar] [CrossRef]
- Zeddam, C.; Belhaneche-Bensemra, N. Application of FT-IR spectroscopy to the specific migration study of an organotin heat stabilizer from rigid poly(vinyl chloride) into food simulants. Polimery 2011, 56, 657–661. [Google Scholar] [CrossRef]
- Wang, M.; Song, X.H.; Jiang, J.C.; Xia, J.L.; Li, M. Binary amide-containing tung-oil-based Ca/Zn stabilizers: Effects on thermal stability and plasticization performance of poly(vinyl chloride) and mechanism of thermal stabilization. Polym. Degrad. Stab. 2017, 143, 106–117. [Google Scholar] [CrossRef]
- Wang, M.; Xia, J.L.; Jiang, J.C.; Li, S.H.; Huang, K.; Mao, W.; Li, M. A novel liquid Ca/Zn thermal stabilizer synthesized from tung-maleic anhydride and its effects on thermal stability and mechanical properties of PVC. Polym. Degrad. Stab. 2016, 133, 136–143. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, D.D.; Wei, Z.; Ma, Y.Q. Synthesis of lanthanum ricinoleate and its effect on thermal stability and mechanical properties in PVC. J. Rare Earth 2014, 32, 1089–1094. [Google Scholar] [CrossRef]
- Grivel, J.C.; Zhao, Y.; Guevara, M.J.S.; Watenphul, A. Studies on the thermal decomposition of lanthanum (III) valerate and lanthanum(III) caproate in argon. Thermochim. Acta 2015, 612, 1–9. [Google Scholar] [CrossRef]
- Xie, L.H.; Li, D.G.; Fu, M.; Zhang, J.; Zhang, L.P.; Zhang, Y.L.; Zhao, P.P. Study on lanthanum-pentaerythritol alkoxide as a thermal stabilizer for rigid polyvinyl chloride. J. Vinyl. Addit. Technol. 2017, 23, 55–61. [Google Scholar] [CrossRef]
- Zhang, D.F.; Yao, S.; Li, S.; Wang, J.; Yao, Y.W. A novel La-containing additive for the long-term thermal stabilization of poly(vinyl chloride). Polym. Degrad. Stab. 2017, 144, 187–194. [Google Scholar] [CrossRef]
- Li, D.G.; Xie, L.H.; Fu, M.; Zhang, J.; Indrawirawan, S.; Zhang, Y.L.; Tang, S.Y. Synergistic effects of lanthanum-pentaerythritol alkoxide with zinc stearates and with beta-diketone on the thermal stability of poly(vinyl chloride). Polym. Degrad. Stab. 2015, 114, 52–59. [Google Scholar] [CrossRef]
