Phosphorus Containing Polyacrylamides as Flame Retardants for Epoxy-Based Composites in Aviation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of BA-DOPO
2.2. Synthesis of TAHHT-DOPO
2.3. Synthesis of TAHHT-DDPO
2.4. Synthesis of TAHHT-DOPO-DDPO
2.5. Characterization
3. Results and Discussion
3.1. Characterization of Phosphorus-Containing Polyacrylamides
3.2. Neat Resin Samples and Carbon Fiber-Reinforced Composites
3.2.1. Material Characterization
3.2.2. Thermal Properties
3.2.3. Flame-Retardant Performance
3.2.4. Fiber Protection
3.3. Synergistic Mixtures with Boehmite
3.3.1. Thermal Properties
3.3.2. Flame-Retardant Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zang, L.; Wagner, S.; Ciesielski, M.; Müller, P.; Döring, M. Novel star-shaped and hyperbranched phosphorus-containing flame retardants in epoxy resins. Polym. Adv. Technol. 2011, 22, 1182–1191. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stöß, K.; Ciesielski, M.; Diederichs, J.; Döring, M.; Krämer, J.; Altstädt, V. A New Halogen-Free Flame Retardant Based on 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide for Epoxy Resins and their Carbon Fiber Composites for the Automotive and Aviation Industries. Macromol. Mater. Eng. 2011, 296, 14–30. [Google Scholar] [CrossRef]
- Döring, M.; Ciesielski, M.; Heinzmann, C. Synergistic flame retardant mixtures in epoxy resins. In Fire and Polymers VI: New advances in flame retardant chemistry and science; Morgan, A.B., Wilkie, C.A., Nelson, G.N., Eds.; American Chemical Society: Washington, DC, USA, 2012; Volume 1118, pp. 295–309. [Google Scholar]
- Ciesielski, M.; Diederichs, J.; Döring, M.; Schäfer, A. Advanced flame retardant epoxy resins for composite materials. In Fire and Polymers V: Materials and Concepts for Fire Retardancy; Wilkie, C.A., Morgan, A.B., Nelson, G.L., Eds.; American Chemical Society: Washington, DC, USA, 2009; Volume 1013, pp. 174–190. [Google Scholar]
- Levchik, S.V.; Camino, G.; Costa, L.; Luda, M.P. Mechanistic study of thermal behaviour and combustion performance of carbon fibre-epoxy resin composites fire retarded with a phosphorous-based curing system. Polym. Degrad. Stab. 1996, 54, 317–322. [Google Scholar] [CrossRef]
- Martin, F.J.; Price, K.R. Flammability of epoxy resins. J. Appl. Polym. Sci. 1968, 12, 143–158. [Google Scholar] [CrossRef]
- Toldy, A.; Szolnoki, B.; Marosi, G. Flame retardancy of fibre-reinforced epoxy resin composites for aerospace applications. Polym. Degrad. Stab. 2011, 96, 371–376. [Google Scholar] [CrossRef]
- Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the Modes of Action of Phosphorus-Based Flame Retardants in Polymeric Systems. Materials 2017, 10, 455. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Krämer, J.; Müller, P.; Altstädt, V.; Zang, L.; Döring, M. Flame retardancy of polymers: The role of specific reactions in the condensed phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Eibl, S.; Reiner, D.; Lehnert, M. Gefährdung durch lungengängige Faserfragmente nach dem Abbrand Kohlenstofffaser verstärkter Kunststoffe (Health hazards by respirable fiber fragments after combustion of carbon fiber reinforced plastic material). Gefahrstoffe - Reinhaltung der Luft 2014, 74, 286. [Google Scholar]
- Eibl, S.; Scholz, N. Besondere Gefährdung beim Abbrand von Carbon-Kunststoffen (Particular hazards related to combustion of carbon fiber reinforced plastic material). Brandschutz, Deutsche Feuerwehr-Zeitung 2014, 6, 423–427. [Google Scholar]
- Hertzberg, T. Dangers relating to fires in carbon-fibre based composite material. Fire Mater. 2005, 29, 231–248. [Google Scholar] [CrossRef]
- Holt, P.F.; Horne, M. Dust from Carbon Fibre. Environ. Res. 1978, 17, 276–283. [Google Scholar] [CrossRef]
- Eibl, S. Potential for the formation of respirable fibers in carbon fiber reinforced plastic materials after combustion. Fire Mater. 2017, 41, 808–816. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Hazard Prevention and Control in the Work Environment: Airborne Dust, WHO/SDE/OEH/99.14; 1999; Available online: http://www.who.int/occupational_health/publications/en/oehairbornedust3.pdf (accessed on 18 December 2017).
- Eibl, S. Flammgeschützte Kohlenstofffaser (Flame retardant carbon fiber). DE 102015010001 A1, 2 February 2017. [Google Scholar]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.; Altstädt, V.; et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends. Polym. Int. 2007, 56, 1404–1414. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Moy, P.Y.; Gregor, A.I.; Pirelli, R.L.; Bright, D.A. Oligomeric Bis-Phosphate Flame Retardants and Compositions Containing The Same. EP2089402 B1, 19 August 2009. [Google Scholar]
- Hamciuc, C.; Vlad-Bubulac, T.; Serbezeanu, D.; Carja, I.-D.; Hamciuc, E.; Lisa, G.; Pérez, V.F. Environmentally friendly fire-resistant epoxy resins based on a new oligophosphonate with high flame retardant efficiency. RSC Adv. 2016, 6, 22764–22776. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Xing, W.; Lu, H. Synthesis and characterization of a DOPO-substitued organophosphorus oligomer and its application in flame retardant epoxy resins. Prog. Org. Coat. 2011, 71, 72–82. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stöß, K.; Ciesielski, M.; Diederichs, J.; Döring, M.; Krämer, J.; Altstädt, V. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Eur. Polym. J. 2011, 47, 1081–1089. [Google Scholar] [CrossRef]
- Müller, P.; Bykov, Y.; Döring, M. New star-shaped phosphorus-containing flame retardants based on acrylates for epoxy resins. Polym. Adv. Technol. 2013, 24, 834–840. [Google Scholar] [CrossRef]
- Minegishi, S.; Komatsu, S.; Kameyama, A.; Nishikubo, T. Novel synthesis of polyphosphonates by the polyaddition of bis(epoxide) with diaryl phosphonates. J. Polym. Sci., Polym. Chem. Ed. 1999, 37, 959–965. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Zhao, Z.; Yan, H. A novel hyperbranched poly(phosphorodiamidate) with high expansion degree and carbonization efficiency used for improving flame retardancy of APP/PP composites. Polym. Degrad. Stab. 2017, 142, 29–41. [Google Scholar] [CrossRef]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Thermal degradation of a reactive flame retardant based on cyclotriphosphazene and its blend with DGEBA epoxy resin. Polym. Degrad. Stab. 2009, 94, 2101–2106. [Google Scholar] [CrossRef]
- Pagliuca, A. Flame Retardant Material and a Cable Having a Cable Sheath Composed of the Same. US 2011/0269888 A1, 3 November 2011. [Google Scholar]
- Sun, T.; Zhuo, Q.; Chen, Y.; Wu, Z. Synthesis of boehmite and its effect on flame retardancy of epoxy resin. High Perform. Polym. 2014, 27, 100–104. [Google Scholar] [CrossRef]
- Reuter, J.; Greiner, L.; Schönberger, F.; Döring, M. Synergistic flame retardant interplay of phosphorus containing flame retardants with aluminum trihydrate depending on the specific surface area in unsaturated polyester resin. J. Appl. Polym. Sci. 2019, 136, 47270. [Google Scholar] [CrossRef]
- Hexcel Inc. Product data sheet RTM6. Available online: www.hexcel.com (accessed on 24 December 2018).
