3.2. Effect of Intrinsic Variables in Electrospinning Process Parameters
The optimal electrospinning process parameters (mainly flow and voltage) to obtain a stable Taylor cone depend on the solution’s intrinsic parameters. When the design of experiments methodology is used to establish this dependence there are several approaches: to employ voltage and flow rate as selected variables [
37,
38], to use the same voltage and flow rate for all solutions [
39,
40], or to adjust these parameters in each solution. As mentioned in the introduction, we selected the last strategy in order to avoid effects due to the poor quality and low production of fibers in the response parameters. It must be taken into account that our goal was to obtain shape memory fibers and therefore, enough material to manipulate the mats was required. Therefore, not only flow rate and voltage were adjusted in each solution but also evaluated as responses in order to study how these variables were affected by the solution properties.
The voltage and flow rate employed to obtain a stable Taylor cone for each solution are shown in
Table 2 and coefficients, P-values, and R
2 for the regression model obtained for voltage are represented in
Table 3. A P-value lower than 0.05 means that the factor has significance within a 95% of confidence [
41].
Only the combined effect of X
1 and X
2 had a significant effect in a 95% of significance.
Table 4 shows the same calculation after removing items with higher P-value. R
2 was 0.92, so 92% of the response was explained by the model.
Figure 1 shows the measured vs predicted voltage.
The combined effect of PCL percentage and solution concentration played an important role as a P-value lower than 0.01 was obtained. In our approach, the voltage was established at the same flow rate for each solution, and then flow rate was readjusted. Therefore, at the same flow rate, solutions with a higher concentration of PCL (the combined effect of X
1·X
