Water Absorption, Hydrothermal Expansion, and Thermomechanical Properties of a Vinylester Resin for Fiber-Reinforced Polymer Composites Subjected to Water or Alkaline Solution Immersion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Water Uptake Test
2.4. Sample Drying
2.5. Characterization
3. Results and Discussion
3.1. Water Uptake and Diffusion
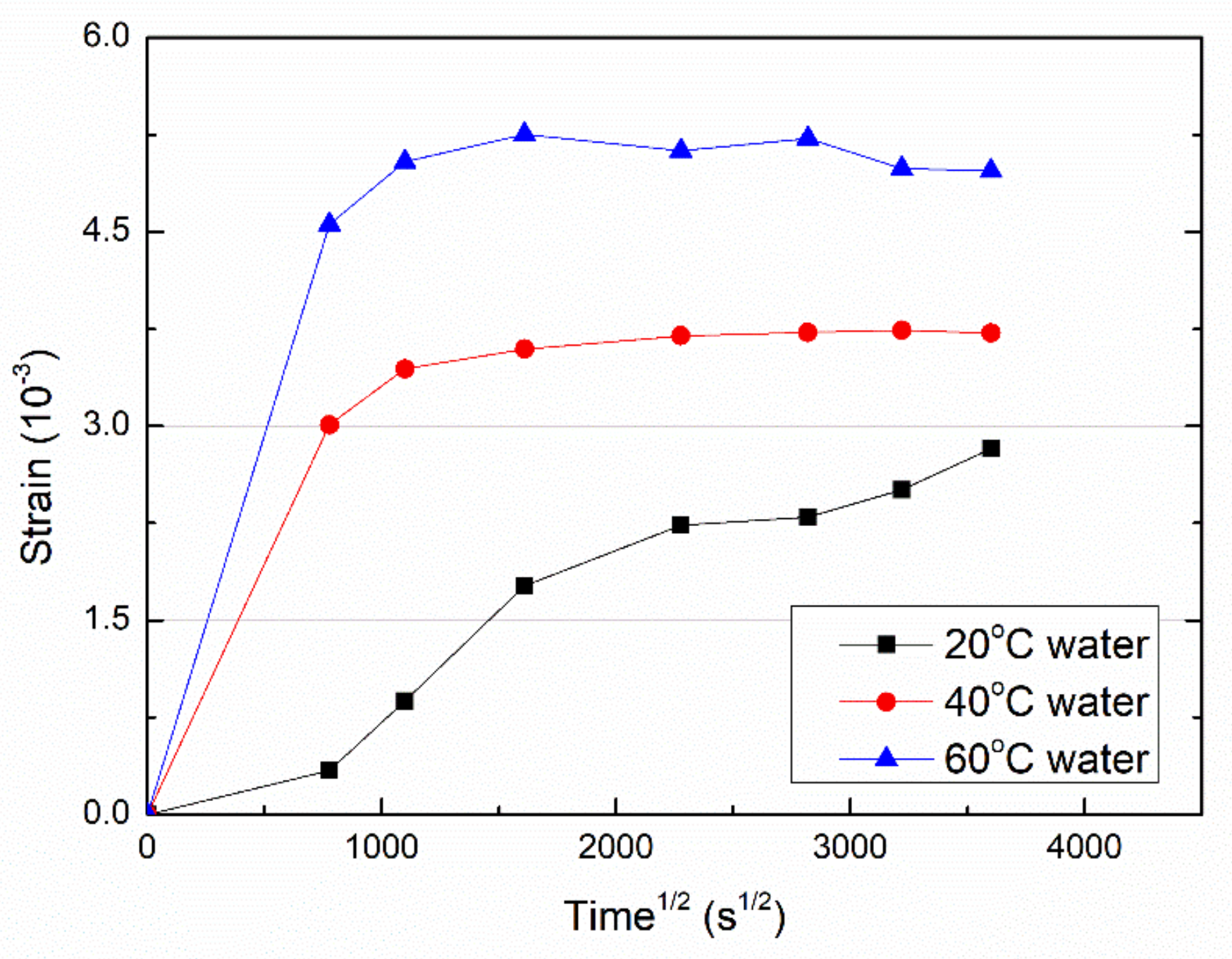
3.2. Hygrothermal Expansion
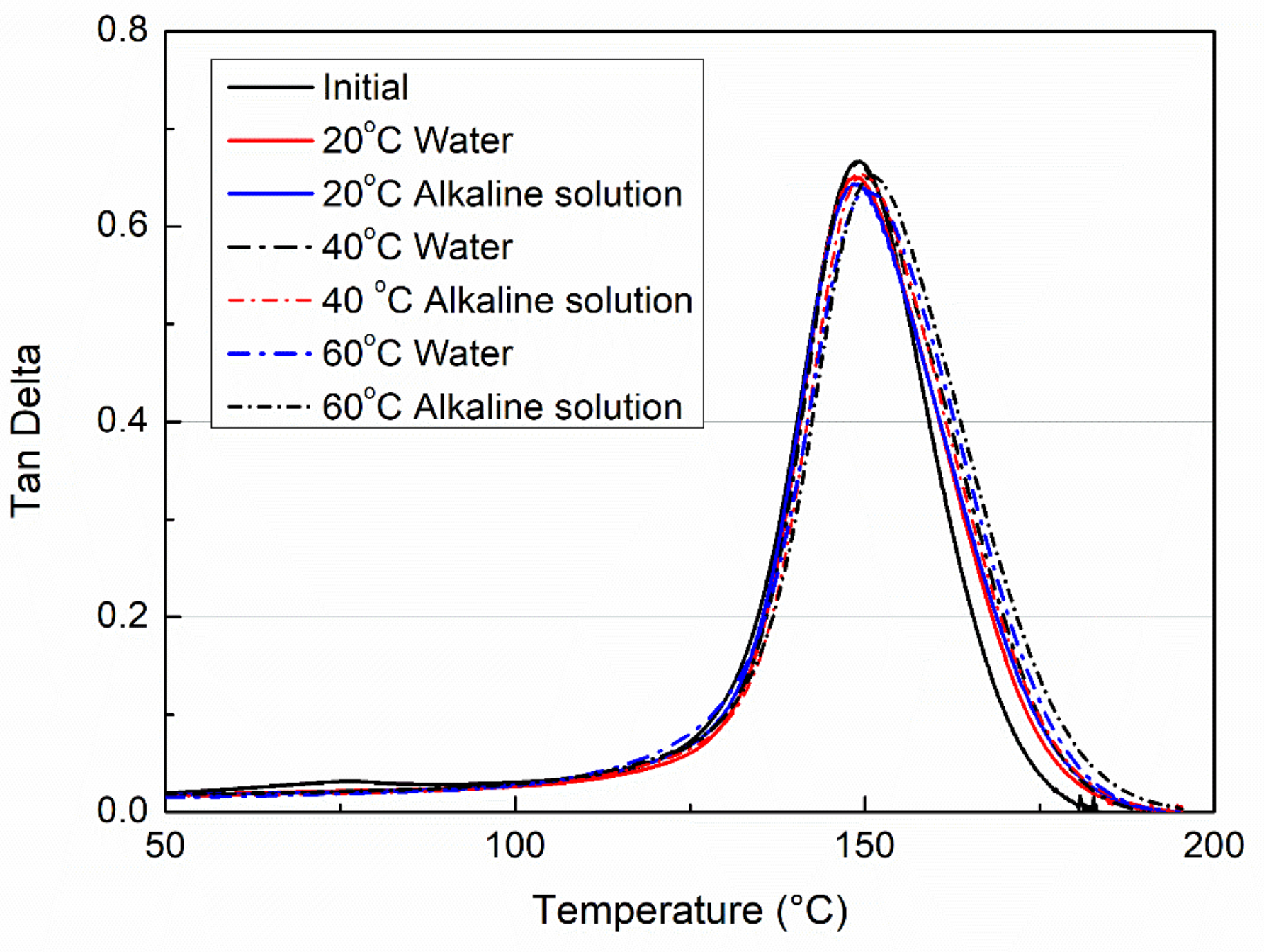
3.3. Glass Transition Temperatures
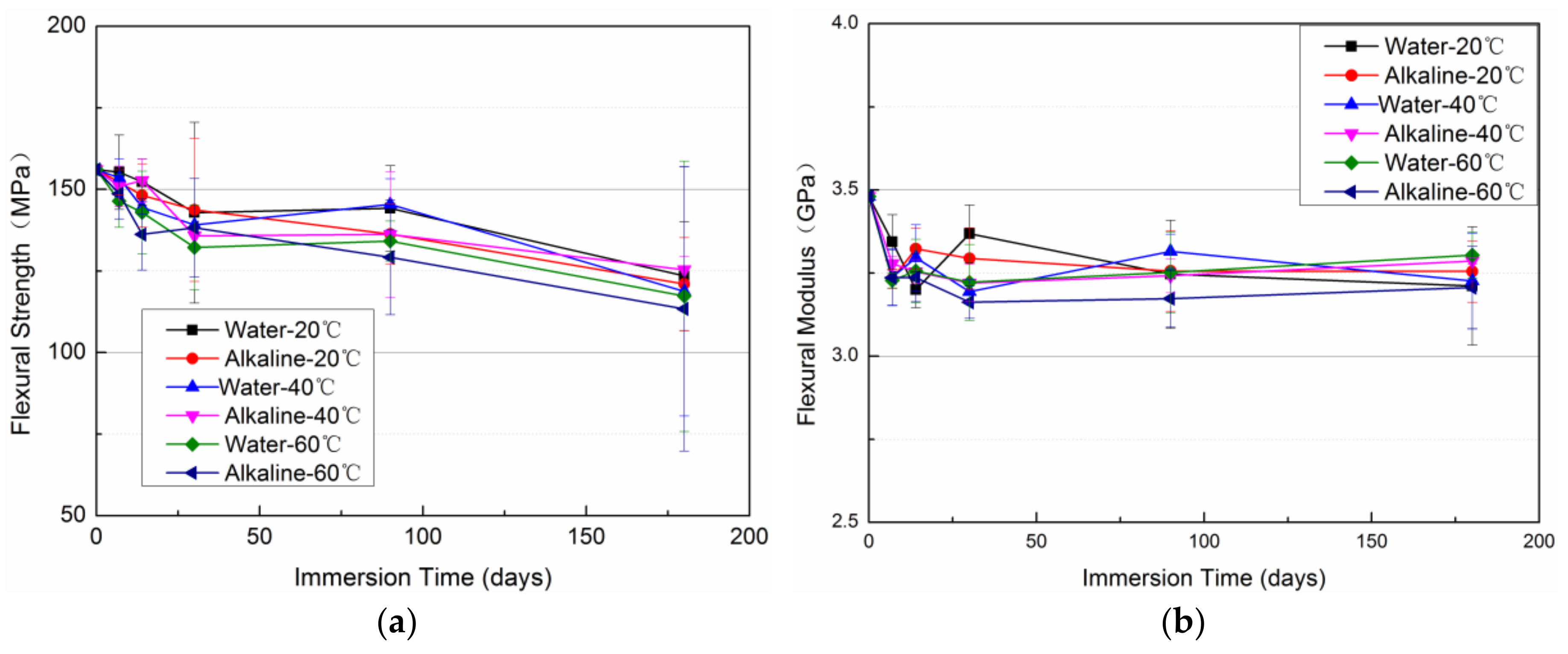
3.4. Evolution of Mechanical Properties
4. Conclusions
- (1)
- Immersion in an alkaline solution causes a mass loss (approx. 0.4%) due to hydrolysis and the leaching of uncured small molecules.
- (2)
- With the same water uptake, the expansion of VE samples increased with the immersion temperatures, which is attributed to the increased relaxation extent of the resin molecular network.
