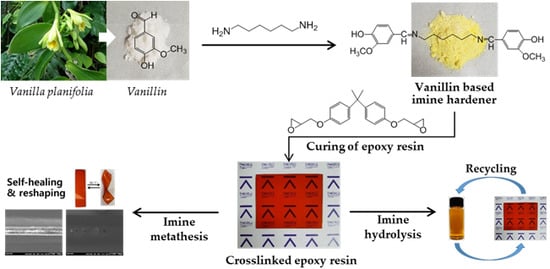
Thermal Healing, Reshaping and Ecofriendly Recycling of Epoxy Resin Crosslinked with Schiff Base of Vanillin and Hexane-1,6-Diamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
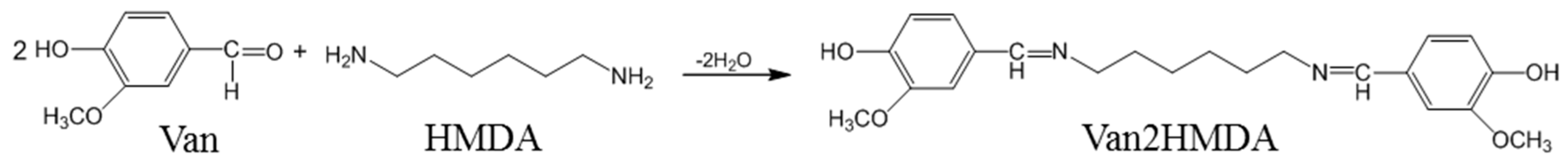
2.2. Preparation of Dihydroxylimine Compound
2.3. Curing of Bisphenol A-Based Epoxy Resin by Van2HMDA
2.4. Recycle of Crosslinked Epoxy Polymer Cured by Van2HMDA
2.5. Methods
3. Results and Discussion
3.1. Preparation of Dihydroxylimine Compound
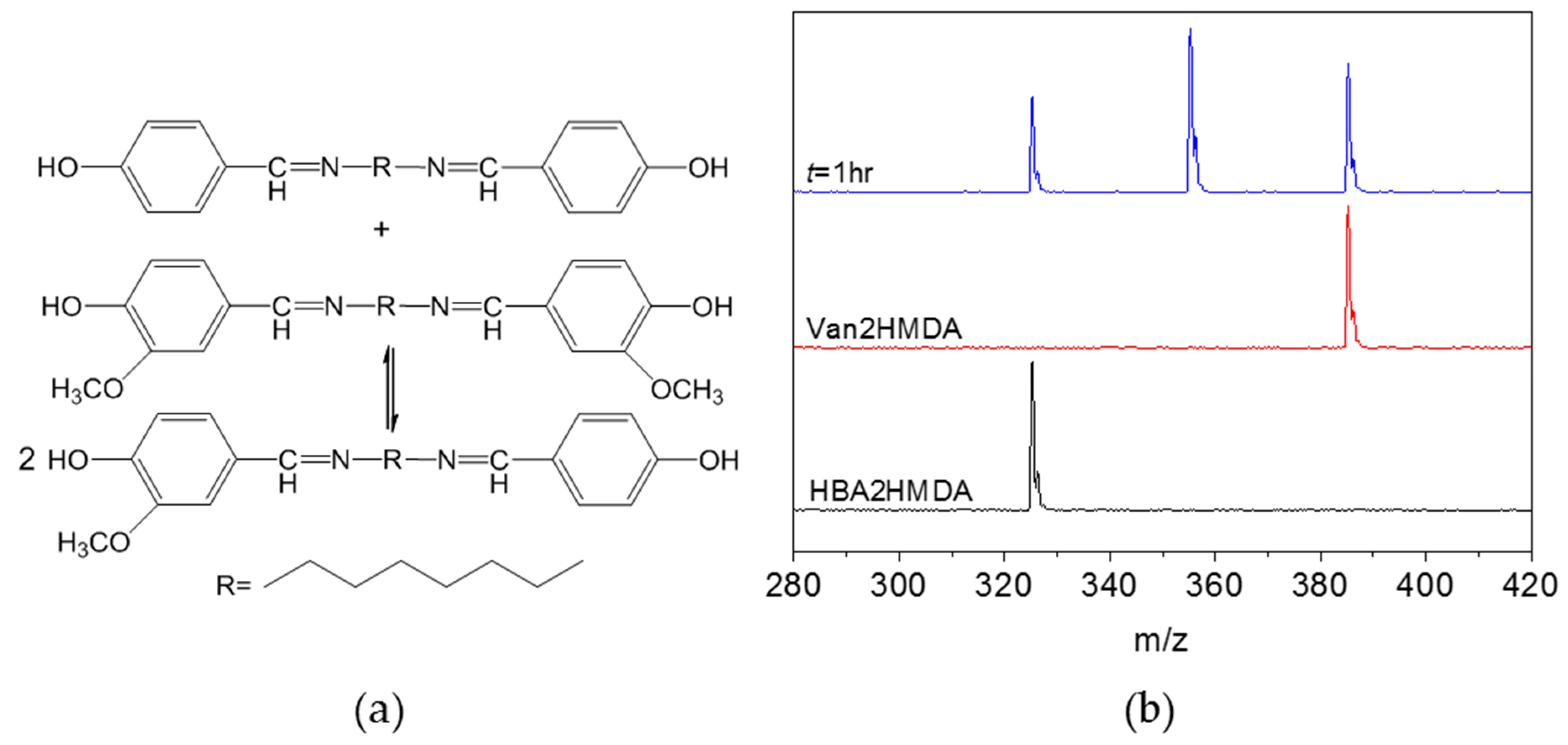
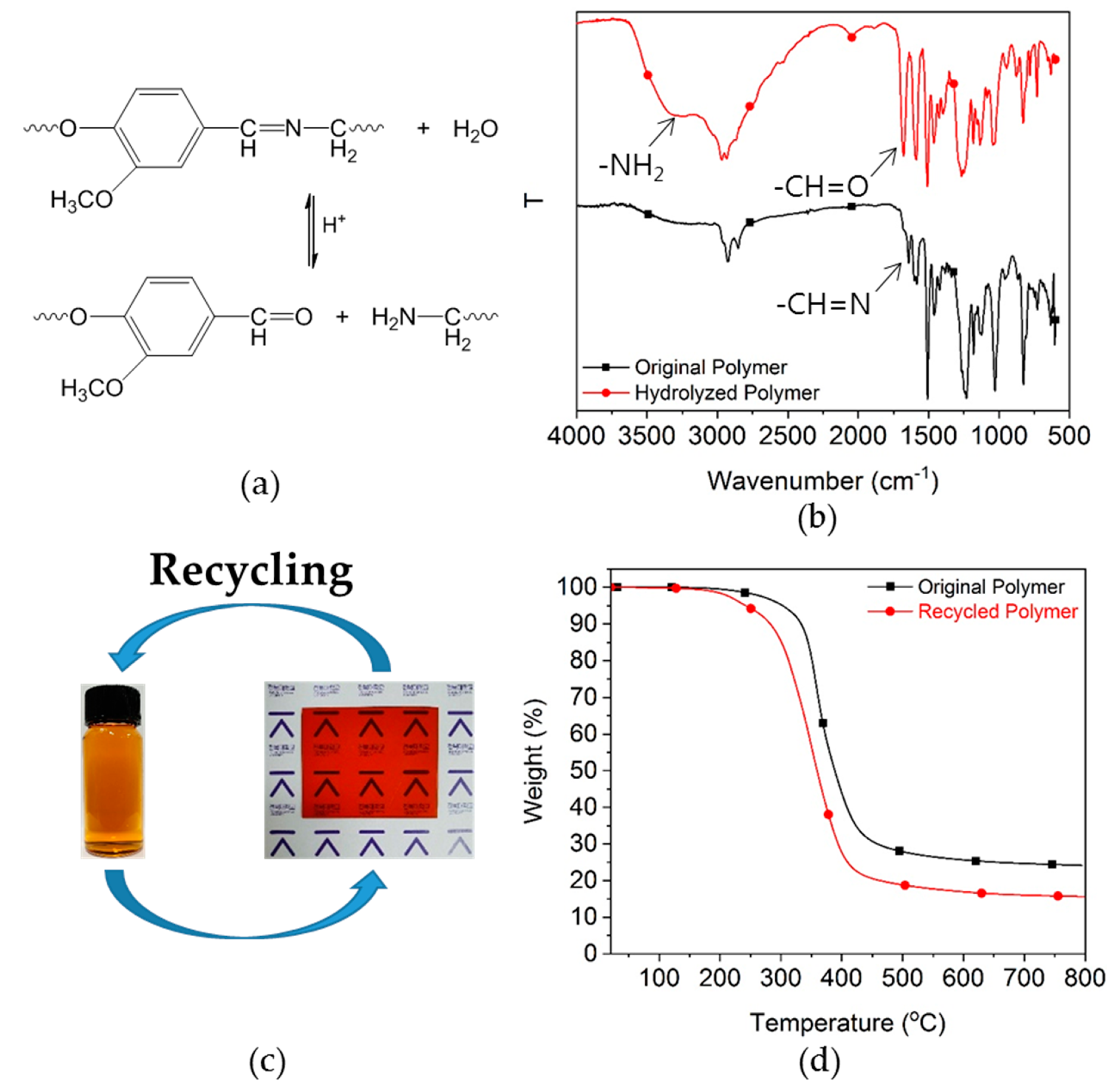
3.2. Imine Metathesis of the Model Compounds
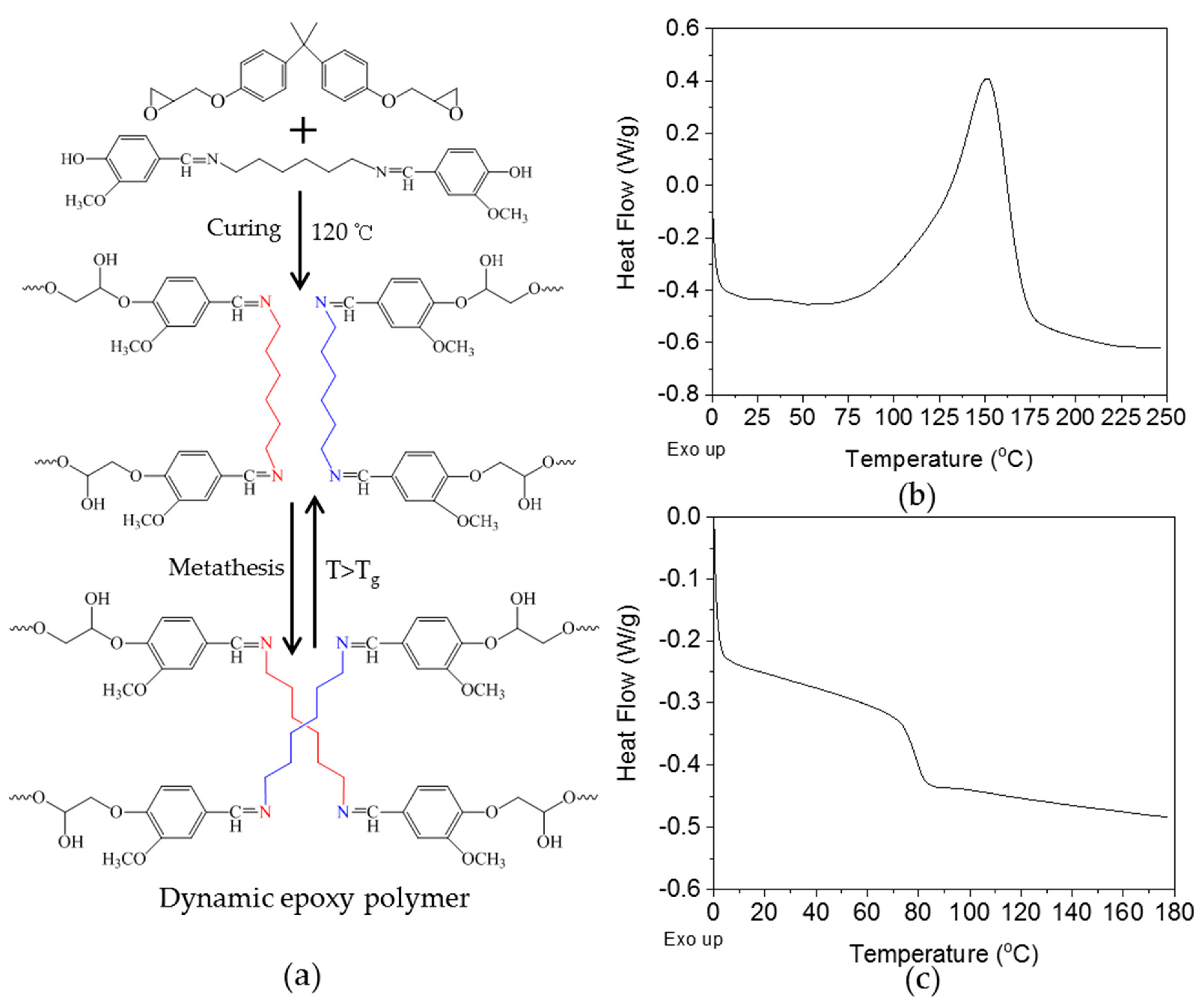
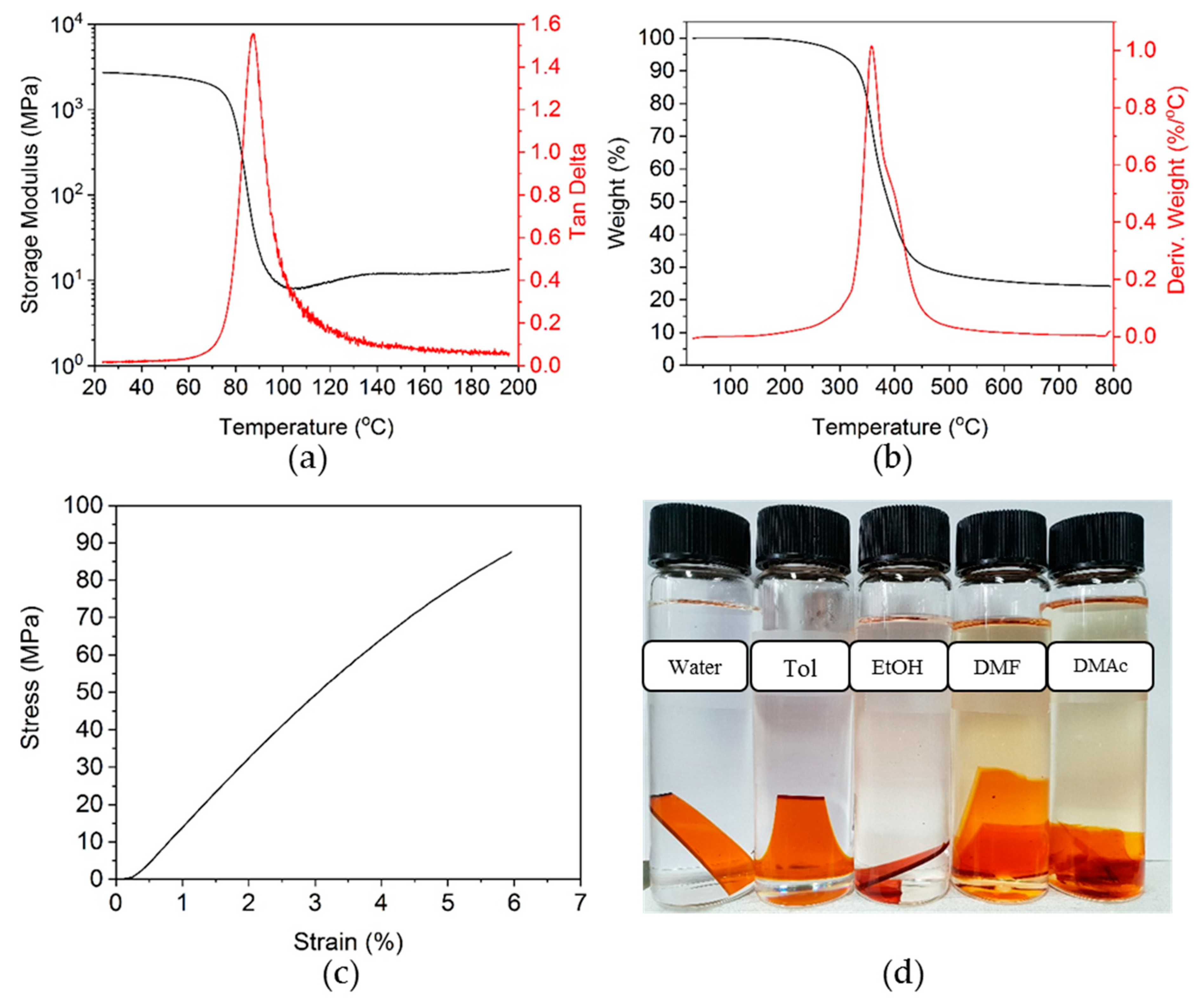
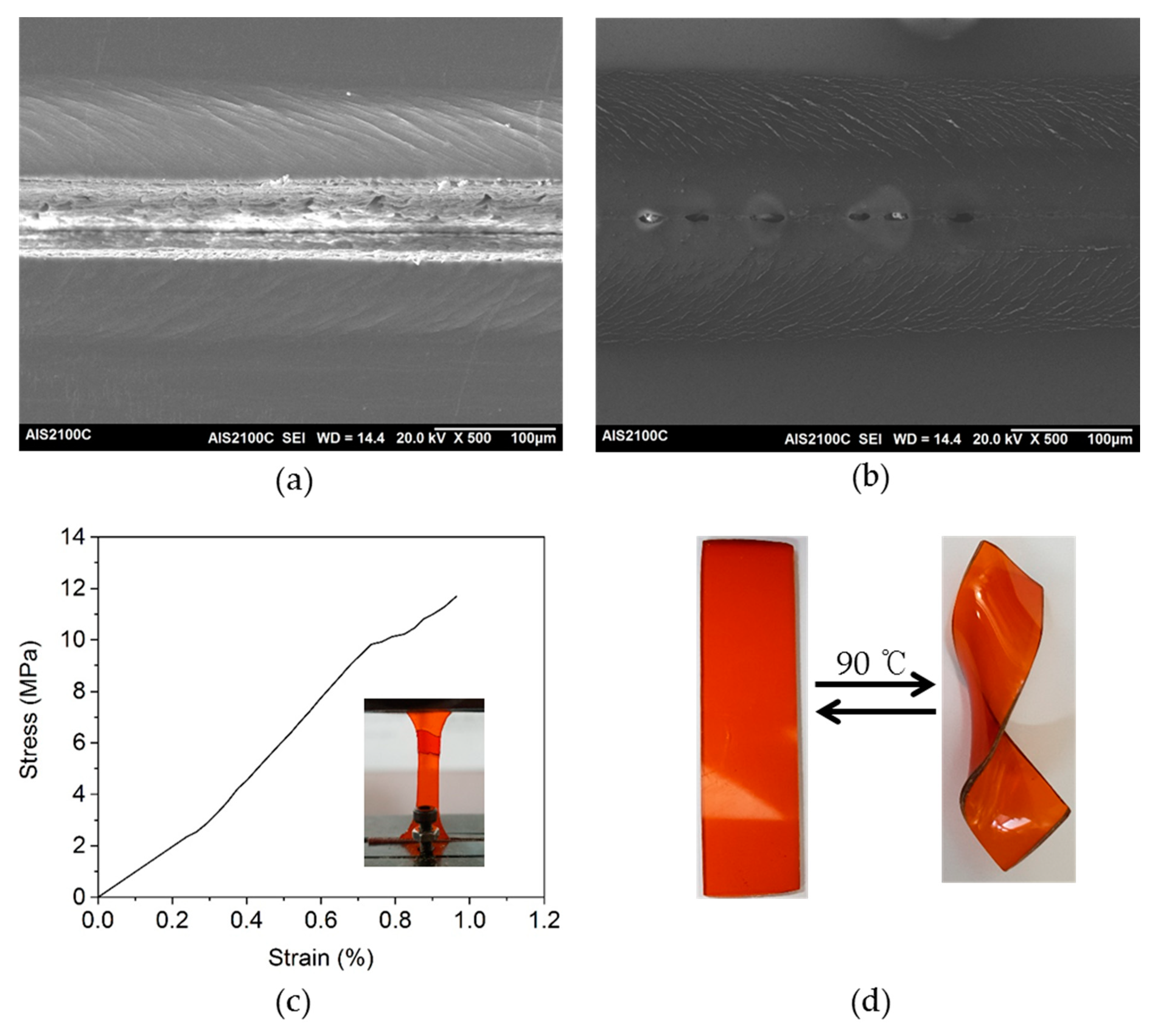
3.3. Curing of Epoxy Resin by the Dihydroxylimine Compound
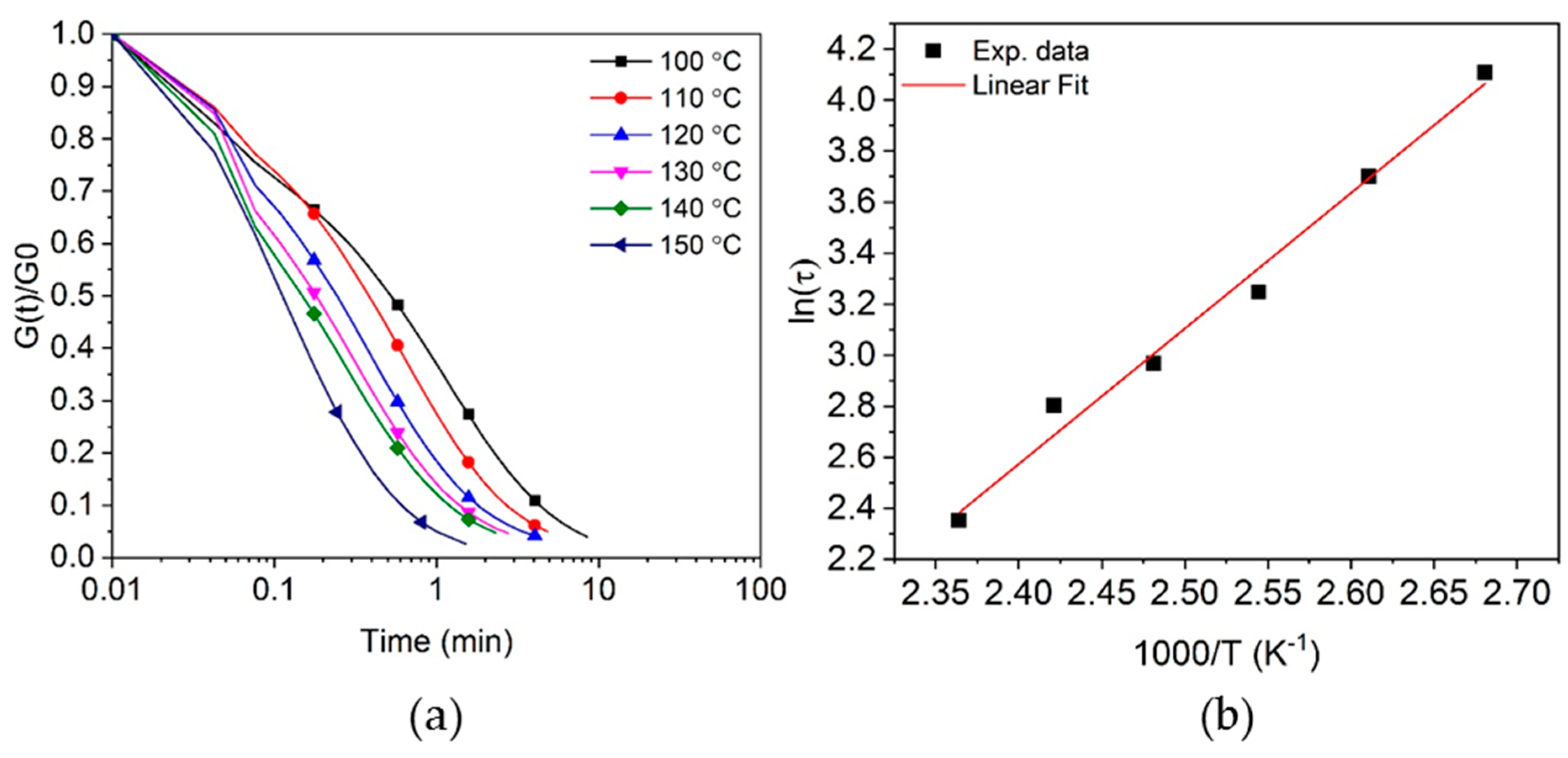
3.4. Stress Relaxation of Crosslinked Epoxy Resin
3.5. Thermal-Healing Properties and Reshaping Ability of the Crosslinked Epoxy Resin
3.6. Ecofriendly Recycling of Crosslinked Epoxy Polymer
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brown, E.N.; White, S.R.; Sottos, N.R. Retardation and repair of fatigue cracks in a microcapsule toughened epoxy composite—Part II: In situ self-healing. Compos. Sci. Technol. 