2 corresponds to the total concentration of PCL in the solutions) required a higher voltage to start the process. High concentration of PCL resulted in high viscosity solutions and therefore higher voltage was required when increasing the PCL concentration [
42].
In the case of X
3, numerous authors have employed organic salts in electrospinning, and usually, the spinnability of the polymer solutions containing organic salts increases as the charge density is higher and therefore the pulling force that suffers the solution at the same voltage increases [
43,
44]. In our case, there was some indication that a higher amount of organic salt decreased the minimum voltage to start the process (as the coefficient for the amount of initiator was negative) but the P-value was higher than 0.05 so the confidence level was below 95%.
The final equation for the necessary voltage can therefore be extracted from the
Table 4 and be expressed as:
In the case of flow rate, values for coefficients, P-values, and R
2 for the regression model obtained are represented in
Table 5.
When all variables were studied, only X
1 (concentration) was significant in the model. Terms related to the amount of initiator or to the combined effect of variables were high enough to be ignored. Therefore, only X
1 and X
2 were maintained, and the regression model was obtained again. The results of such calculation are shown in
Table 6.
Considering only concentration and PCL percentage, both of them had significance inside the 95% level of confidence. The R2 obtained implies that only 78% of the response was explained by the model.
In our system, an increase of the flow rate was necessary to get a stable process when either the amount of PCL or concentration were augmented. In the case of concentration, this can be explained as a higher proportion of the drop being electrospun in the exit of the needle, and therefore to maintain the stability higher flow rate had to be applied. The same effect took place when the PCL amount was increased, as more material was electrospun (PCL was the only high molecular weight component in the system capable of being spun into fibers).
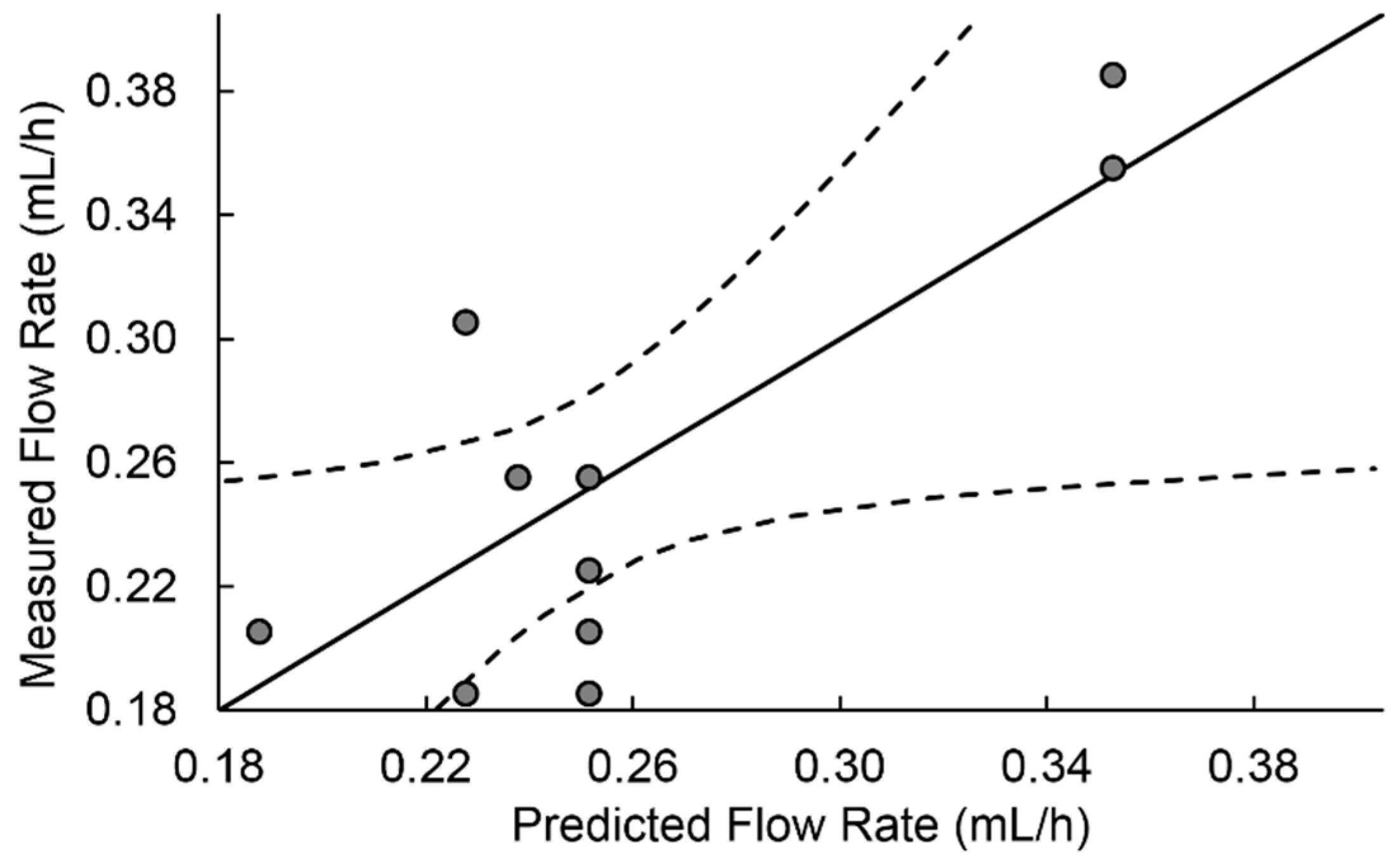
Figure 2 shows the measured versus predicted flow rate values with a regression line and 95% confidence curves for the regression model employing the terms in
Table 6.
The values of variables with no significance were removed, giving rise to the equation for flow rate shown below.
It must be taken into consideration that the flow rate was changed along with voltage, and that both variables were strongly dependent [
45]. In a general way, a higher voltage results in a higher jet pull rate, and to obtain a uniform process high flow rates are required [
37].
3.3. Curing and Thermal Characterization of the Electrospun Mats
After the electrospinning process, samples were separated from the collector and cured under UV irradiation. The degree of curing was determined by ATR-FTIR.
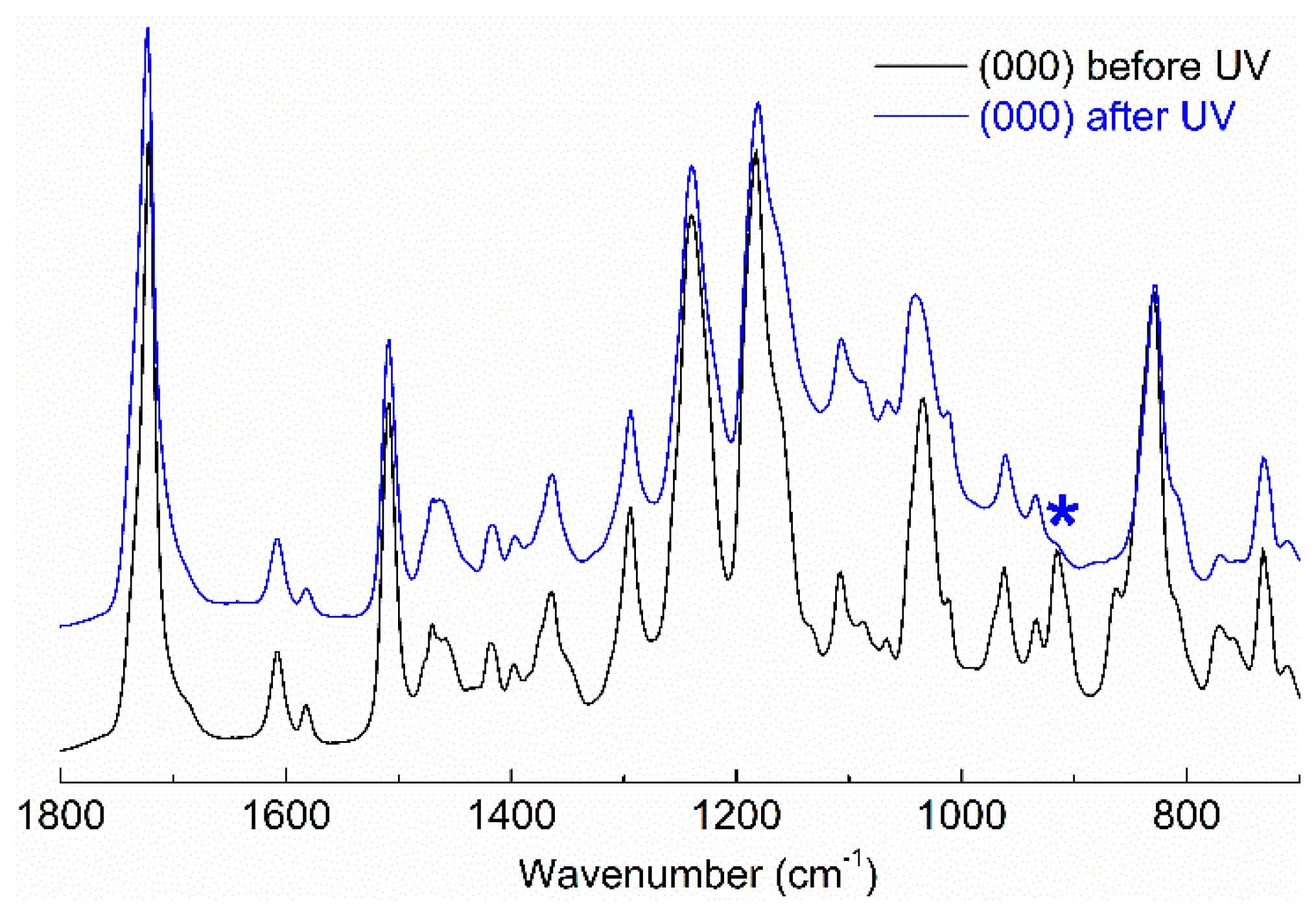
Figure 3 shows the scale expanded infrared spectra of the central point of the sample before and after UV irradiation. The spectra were characterized by the bands at 1735 cm
−1 (C=O st of the PCL), 1200–1300 cm
−1 (C-O st of PCL and C-O-C st of epoxy ether), 910 cm
−1 (bending of epoxy ring) and 830 cm
−1 (=CH oop bending of epoxy aromatic ring). As can be seen in the
Figure 3, the band at 910 cm
−1 corresponding to the bending of the epoxy group disappeared after curing, indicating that the epoxy ring was opened after UV irradiation, confirming that the photopolymerization took place. All the samples displayed a similar reduction for this absorption. From the infrared spectra the conversion of the curing reaction was calculated and values higher than 95% were obtained in all the samples. As a consequence of the high curing degree the fibers maintained the shape above the melting temperature of the PCL.
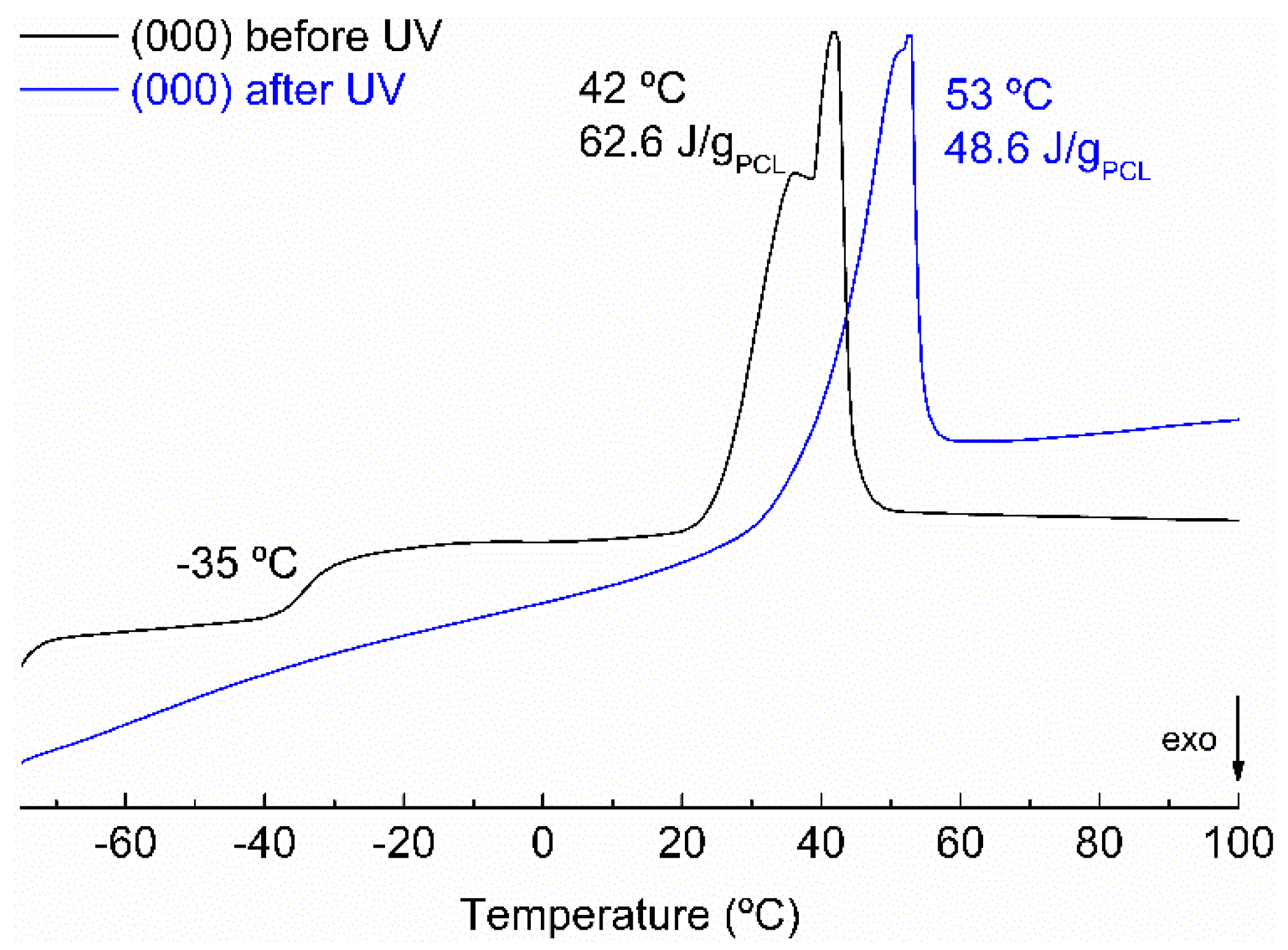
The melting behaviour after curing was also analyzed for all the samples by carrying out DSC experiments.