- Schuster, T.J.; Eibl, S.; Gudladt, H.-J. Influence of carbon nanotubes on thermal response and reaction to fire properties of carbon fibre-reinforced plastic material. J. Compos. Mater. 2017, 52, 567–579. [Google Scholar] [CrossRef]
- Prüfungen zur Beurteilung der Brandgefahr - Teil 11-10: Prüfflammen - Prüfverfahren mit einer 50-W-Prüfflamme horizontal und vertikal (IEC 60695-11-10:2013), 2015; DIN EN 60695-11-10.
- Babrauskas, V. Development of the cone calorimeter-A bench-scale heat release rate apparatus based on oxygen consumption. Fire. Mater. 1984, 8, 81–95. [Google Scholar] [CrossRef]
- Reaction-to-fire Tests-Heat release, Smoke Production and Mass Loss Rate; ISO: Geneva, Switzerland, 2002; ISO 5660-1,2.
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Aerospace series-carbon fiber reinforced plastics-unidirectional laminates; determination of apparent interlaminar shear strength, 1997; DIN EN 2563:1997-03.
- Weil, E.D.; Zhu, W.; Patel, N.; Mukhopadhyay, S.M. A systems approach to flame retardancy and comments on modes of action. Polym. Degrad. Stab. 1996, 54, 125–136. [Google Scholar] [CrossRef]