2005, 65, 2474–2480. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Rong, M.Z.; Zhang, M.Q.; Yang, G.C. Self-healing epoxy composites—Preparation and effect of the healant consisting of microencapsulated epoxy and latent curing agent. Compos Sci Technol. 2007, 67, 201–212. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Broekhuis, A.A.; Picchioni, F. Reversible polymer networks containing covalent and hydrogen bonding interactions. Eur. Polym. J. 2014, 50, 127–134. [Google Scholar] [CrossRef]
- Dello Iacono, S.; Martone, A.; Pastore, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. Thermally activated multiple self-healing diels-alder epoxy system. Polym. Eng. Sci. 2017, 57, 674–679. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.M.; Dong, X.; Liu, X.G.; Xu, J.J.; Wang, D.J. Facile Fabrication of Fast Recyclable and Multiple Self-healing Epoxy Materials through Diels-Alder Adduct Cross-linker. J. Polym. Sci. Pol. Chem. 2015, 53, 2094–2103. [Google Scholar] [CrossRef]
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 2015, 79, 187–194. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.J.; Qi, H.J. Solvent Assisted Pressure-Free Surface Welding and Reprocessing of Malleable Epoxy Polymers. Macromolecules 2016, 49, 5527–5537. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Shape memory epoxy vitrimers based on DGEBA crosslinked with dicarboxylic acids and their blends with citric acid. Rsc. Adv. 2016, 6, 88647–88655. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Wang, Y.; Zhu, J.; Yu, J.R.; Hu, Z.M. Bio-Based Epoxy Vitrimers: Reprocessibility, Controllable Shape Memory, and Degradability. J. Polym. Sci. Pol. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- de Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabanero, G.; Rodriguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Lei, Z.Q.; Xiang, H.P.; Yuan, Y.J.; Rong, M.Z.; Zhang, M.Q. Room-Temperature Self-Healable and Remoldable Cross-linked Polymer Based on the Dynamic Exchange of Disulfide Bonds. Chem. Mater. 2014, 26, 2038–2046. [Google Scholar] [CrossRef]
- Gao, W.T.; Bie, M.Y.; Liu, F.; Chang, P.S.; Quan, Y.W. Self-Healable and Reprocessable Polysulfide Sealants Prepared from Liquid Polysulfide Oligomer and Epoxy Resin. Acs. Appl. Mater. Inter. 2017, 9, 15798–15808. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.; Goossens, H.; Klumperman, B. Self-Healing Materials Based on Disulfide Links. Macromolecules 2011, 44, 2536–2541. [Google Scholar] [CrossRef]