Figure 4 shows the second heat scan for an electrospun sample before and after curing. Before curing, the thermogram showed a glass transition at −35 °C and a melting transition at 42 °C that could be assigned to DGEBA T
g and PCL melting respectively. After curing, the T
g of epoxy disappeared and the PCL melting temperature increased due to the fact that before irradiation DGEBA was partly soluble in the PCL, lowering the melting point. All the samples showed a similar behaviour. After curing, all the samples showed melting temperatures close to that of pure PCL and their crystallization degree was between 23 and 40%.
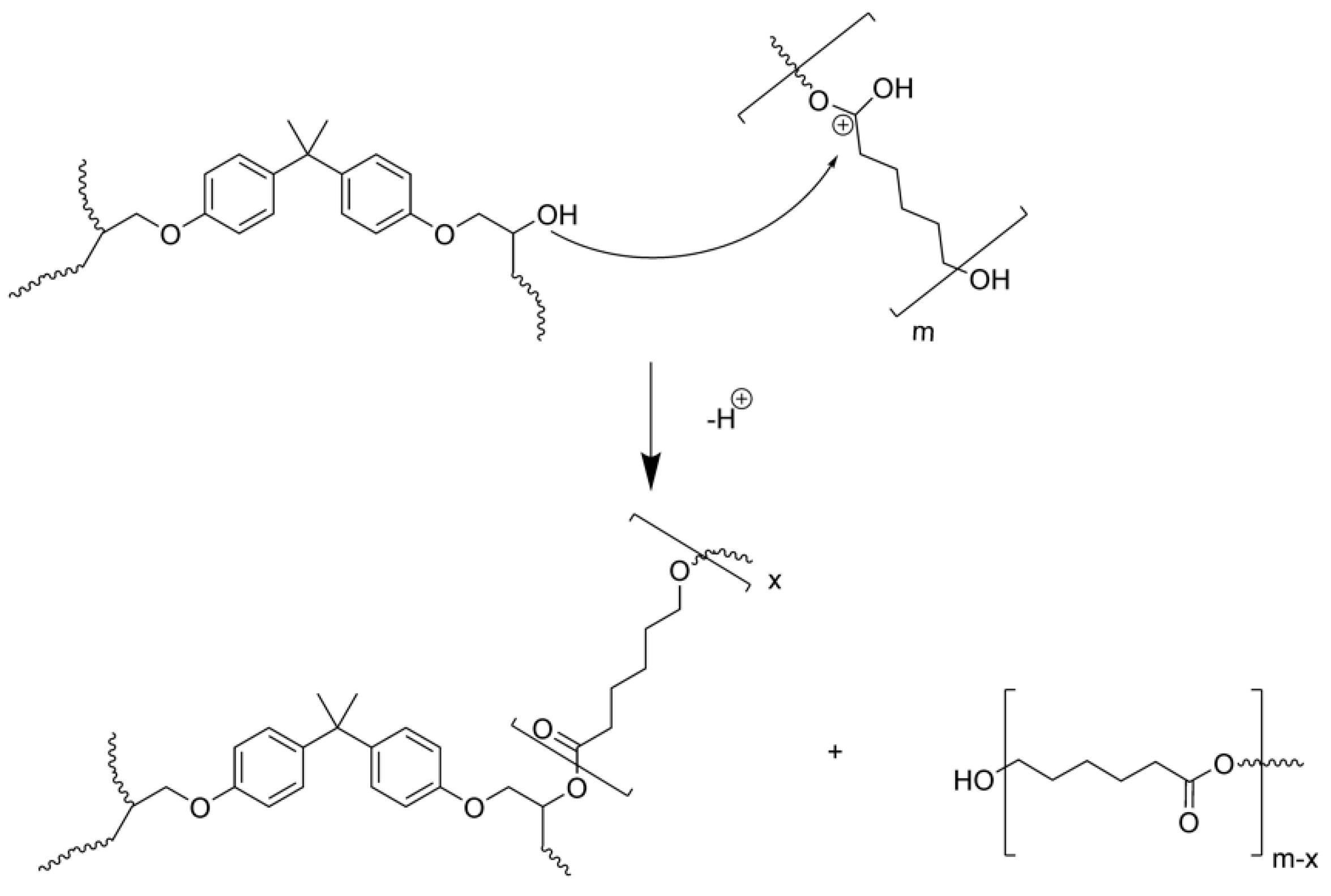
The final structure was determined to be a thermoset network with the PCL chemically integrated, as the gel content of the mats was around 90% (when the epoxy monomer is only up to 60%). Arnebold et al demonstrated that in the acid conditions of this kind of photopolymerization a transesterification reaction can happen [
46] (
Scheme 1) and this was confirmed in our system by GPC as the molecular weight of the extracted PCL was lower than the original PCL.