- Petreus, O.; Popescu, F.N.; Cascaval, C.N. Action of some organophosphonic compounds on a diglycidyl ether-bisphenol-A epoxy resin. Die Angewandte Makromolekulare Chemie 1994, 222, 13–23. [Google Scholar] [CrossRef]
- Rose, N.; Le Bras, M.; Delobel, R.; Costes, B.; Henry, Y. Thermal oxidative degradation of an epoxy resin. Polym. Degrad. Stab. 1993, 42, 307–316. [Google Scholar] [CrossRef]
- Rose, N.; Le Bras, M.; Bourbigot, S.; Delobel, R. Thermal oxidative degradation of epoxy resins: evaluation of their heat resistance using invariant kinetic parameters. Polym. Degrad. Stab. 1994, 45, 387–397. [Google Scholar] [CrossRef]
- Nabaltec AG. Actilox Boehmite Product Data Sheet. Available online: https://nabaltec.de/fileadmin/user_upload/03_produkte/03-3_boehmit/nabaltec_tds_actilox-as1.pdf (accessed on 5 February 2019).
- Sasol GmbH. DISPERAL/DISPAL Product Data Sheet. Available online: www.sasoltechdata.com/tds/DISPERAL_DISPAL.pdf (accessed on 5 February 2019).
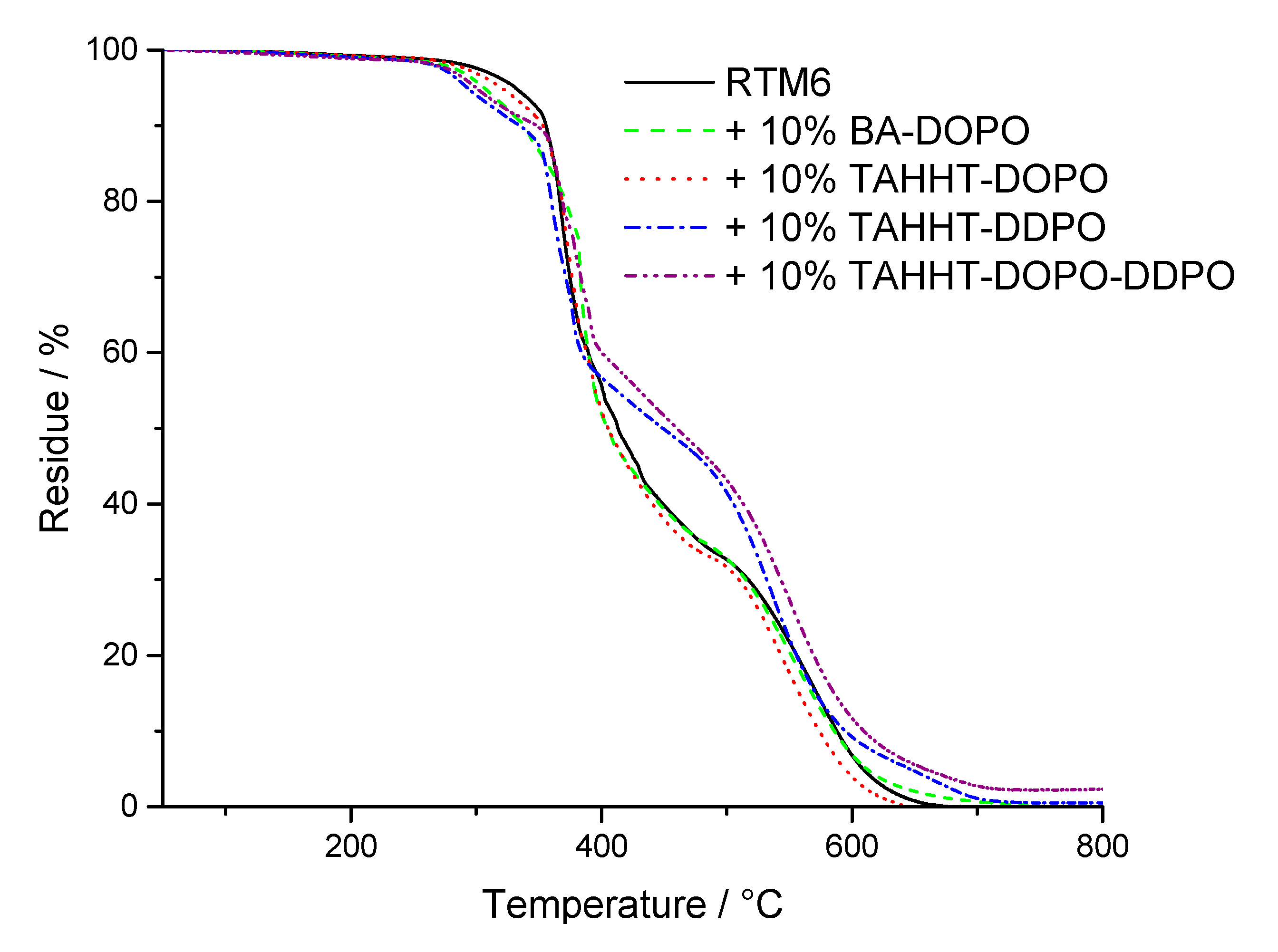
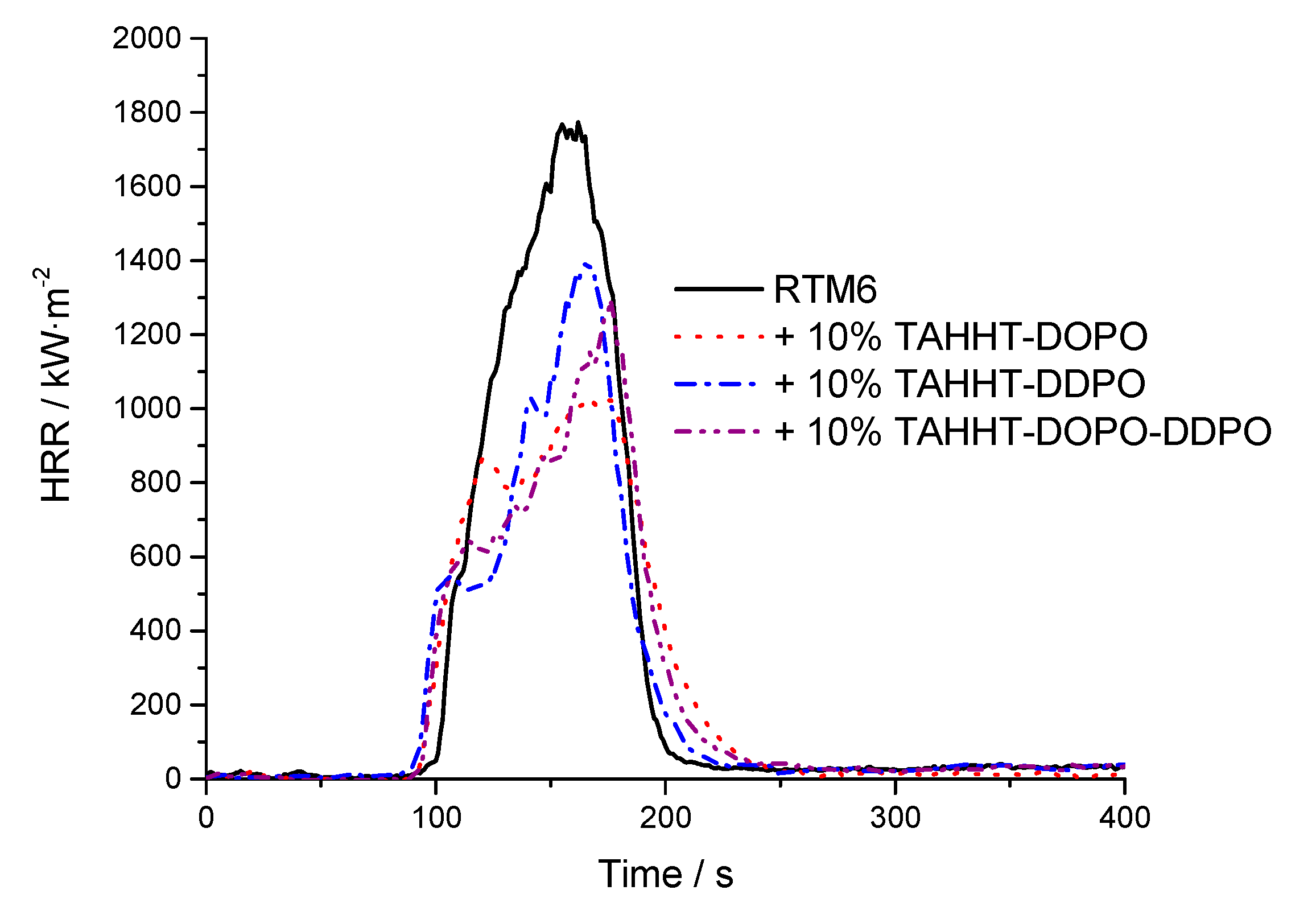
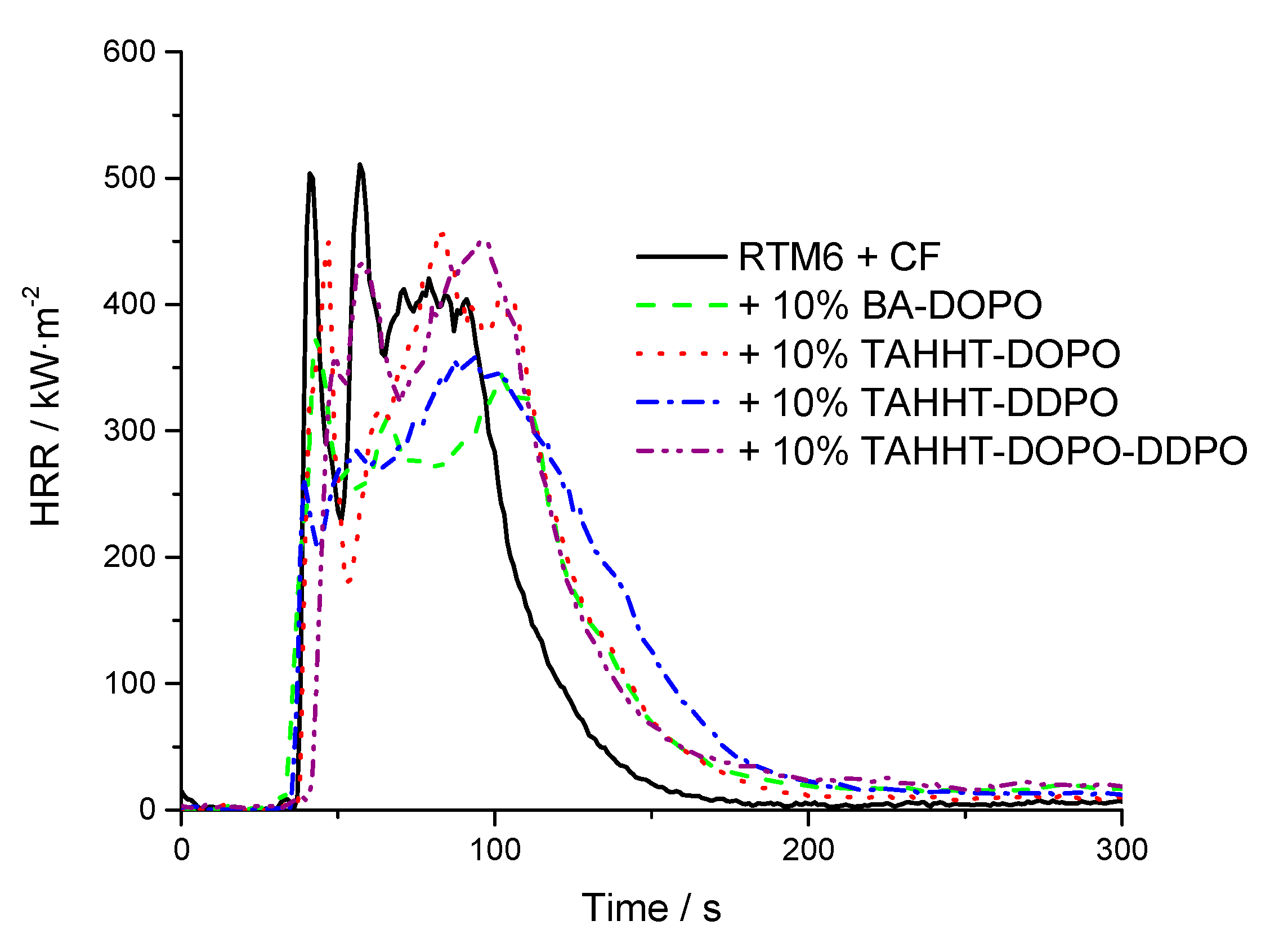
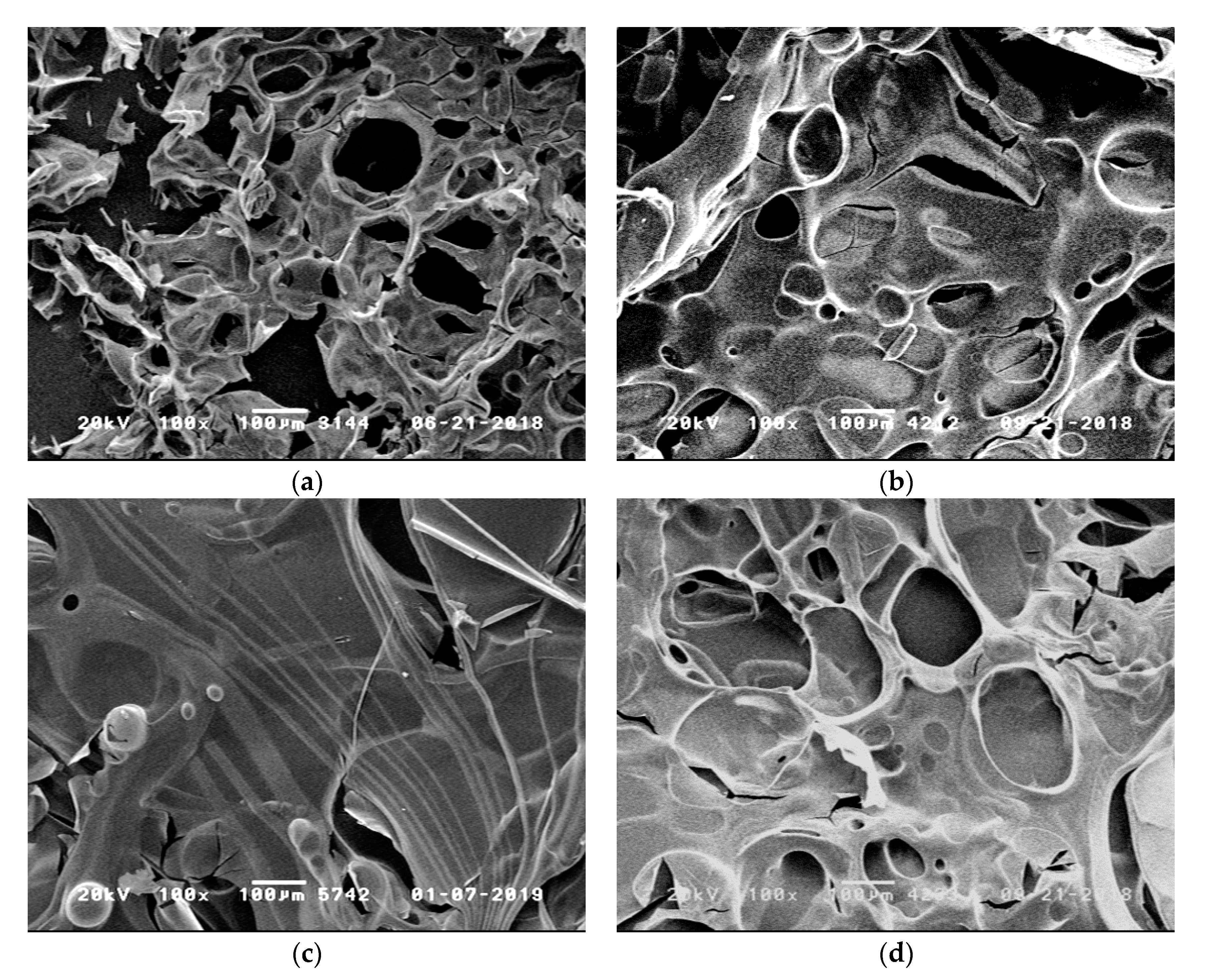

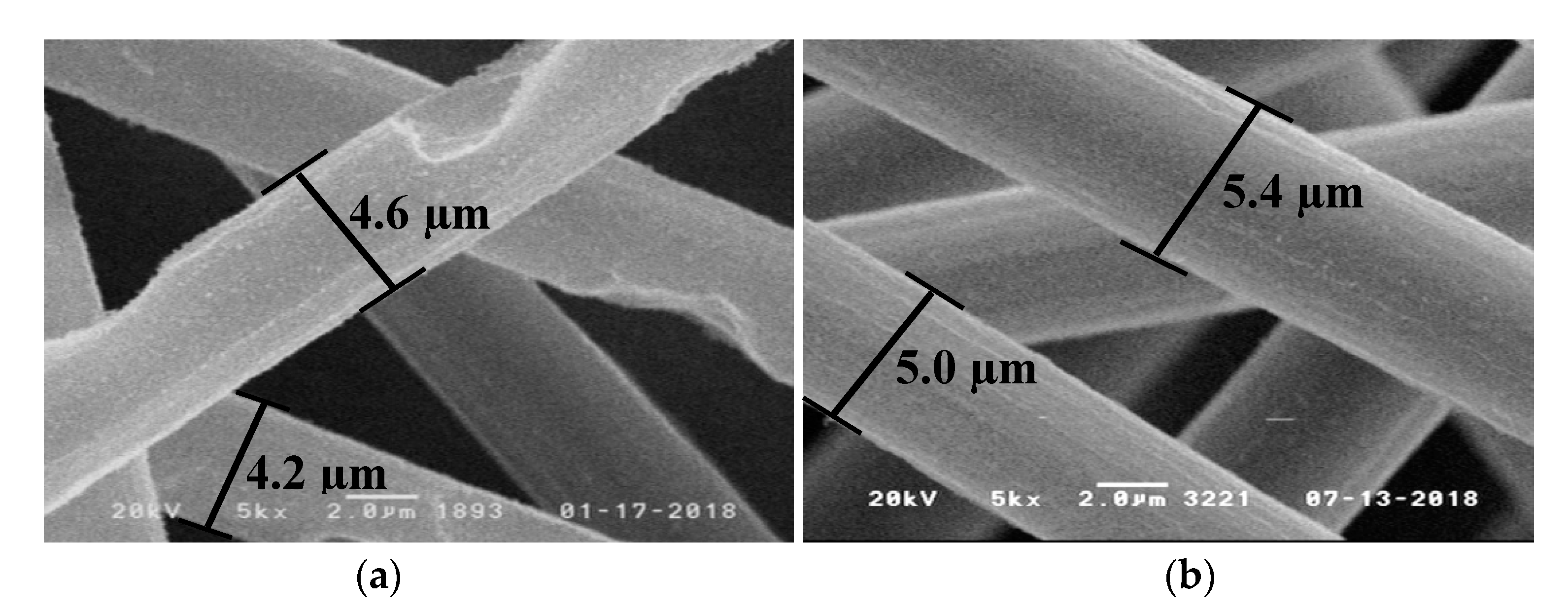
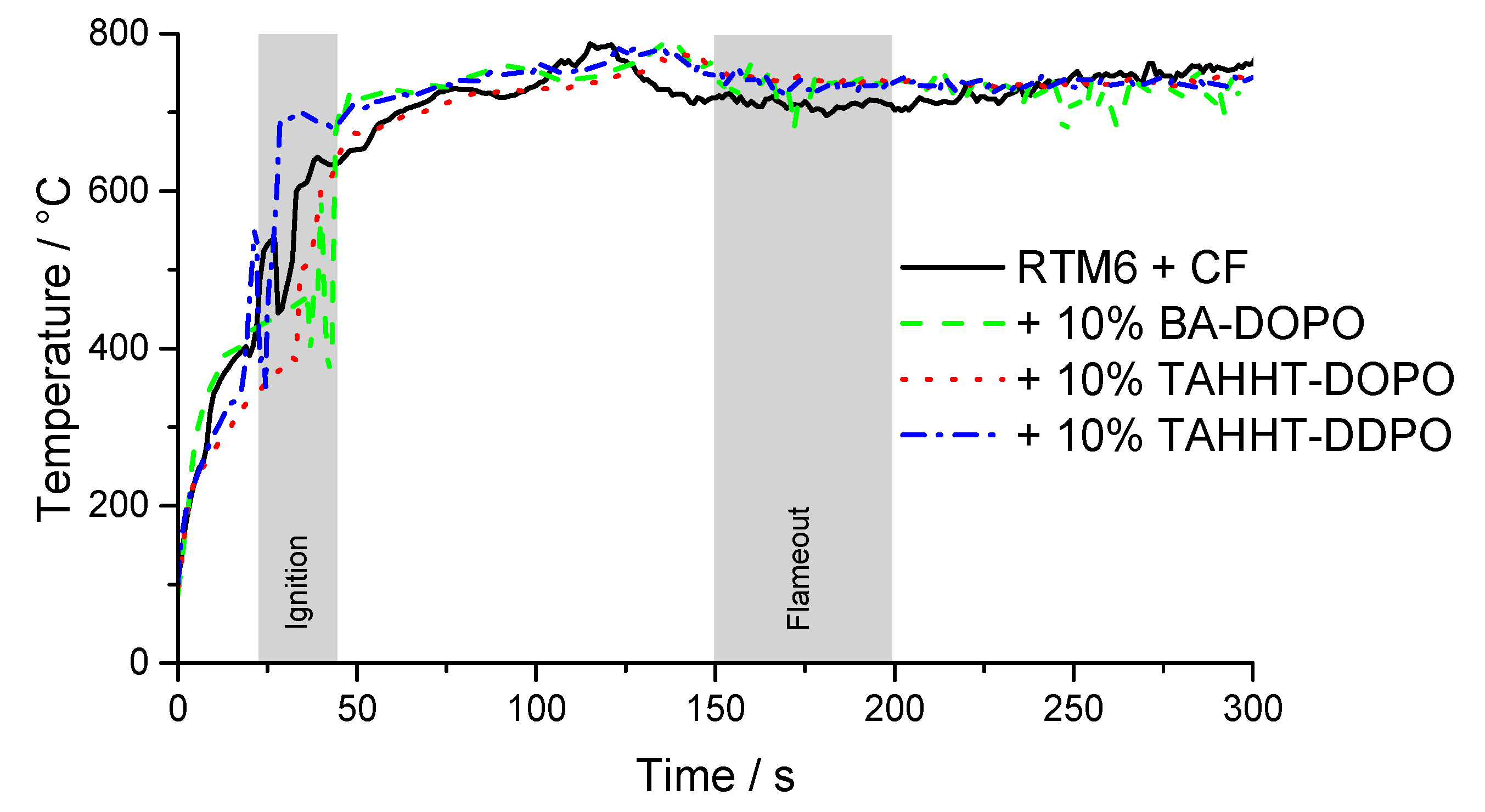

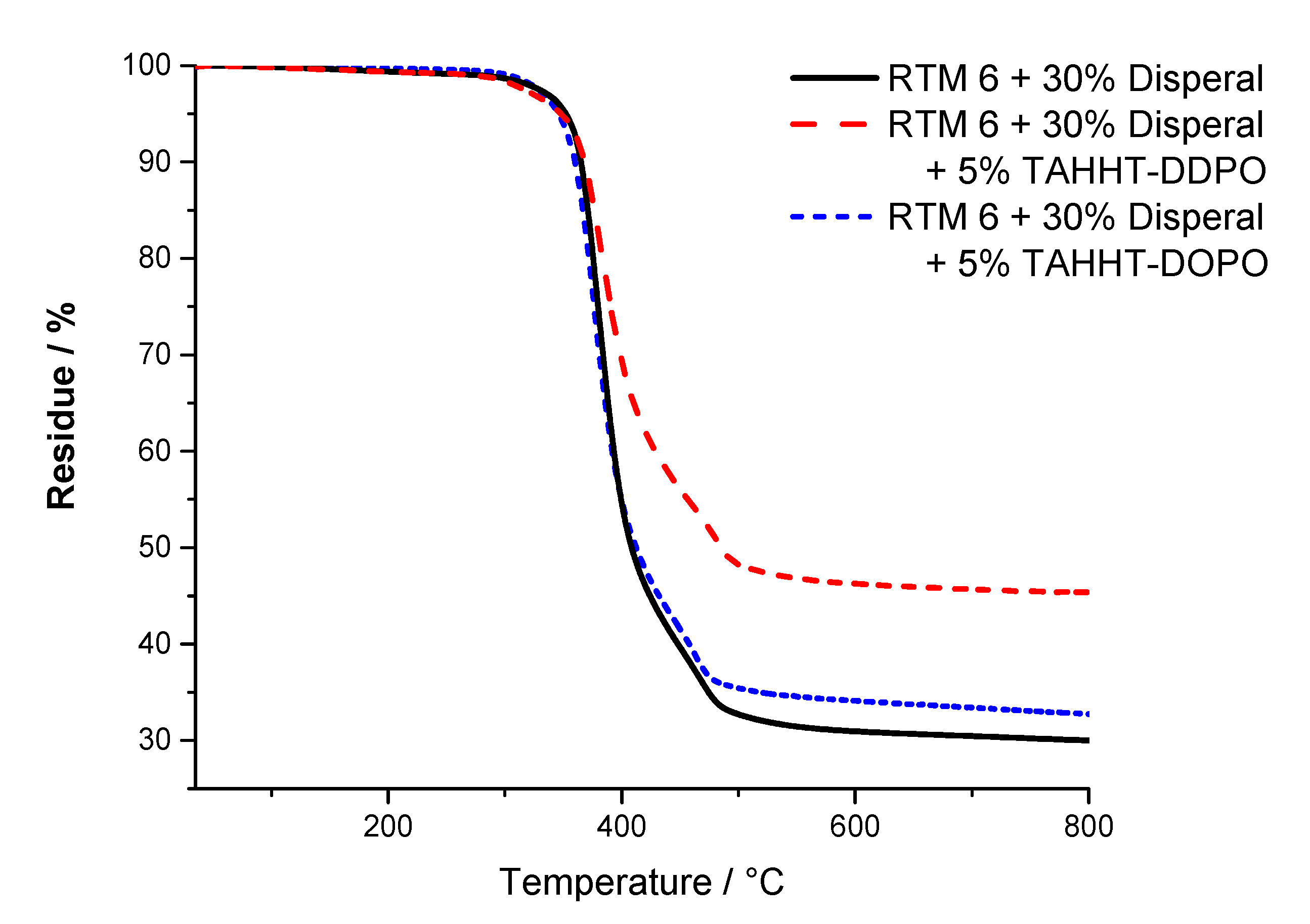
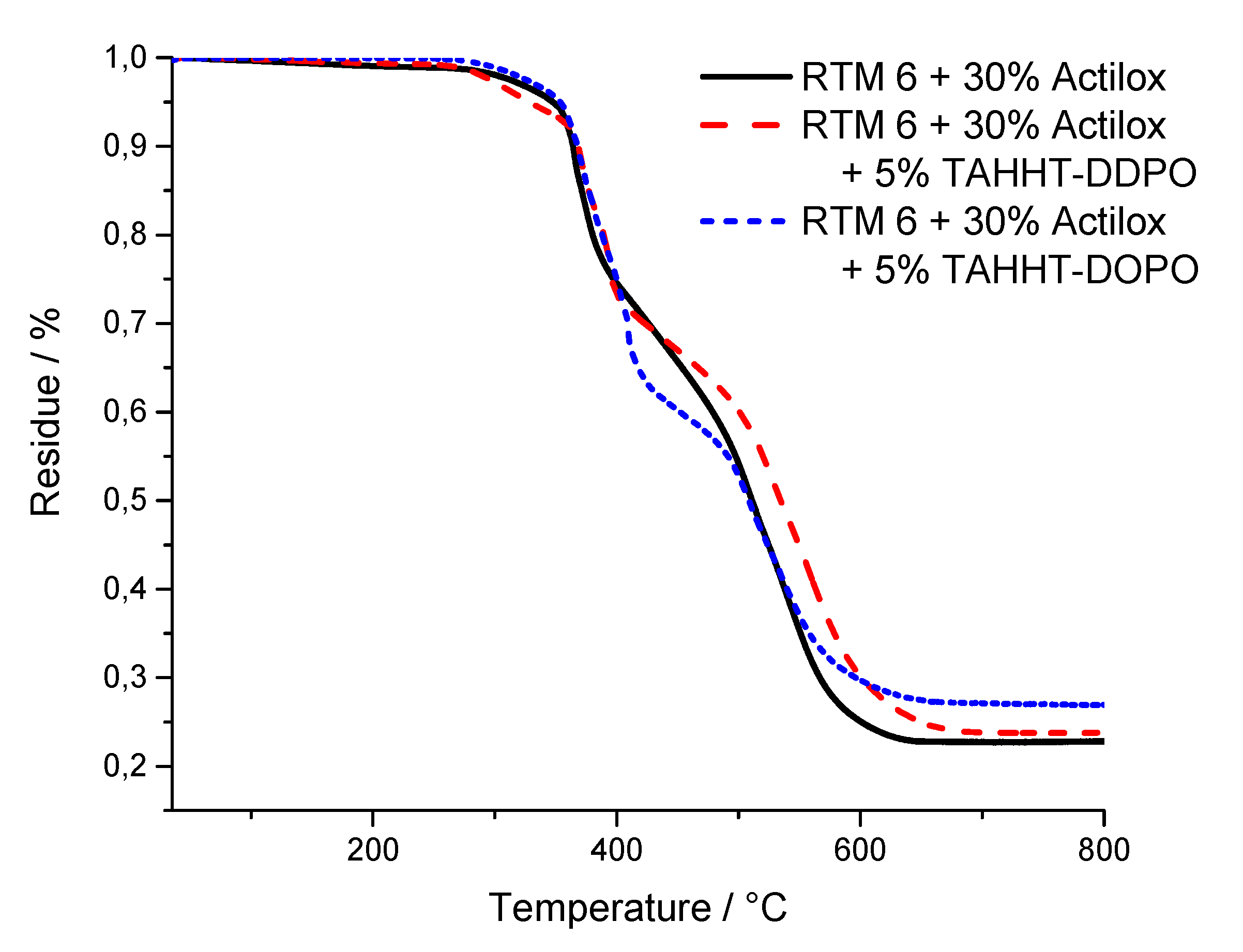

| Flame Retardant in RTM6-Matrix | Reinforcement | Thickness/mm |
|---|---|---|
| - | - | 4 |
| 10% TAHHT-DOPO | - | 4 |
| 10% TAHHT-DDPO | - | 4 |
| 10% TAHHT-DOPO-DDPO | - | 4 |
| - | CF | 2 |
