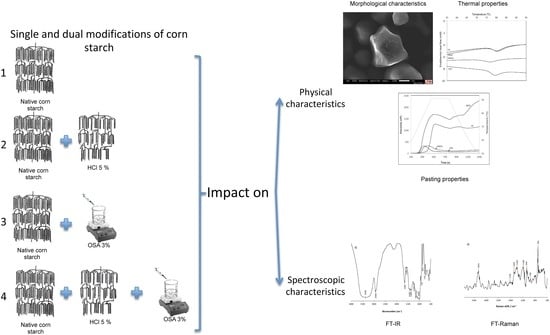
Effect of Dual Modification on the Spectroscopic, Calorimetric, Viscosimetric and Morphological Characteristics of Corn Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
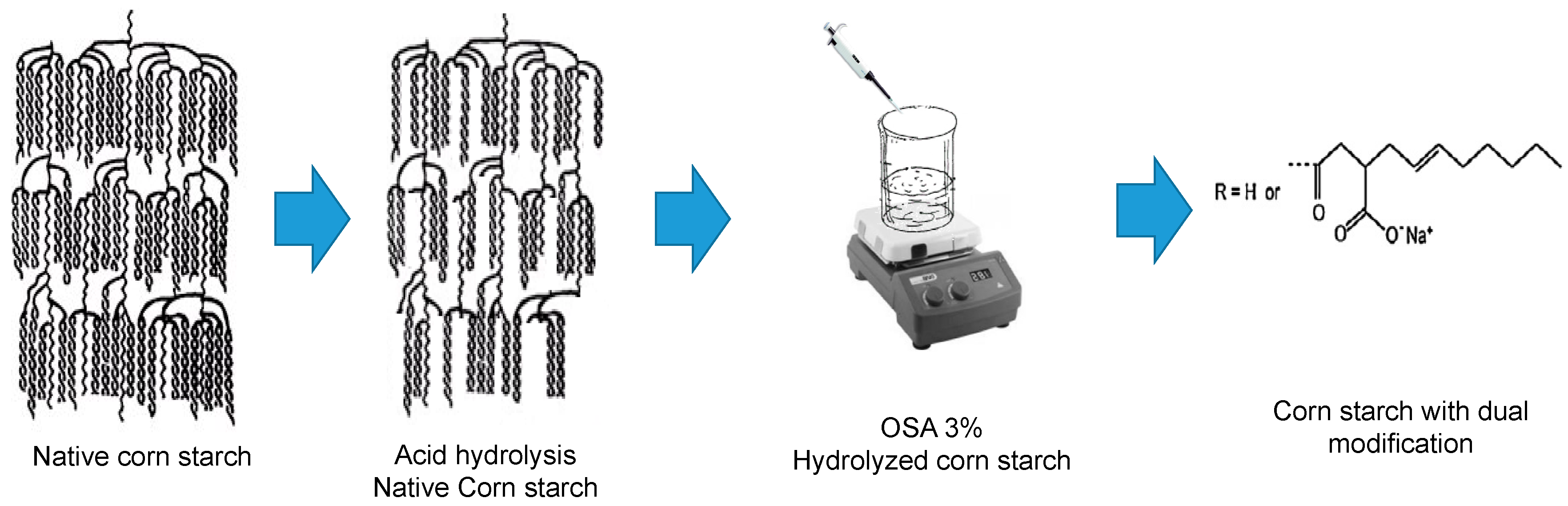
2.2. Acid Hydrolysis Pretreatment
2.3. Succinylation Treatment
2.4. Degree of Substitution
2.5. Physicochemical Characterization of the Starches
2.5.1. Peak Viscosity (PV)
2.5.2. Thermal Properties of the Starches
2.5.3. Scanning Electron Microscopy (SEM)
2.5.4. FTIR Spectroscopy
2.5.5. Raman Spectroscopy
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Dual Modification on the Degree of Substitution
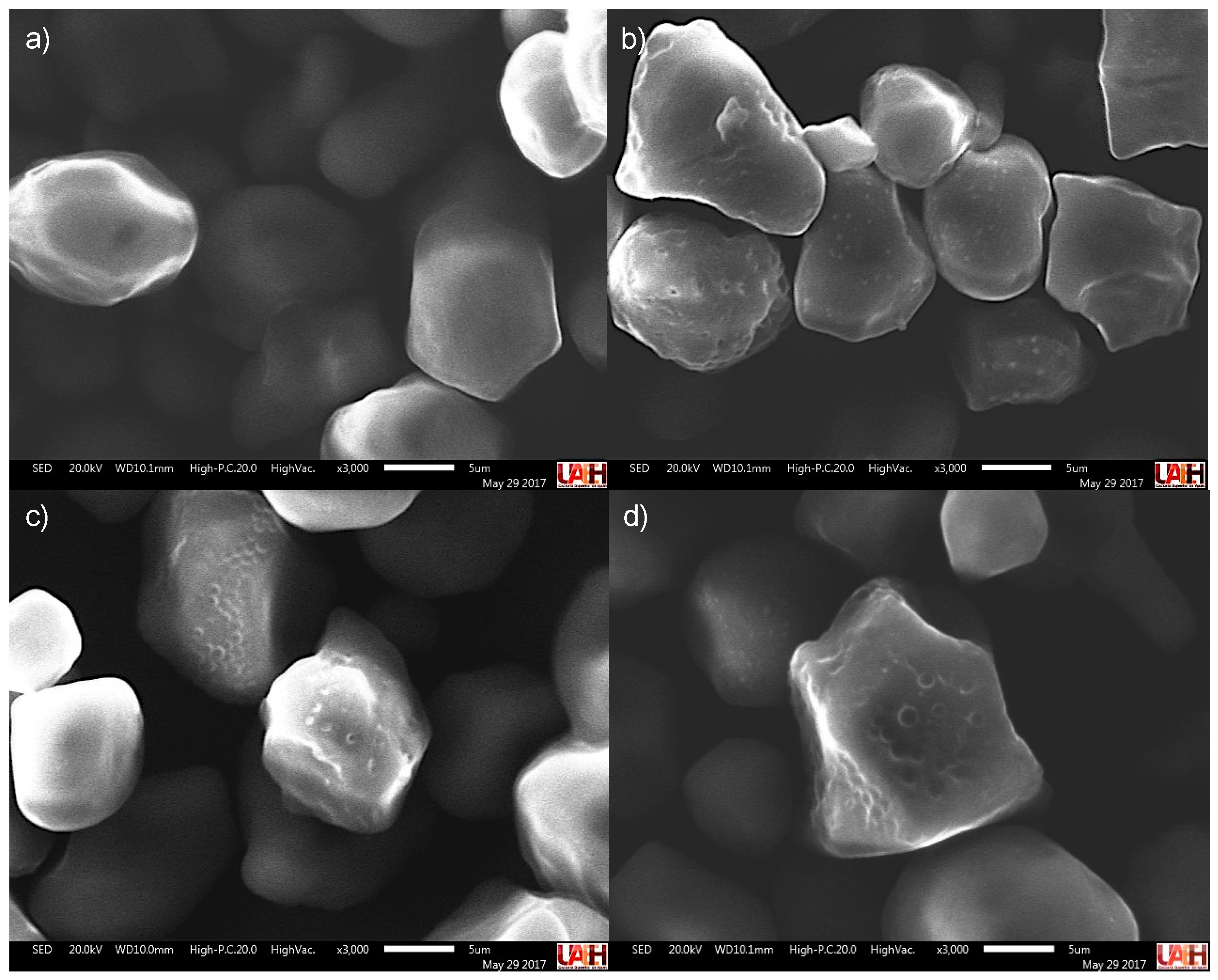
3.2. Effect of the Dual Modification on the Morphology of Corn Starches
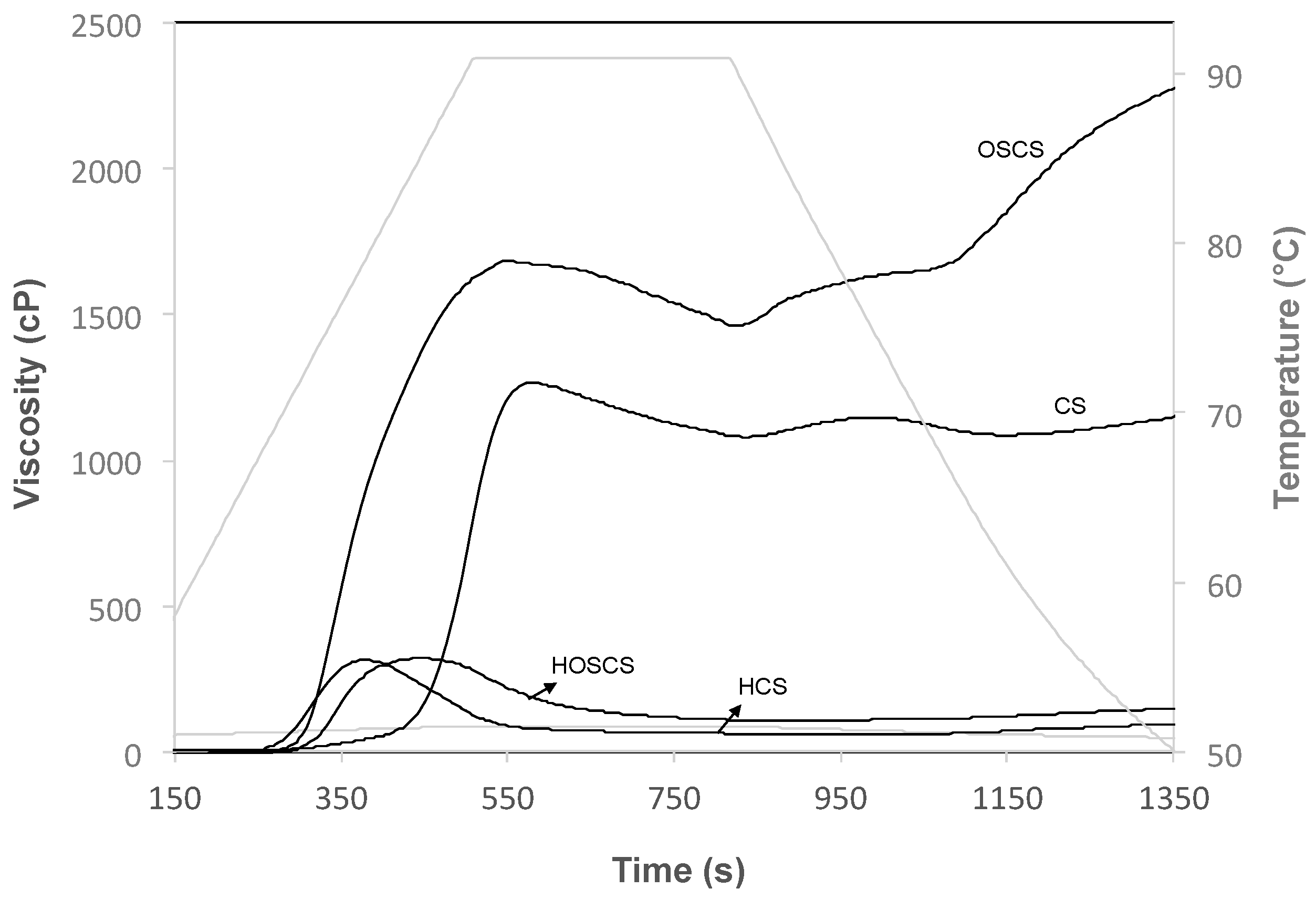
3.3. Peak Viscosity (PV)
3.4. Differential Scanning Calorimetry Analysis
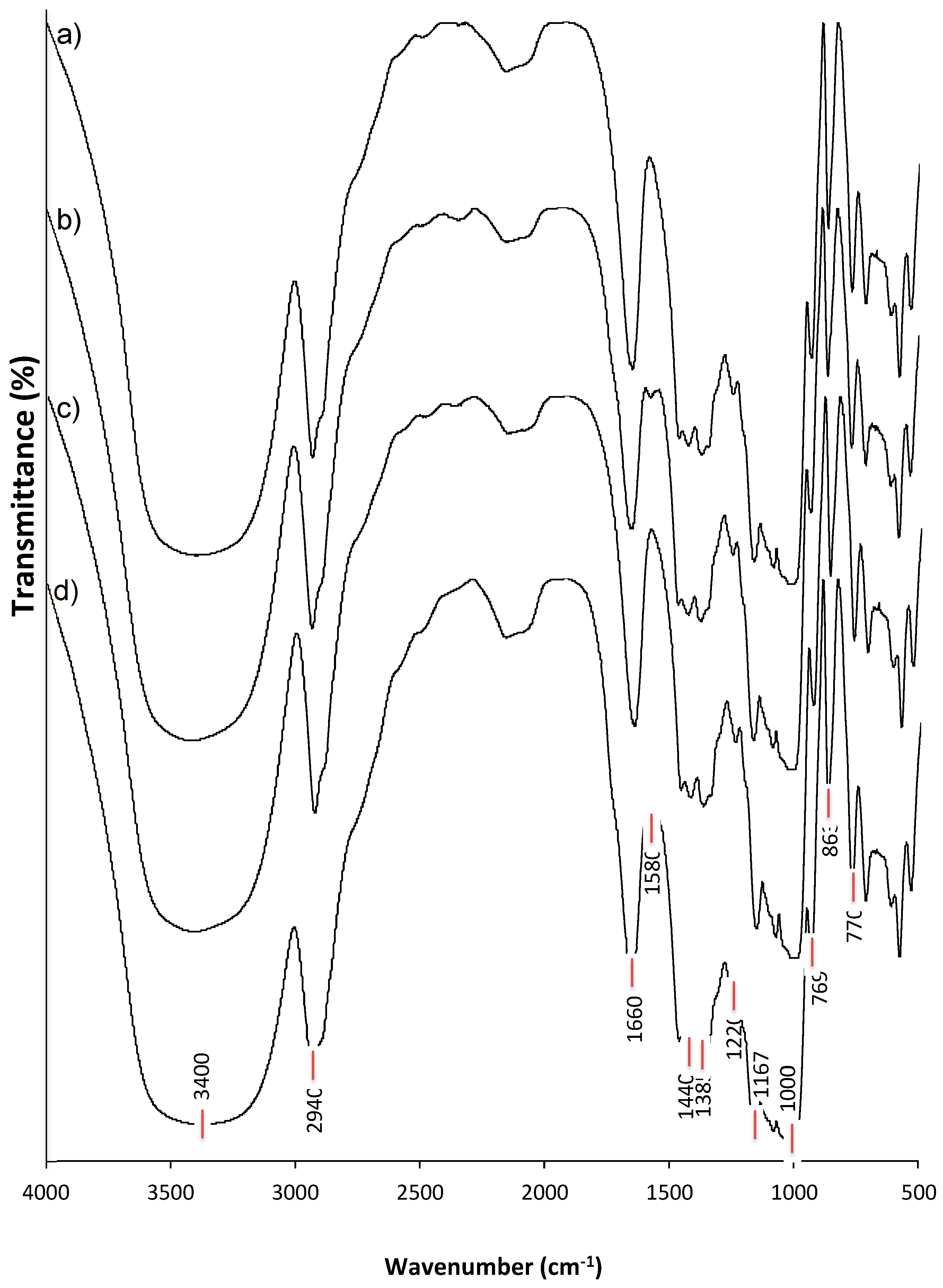
3.5. FTIR Analysis of Structural Alterations due to the Dual Modification of Starch
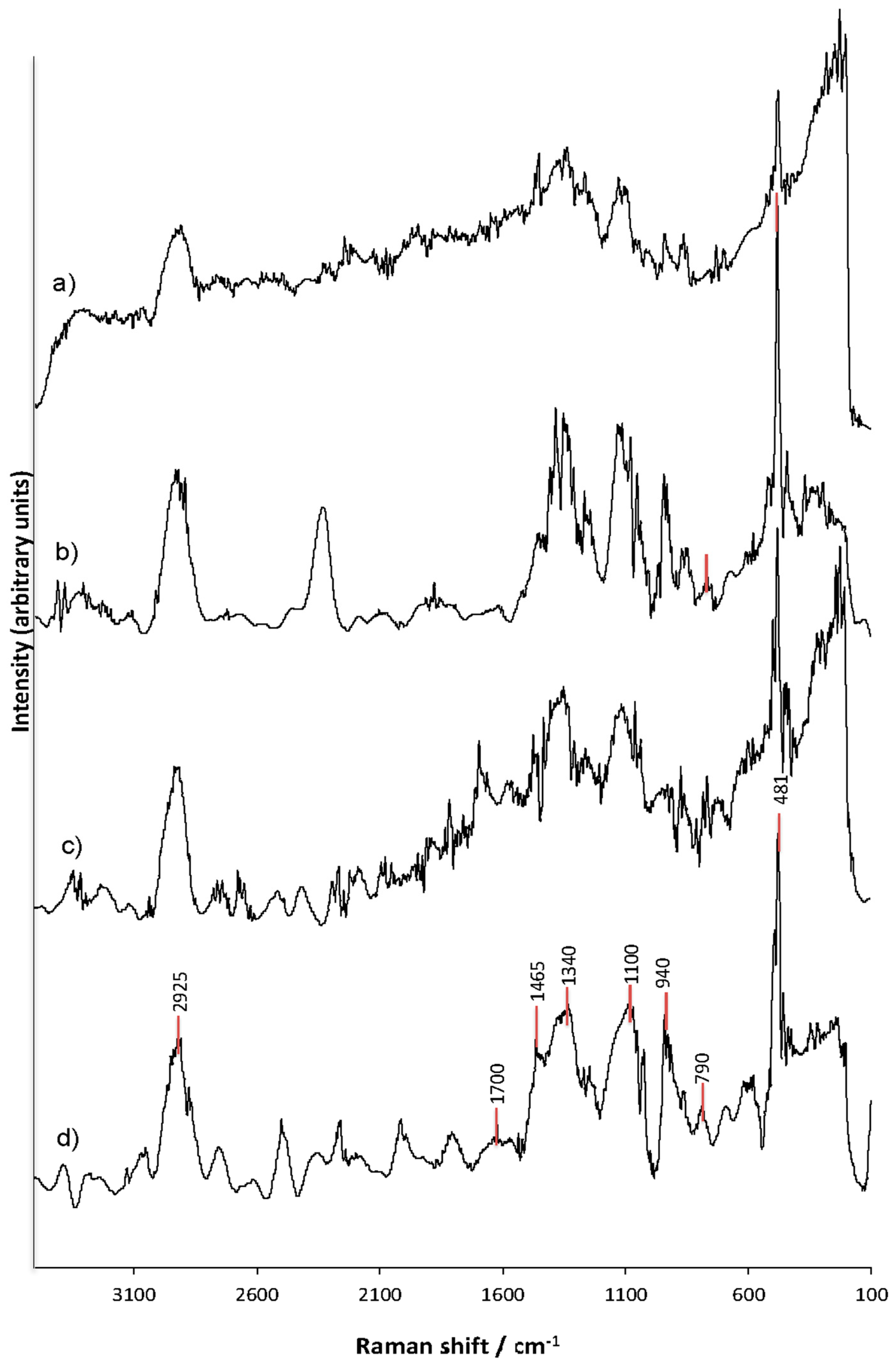
3.6. Raman Spectroscopy Analysis (FT-Raman) of the Structural Changes of Modified Starches
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Shi, M.; Gao, Q.; Liu, Y. Changes in the Structure and Digestibility of Wrinkled Pea Starch with Malic Acid Treatment. Polymers 2018, 10, 1359. [Google Scholar] [CrossRef]
- Pradyawong, S.; Juneja, A.; Sadiq, M.; Noomhorm, A.; Singh, V. Comparison of Cassava Starch with Corn as a Feedstock for Bioethanol Production. Energies 2018, 11, 3476. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crop. Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Liu, C.; Shao, Y.; Jia, D. Chemically modified starch reinforced natural rubber composites. Polym. J. 2008, 49, 2176–2181. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L.; Wang, S.; Copeland, L. Effect of acid hydrolysis on starch structure and functionality: A review. Crit. Rev. Food Sci. 2015, 55, 1081–1097. [Google Scholar] [CrossRef]
- Mehboob, S.; Ali, T.M.; Alam, F.; Hasnain, A. Dual modification of native white sorghum (Sorghum bicolor) starch via acid hydrolysis and succinylation. LWT-Food Sci. Technol. 2015, 64, 459–467. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, J.O. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Jayakody, L.; Hoover, R. The effect he effect of lintnerization on cereal starch granules. Food Res. Int. 2002, 35, 665–680. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Wang, S.; Copeland, L. Effects of hydrothermal-alkali and freezing-thawing pre-treatments on modification of corn starch with octenyl succinic anhydride. Carbohydr. Polym. 2017, 175, 361–369. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.C. Structure and preparation of octenyl succinic esters of granular starch, microporous starch and soluble maltodextrin. Carbohydr. Polym. 2011, 83, 520–527. [Google Scholar] [CrossRef]
- Passauer, L.; Bender, H. Functional group analysis of starches reacted with urea-phosphoric acid—Correlation of wet chemical measures with FT Raman spectroscopy. Carbohydr. Polym. 2017, 168, 356–364. [Google Scholar] [CrossRef]
- Flores-Silva, P.C.; Alvarez-Ramirez, J.; Bello-Perez, L.A. Effect of Dual Modification Order with Ultrasound and Hydrothermal Treatments on Starch Digestibility. Starch-Stärke 2018, 70, 1700284. [Google Scholar] [CrossRef]
