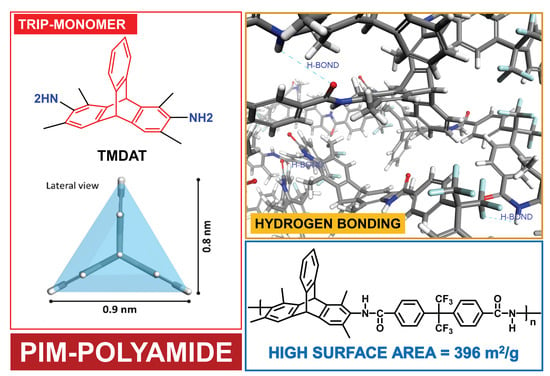
Synthesis and Gas-Permeation Characterization of a Novel High-Surface Area Polyamide Derived from 1,3,6,8-Tetramethyl-2,7-diaminotriptycene: Towards Polyamides of Intrinsic Microporosity (PIM-PAs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.3. Polymer Synthesis
2.4. Film Preparation
2.5. Pure- and Mixed-Gas Permeation Coefficients
3. Results and Discussion
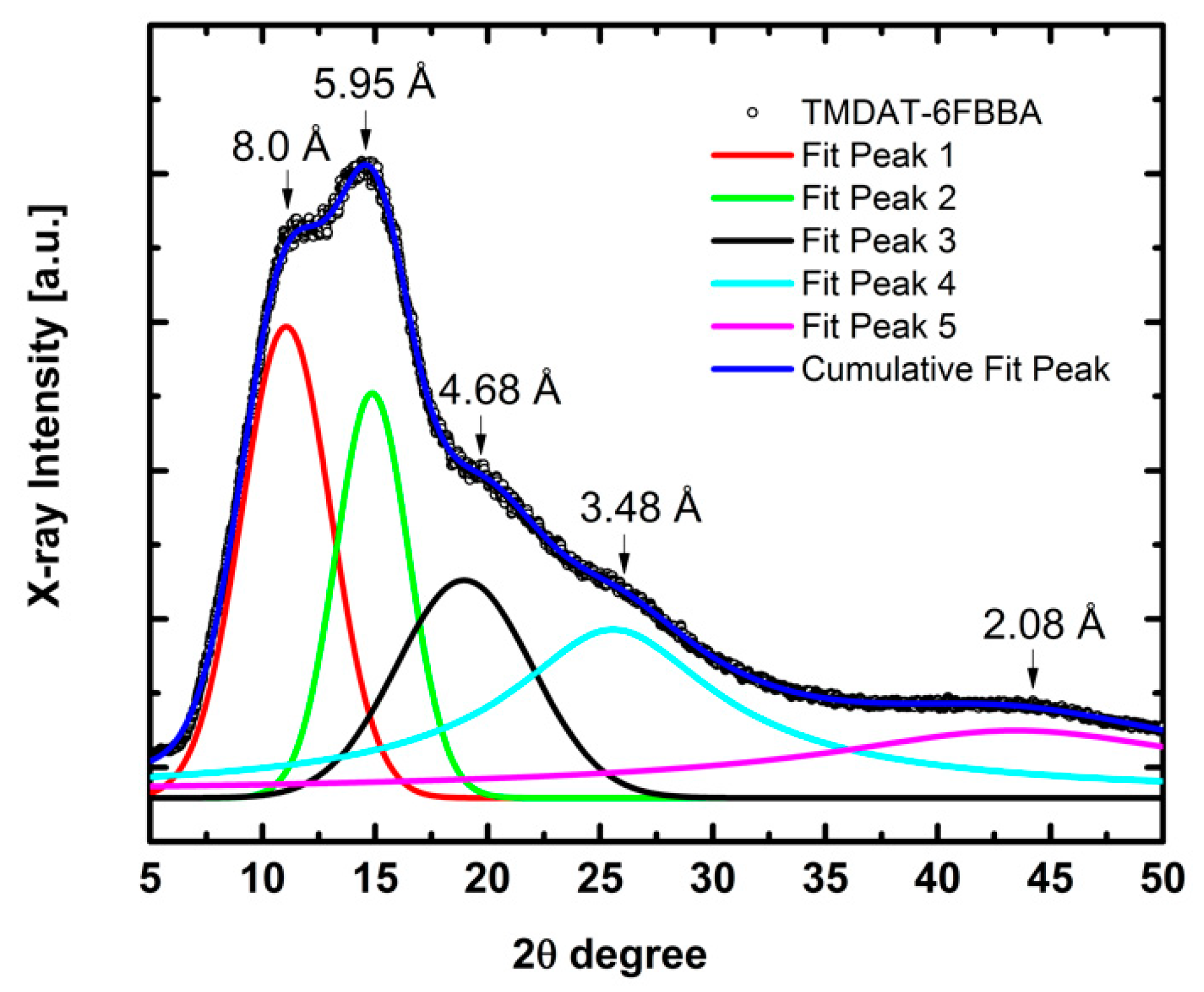
3.1. Polymer Characterization
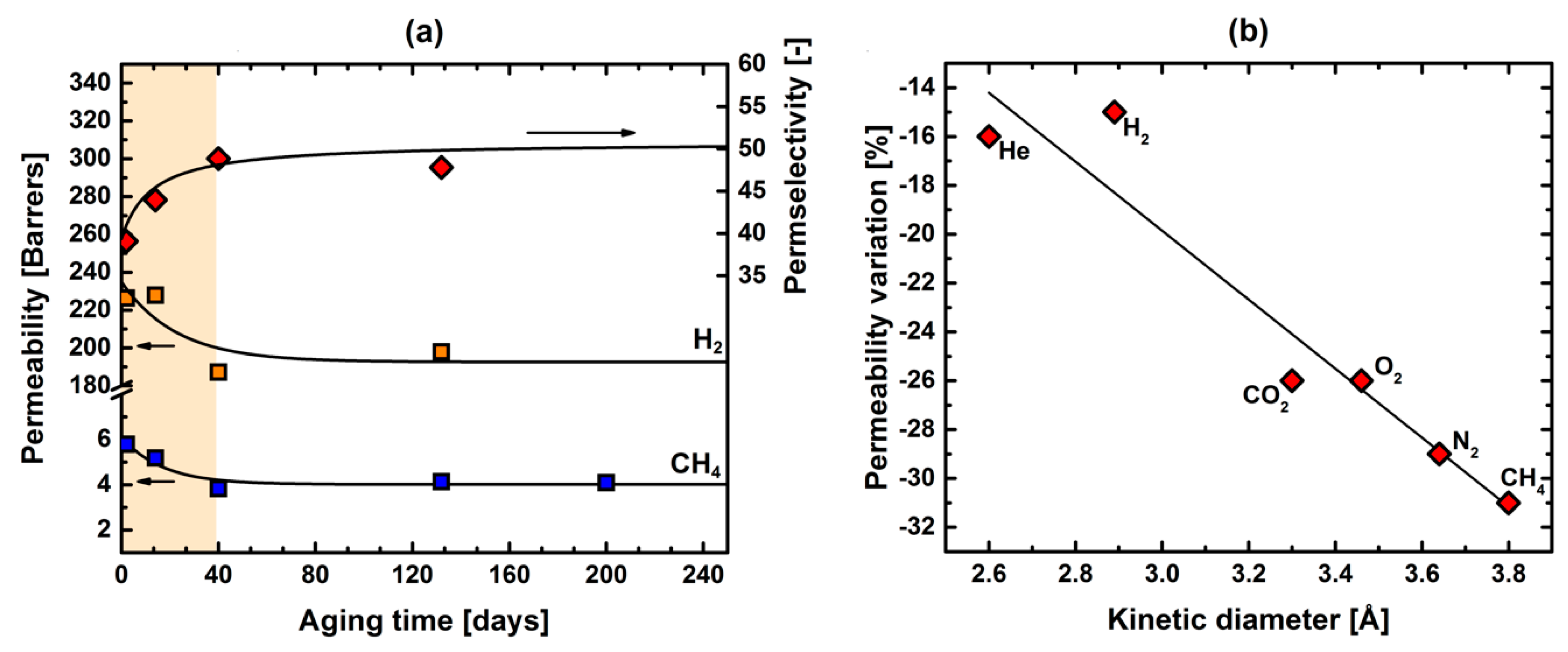
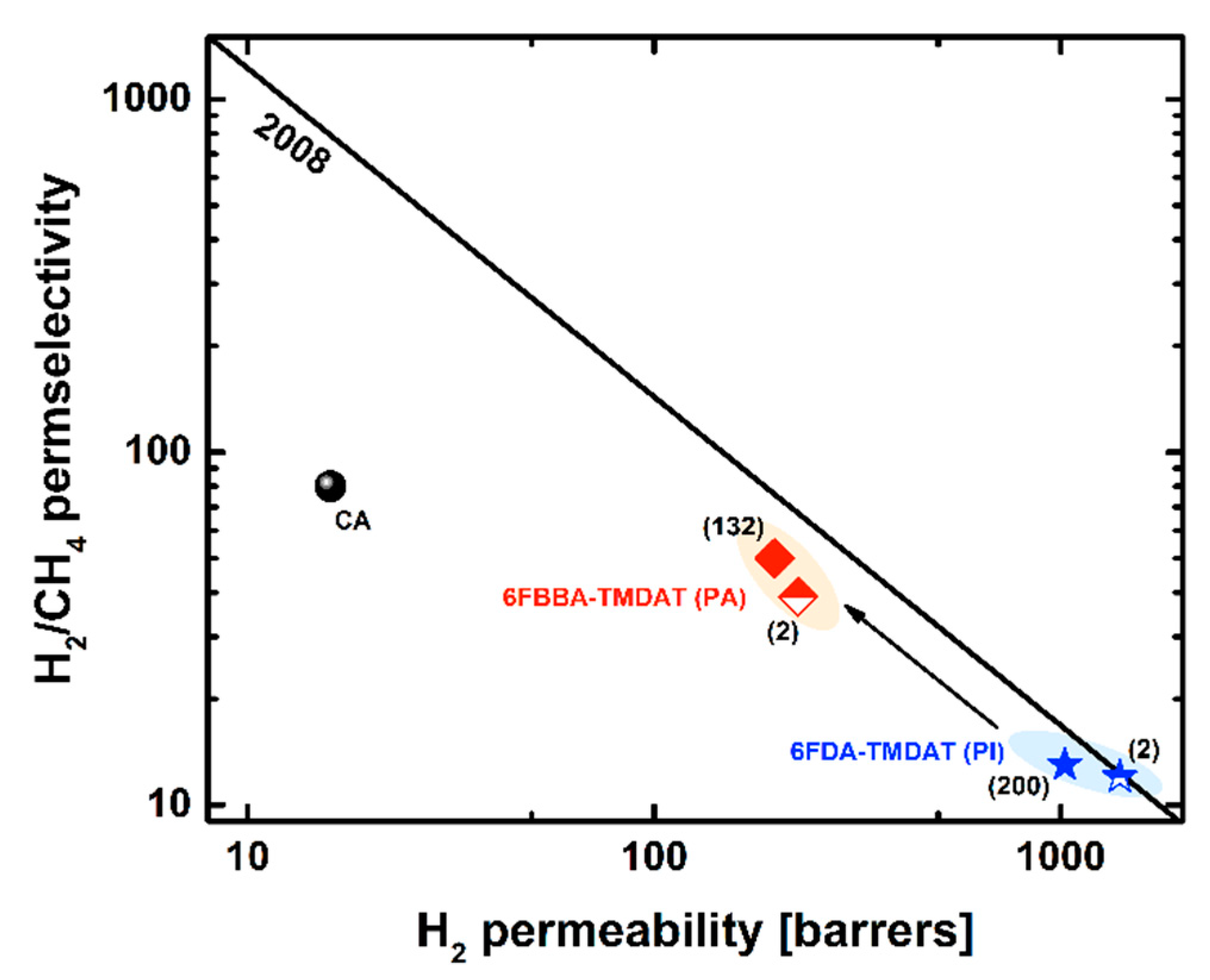
3.2. Pure-Gas Sorption, Permeation, and Physical-Aging
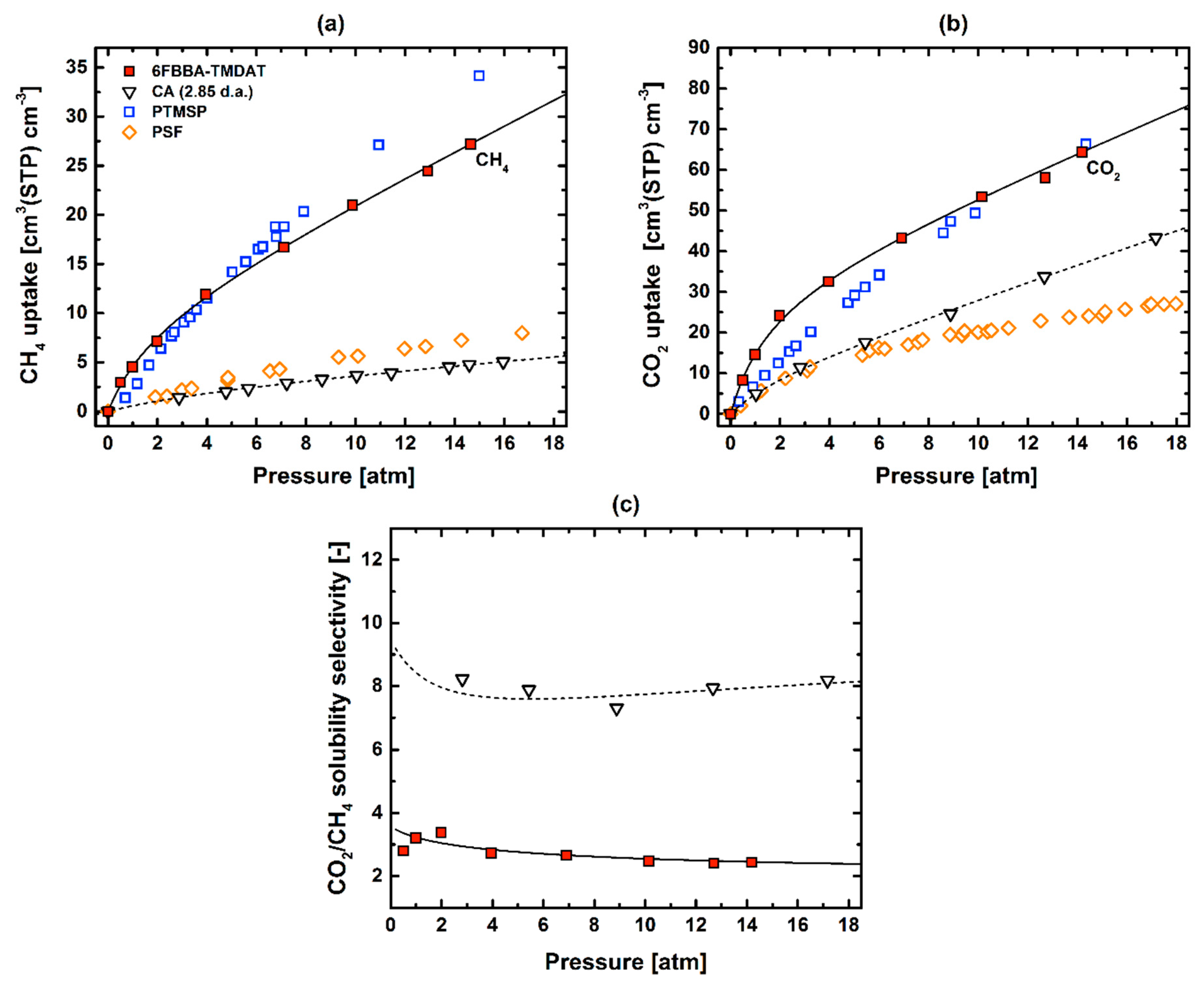
3.3. CO2–CH4 Mixed-Gas Permeation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mera, H.; Takata, T. High-performance fibers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Loeb, S.; Sourirajan, S. Sea water demineralization by means of an osmotic membrane. In Saline Water Conversion II; Gould, R.F., Ed.; American Chemical Society: Washington, DC, USA, 1963; Volume 38, pp. 117–132. [Google Scholar]
- Baker, R.W. Reverse osmosis. In Membrane Technology and Applications, 3rd ed.; Baker, R.W., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Qian, H.; Zheng, J.; Zhang, S. Preparation of microporous polyamide networks for carbon dioxide capture and nanofiltration. Polymer 2013, 54, 557–564. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I. Exploring aramid as emerging contender for CO2 capture. Chin. J. Chem. Eng. 2016, 24, 850–855. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, F.; Liu, Q.; Zong, C.; Yang, W.; Cao, X.; Jin, W.; Xu, N. Microporous polyamide membranes for molecular sieving of nitrogen from volatile organic compounds. Angew. Chem. Int. Ed. 2017, 56, 5755–5759. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Su, Q.; Antonietti, M.; Thomas, A. Exploring polymers of intrinsic microporosity – microporous, soluble polyamide and polyimide. Macromol. Rapid Commun. 2007, 28, 1871–1876. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, H.; Jin, Y.; Zhang, W. Porous poly(aryleneethynylene) networks through alkyne metathesis. Chem. Mater. 2013, 25, 3718–3723. [Google Scholar] [CrossRef]