| 10% BA-DOPO | CF | 2 |
| 10% TAHHT-DOPO | CF | 2 |
| 10% TAHHT-DDPO | CF | 2 |
| 10% TAHHT-DOPO-DDPO | CF | 2 |
| 35% Actilox B30 | - | 4 |
| 30% Actilox B30 + 5% TAHHT-DDPO | - | 4 |
| 30% Actilox B30 + 5% TAHHT-DOPO | - | 4 |
| 35% Disperal 40 | - | 4 |
| 30% Disperal 40 + 5% TAHHT-DDPO | - | 4 |
| 30% Disperal 40 + 5% TAHHT-DOPO | - | 4 |
| Sample | Tmax/°C | T1%/°C | T5%/°C | Residue/% |
|---|---|---|---|---|
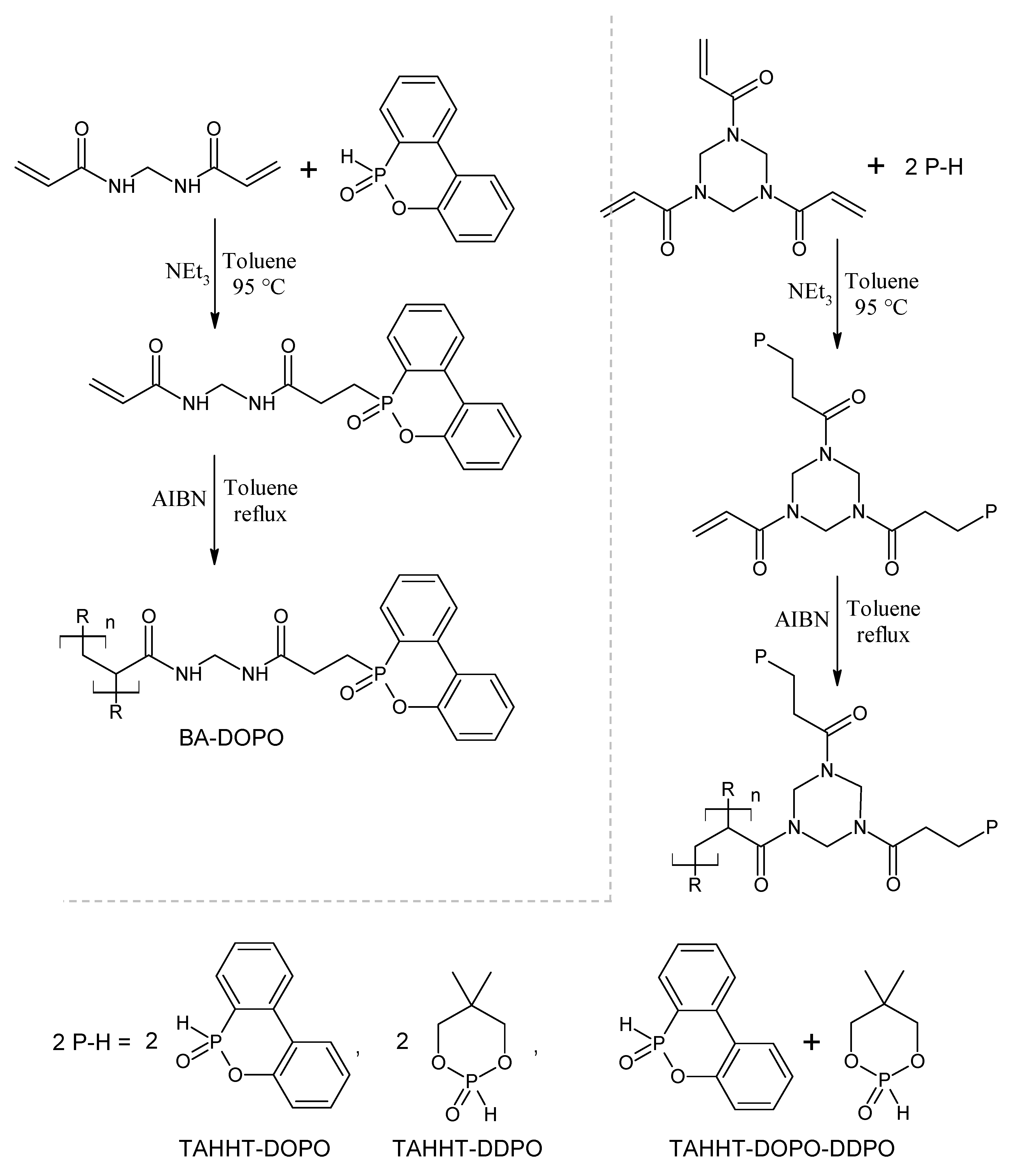
| BA-DOPO, N2 | 323 | 220 | 281 | 15 (800 °C) |
| TAHHT-DOPO | 337 | 231 | 307 | 10 (800 °C) |