- Lu, Y.X.; Guan, Z.B. Olefin Metathesis for Effective Polymer Healing via Dynamic Exchange of Strong Carbon-Carbon Double Bonds. J. Am. Chem. Soc. 2012, 134, 14226–14231. [Google Scholar] [CrossRef]
- Lai, J.C.; Mei, J.F.; Jia, X.Y.; Li, C.H.; You, X.Z.; Bao, Z.A. A Stiff and Healable Polymer Based on Dynamic-Covalent Boroxine Bonds. Adv. Mater. 2016, 28, 8277–8282. [Google Scholar] [CrossRef]
- Cromwell, O.R.; Chung, J.; Guan, Z.B. Malleable and Self-Healing Covalent Polymer Networks through Tunable Dynamic Boronic Ester Bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495. [Google Scholar] [CrossRef]
- Kuhl, N.; Bode, S.; Bose, R.K.; Vitz, J.; Seifert, A.; Hoeppener, S.; Garcia, S.J.; Spange, S.; van der Zwaag, S.; Hager, M.D.; et al. Acylhydrazones as Reversible Covalent Crosslinkers for Self-Healing Polymers. Adv. Funct. Mater. 2015, 25, 3295–3301. [Google Scholar] [CrossRef]
- Layer, R.W. Chemistry of Imines. Chem. Rev. 1963, 63, 489. [Google Scholar] [CrossRef]
- Ciaccia, M.; Cacciapaglia, R.; Mencarelli, P.; Mandolini, L.; Di Stefano, S. Fast transimination in organic solvents in the absence of proton and metal catalysts. A key to imine metathesis catalyzed by primary amines under mild conditions. Chem. Sci. 2013, 4, 2253–2261. [Google Scholar] [CrossRef]
- Taynton, P.; Zhu, C.P.; Loob, S.; Shoemaker, R.; Pritchard, J.; Jin, Y.H.; Zhang, W. Re-healable polyimine thermosets: Polymer composition and moisture sensitivity. Polym. Chem.-UK 2016, 7, 7052–7056. [Google Scholar] [CrossRef]
- Chao, A.; Negulescu, J.; Zhang, D.H. Dynamic Covalent Polymer Networks Based on Degenerative Imine Bond Exchange: Tuning the Malleability and Self-Healing Properties by Solvent. Macromolecules 2016, 49, 6277–6284. [Google Scholar] [CrossRef]
- Lei, Z.Q.; Xie, P.; Rong, M.Z.; Zhang, M.Q. Catalyst-free dynamic exchange of aromatic Schiff base bonds and its application to self-healing and remolding of crosslinked polymers. J. Mater. Chem. A 2015, 3, 19662–19668. [Google Scholar] [CrossRef]
- Stuparu, M.C.; Khan, A.; Hawker, C.J. Phase separation of supramolecular and dynamic block copolymers. Polym. Chem.-UK 2012, 3, 3033–3044. [Google Scholar] [CrossRef]
- Rao, J.Y.; Khan, A. Using reversibility of the dynamic covalent bond to create porosity in highly ordered polymer thin films under mild conditions and nano-pore functionalization in the gas phase. Polym. Chem.-UK 2013, 4, 2691–2695. [Google Scholar] [CrossRef]