- Li, M.; Duan, C.; Wang, H.O.; Liu, Z.G.; Wang, M.T.; Hu, Y.H. Lanthanum histidine with pentaerythritol and zinc stearate as thermal stabilizers for poly(vinyl chloride). J. Appl. Polym. Sci. 2016, 133, 42878. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.D.; Wang, X.B.; Li, K.S.; Liu, X.R. Synthesis of the complex of lanthanum (III) with N-(2-amino ethyl) maleamic acid radical and its application to PVC as thermal stabilizer. Polym. Degrad. Stab. 2016, 124, 87–94. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, R.R.; Yassin, A.A. Organic thermal stabilizers for rigid poly(vinyl chloride). Part X. N-acryloyl-N′-p-substituted phenylthiourea derivatives. Polym. Degrad. Stabil. 2003, 82, 387–393. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, R.R.; Yassin, A.A. Organic thermal stabilizers for rigid poly(vinyl chloride) VIII. Phenylurea and phenylthiourea derivatives. Polym. Degrad. Stabil. 2003, 81, 37–45. [Google Scholar] [CrossRef]
- Wu, B.Z.; Wang, Y.; Chen, S.; Wang, M.Y.; Ma, M.; Shi, Y.Q.; Wang, X. Bis-uracil based high efficient heat stabilizers used in super transparent soft poly (vinyl chloride). Polym. Degrad. Stabil. 2018, 149, 143–151. [Google Scholar] [CrossRef]
- Xu, X.P.; Chen, S.; Shi, Y.Q.; Wu, B.Z.; Ma, M.; Wang, X. Novel organic antibacterial thermal stabilizers for transparent poly(vinyl chloride). J. Therm. Anal. Calorim. 2015, 122, 1435–1444. [Google Scholar] [CrossRef]
- Rocha, M.; Piro, O.E.; Echeverria, G.A.; Pastoriza, A.C.; Sgariglia, M.A.; Soberon, J.R.; Gil, D.M. Co(II), Ni(II) and Cu(II) ternary complexes with sulfadiazine and dimethylformamide: Synthesis, spectroscopic characterization, crystallographic study and antibacterial activity. J. Mol. Struct. 2019, 1176, 605–613. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zhou, L.; Yang, W.C.; Zhou, L.N.; Bao, Y.; Zhang, M.J.; Hou, B.H.; Xu, Z.; Yin, Q.X. Effects of Hydrogen Bond Acceptor Ability of Solvents on Molecular Self-Assembly of Sulfadiazine Solvates. J. Pharm. Sci. USA 2018, 107, 2823–2828. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, J.; Shi, X.M.; Jiang, G.D. Influence of UV absorber on photodegradation processes of poly(vinyl chloride) with different average degrees of polymerization. Polym. Eng. Sci. 2007, 47, 1480–1490. [Google Scholar] [CrossRef]
- Xu, X.P.; Chen, S.; Tang, W.; Qu, Y.J.; Wang, X. Investigation of basic zinc cyanurate as a novel thermal stabilizer for poly(vinyl chloride) and its synergistic effect with calcium stearate. Polym. Degrad. Stab. 2014, 99, 211–218. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Z.Y.; Liu, Z.G.; Hu, Y.H.; Wang, M.T.; Wang, H.O. Effect of lanthanum cyanurate as novel organic thermal stabilizers for polyvinyl chloride. Polym. Eng. Sci. 2013, 53, 1706–1711. [Google Scholar] [CrossRef]
- Dong, T.B.; Li, D.G.; Li, Y.P.; Han, W.Y.; Zhang, L.P.; Xie, G.; Sunarso, J.; Liu, S.M. Design and synthesis of polyol ester-based zinc metal alkoxides as a bi-functional thermal stabilizer for poly(vinyl chloride). Polym. Degrad. Stab. 2019, 159, 125–132. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Chen, S.; Ma, M.; Wu, B.Z.; Ying, J.; Xu, X.P.; Wang, X. Highly efficient and antibacterial zinc norfloxacin thermal stabilizer for poly(vinyl chloride). RSC Adv. 2016, 6, 97491–97502. [Google Scholar] [CrossRef]
- Ameen, I.; Tripathi, A.K.; Siddiqui, A.; Kapil, G.; Pandey, S.S.; Tripathi, U.N. Synthesis, characterizations and photo-physical properties of novel lanthanum(III) complexes. J. Taibah Univ. Sci. 2018, 12, 796–808. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.G.; Liu, H.; Zhang, J. Insights into the use of zinc-mannitol alkoxide as a novel thermal stabilizer for rigid poly(vinyl chloride). J. Appl. Polym. Sci. 2015, 132, 42038. [Google Scholar] [CrossRef]
- Khaleghi, M.; Didehban, K.; Shabanian, M. Effect of new melamine-terephthaldehyde resin modified graphene oxide on thermal and mechanical properties of PVC. Polym. Test. 2017, 63, 382–391. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.C.; Zhang, J.; Zhu, W.Q.; Tian, T.S. Remarkably improved toughness and thermal stability of poly (vinyl chloride) (PVC)/poly (alphamethyl-styrene-acrylonitrile) (alpha-MSAN) blend with the assistance of two impact modifiers. Polym. Test. 2016, 51, 1–5. [Google Scholar] [CrossRef]
- Chen, S.; Xu, X.P.; Zhang, J.H.; Tang, W.; Qu, Y.J.; Wang, X. Efficiency and mechanism for the stabilizing action of N,N’-bis(phenylcarbamoyl)alkyldiamines as thermal stabilizers and co-stabilizers for poly(vinyl chloride). Polym. Degrad. Stab. 2014, 105, 178–184. [Google Scholar] [CrossRef]
- Xu, X.P.; Chen, S.; Tang, W.; Qu, Y.J.; Wang, X. Synthesis and application of uracil derivatives as novel thermal stabilizers for rigid poly(vinyl chloride). Polym. Degrad. Stab. 2013, 98, 659–665. [Google Scholar] [CrossRef]
- Chen, G.; Gu, L.Q.; Yao, Y.W. A novel zinc-containing additive for the long-term thermal stabilization of poly(vinyl chloride). Polym. Degrad. Stab. 2014, 107, 113–119. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.W.; Xin, J.N.; Huang, K.; Li, S.H.; Wang, M.; Xia, J.L. Design of green zinc-based thermal stabilizers derived from tung oil fatty acid and study of thermal stabilization for PVC. J. Appl. Polym. Sci. 2014, 105, 178–184. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.W.; Huang, K.; Li, S.H.; Jiang, J.C.; Xia, J.L. Mixed calcium and zinc salts of dicarboxylic acids derived from rosin and dipentene: Preparation and thermal stabilization for PVC. RSC Adv. 2014, 4, 63576–63585. [Google Scholar] [CrossRef]
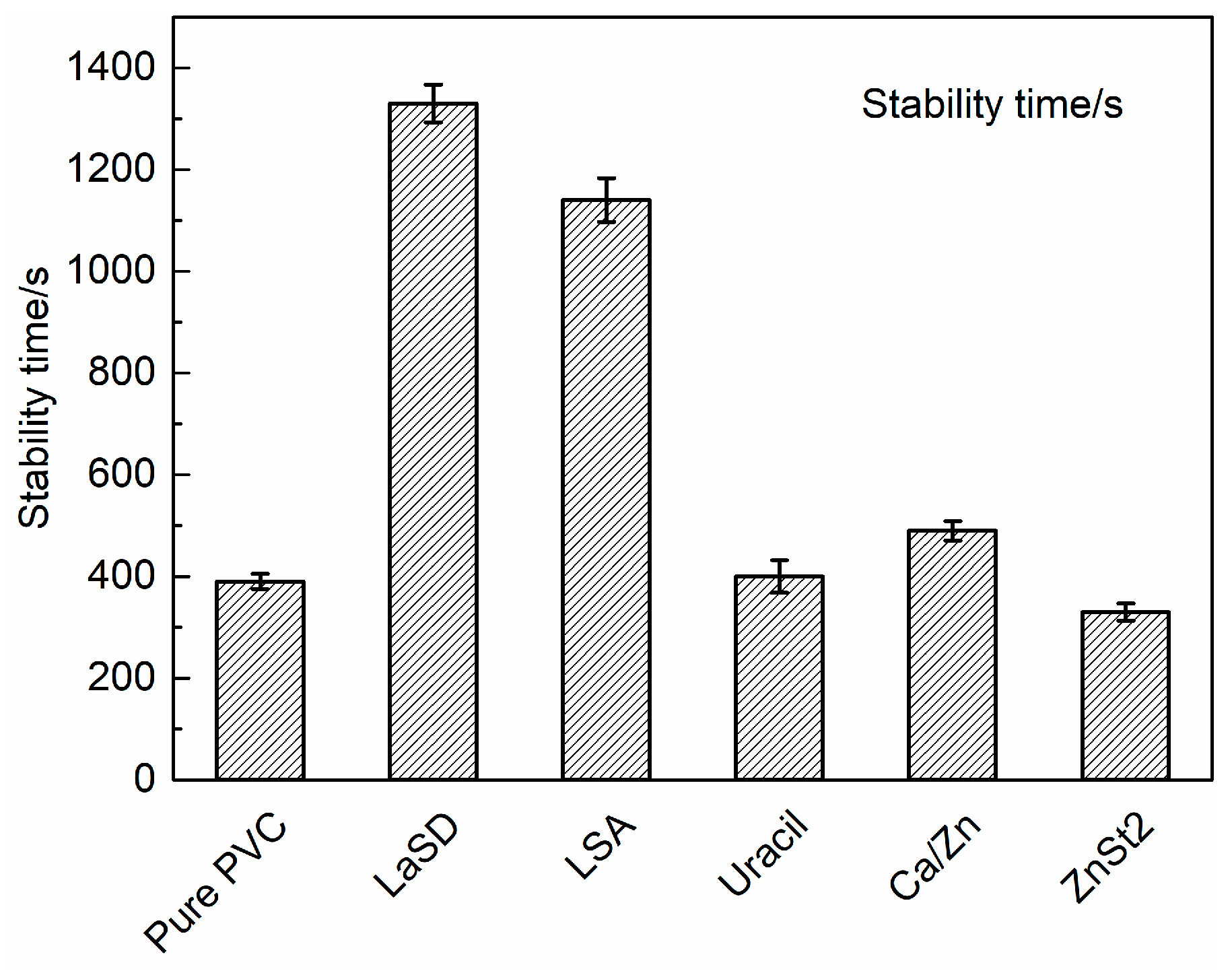


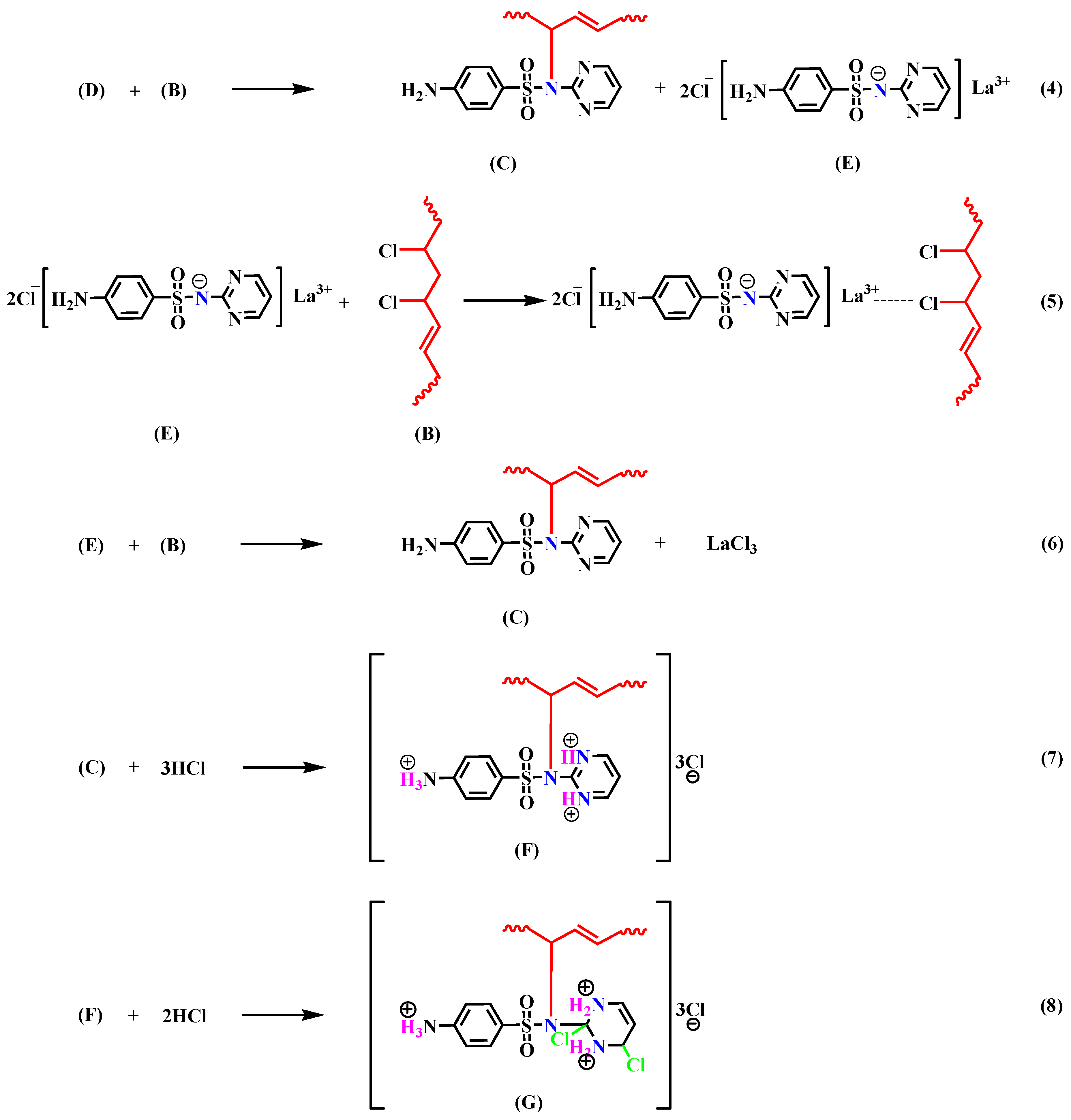


| Element | La | C | H | N | S | Molecular formula |
|---|---|---|---|---|---|---|
| Theory | 15.69 | 40.63 | 3.05 | 18.96 | 10.84 | La(C10H9N4O2S)3 |
| Reality | 15.21 | 40.01 | 3.34 | 18.33 | 10.43 | La(C10H9N4O2S)3 |