| TAHHT-DDPO | 287 | 181 | 255 | 19 (800 °C) |
| TAHHT-DOPO-DDPO | 315 | 220 | 268 | 9 (800 °C) |
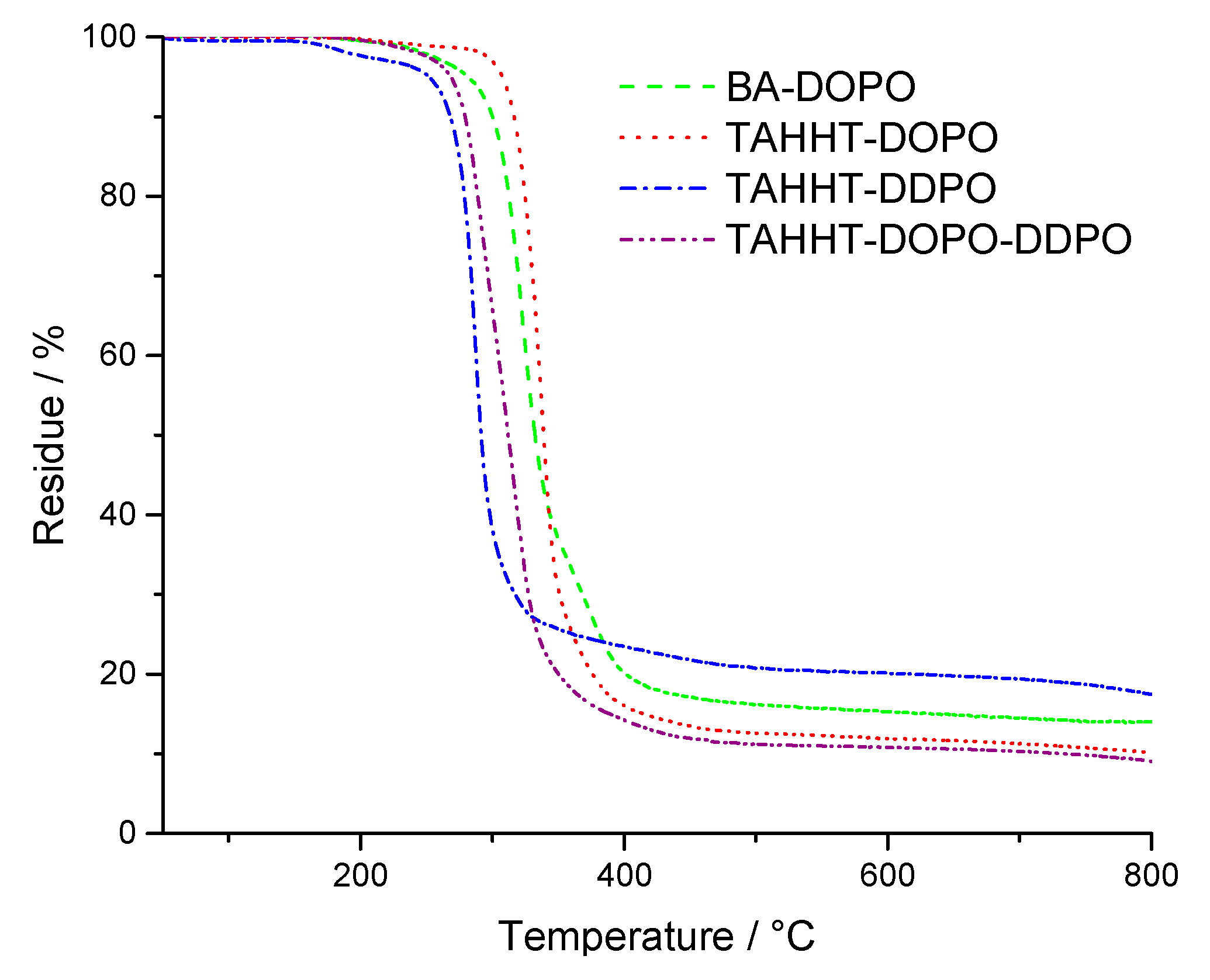
| BA-DOPO, air | I 335 | II 549 | III 717 | 178 | 290 | 25 (453 °C) | 7 (800 °C) |
| TAHHT-DOPO | I 237 | II 300 | 237 | 300 | 25 (544 °C) | 6 (800 °C) |
| TAHHT-DDPO | I 280 | II 526 | III 699 | 209 | 250 | 33 (419 °C) | 14 (800 °C) |
| TAHHT-DOPO-DDPO | I 317 | II 472 | 213 | 268 | 22 (420 °C) | 8 (800 °C) |
| Sample | Tg(Max(tan(δ))) 1/°C | Moisture uptake/% | