Hierarchical Structure and Thermal Property of Starch-Based Nanocomposites with Different Amylose/Amylopectin Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Different Amylose Content Starch-Based Nanocomposites
2.3. Characterization
2.3.1. Morphology
2.3.2. Transmission Electron Microscopy Analysis
2.3.3. Small-Angle X-Ray Scattering (SAXS) Analysis
2.3.4. Wide angle X-ray Diffraction (XRD) analysis
2.3.5. Thermal Property
2.3.6. Statistical Analysis
3. Results and Discussion
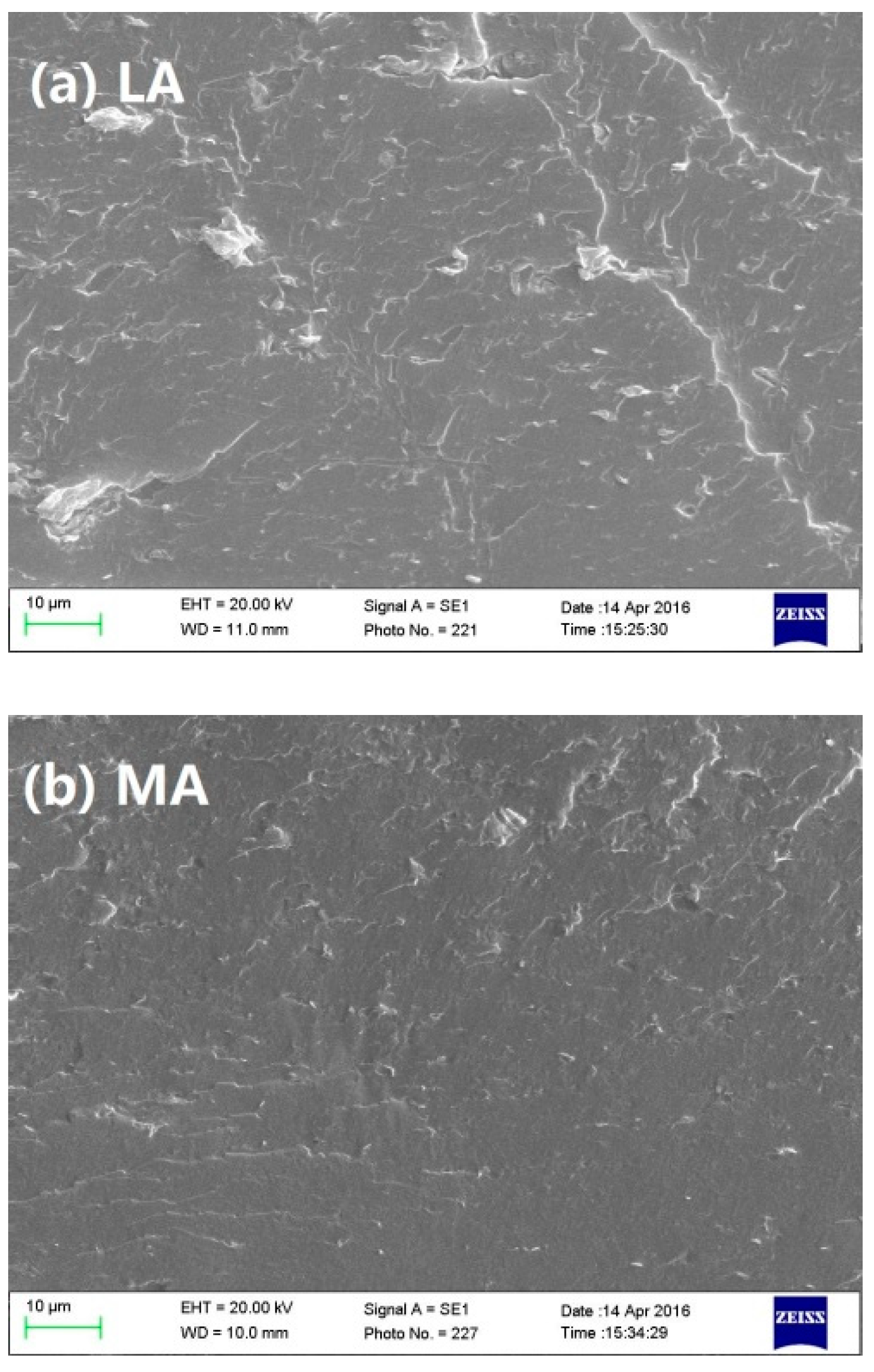
3.1. Morphological Characterization
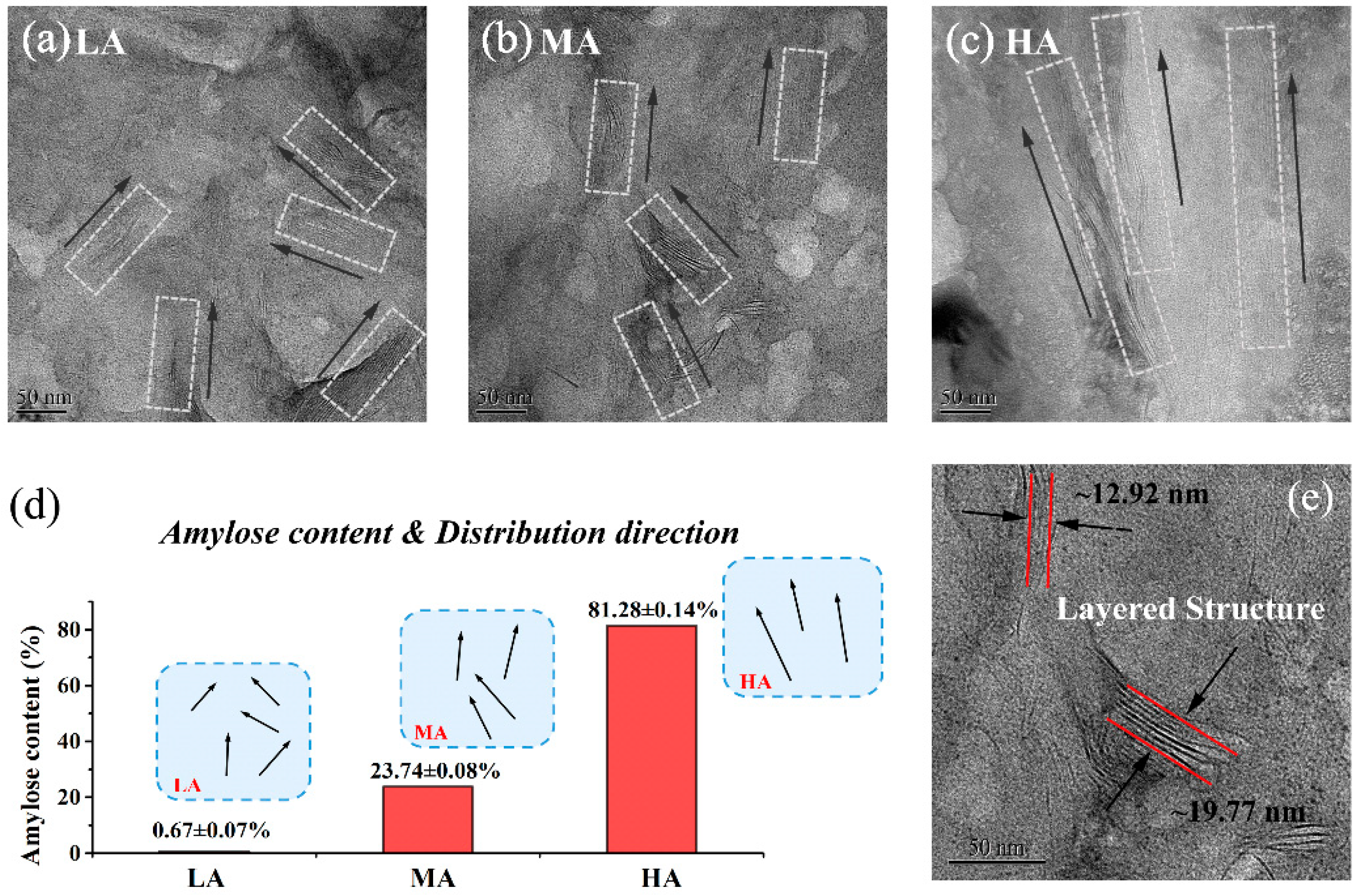
3.2. Dispersion of the Nanofiller
3.3. Microregion Structures
3.4. Crystalline Structure
3.5. Thermal Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moreira, M.A.; André, L.C.; Cardeal, Z.d.L. Analysis of plasticizer migration to meat roasted in plastic bags by SPME-GC/MS. Food Chem. 2015, 178, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, C.; Gouanvé, F.; Espuche, E. Effect of a plasticizer on the structure of biodegradable starch/clay nanocomposites: Thermal, water-sorption, and oxygen-barrier properties. J. Appl. Polym. Sci. 2009, 4, 2044–2056. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioğlu, A.; Altınkaya, S.A. Development of cellulose acetate based antimicrobial food packaging materials for controlled release of lysozyme. J. Food Eng. 2009, 90, 453–462. [Google Scholar] [CrossRef]
- Attaran, S.A.; Hassan, A.; Wahit, M.U. Materials for food packaging applications based on bio-based polymer nanocomposites: A review. J. Thermoplast. Compos. Mater. 2015, 30, 143–173. [Google Scholar] [CrossRef]