| Materials | T5% (oC) | T10% (°C) | Tf (°C) | Wf (%) |
|---|---|---|---|---|
| PVC | 242.1 | 256.8 | 288.3 | 70.5 |
| PVC/LaSD (1.0 wt %) | 244.1 | 258.6 | 291.2 | 66.9 |
| PVC/LaSD (3.0 wt %) | 245.3 | 259.2 | 292.9 | 65.7 |
| PVC/LaSD (5.0 wt %) | 246.6 | 261.1 | 294.5 | 61.9 |
| Sample | β (K/ min) | Tmax/ K | Ea/ (kJ/ mol) |
|---|---|---|---|
| PVC/LaSD | 10 | 564.1 | 106.6 |
| 15 | 574.3 | ||
| 20 | 579.5 | ||
| 25 | 586.2 | ||
| PVC/LaSD/ESBO | 10 | 568.1 | 112.1 |
| 15 | 577.3 | ||
| 20 | 581.6 | ||
| 25 | 589.7 | ||
| PVC/LaSD/ESBO/CaSt2 | 10 | 566.6 | 119.9 |
| 15 | 575.3 | ||
| 20 | 581.3 | ||
| 25 | 586.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Ma, X.; Li, B.; Jin, Z.; Xu, Y.; Fang, C.; Zhou, X.; Ge, Y.; Ye, F. Development and Investigation of Lanthanum Sulfadiazine with Calcium Stearate and Epoxidised Soyabean Oil as Complex Thermal Stabilizers for Stabilizing Poly(vinyl chloride). Polymers 2019, 11, 531. https://doi.org/10.3390/polym11030531
Ye Q, Ma X, Li B, Jin Z, Xu Y, Fang C, Zhou X, Ge Y, Ye F. Development and Investigation of Lanthanum Sulfadiazine with Calcium Stearate and Epoxidised Soyabean Oil as Complex Thermal Stabilizers for Stabilizing Poly(vinyl chloride). Polymers. 2019; 11(3):531. https://doi.org/10.3390/polym11030531
Chicago/Turabian StyleYe, Qiufeng, Xiaotao Ma, Bobin Li, Zhe Jin, Yingying Xu, Cheng Fang, Xiaoya Zhou, Yeqian Ge, and Feng Ye. 2019. "Development and Investigation of Lanthanum Sulfadiazine with Calcium Stearate and Epoxidised Soyabean Oil as Complex Thermal Stabilizers for Stabilizing Poly(vinyl chloride)" Polymers 11, no. 3: 531. https://doi.org/10.3390/polym11030531
APA StyleYe, Q., Ma, X., Li, B., Jin, Z., Xu, Y., Fang, C., Zhou, X., Ge, Y., & Ye, F. (2019). Development and Investigation of Lanthanum Sulfadiazine with Calcium Stearate and Epoxidised Soyabean Oil as Complex Thermal Stabilizers for Stabilizing Poly(vinyl chloride). Polymers, 11(3), 531. https://doi.org/10.3390/polym11030531