- Ben, T.; Qiu, S. Porous aromatic frameworks: Synthesis, structure and functions. CrystEngComm 2013, 15, 17–26. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of gases and vapors in glassy and rubbery polymers. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Freeman, B.D., Pinnau, I., Eds.; Wiley: Chichester, UK, 2006. [Google Scholar]
- Espeso, J.; Lozano, A.E.; de la Campa, J.G.; de Abajo, J. Effect of substituents on the permeation properties of polyamide membranes. J. Membr. Sci. 2006, 280, 659–665. [Google Scholar] [CrossRef]
- Díez, B.; Cuadrado, P.; Marcos-Fernández, Á.; Prádanos, P.; Tena, A.; Palacio, L.; Lozano, Á.E.; Hernández, A. Helium recovery by membrane gas separation using poly(o-acyloxyamide)s. Ind. Eng. Chem. Res. 2014, 53, 12809–12818. [Google Scholar] [CrossRef]
- de Abajo, J.; de la Campa, J.G.; Lozano, A.E. Designing aromatic polyamides and polyimides for gas separation membranes. Macromol. Symp. 2003, 199, 293–306. [Google Scholar] [CrossRef]
- Santiago-Garcia, J.L.; Pérez-Francisco, J.M.; Zolotukhin, M.G.; Vázquez-Torres, H.; Aguilar-Vega, M.; González-Díaz, M.O. Gas transport properties of novel aromatic poly- and copolyamides bearing bulky functional groups. J. Membr. Sci. 2017, 522, 333–342. [Google Scholar] [CrossRef]
- Morisato, A.; Ghosal, K.; Freeman, B.D.; Chern, R.T.; Alvarez, J.C.; de la Campa, J.G.; Lozano, A.E.; de Abajo, J. Gas separation properties of aromatic polyamides containing hexafluoroisopropylidene groups. J. Membr. Sci. 1995, 104, 231–241. [Google Scholar] [CrossRef]
- Calle, M.; Lozano, A.E.; de Abajo, J.; de la Campa, J.G.; Álvarez, C. Design of gas separation membranes derived of rigid aromatic polyimides. 1. Polymers from diamines containing di-tert-butyl side groups. J. Membr. Sci. 2010, 365, 145–153. [Google Scholar] [CrossRef]
- Carrera-Figueiras, C.; Aguilar-Vega, M. Gas permeability and selectivity of hexafluoroisopropylidene aromatic isophthalic copolyamides. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2625–2638. [Google Scholar] [CrossRef]
- Guzmán-Lucero, D.; Palomeque-Santiago, J.; Camacho-Zúñiga, C.; Ruiz-Treviño, F.; Guzmán, J.; Galicia-Aguilar, A.; Aguilar-Lugo, C. Gas permeation properties of soluble aromatic polyimides based on 4-fluoro-4,4′-diaminotriphenylmethane. Materials 2015, 8, 1951–1965. [Google Scholar] [CrossRef]
- López-Nava, R.; Vázquez-Moreno, F.S.; Palí-Casanova, R.; Aguilar-Vega, M. Gas permeability coefficients of isomeric aromatic polyamides obtained from 4,4′-(9-fluorenylidene) diamine and aromatic diacid chlorides. Polym. Bull. 2002, 49, 165–172. [Google Scholar] [CrossRef]
- Plaza-Lozano, D.; Comesaña-Gándara, B.; de la Viuda, M.; Seong, J.G.; Palacio, L.; Prádanos, P.; de la Campa, J.G.; Cuadrado, P.; Lee, Y.M.; Hernández, A.; et al. New aromatic polyamides and polyimides having an adamantane bulky group. Mater. Today Commun. 2015, 5, 23–31. [Google Scholar] [CrossRef]
- Bisoi, S.; Bandyopadhyay, P.; Bera, D.; Banerjee, S. Effect of bulky groups on gas transport properties of semifluorinated poly(ether amide)s containing pyridine moiety. Eur. Polym. J. 2015, 66, 419–428. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Banerjee, S. Spiro[fluorene-9,9′-xanthene] containing fluorinated poly(ether amide)s: Synthesis, characterization and gas transport properties. Eur. Polym. J. 2015, 69, 140–155. [Google Scholar] [CrossRef]
- Bera, D.; Bandyopadhyay, P.; Ghosh, S.; Banerjee, S.; Padmanabhan, V. Highly gas permeable aromatic polyamides containing adamantane substituted triphenylamine. J. Membr. Sci. 2015, 474, 20–31. [Google Scholar] [CrossRef]
- Bera, D.; Bandyopadhyay, P.; Ghosh, S.; Banerjee, S. Gas transport properties of aromatic polyamides containing adamantyl moiety. J. Membr. Sci. 2014, 453, 175–191. [Google Scholar] [CrossRef]
- Bisoi, S.; Mandal, A.K.; Padmanabhan, V.; Banerjee, S. Aromatic polyamides containing trityl substituted triphenylamine: Gas transport properties and molecular dynamics simulations. J. Membr. Sci. 2017, 522, 77–90. [Google Scholar] [CrossRef]