- González-Cruz, L.; Montañez-Soto, J.L.; Conde-Barajas, E.; Negrete-Rodríguez, M.L.X.; Flores-Morales, A.; Bernardino-Nicanor, A. Spectroscopic, calorimetric and structural analyses of the effects of hydrothermal treatment of rice beans and the extraction solvent on starch characteristics. Int. J. Biol. Macromol. 2018, 107, 965–972. [Google Scholar] [CrossRef]
- Bernardino-Nicanor, A.; Acosta-García, G.; Güemes-Vera, N.; Montañez-Soto, J.L.; Vivar-Vera, M.A.; González-Cruz, L. Fourier transform infrared and Raman spectroscopic study of the effect of the thermal treatment and extraction methods on the characteristics of ayocote bean starches. J. Food Sci. Techol. 2017, 54, 933–943. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cui, F.; Ping, L.; Song, J.; Ravee, Y.; Wang, Y. Production of octenyl succinic anhydride-modified waxy corn starch and its characterization. J. Agric. Food Chem. 2008, 56, 11499–11506. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, A.K.; Yadav, D.N.; Arora, S.; Vishwakarma, R.K. Impact of octenyl succinylation on rheological, pasting, thermal and physicochemical properties of pearl millet (Pennisetum typhoides) starch. LWT-Food Sci. Technol. 2016, 73, 52–59. [Google Scholar] [CrossRef]
- Chung, H.J.; Lee, S.E.; Han, J.A.; Lim, S.T. Physical properties of dry-heated octenyl succinylated waxy corn starches and its application in fat-reduced muffin. J. Cereal Sci. 2010, 52, 496–501. [Google Scholar] [CrossRef]
- Fonseca-Florido, H.A.; Vázquez-García, H.G.; Méndez-Montealvo, G.; Basilio-Cortés, U.A.; Navarro-Cortés, R.; Rodríguez-Marín, M.L.; Castro-Rosas, J.; Gómez-Aldapa, C.A. Effect of acid hydrolysis and OSA esterification of waxy cassava starch on emulsifying properties in Pickering-type emulsions. LWT-Food Sci. Technol. 2018, 91, 258–264. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohydr. Polym. 2006, 66, 521–527. [Google Scholar] [CrossRef]