3.4. Effect of Intrinsic Variables in Diameter Distribution and Morphology of Electrospun Mats
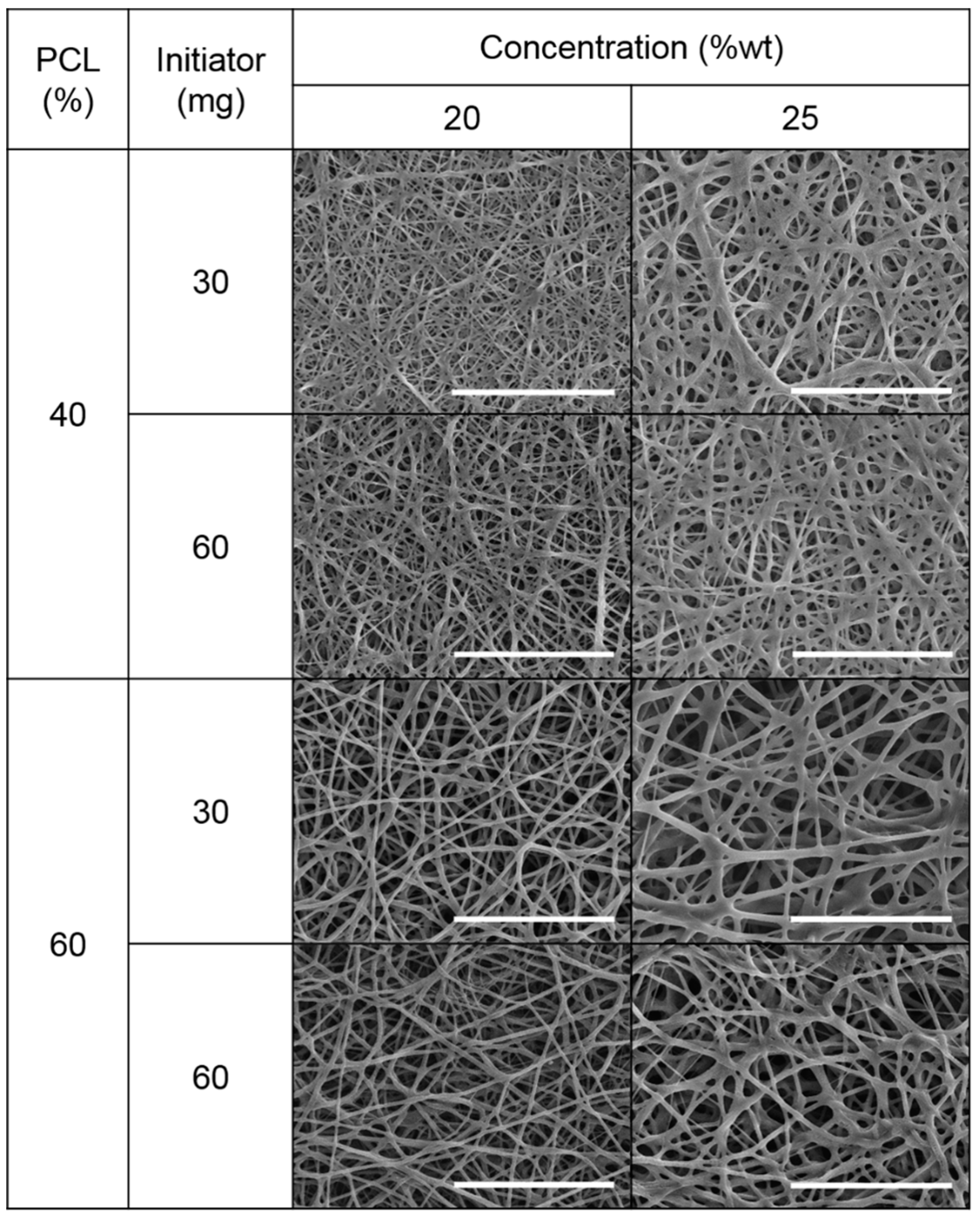
Figure 5 shows the SEM images of the electrospun mats obtained in the different experiments after the UV curing.
In all mats well-formed fibers without beads were observed. This corroborated that the selected extreme conditions were appropriate for obtaining fibers without beads.
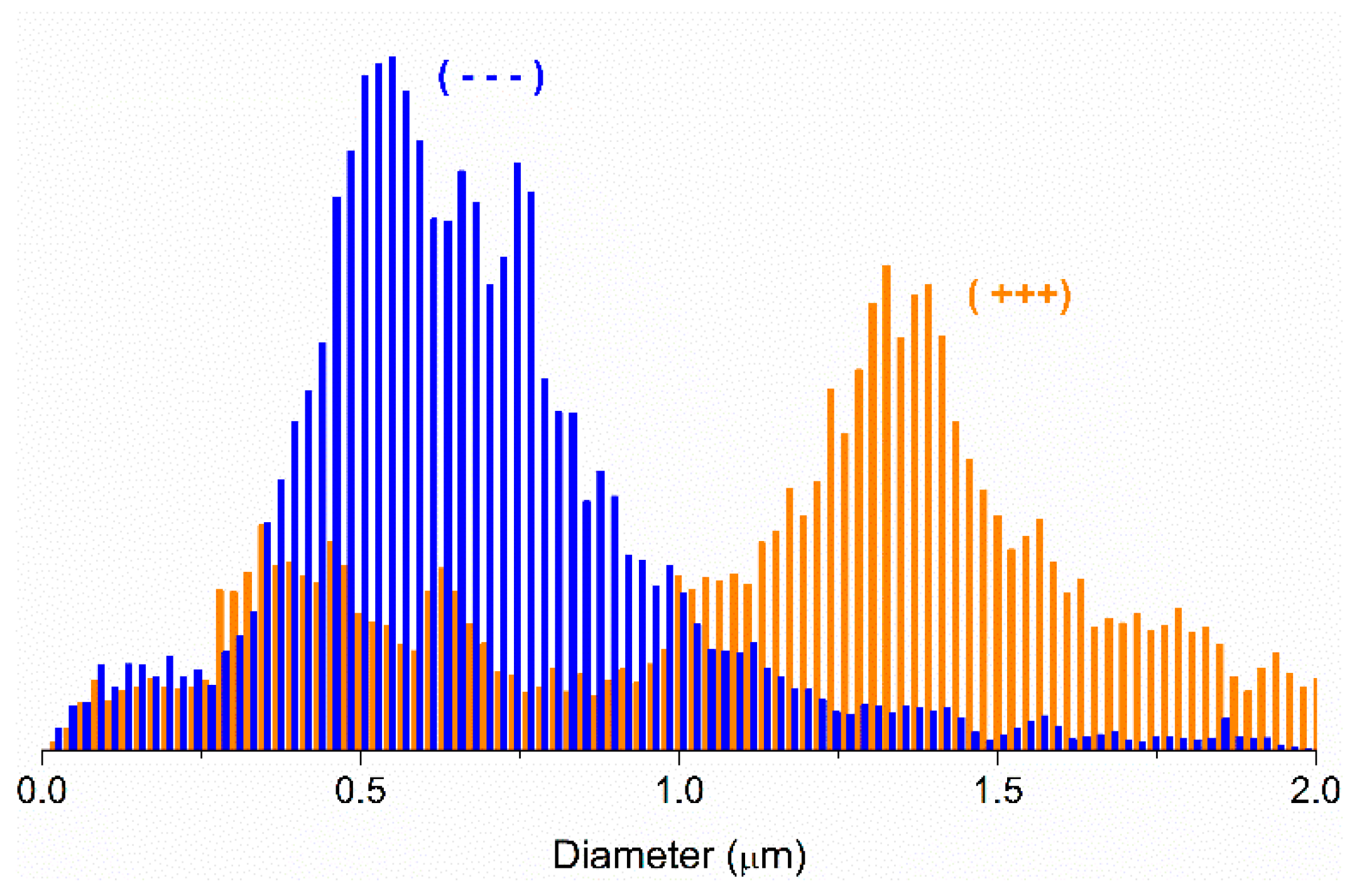
From these images and using image J software, the fiber diameter and distribution was measured. As an example, in
Figure 6 the diameter size distribution for samples (- - -) and (+++) is shown. As can be seen, for both samples the diameter did not show a normal distribution which was in accordance with previous data [
27].
The mean diameter of the fibers electrospun in the different experiments is shown in
Table 2. As can be seen the diameter of the fibers could be customized by changing different parameters obtaining fibers with diameters ranging from 0.70 to 1.40 μm.
In order to study the effect of the different variables in the diameter of the fiber DoE methodology was applied and the results are shown in
Table 7. From the P-values in
Table 7, it was clear that concentration (X
1) played an important role in the fiber diameter, and that it was the intrinsic variable that had the greatest effect
The terms with no significance were removed and the results are shown in
Table 8. As observed, the concentration (X
1) was the main variable that defined the diameter of the fibers. As stated by many authors, and in agreement with the results obtained in this work, the diameter of the fibers increases with the polymer concentration in the electrospun solution as the amount of material capable of being spun is higher [
47]. In addition, from the results of
Table 2, it was clear that higher flow rates were needed to obtain fibers at higher concentrations and thus the flow rate can be also responsible for the increase observed in the diameter of the fibers.
Fiber diameter also increased with PCL concentration (X
2), possibly because PCL was the only high molecular weight component of the mixture. As the PCL was above the critical concentration, increasing it meant more entanglements were possible and the stretching of the fibers due to the electric force lowered and thus fibers with higher diameters were obtained [
48]. In addition, at the same PCL percentage, increasing the amount of initiator (organic salt) reduced the diameter of the obtained fibers. This was also observed by other authors and can be explained by the high charge density originated by the addition of the salt. [
34,
47,
49].
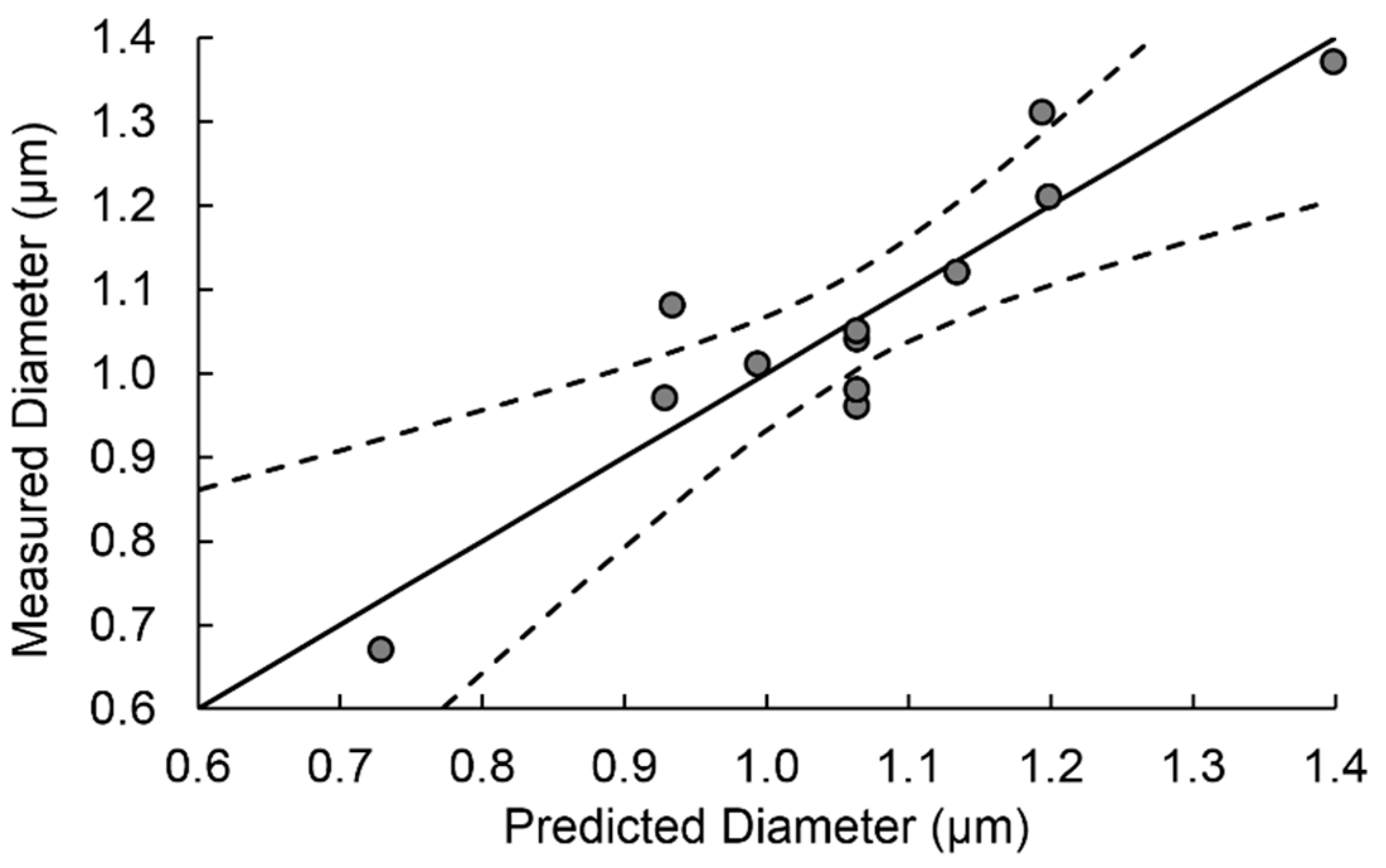
It has to be noted that R
2 was 0.984. Therefore, the measured diameter was property explained by the model.
Figure 7 shows the measured vs predicted diameters obtained by the regression analysis.