- Rao, J.Y.; De, S.; Khan, A. Synthesis and self-assembly of dynamic covalent block copolymers: Towards a general route to pore-functionalized membranes. Chem. Commun. 2012, 48, 3427–3429. [Google Scholar] [CrossRef]
- Abu-Omar, S.Z.M.M. Recyclable and Malleable Epoxy Thermoset Bearing Aromatic Imine Bonds. Macromolecules 2018, 51, 9816–9824. [Google Scholar] [CrossRef]
- Koike, T. Progress in Development of Epoxy Resin Systems Based on Wood Biomass in Japan. Polym. Eng. Sci. 2012, 52, 701–717. [Google Scholar] [CrossRef]
- Aouf, C.; Lecomte, J.; Villeneuve, P.; Dubreucq, E.; Fulcrand, H. Chemo-enzymatic functionalization of gallic and vanillic acids: Synthesis of bio-based epoxy resins prepolymers. Green. Chem. 2012, 14, 2328–2336. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green. Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- da Silva, E.A.B.; Zabkova, M.; Araujo, J.D.; Cateto, C.A.; Barreiro, M.F.; Belgacem, M.N.; Rodriques, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Krassig, H.; Greber, G. Uber Die Umsetzungen Von Terephthalaldehyd Mit Aliphatischen Diaminen. 431. Mitteilung Uber Makromolekulare Verbindungen. Makromolekul Chem. 1956, 17, 131–153. [Google Scholar] [CrossRef]