- Fonseca-Florido, H.A.; Gómez-Aldapa, C.A.; López-Echevarría, G.; Velazquez, G.; Morales-Sánchez, E.; Castro-Rosas, J.; Méndez-Montealvo, G. Effect of granular disorganization and the water content on the rheological properties of amaranth and achira starch blends. LWT-Food Sci. Technol. 2018, 87, 280–286. [Google Scholar] [CrossRef]
- Bai, Y.; Kaufman, R.C.; Wilson, J.D.; Shi, Y.C. Position of modifying groups on starch chains of octenylsuccinic anhydride-modified waxy maize starch. Food Chem. 2014, 153, 193–199. [Google Scholar] [CrossRef]
- Bello-Flores, C.A.; Nuñez-Santiago, M.C.; San Martín-Gonzalez, M.F.; BeMiller, J.N.; Bello-Pérez, L.A. Preparation and characterization of octenylsuccinylated plantain starch. Int. J. Biol. Macromol. 2014, 70, 334–339. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch. Food Chem. 2014, 151, 154–160. [Google Scholar] [CrossRef]
- Ye, F.; Miao, M.; Huang, C.; Lu, K.; Jiang, B.; Zhang, T. Elucidation of Substituted Ester Group Position in Octenylsuccinic Anhydride Modified Sugary Maize Soluble Starch. J. Agric. Food Chem. 2014, 62, 11696–11705. [Google Scholar] [CrossRef]
- Utrilla-Coello, R.G.; Hernández-Jaimes, C.; Carrillo-Navas, H.; González, F.; Rodríguez, E.; Bello-Perez, L.A.; Alvarez-Ramirez, J. Acid hydrolysis of native corn starch: Morphology, crystallinity, rheological and thermal properties. Carbohydr. Polym. 2014, 103, 596–602. [Google Scholar] [CrossRef]