The final equation for the obtained diameter can be expressed as:
3.5. Effect of Intrinsic Variables in Shape Memory Properties
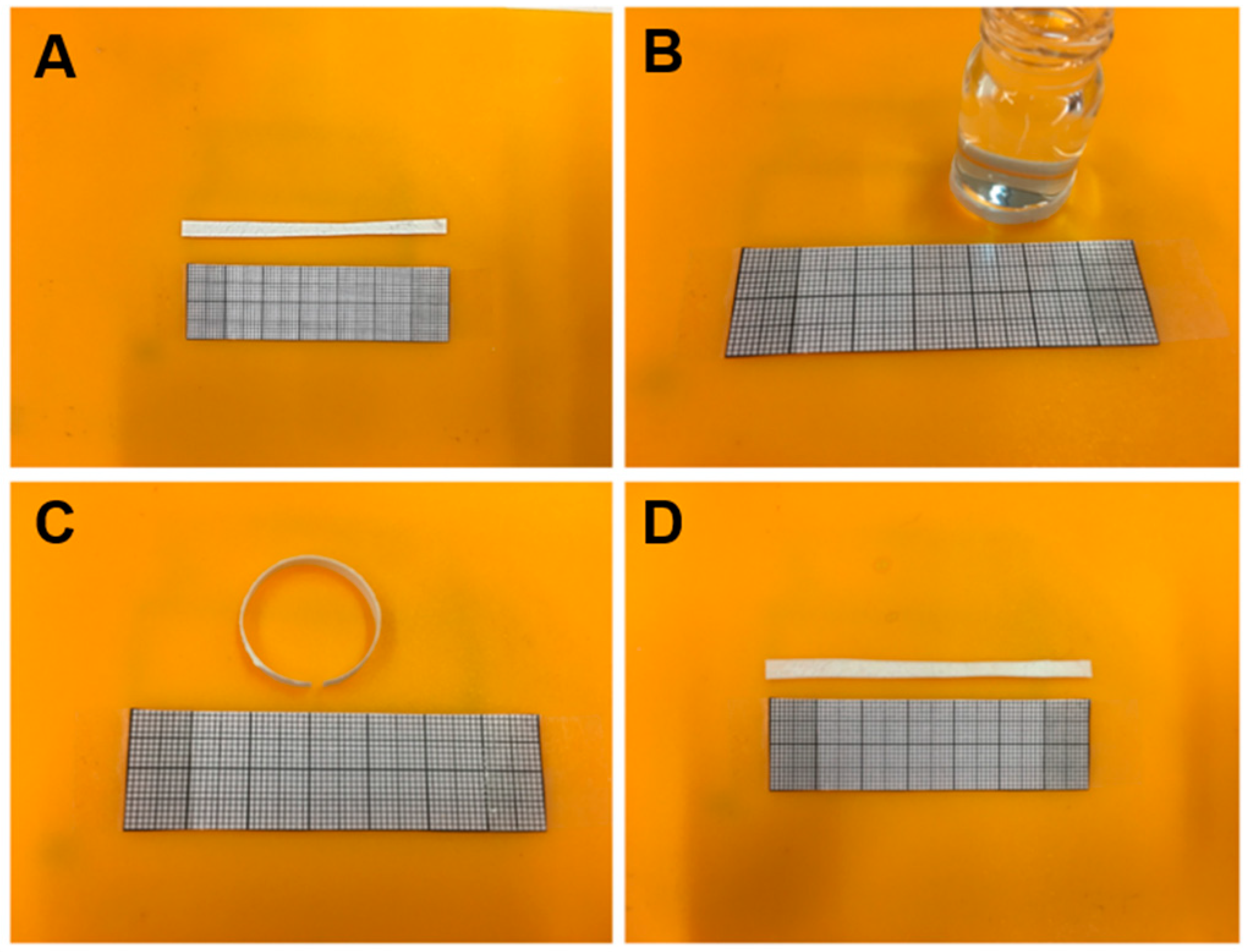
The shape memory effect of the electrospun samples was measured by a manual folding-deploy test as described in
Section 2.4.
Figure 8 shows photographs of the process. As recorded in
Table 2, all samples showed a recovery and fixity ratio between 95 and 100%, so all samples displayed excellent shape memory properties. Other authors obtained similar results in PCL based systems employing sol-gel reaction [
50] or blends with other polymers such as elastomeric polyurethanes [
24].
The results of the DoE analysis for the fixity and recovery ratios are shown in
Table 9 and
Table 10. From the results obtained in
Table 9, it was clear that in the intrinsic range studied, no significant differences were obtained relating to the fixity ratio. It must be taken into consideration that the fixation process is related with the melting and crystallization of the PCL and therefore it can be argued that the concentration of this component will have an influence on the fixity ratio. However, as stated before, no influence of this variable was observed. This unexpected result can be explained taking into consideration that in the range of studied concentrations the degree of crystallinity was high enough (in DSC values ranging from 23 to 40% were obtained, as reported in the previous section) to maintain the temporary form in a good level in all the samples.
The recovery ratio was significant in a 95% level of confidence (P-value of 0.018) when only concentration was employed in the model (
Table 10). However, R
2 remained low (0.82) and therefore a great part of the response was not explained by the model. The recovery process is related to the netpoints provided by the photocuring of the epoxy resin, and therefore a relation with the epoxy and/or the photo-initiator amount were expected. However, the data did not show any dependence with these parameters probably because, as reported by FTIR analysis, the curing degree of all samples was very high.
The data showed that there was a dependency of the recovery ratio on the solution concentration. This was not easy to explain. However, it must be taken into account that when increasing the solution concentration, the diameter of the fibers increased. Therefore, it seems that fibers with higher diameter showed slightly better recovery behaviour.