- (3)
- Immersion in both water and an alkaline solution leads to a remarkable decrease of the glass transition temperatures of the VE samples, while they can be recovered almost completely by drying.
- (4)
- The tensile strength and elongation at break can be enhanced for a short term (less than 6 months) of immersion but will be degraded by an extended immersion period.
Author Contributions
Funding
Conflicts of Interest
References
- Da Silva, L.V.; da Silva, F.W.; Tarpani, J.R.; Forte, M.M.D.; Amico, S.C. Ageing effect on the tensile behavior of pultruded cfrp rods. Mater. Des. 2016, 110, 245–254. [Google Scholar] [CrossRef]
- Ramirez, G.; Engelhardt, M.D. Experimental investigation of a large-scale composite riser tube under external pressure. J. Press. Vessel Technol. 2009, 131, 051205. [Google Scholar] [CrossRef]
- Berg, A.C.; Bank, L.C.; Oliva, M.G.; Russell, J.S. Construction and cost analysis of an frp reinforced concrete bridge deck. Constr. Build. Mater. 2006, 20, 515–526. [Google Scholar] [CrossRef]
- Yu, Y.H.; Yang, X.P.; Wang, L.L.; Liu, H.L. Hygrothermal aging on pultruded carbon fiber/vinyl ester resin composite for sucker rod application. J. Reinf. Plast. Compos. 2006, 25, 149–160. [Google Scholar] [CrossRef]
- Pan, Y.; Xian, G.; Li, H. Effects of freeze-thaw cycles on the behavior of the bond between cfrp plates and concrete substrates. J. Compos. Constr. 2018, 22, 04018011. [Google Scholar] [CrossRef]
- Ozel, M.; Bank, L.C.; Arora, D.; Gonenc, O.; Gremel, D.; Nelson, B.; McMonigal, D. Comparison between frp rebar, frp grid and steel rebar reinforced concrete beams. In Fibre-Reinforcement Polymer: Reinforcement for Concrete Structures; World Scientific: Singapore, 2003; pp. 1067–1076. [Google Scholar]