- Falade, K.O.; Ayetigbo, O.E. Effects of tempering (annealing), acid hydrolysis, low-citric acid substitution on chemical and physicochemical properties of starches of four yam (Dioscorea spp.) cultivars. J. Food Sci. Techol. 2017, 54, 1455–1456. [Google Scholar] [CrossRef]
- Ortega-Ojeda, F.E.; Larsson, H.; Eliasson, A.C. Ortega-Ojeda, F.E., Larsson, H.; Eliasson, A.C. Gel formation in mixtures of hydrophobically modified potato and high amylopectin potato starch. Carbohydr. Polym. 2005, 59, 313–317. [Google Scholar] [CrossRef]
- Song, X.; He, G.; Ruan, H.; Chen, Q. Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch-Stärke 2006, 58, 109–117. [Google Scholar] [CrossRef]
- Zhu, W.; Xie, H.L.; Song, X.Y.; Ren, H.T. Production and physicochemical properties of 2-octen-1-ylsuccinic derivatives from waxy corn starch. J. Food Sci. 2011, 76, C362–C367. [Google Scholar] [CrossRef]
- Betancur-Ancona, D.; García-Cervera, E.; Cañizares-Hernández, E.; Chel-Guerrero, L. Chemical modification of jack bean (Canavalia ensiformis) starch by succinylation. Starch-Stärke 2002, 54, 540–546. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Effect of octenylsuccinylation on physicochemical and functional properties of waxy maize and amaranth starches. Carbohydr. Polym. 2007, 68, 447–456. [Google Scholar] [CrossRef]
- Lawal, O.S. Succinyl and acetyl starch derivatives of a hybrid maize: Physicochemical characteristics and retrogradation properties monitored by differential scanning calorimetry. Carbohydr. Res. 2004, 339, 2673–2682. [Google Scholar] [CrossRef]
- Bao, J.; Xing, J.; Phillips, D.L.; Corke, H. Physical properties of octenyl succinic anhydride modified rice, wheat, and potato starches. J. Agric. Food Chem. 2003, 51, 2283–2287. [Google Scholar] [CrossRef]
- Klein, B.; Pinto, V.Z.; Vanier, N.L.; da Rosa Zavareze, E.; Colussi, R.; do Evangelho, J.A.; Gutkoski, L.C.; Dias, A.R.G. Effect of single and dual heat–moisture treatments on properties of rice, cassava, and pinhao starches. Carbohydr. Polym. 2013, 98, 1578–1584. [Google Scholar] [CrossRef]
- Pinto, V.Z.; Vanier, N.L.; Klein, B.; Zavareze, E.D.R.; Elias, M.C.; Gutkoski, L.C.; Helbig, E.; Dias, A.R.G. Physicochemical, crystallinity, pasting and thermal properties of heat-moisture-treated pinhão starch. Starch-Stärke 2012, 64, 855–863. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Li, G.Y.; Xie, Q.T.; Zhang, B.; Li, X.M.; Pan, Y.; Chen, H.Q. Evaluation studies on the combined effect of hydrothermal treatment and octenyl succinylation on the physic-chemical, structural and digestibility characteristics of sweet potato starch. Food Chem. 2018, 256, 413–418. [Google Scholar] [CrossRef]
- Pascoal, A.M.; Di-Medeiros, M.C.B.; Batista, K.A.; Leles, M.I.; Lião, L.M.; Fernandes, K.F. Extraction and chemical characterization of starch from S. lycocarpum fruits. Carbohydr. Polym. 2013, 98, 1304–1310. [Google Scholar] [CrossRef]
- Dutta, H.; Paul, S.K.; Kalita, D.; Mahanta, C.L. Effect of acid concentration and treatment time on acid–alcohol modified jackfruit seed starch properties. Food Chem. 2011, 128, 284–291. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Q.; Luo, F.X.; Fu, X.; Jiang, H.; Jane, J.L. Effects of octenylsuccinylation on the structure and properties of high-amylose maize starch. Carbohydr. Polym. 2011, 84, 1276–1281. [Google Scholar] [CrossRef]
- Guo, J.; Liu, L.; Lian, X.; Li, L.; Wu, H. The properties of different cultivars of Jinhai sweet potato starches in China. Int. J. Biol. Macromol. 2014, 67, 1–6. [Google Scholar] [CrossRef]
- Parvinzadeh, G.M.; Stir, M.; Bourquin, M.; Hulliger, J. Mineralization of calcium phosphate crystals in starch template inducing a brushite kidney stone biomimetic composite. Cryst. Growth Des. 2013, 13, 2166–2173. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, A.; Singh, N.; Sodhi, N.S. Effect of shearing on functional properties of starches isolated from Indian kidney beans. Starch-Stärke 2013, 65, 808–813. [Google Scholar] [CrossRef]
- Capek, P.; Dra’bik, M.; Turjan, J. Characterization of starch and its mono and hybrid derivatives by thermal analysis and FT-IR spectroscopy. J. Therm. Anal. Calorim. 2009, 99, 667–673. [Google Scholar] [CrossRef]
- Phillips, D.L.; Xing, J.; Chong, C.K.; Liu, H.; Corke, H. Determination of the degree of succinylation in diverse modified starches by Raman spectroscopy. J. Agric. Food Chem. 2000, 48, 5105–5108. [Google Scholar] [CrossRef]
- Wetzel, D.L.; Shi, Y.C.; Schmidt, U. Confocal Raman and AFM imaging of individual granules of octenyl succinate modified and natural waxy maize starch. Vib. Spectrosc. 2010, 53, 173–177. [Google Scholar] [CrossRef]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched cassava starch crystallinity determination by Raman spectroscopy: Correlation of features in Raman spectra with X-ray diffraction and 13C CP/MAS NMR spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef]
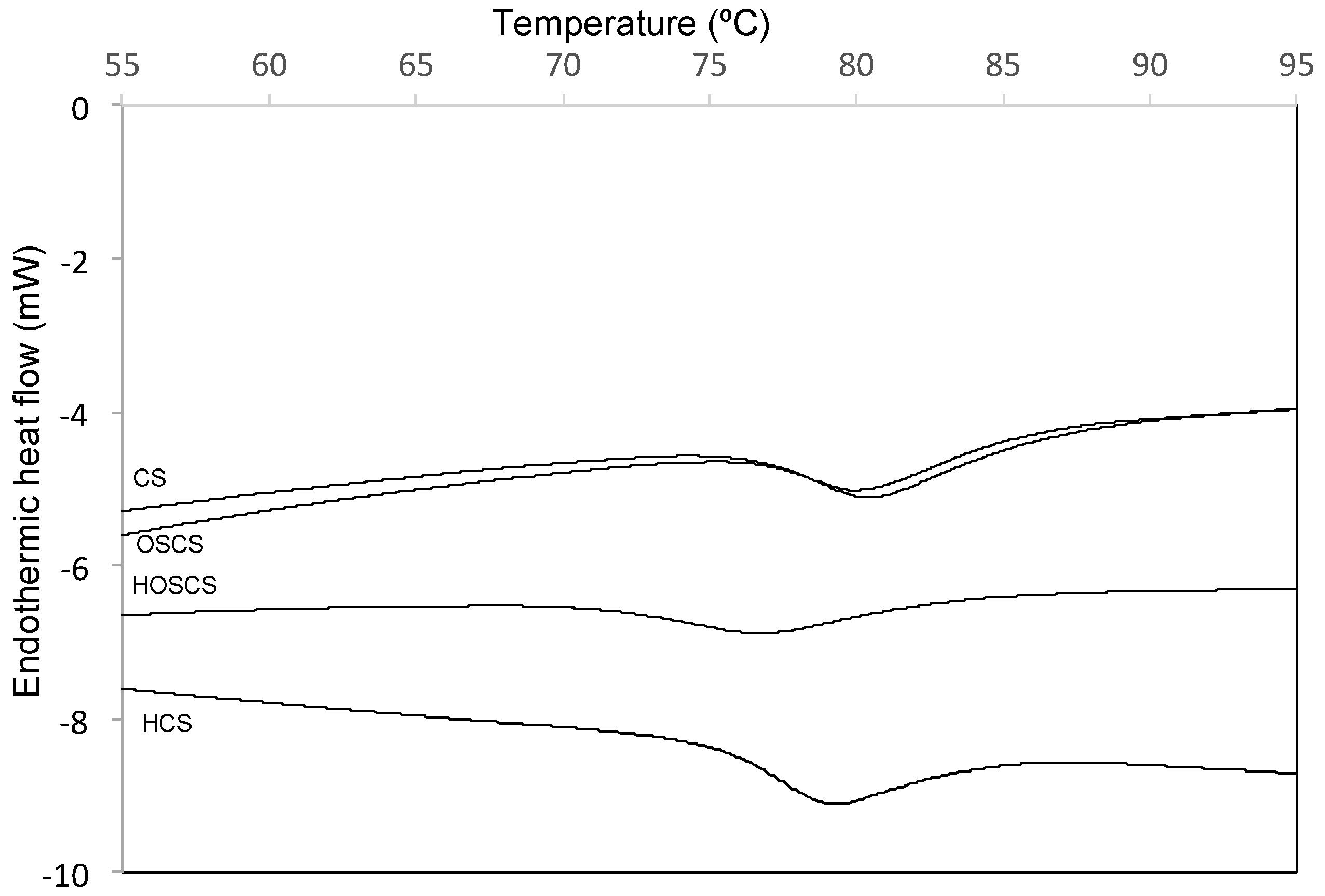
| Starch Sample | To | Tp | Tc | Tc–To | ΔH |
|---|---|---|---|---|---|
| CS | 76.69 ± 0.18 a | 80.45 ± 0.16 a | 85.46 ± 0.12 a | 8.77 | 10.94 ± 0.14 a |