- Winkler, H.; Vorwerg, W.; Rihm, R. Thermal and mechanical properties of fatty acid starch esters. Carbohydr. Polym. 2014, 102, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.; Vorwerg, W.; Wetzel, H. Synthesis and properties of fatty acid starch esters. Carbohydr. Polym. 2013, 98, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Kaur, M.; Singh, N.; Lim, S.T. A comparison of native and oxidized normal and waxy corn starches: Physicochemical, thermal, morphological and pasting properties. LWT Food Sci. Technol. 2008, 41, 1000–1010. [Google Scholar] [CrossRef]
- Whitney, K.; Reuhs, B.L.; Martinez, M.O.; Simsek, S. Analysis of octenylsuccinate rice and tapioca starches: Distribution of octenylsuccinic anhydride groups in starch granules. Food Chem. 2016, 211, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the morphology, crystalline structure and thermal properties of yellow ginger starch acetates with different degrees of substitution. Thermochim. Acta 2009, 495, 57–62. [Google Scholar] [CrossRef]
- López, O.V.; García, M.A.; Zaritzky, N.E. Film forming capacity of chemically modified corn starches. Carbohydr. Polym. 2008, 73, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, X.; Huang, C.; Chen, L.; Li, L. Plasticization effect of triacetin on structure and properties of starch ester film. Carbohydr. Polym. 2013, 94, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Johansson, D.; Johansson, K.; Svegmark, K. Material Properties and Molecular Aspects of Highly Acetylated Starch-Based Films. J. Renew. Mater. 2014, 2, 134–144. [Google Scholar] [CrossRef]
- Paul, M.A.; Alexandre, M.; Degée, P.; Henrist, C.; Rulmont, A.; Dubois, P. New nanocomposite materials based on plasticized poly(L-lactide) and organo-modified montmorillonites—Thermal and morphological study. Polymer 2003, 44, 443–450. [Google Scholar] [CrossRef]
- Tang, X.Z.; Alavi, S.; Herald, T.J. Barrier and Mechanical Properties of Starch-Clay Nanocomposite Films. Cereal Chem. 2008, 85, 433–439. [Google Scholar] [CrossRef]