- Xian, G.; Hong, B.; Wang, Z.; Li, H. Durability study of cfrp pultruded plates subjected to water immersion. In Proceedings of the 7th International Conference on FRP Composites in Civil Engineering, Vancouver, BC, Canada, 20–22 August 2014. [Google Scholar]
- Hong, B.; Xian, G.; Wang, Z. Durability study of pultruded carbon fiber reinforced polymer plates subjected to water immersion. Adv. Struct. Eng. 2018, 21, 571–579. [Google Scholar] [CrossRef]
- Hong, B.; Xian, G.; Li, H. Effects of water or alkali solution immersion on the water uptake and physicomechanical properties of polyurethane. Polym. Eng. Sci. 2018, 58, 2276–2287. [Google Scholar] [CrossRef]
- Hong, B.; Xian, G. Ageing of a thermosetting polyurethane and its pultruded carbon fiber plates subjected to seawater immersion. Constr. Build. Mater. 2018, 165, 514–522. [Google Scholar] [CrossRef]
- Portnov, G.; Bakis, C.E.; Lackey, E.; Kulakov, V. Frp reinforcing bars—Designs and methods of manufacture (review of patents). Mech. Compos. Mater. 2013, 49, 381–400. [Google Scholar] [CrossRef]
- Alvarez, V.; Vazquez, A.; De La Osa, O. Cyclic water absorption behavior of glass-vinylester and glass-epoxy composites. J. Compos. Mater. 2007, 41, 1275–1289. [Google Scholar] [CrossRef]
- Afshar, A.; Alkhader, M.; Korach, C.S.; Chiang, F.-P. Effect of long-term exposure to marine environments on the flexural properties of carbon fiber vinylester composites. Compos. Struct. 2015, 126, 72–77. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Yang, Y.; Xian, G. Flexural fatigue behavior of a pultruded basalt fiber reinforced epoxy plate subjected to elevated temperatures exposure. Polym. Compos. 2018, 39, 1731–1741. [Google Scholar] [CrossRef]