- Bera, D.; Padmanabhan, V.; Banerjee, S. Highly gas permeable polyamides based on substituted triphenylamine. Macromolecules 2015, 48, 4541–4554. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Bera, D.; Banerjee, S. Synthesis, characterization and gas transport properties of semifluorinated new aromatic polyamides. Sep. Purif. Technol. 2013, 104, 138–149. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Bera, D.; Ghosh, S.; Banerjee, S. Di-tert-butyl containing semifluorinated poly(ether amide)s: Synthesis, characterization and gas transport properties. J. Membr. Sci. 2013, 447, 413–423. [Google Scholar] [CrossRef]
- Budd, P.M.; Elabas, E.S.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E.; Wang, D. Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas permeation properties, physical aging, and its mitigation in high free volume glassy polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, X.; Ghanem, B.S.; Alghunaimi, F.; Pinnau, I.; Han, Y. Polymers of intrinsic microporosity for energy-intensive membrane-based gas separations. Mater. Today Nano 2018, 3, 69–95. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-tuned intrinsically ultramicroporous polymers redefine the permeability/selectivity upper bounds of membrane-based air and hydrogen separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef]
- Ding, Y.; Bikson, B. Soluble aromatic polyamides containing the phenylindane group and their gas transport characteristics. Polymer 2002, 43, 4709–4714. [Google Scholar] [CrossRef]
- Bisoi, S.; Mandal, A.K.; Singh, A.S.B.; Banerjee, S. Gas separation properties of Troeger’s base-bridged polyamides. e-Polymers 2017, 27, 283–293. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Swaidan, R.; Litwiller, E.; Pinnau, I. Ultra-microporous triptycene-based polyimide membranes for high-performance gas separation. Adv. Mater. 2014, 26, 3688–3692. [Google Scholar] [CrossRef]
- Carta, M.; Croad, M.; Malpass-Evans, R.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; Friess, K.; Lanč, M.; McKeown, N.B. Triptycene induced enhancement of membrane gas selectivity for microporous Tröger’s base polymers. Adv. Mater. 2014, 26, 3526–3531. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Li, B.; Tan, B.; Chen, C.-F.; Xu, H.-B.; Yang, X.-L. Triptycene-based microporous polymers: Synthesis and their gas storage properties. ACS Macro Lett. 2012, 1, 190–193. [Google Scholar] [CrossRef]
- Swaidan, R.; Al-Saeedi, M.; Ghanem, B.; Litwiller, E.; Pinnau, I. Rational design of intrinsically ultramicroporous polyimides containing bridgehead-substituted triptycene for highly selective and permeable gas separation membranes. Macromolecules 2014, 47, 5104–5114. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Alghunaimi, F.; Wang, Y.; Genduso, G.; Pinnau, I. Synthesis of highly gas-permeable polyimides of intrinsic microporosity derived from 1,3,6,8-tetramethyl-2,7-diaminotriptycene. ACS Omega 2018, 3, 11874–11882. [Google Scholar] [CrossRef]
- Chen, C.-F.; Ma, Y.-X. Synthesis and reactions of triptycenes and their derivatives. In Iptycenes Chemistry: From Synthesis to Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 13–77. [Google Scholar]
- Alghunaimi, F.; Ghanem, B.; Alaslai, N.; Swaidan, R.; Litwiller, E.; Pinnau, I. Gas permeation and physical aging properties of iptycene diamine-based microporous polyimides. J. Membr. Sci. 2015, 490, 321–327. [Google Scholar] [CrossRef]
- Hoffmeister, E.; Kropp, J.E.; McDowell, T.L.; Michel, R.H.; Rippie, W.L. Triptycene polymers. J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 55–72. [Google Scholar] [CrossRef]
- Kasashima, Y.; Kaneda, T.; Akutsu, F.; Naruchi, K.; Miura, M. Synthesis and properties of aromatic polyamides and polyimides from 9, 10-dihydro-9,10-o-benzenoanthracene-1,4-diamine. Polym. J. 1994, 26, 1179–1185. [Google Scholar] [CrossRef]
- Kasashima, Y.; Kaneda, T.; Saito, G.; Akutsu, F.; Naruchi, K.; Miura, M. Synthesis and properties of aromatic polyamides from 2,7-triptycenediamine. Macromol. Chem. Phys. 1994, 195, 2693–2697. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Chou, J.-S.; Guo, W.; Tsai, T.-H. Synthesis and characterization of novel organosoluble and thermally stable polyamides bearing triptycene in their backbones. J. Polym. Res. 2012, 19, 9902. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chiu, Y.-T. Electrosynthesis and electrochromic properties of poly(amide-triarylamine)s containing triptycene units. RSC Adv. 2015, 5, 90941–90951. [Google Scholar] [CrossRef]