| HCS | 65.09 ± 0.28 b | 69.45 ± 0.14 c | 74.93 ± 0.19 b | 9.84 | 10.57 ± 0.18 b |
| OSCS | 75.91 ± 0.18 a | 79.08 ± 0.12 b | 84.95 ± 0.16 a | 9.04 | 10.07 ± 0.14 b |
| HOSCS | 60.52 ± 0.27 c | 67.09 ± 0.15 d | 73.99 ± 0.14 c | 13.47 | 9.14 ± 0.15 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basilio-Cortés, U.A.; González-Cruz, L.; Velazquez, G.; Teniente-Martínez, G.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Bernardino-Nicanor, A. Effect of Dual Modification on the Spectroscopic, Calorimetric, Viscosimetric and Morphological Characteristics of Corn Starch. Polymers 2019, 11, 333. https://doi.org/10.3390/polym11020333
Basilio-Cortés UA, González-Cruz L, Velazquez G, Teniente-Martínez G, Gómez-Aldapa CA, Castro-Rosas J, Bernardino-Nicanor A. Effect of Dual Modification on the Spectroscopic, Calorimetric, Viscosimetric and Morphological Characteristics of Corn Starch. Polymers. 2019; 11(2):333. https://doi.org/10.3390/polym11020333
Chicago/Turabian StyleBasilio-Cortés, Ulin Antobelli, Leopoldo González-Cruz, Gonzalo Velazquez, Gerardo Teniente-Martínez, Carlos Alberto Gómez-Aldapa, Javier Castro-Rosas, and Aurea Bernardino-Nicanor. 2019. "Effect of Dual Modification on the Spectroscopic, Calorimetric, Viscosimetric and Morphological Characteristics of Corn Starch" Polymers 11, no. 2: 333. https://doi.org/10.3390/polym11020333
APA StyleBasilio-Cortés, U. A., González-Cruz, L., Velazquez, G., Teniente-Martínez, G., Gómez-Aldapa, C. A., Castro-Rosas, J., & Bernardino-Nicanor, A. (2019). Effect of Dual Modification on the Spectroscopic, Calorimetric, Viscosimetric and Morphological Characteristics of Corn Starch. Polymers, 11(2), 333. https://doi.org/10.3390/polym11020333