- Lu, Z.; Xian, G. Combined effects of sustained tensile loading and elevated temperatures on the mechanical properties of pultruded bfrp plates. Constr. Build. Mater. 2017, 150, 310–320. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Xian, G.; Li, H. Effects of surface treatment of carbon fiber: Tensile property, surface characteristics, and bonding to epoxy. Polym. Compos. 2016, 37, 2921–2932. [Google Scholar] [CrossRef]
- Kafodya, I.; Xian, G.; Li, H. Durability study of pultruded cfrp plates immersed in water and seawater under sustained bending: Water uptake and effects on the mechanical properties. Compos. Part B Eng. 2015, 70, 138–148. [Google Scholar] [CrossRef]
- Helbling, C.S.; Karbhari, V.M. Investigation of the sorption and tensile response of pultruded e-glass/vinylester composites subjected to hygrothermal exposure and sustained strain. J. Reinf. Plast. Compos. 2008, 27, 613–638. [Google Scholar] [CrossRef]
- Yang, S.; Liu, W.; Fang, Y.; Huo, R. Influence of hygrothermal aging on the durability and interfacial performance of pultruded glass fiber-reinforced polymer composites. J. Mater. Sci. 2019, 54, 2102–2121. [Google Scholar] [CrossRef]
- Benmokrane, B.; Ali, A.H.; Mohamed, H.M.; ElSafty, A.; Manalo, A. Laboratory assessment and durability performance of vinyl-ester, polyester, and epoxy glass-frp bars for concrete structures. Compos. Part B Eng. 2017, 114, 163–174. [Google Scholar] [CrossRef]
- Benmokrane, B.; Elgabbas, F.; Ahmed, E.A.; Cousin, P. Characterization and comparative durability study of glass/vinylester, basalt/vinylester, and basalt/epoxy frp bars. J. Compos. Constr. 2015, 19, 04015008. [Google Scholar] [CrossRef]
- Ramirez, F.A.; Carlsson, L.A.; Acha, B.A. Evaluation of water degradation of vinylester and epoxy matrix composites by single fiber and composite tests. J. Mater. Sci. 2008, 43, 5230–5242. [Google Scholar] [CrossRef]