- Mondal, S.; Das, N. Triptycene based organosoluble polyamides: Synthesis, characterization and study of the effect of chain flexibility on morphology. RSC Adv. 2014, 4, 61383–61393. [Google Scholar] [CrossRef]
- Bera, R.; Mondal, S.; Das, N. Nanoporous triptycene based network polyamides (TBPs) for selective CO2 uptake. Polymer 2017, 111, 275–284. [Google Scholar] [CrossRef]
- Luo, S.; Liu, J.; Lin, H.; Kazanowska, B.A.; Hunckler, M.D.; Roeder, R.K.; Guo, R. Preparation and gas transport properties of triptycene-containing polybenzoxazole (PBO)-based polymers derived from thermal rearrangement (TR) and thermal cyclodehydration (TC) processes. J. Mater. Chem. A 2016, 4, 17050–17062. [Google Scholar] [CrossRef]
- Ellison, H.; Hey, D.H. The action of benzaldehyde on o-, m-, and p-xylene in the presence of aluminium chloride. J. Chem. Soc. (Resumed) 1938, 1847–1853. [Google Scholar] [CrossRef]
- Yamazaki, N.; Matsumoto, M.; Higashi, F. Studies on reactions of the n-phosphonium salts of pyridines. XIV. Wholly aromatic polyamides by the direct polycondensation reaction by using phosphites in the presence of metal salts. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 1373–1380. [Google Scholar] [CrossRef]
- O’Brien, K.C.; Koros, W.J.; Barbari, T.A.; Sanders, E.S. A new technique for the measurement of multicomponent gas transport through polymeric films. J. Membr. Sci. 1986, 29, 229–238. [Google Scholar] [CrossRef]
- Alghunaimi, F.; Ghanem, B.; Alaslai, N.; Mukaddam, M.; Pinnau, I. Triptycene dimethyl-bridgehead dianhydride-based intrinsically microporous hydroxyl-functionalized polyimide for natural gas upgrading. J. Membr. Sci. 2016, 520, 240–246. [Google Scholar] [CrossRef]
- Vopička, O.; De Angelis, M.G.; Sarti, G.C. Mixed gas sorption in glassy polymeric membranes: I. CO2/CH4 and n-C4/CH4 mixtures sorption in poly(1-trimethylsilyl-1-propyne) (PTMSP). J. Membr. Sci. 2014, 449, 97–108. [Google Scholar] [CrossRef]
- Puleo, A.C.; Paul, D.R.; Kelley, S.S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Membr. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Erb, A.J.; Paul, D.R. Gas sorption and transport in polysulfone. J. Membr. Sci. 1981, 8, 11–22. [Google Scholar] [CrossRef]
- Koros, W.J.; Chan, A.H.; Paul, D.R. Sorption and transport of various gases in polycarbonate. J. Membr. Sci. 1977, 2, 165–190. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Al-Saeedi, M.; Litwiller, E.; Pinnau, I. Role of intrachain rigidity in the plasticization of intrinsically microporous triptycene-based polyimide membranes in mixed-gas CO2/CH4 separations. Macromolecules 2014, 47, 7453–7462. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Genduso, G.; Ghanem, B.S.; Pinnau, I. Experimental mixed-gas permeability, sorption and diffusion of CO2–CH4 mixtures in 6FDA-mPDA polyimide membrane: Unveiling the effect of competitive sorption on permeability selectivity. Membranes 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Genduso, G.; Litwiller, E.; Ma, X.; Zampini, S.; Pinnau, I. Mixed-gas sorption in polymers via a new barometric test system: Sorption and diffusion of CO2-CH4 mixtures in polydimethylsiloxane (PDMS). J. Membr. Sci. 2019. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
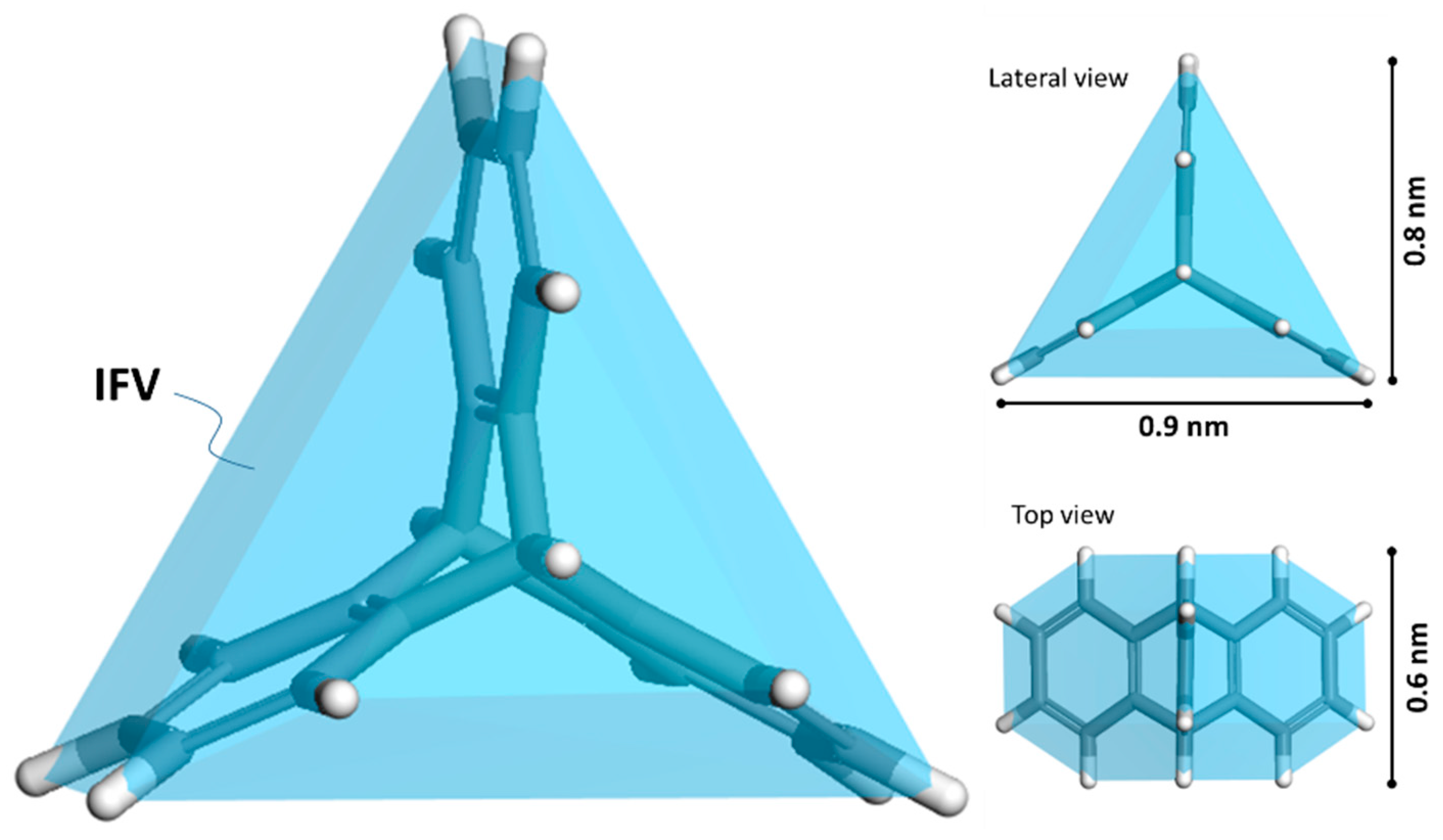
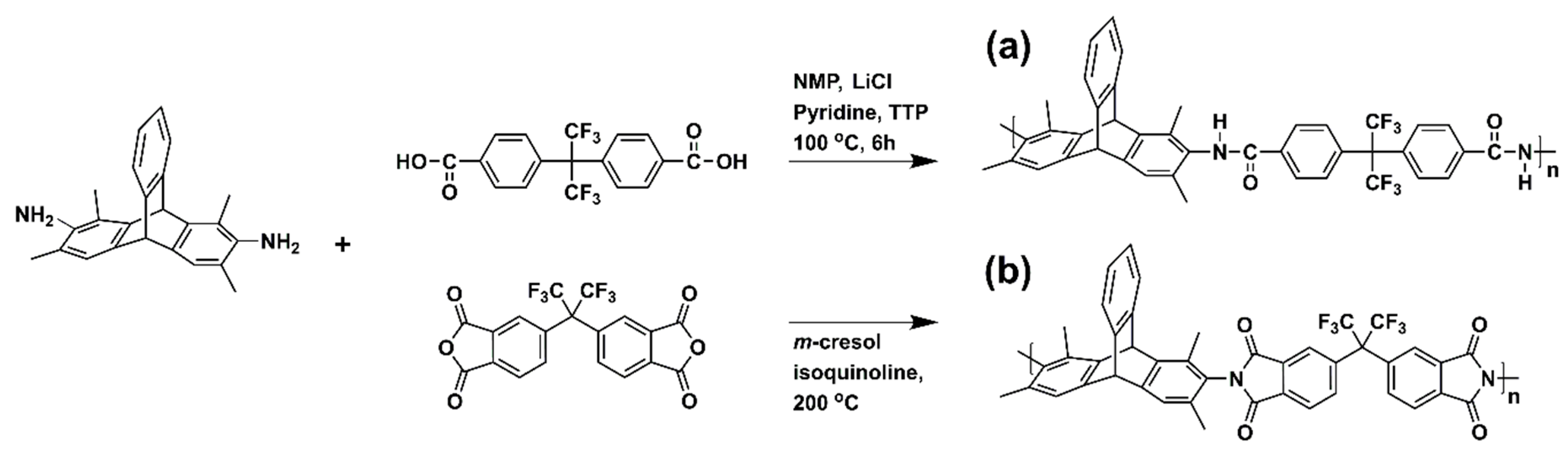
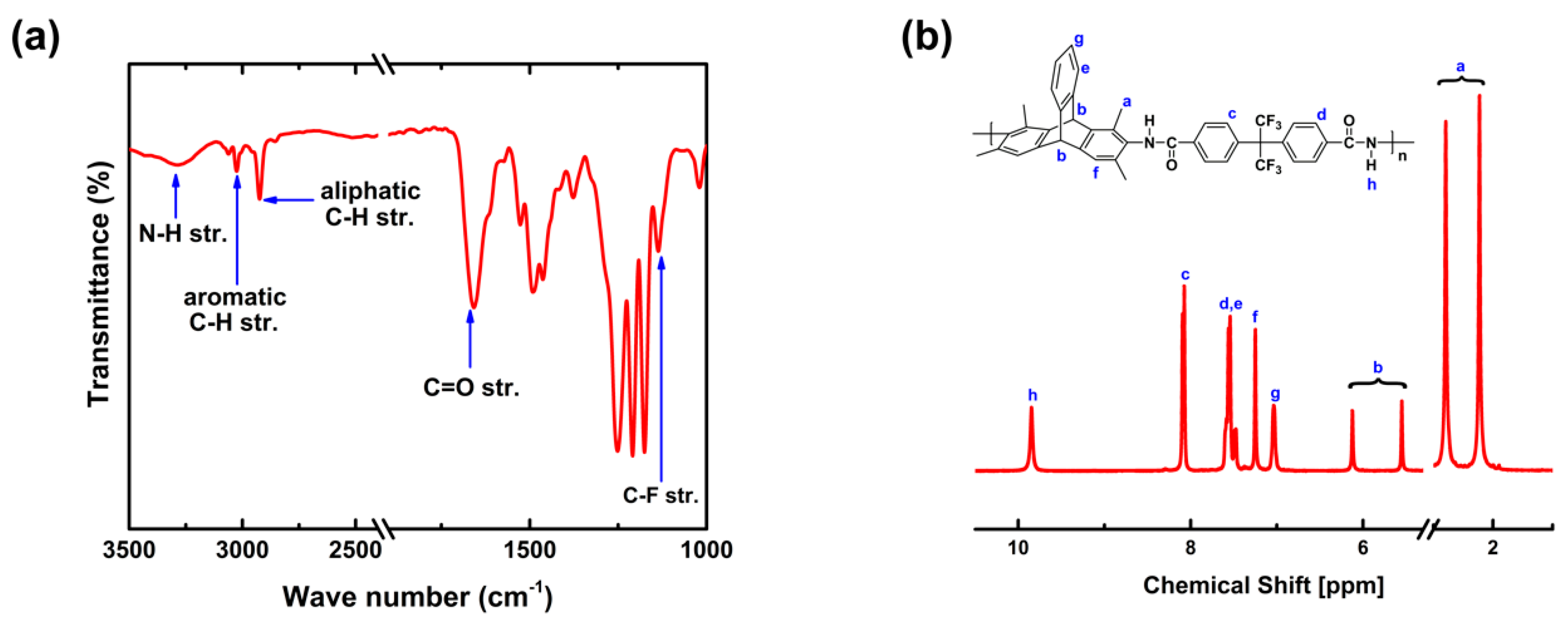
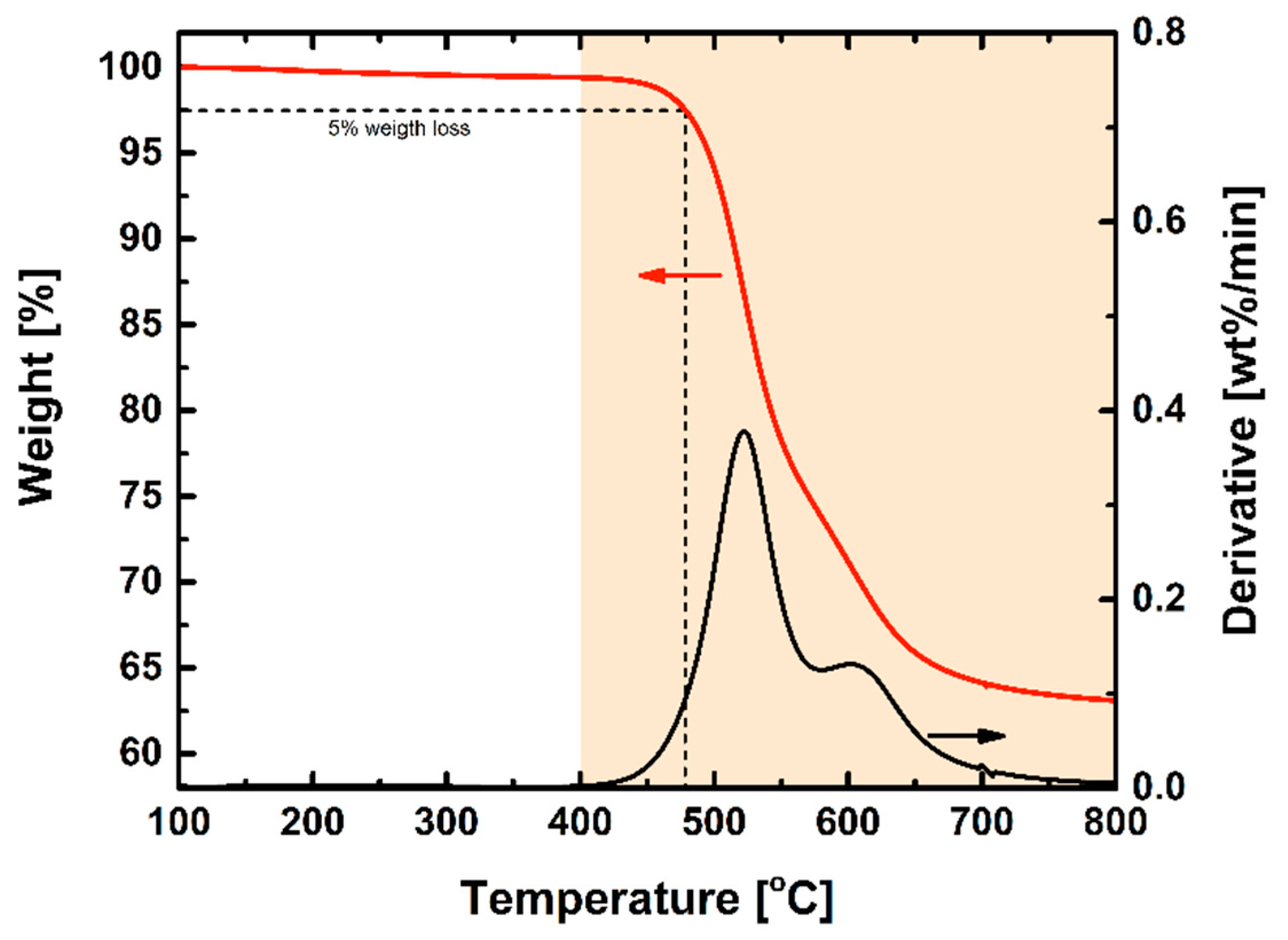
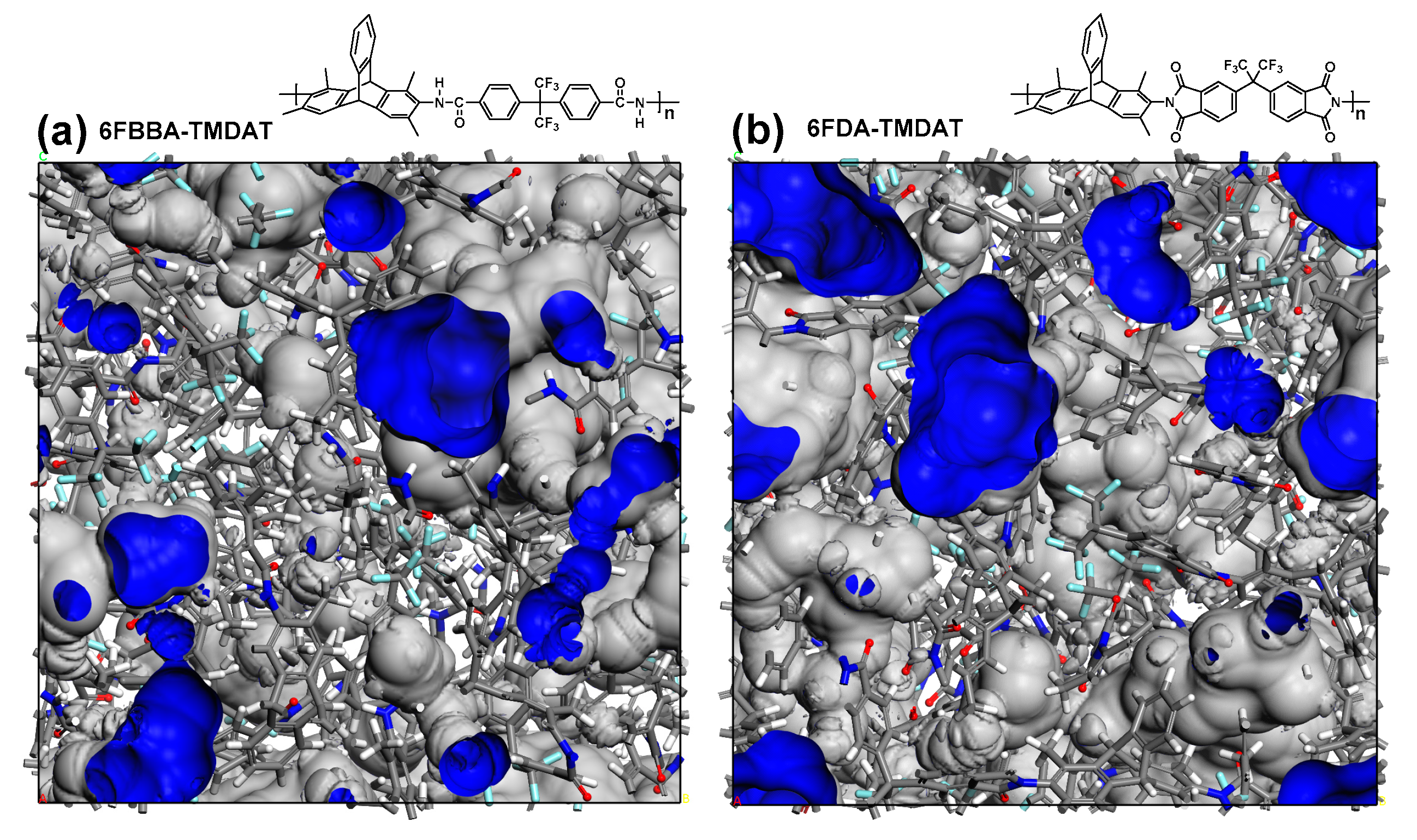
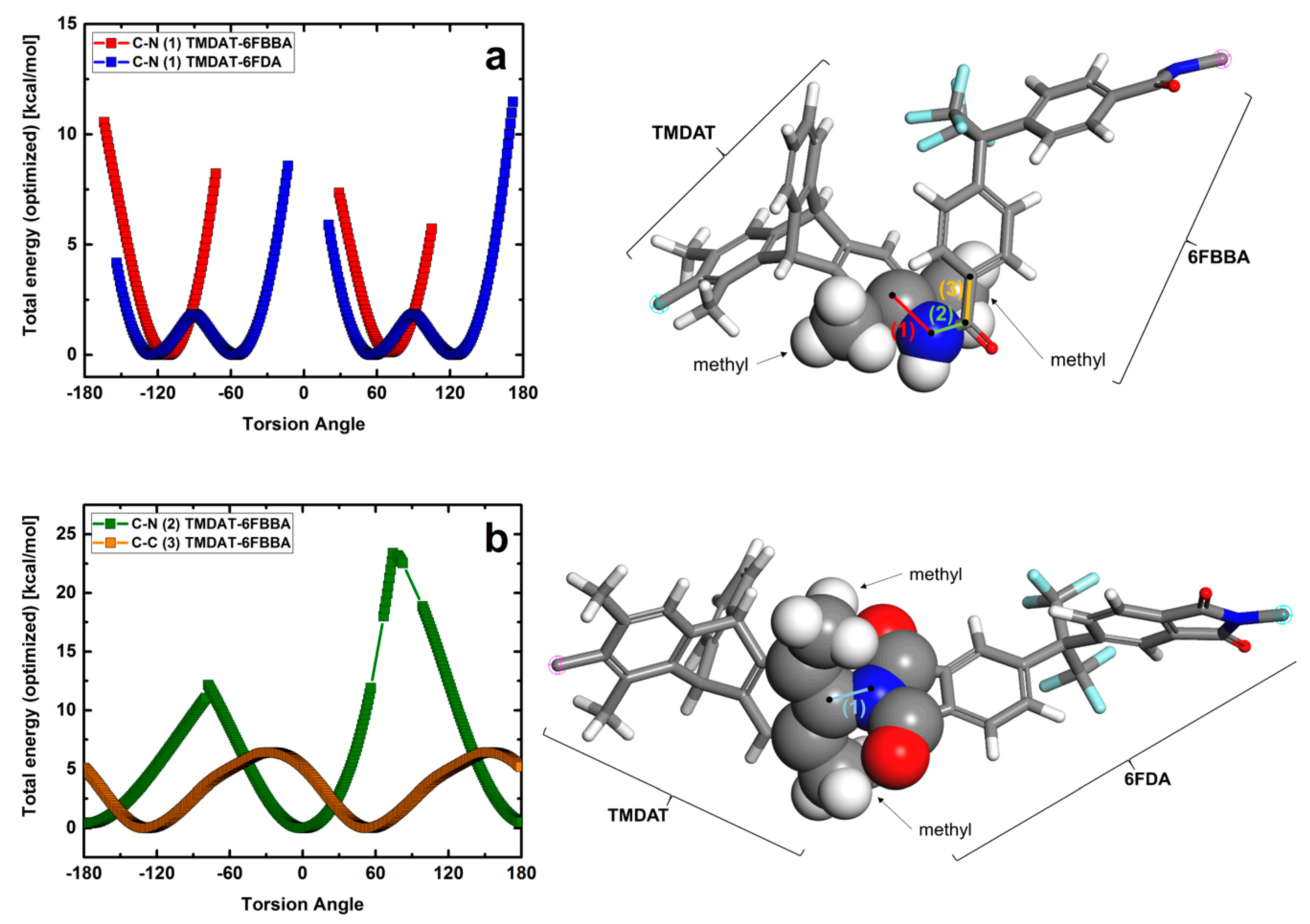
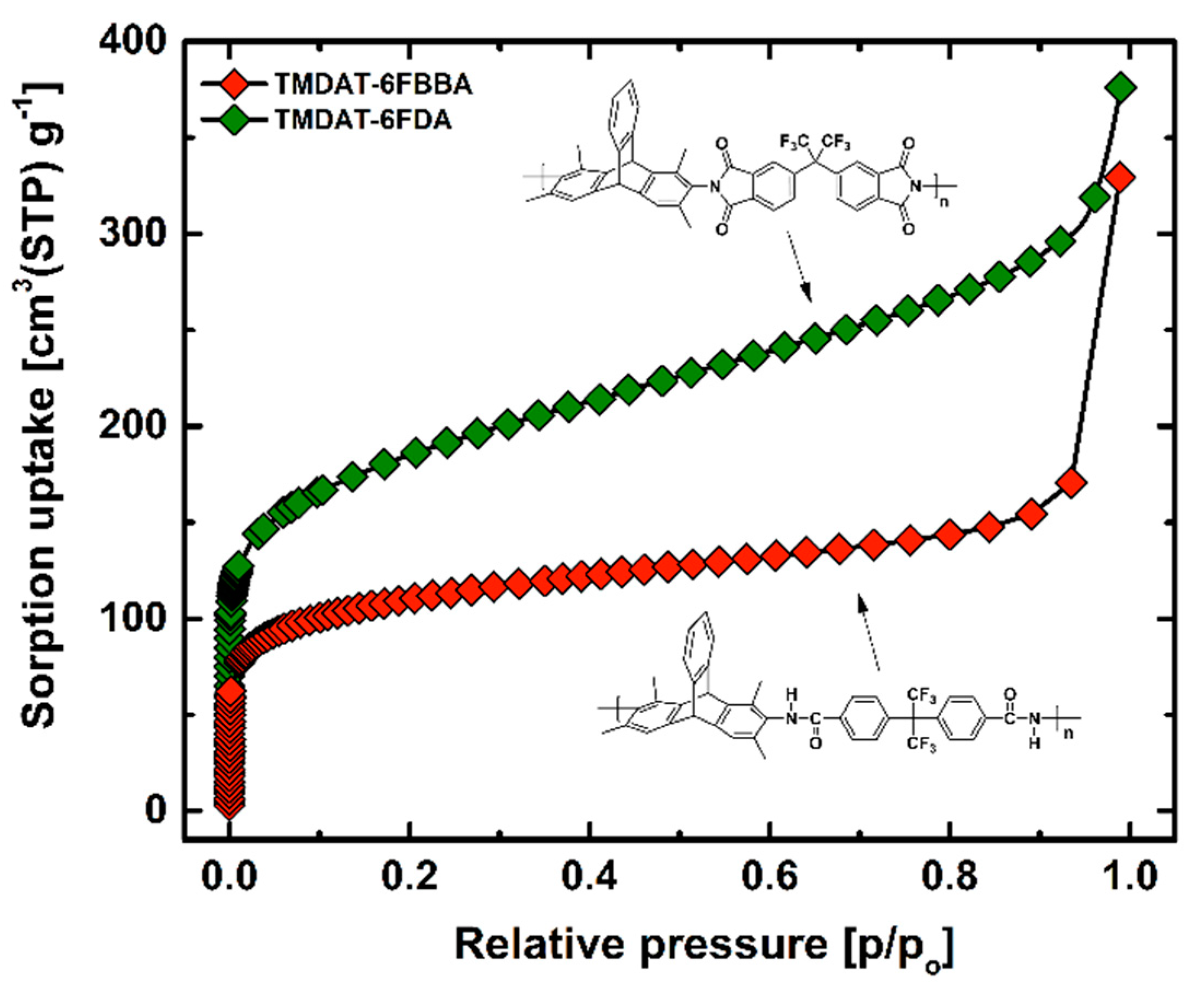

| Polymer | Td,5% [°C] | SBET [m2 g−1] | [g cm−3] | FFV [–] |
|---|---|---|---|---|
| 6FBBA-TMDAT [this work] | 480 | 396 | 1.15 ± 0.04 | 0.217 |
| 6FDA-TMDAT [45] | 470 * | 620 * | 1.13 ± 0.04 | 0.254 |
| Aging | He | H2 | N2 | O2 | CH4 | CO2 | H2/CH4 | O2/N2 | CO2/CH4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polymer | [days] | Pure-Gas Permeability [barrer] | Ideal Selectivity [–] | |||||||
| 6FBBA-TMDAT [This work] | 2 | 185 | 226 | 7.1 | 33 | 5.8 | 144 | 39 | 4.7 | 25 |
| 6FBBA-TMDAT [This work] | 14 | 178 | 228 | 6.4 | 30 | 5.2 | 133 | 44 | 4.8 | 26 |
| 6FBBA-TMDAT [This work] | 40 | 150 | 187 | 4.8 | 24 | 3.8 | 105 | 49 | 4.9 | 27 |
| 6FBBA-TMDAT [This work] | 132 | 160 | 198 | 5.2 | 25 | 4.1 | 109 | 48 | 4.8 | 26 |
| 6FDA-TMDAT [45] | fresh | 758 | 1400 | 113 | 374 | 121 | 1727 | 12 | 3.3 | 14 |
| 6FDA-TMDAT [45] | 200 | 601 | 1024 | 70 | 261 | 76 | 1150 | 13 | 3.7 | 15 |
| CA (D.S. 2.84) [61] | fresh | 20 | 16 | 0.2 | 1.5 | 0.2 | 6.6 | 80 | 6.3 | 33 |
| S, CH4 | S, CO2 | D, CH4 | D, CO2 | αS | αD | |
|---|---|---|---|---|---|---|
| Polymer | [cm3(STP) cm−3 atm−1] | [cm2 s−1] | [–] | [–] | ||
| 6FBBA-TMDAT [This work] | 3.6 | 12.2 | 9.1 × 10−9 | 7.1 × 10−8 | 3.4 | 7.8 |
| CA (D.S. 2.84) [61] | 0.5 | 4.2 | 5.1 × 10−9 | 1.8 × 10−8 | 8.1 | 3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genduso, G.; Ghanem, B.S.; Wang, Y.; Pinnau, I. Synthesis and Gas-Permeation Characterization of a Novel High-Surface Area Polyamide Derived from 1,3,6,8-Tetramethyl-2,7-diaminotriptycene: Towards Polyamides of Intrinsic Microporosity (PIM-PAs). Polymers 2019, 11, 361. https://doi.org/10.3390/polym11020361
Genduso G, Ghanem BS, Wang Y, Pinnau I. Synthesis and Gas-Permeation Characterization of a Novel High-Surface Area Polyamide Derived from 1,3,6,8-Tetramethyl-2,7-diaminotriptycene: Towards Polyamides of Intrinsic Microporosity (PIM-PAs). Polymers. 2019; 11(2):361. https://doi.org/10.3390/polym11020361
Chicago/Turabian StyleGenduso, Giuseppe, Bader S. Ghanem, Yingge Wang, and Ingo Pinnau. 2019. "Synthesis and Gas-Permeation Characterization of a Novel High-Surface Area Polyamide Derived from 1,3,6,8-Tetramethyl-2,7-diaminotriptycene: Towards Polyamides of Intrinsic Microporosity (PIM-PAs)" Polymers 11, no. 2: 361. https://doi.org/10.3390/polym11020361
APA StyleGenduso, G., Ghanem, B. S., Wang, Y., & Pinnau, I. (2019). Synthesis and Gas-Permeation Characterization of a Novel High-Surface Area Polyamide Derived from 1,3,6,8-Tetramethyl-2,7-diaminotriptycene: Towards Polyamides of Intrinsic Microporosity (PIM-PAs). Polymers, 11(2), 361. https://doi.org/10.3390/polym11020361