- Nejad, H.M.; Ganster, J.; Volert, B. Starch esters with improved mechanical properties through melt compounding with nanoclays. J. Appl. Polym. Sci. 2010, 118, 503–510. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Mohan, T.P.; Kanny, K. Thermoforming studies of corn starch-derived biopolymer film filled with nanoclays. J. Plast. Film Sheet. 2015, 32, 163–188. [Google Scholar] [CrossRef]
- Eğri, Ö.; Salimi, K.; Eğri, S.; Pişkin, E.; Rzayev, Z.M.O. Fabrication and characterization of novel starch-grafted poly l-lactic acid/montmorillonite organoclay nanocomposites. Carbohydr. Polym. 2016, 137, 111–118. [Google Scholar] [CrossRef]
- Olivato, J.B.; Marini, J.; Pollet, E.; Yamashita, F.; Grossmann, M.V.E.; Avérous, L. Elaboration, morphology and properties of starch/polyester nano-biocomposites based on sepiolite clay. Carbohydr. Polym. 2015, 118, 250–256. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. Starch nano-biocomposites based on needle-like sepiolite clays. Carbohydr. Polym. 2010, 80, 145–153. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abdel-Aziz, M.S.; El-sayed, S.M. Chitosan nanocomposite films based on Ag-NP and Au-NP biosynthesis by Bacillus Subtilis as packaging materials. Int. J. Biol. Macromol. 2014, 69, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Rhim, J.W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, R.; Mohammadi, N. Starch-based nanocomposites: A comparative performance study of cellulose whiskers and starch nanoparticles. Carbohydr. Polym. 2014, 106, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Chen, L.; Li, L.; Li, B.; Zhu, J. Tunable d-Limonene Permeability in Starch-Based Nanocomposite Films Reinforced by Cellulose Nanocrystals. J. Agric. Food Chem. 2018, 66, 979–987. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Mchugh, T.H. Nanocomposites in Food Packaging—A Review. Intech 2011, 54–78. [Google Scholar]
- Madhumitha, G.; Fowsiya, J.; Mohana, R.S.; Thakur, V.K. Recent advances in starch–clay nanocomposites. Int. J. Polym. Anal. Charact. 2018, 23, 331–345. [Google Scholar] [CrossRef]