- Fichera, M.; Carlsson, L.A. Moisture transport in unidirectional carbon/vinylester panels with imperfect fiber/matrix interface. J. Compos. Mater. 2016, 50, 751–760. [Google Scholar] [CrossRef]
- Figliolini, A.M.; Carlsson, L.A. Mechanical properties of carbon fiber/vinylester composites exposed to marine environments. Polym. Compos. 2014, 35, 1559–1569. [Google Scholar] [CrossRef]
- Lu, Z.; Xian, G. Resistance of basalt fibers to elevated temperatures and water or alkaline solution immersion. Polym. Compos. 2018, 39, 2385–2393. [Google Scholar] [CrossRef]
- Li, H.; Xian, G.; Lin, Q.; Zhang, H. Freeze-thaw resistance of unidirectional-fiber-reinforced epoxy composites. J. Appl. Polym. Sci. 2012, 123, 3781–3788. [Google Scholar] [CrossRef]
- Wrosch, M.; Xian, G.J.; Karbhari, V.M. Moisture absorption and desorption in a uv cured urethane acrylate adhesive based on radiation source. J. Appl. Polym. Sci. 2008, 107, 3654–3662. [Google Scholar] [CrossRef]
- Yang, Q.A.; Xian, G.J.; Karbhari, V.M. Hygrothermal ageing of an epoxy adhesive used in frp strengthening of concrete. J. Appl. Polym. Sci. 2008, 107, 2607–2617. [Google Scholar] [CrossRef]
- Xian, G.J.; Karbhari, V.M. Dmta based investigation of hygrothermal ageing of an epoxy system used in rehabilitation. J. Appl. Polym. Sci. 2007, 104, 1084–1094. [Google Scholar] [CrossRef]
- Xian, G.; Karbhari, V.M. Segmental relaxation of water-aged ambient cured epoxy. Polym. Degrad. Stab. 2007, 92, 1650–1659. [Google Scholar] [CrossRef]
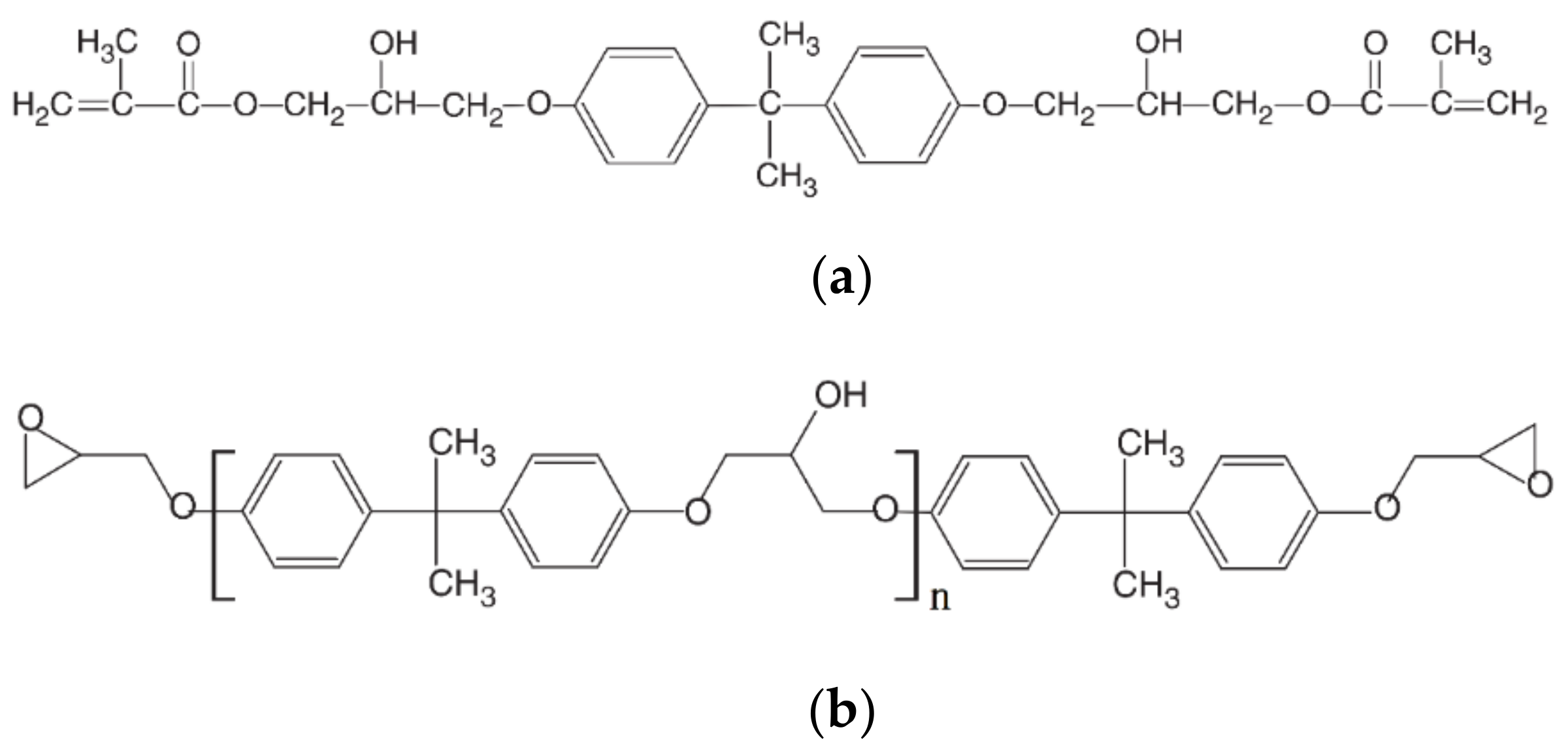
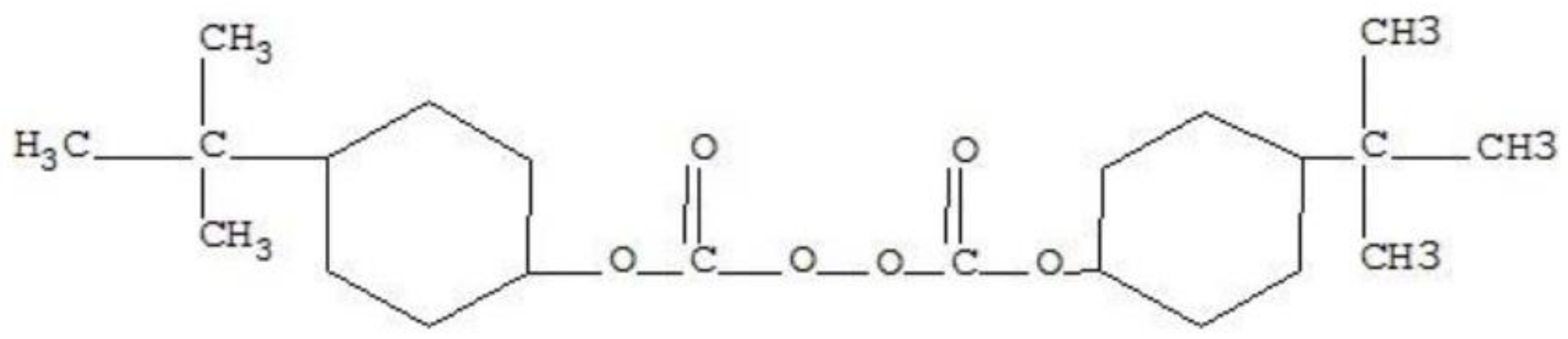
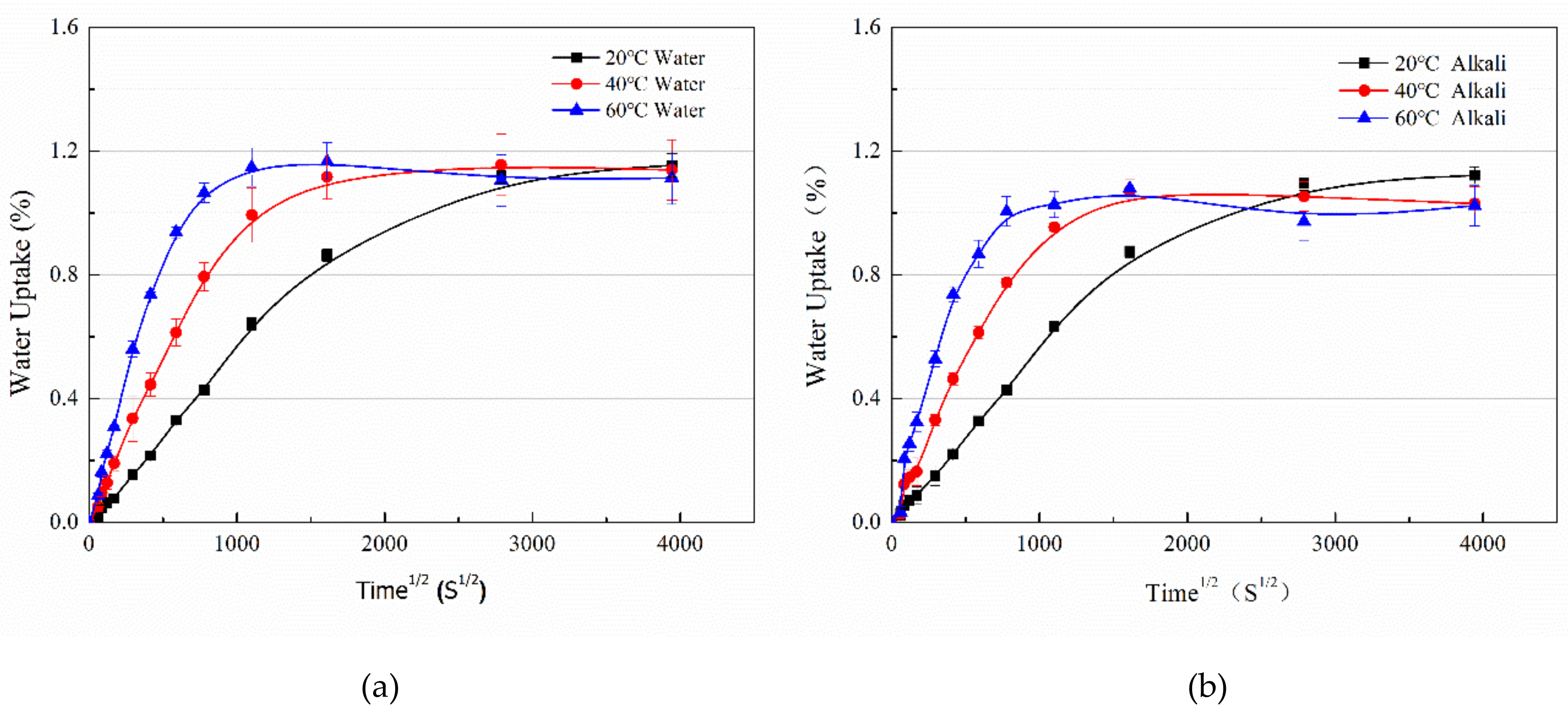


| Immersion Media | Temp. (°C) | M∞ (%) | K (10−6) | D (10−6 mm2/s) |