- Wilhelms, N.; Kulchat, S.; Lehn, J.M. Organocatalysis of C=N/C=N and C=C/C=N Exchange in Dynamic Covalent Chemistry. Helv. Chim. Acta 2012, 95, 2635–2651. [Google Scholar] [CrossRef]
- Muzammil, E.M.; Khan, A.; Stuparu, M.C. Post-polymerization modification reactions of poly(glycidyl methacrylate)s. Rsc. Adv. 2017, 7, 55874–55884. [Google Scholar] [CrossRef]
- Stuparu, M.C.; Khan, A. Thiol-epoxy “click” chemistry: Application in preparation and postpolymerization modification of polymers. J. Polym. Sci. Pol. Chem. 2016, 54, 3057–3070. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, D.G.; Yeo, H.; Rao, J.; Zhu, Z.; Shin, J.; Jeong, K.; Kim, S.; Jung, H.W.; Khan, A. Proton Transfer Hydrogels: Versatility and Applications. J. Am. Chem. Soc. 2018, 140, 6700–6709. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, N.; Rao, J.Y.; Sanyal, A.; Khan, A. Designing functionalizable hydrogels through thiol-epoxy coupling chemistry. Chem. Commun. 2013, 49, 11191–11193. [Google Scholar] [CrossRef]
- Calvert, P. Introduction to Physical Polymer Science—Sperling, L.H. Nature 1987, 326, 216. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Chen, X.; Jin, K.L.; Torkelson, J.M. Vitrimers Designed Both To Strongly Suppress Creep and To Recover Original Cross-Link Density after Reprocessing: Quantitative Theory and Experiments. Macromolecules 2018, 51, 5537–5546. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, V.-D.; Shin, S.-R.; Lee, D.-S.; Kang, I. Thermal Healing, Reshaping and Ecofriendly Recycling of Epoxy Resin Crosslinked with Schiff Base of Vanillin and Hexane-1,6-Diamine. Polymers 2019, 11, 293. https://doi.org/10.3390/polym11020293
Mai V-D, Shin S-R, Lee D-S, Kang I. Thermal Healing, Reshaping and Ecofriendly Recycling of Epoxy Resin Crosslinked with Schiff Base of Vanillin and Hexane-1,6-Diamine. Polymers. 2019; 11(2):293. https://doi.org/10.3390/polym11020293
Chicago/Turabian StyleMai, Van-Dung, Se-Ra Shin, Dai-Soo Lee, and Ilho Kang. 2019. "Thermal Healing, Reshaping and Ecofriendly Recycling of Epoxy Resin Crosslinked with Schiff Base of Vanillin and Hexane-1,6-Diamine" Polymers 11, no. 2: 293. https://doi.org/10.3390/polym11020293
APA StyleMai, V.-D., Shin, S.-R., Lee, D.-S., & Kang, I. (2019). Thermal Healing, Reshaping and Ecofriendly Recycling of Epoxy Resin Crosslinked with Schiff Base of Vanillin and Hexane-1,6-Diamine. Polymers, 11(2), 293. https://doi.org/10.3390/polym11020293