- Karimi, S.; Dufresne, A.; Tahir, P.M.; Karimi, A.; Abdulkhani, A. Biodegradable starch-based composites: Effect of micro and nanoreinforcements on composites properties. J. Mater. Sci. 2014, 49, 4513–4521. [Google Scholar] [CrossRef]
- Huang, J.; Schols, H.A.; Klaver, R.; Jin, Z.; Voragen, A.G.J. Acetyl substitution patterns of amylose and amylopectin populations in cowpea starch modified with acetic anhydride and vinyl acetate. Carbohydr. Polym. 2007, 67, 542–550. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Zhang, B.; Qiao, D.; Pu, H.; Liu, S.; Li, L. Structural features and thermal property of propionylated starches with different amylose/amylopectin ratio. Int. J. Biol. Macromol. 2017, 97, 123–130. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, J.; Zhang, S.; Li, X.; Zhang, B.; Pu, H.; Li, L.; Wang, Q. Hierarchical structure and thermal behavior of hydrophobic starch-based films with different amylose contents. Carbohydr. Polym. 2018, 181, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Jiménez, A.; Cháfer, M.; Gónzalez, C.; Chiralt, A. Effect of amylose:amylopectin ratio and rice bran addition on starch films properties. Carbohydr. Polym. 2014, 111, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Mondragón, M.; Mancilla, J.E.; Rodríguez, G.F.J. Nanocomposites from plasticized high-amylopectin, normal and high-amylose maize starches. Polym. Eng. Sci. 2008, 48, 1261–1267. [Google Scholar] [CrossRef]
- Liu, H.; Chaudhary, D.; Yusa, S.; Tadé, M.O. Preparation and characterization of sorbitol modified nanoclay with high amylose bionanocomposites. Carbohydr. Polym. 2011, 85, 97–104. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Huang, C.; Zhu, J.; Lin, A.H.-M.; Chen, L.; Li, L. Inhibition of plasticizer migration from packaging to foods during microwave heating by controlling the esterified starch film structure. Food Control 2016, 66, 130–136. [Google Scholar] [CrossRef]
- Menzel, C.; Andersson, M.; Andersson, R.; Vazquez, G.J.L.; Daniel, G.; Langton, M.; Gällstedt, M.; Koch, K. Improved material properties of solution-cast starch films: Effect of varying amylopectin structure and amylose content of starch from genetically modified potatoes. Carbohydr. Polym. 2015, 130, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. New approach to elaborate exfoliated starch-based nanobiocomposites. Biomole 2008, 9, 896–900. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Dole, P.; Avérous, L. Starch-based nano-biocomposites: Plasticizer impact on the montmorillonite exfoliation process. Carbohydr. Polym. 2010, 79, 941–947. [Google Scholar] [CrossRef]
- Majdzadeh, A.K.; Navarchian, A.H.; Sadeghi, F. Optimization of mechanical properties of thermoplastic starch/clay nanocomposites. Carbohydr. Polym. 2010, 79, 547–554. [Google Scholar] [CrossRef]
- Saha, N.R.; Sarkar, G.; Roy, I.; Bhattacharyya, A.; Rana, D.; Dhanarajan, G.; Banerjee, R.; Sen, R.; Mishra, R.; Chattopadhyay, D. Nanocomposite films based on cellulose acetate/polyethylene glycol/modified montmorillonite as nontoxic active packaging material. RSC Adv. 2016, 6, 92569–92578. [Google Scholar] [CrossRef]
- Chung, Y.L.; Ansari, S.; Estevez, L.; Hayrapetyan, S.; Giannelis, E.P.; Lai, H.M. Preparation and properties of biodegradable starch-clay nanocomposites. Carbohydr. Polym. 2010, 79, 391–396. [Google Scholar] [CrossRef]
- Dennis, H.R.; Hunter, D.L.; Chang, D.; Kim, S.; White, J.L.; Cho, J.W.; Paul, D.R. Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer 2001, 42, 9513–9522. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S.; Herald, T.J. Effects of plasticizers on the structure and properties of starch–clay nanocomposite films. Carbohydr. Polym. 2008, 74, 552–558. [Google Scholar] [CrossRef]
- Cui, Y.; Kumar, S.; Kona, B.R.; van Houcke, D. Gas barrier properties of polymer/clay nanocomposites. RSC Adv. 2015, 5, 63669–63690. [Google Scholar] [CrossRef]
- Cai, Y.; Qi, H.J.Y.; Liu, Y.; He, X. Sorption/Desorption Behavior and Mechanism of NH4(+) by Biochar as a Nitrogen Fertilizer Sustained-Release Material. J. Agric. Food Chem. 2016, 64, 4958–4964. [Google Scholar] [CrossRef] [PubMed]
- Maio, L.D.; Scarfato, P.; Milana, M.R.; Feliciani, R.; Denaro, M.; Padula, G.; Incarnato, L. Bionanocomposite Polylactic Acid/Organoclay Films: Functional Properties and Measurement of Total and Lactic Acid Specific Migration. Packag. Technol. Sci. 2014, 27, 535–547. [Google Scholar] [CrossRef]



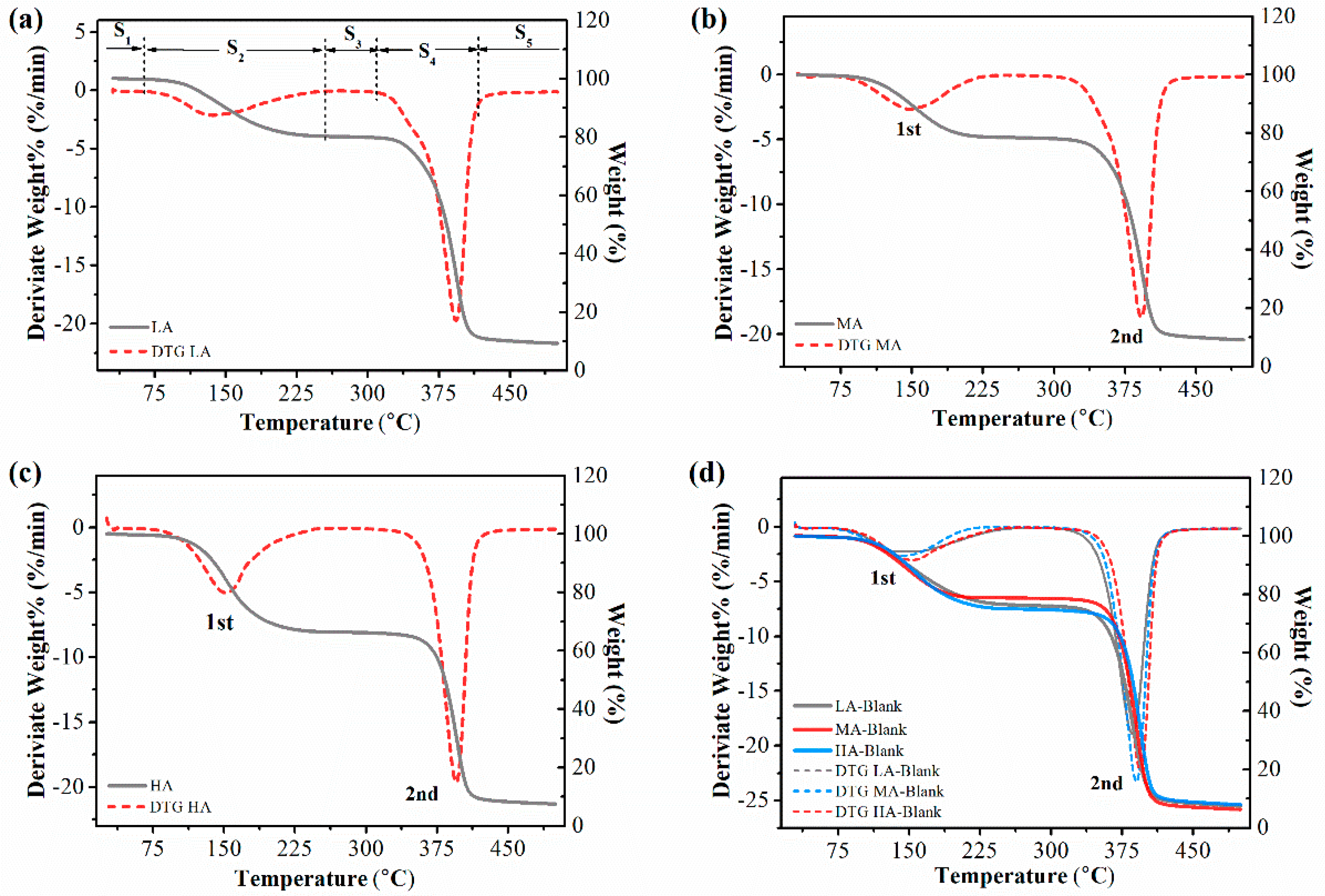
| Sample | Peak 2 | Sample | Peak 2′ | ΔT |
|---|---|---|---|---|
| LA-Blank | 386.12 ± 0.81 | LA | 392.92 ± 1.04 | 6.80 ± 0.73 a |
| MA-Blank | 390.55 ± 1.25 | MA | 392.96 ± 1.16 | 2.40 ± 1.87 b |
| HA-Blank | 393.35 ± 0.95 | HA | 394.56 ± 1.35 | 1.21 ± 0.47 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhu, J.; Liu, Y.; Zou, S.-Y.; Li, L. Hierarchical Structure and Thermal Property of Starch-Based Nanocomposites with Different Amylose/Amylopectin Ratio. Polymers 2019, 11, 342. https://doi.org/10.3390/polym11020342
Zhang S, Zhu J, Liu Y, Zou S-Y, Li L. Hierarchical Structure and Thermal Property of Starch-Based Nanocomposites with Different Amylose/Amylopectin Ratio. Polymers. 2019; 11(2):342. https://doi.org/10.3390/polym11020342
Chicago/Turabian StyleZhang, Shuyan, Jie Zhu, Yujia Liu, Shui-Yang Zou, and Lin Li. 2019. "Hierarchical Structure and Thermal Property of Starch-Based Nanocomposites with Different Amylose/Amylopectin Ratio" Polymers 11, no. 2: 342. https://doi.org/10.3390/polym11020342
APA StyleZhang, S., Zhu, J., Liu, Y., Zou, S.-Y., & Li, L. (2019). Hierarchical Structure and Thermal Property of Starch-Based Nanocomposites with Different Amylose/Amylopectin Ratio. Polymers, 11(2), 342. https://doi.org/10.3390/polym11020342