|---|---|---|---|---|
| Water | 20 | 1.07 | 21.41 | 0.78 |
| 40 | 1.11 | 8.54 | 2.84 | |
| 60 | 1.15 | −9.57 | 8.70 | |
| Alkaline solution | 20 | 1.11 | 3.09 | 0.77 |
| 40 | 1.08 | −9.96 | 2.63 | |
| 60 | 1.05 | −11.15 | 7.24 |
| Immerse Conditions | Mass Loss (%) at | ||
|---|---|---|---|
| 20 °C | 40 °C | 60 °C | |
| 3 months water | 0.07 | 0.11 | 0.23 |
| 3 months Alkaline | 0.13 | 0.15 | 0.39 |
| 6 months water | 0.09 | 0.19 | 0.24 |
| 6 months Alkaline | 0.17 | 0.31 | 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Liu, Y.; Miao, Y.; Xian, G. Water Absorption, Hydrothermal Expansion, and Thermomechanical Properties of a Vinylester Resin for Fiber-Reinforced Polymer Composites Subjected to Water or Alkaline Solution Immersion. Polymers 2019, 11, 505. https://doi.org/10.3390/polym11030505
Yin X, Liu Y, Miao Y, Xian G. Water Absorption, Hydrothermal Expansion, and Thermomechanical Properties of a Vinylester Resin for Fiber-Reinforced Polymer Composites Subjected to Water or Alkaline Solution Immersion. Polymers. 2019; 11(3):505. https://doi.org/10.3390/polym11030505
Chicago/Turabian StyleYin, Xiaoli, Yancong Liu, Yufei Miao, and Guijun Xian. 2019. "Water Absorption, Hydrothermal Expansion, and Thermomechanical Properties of a Vinylester Resin for Fiber-Reinforced Polymer Composites Subjected to Water or Alkaline Solution Immersion" Polymers 11, no. 3: 505. https://doi.org/10.3390/polym11030505
APA StyleYin, X., Liu, Y., Miao, Y., & Xian, G. (2019). Water Absorption, Hydrothermal Expansion, and Thermomechanical Properties of a Vinylester Resin for Fiber-Reinforced Polymer Composites Subjected to Water or Alkaline Solution Immersion. Polymers, 11(3), 505. https://doi.org/10.3390/polym11030505





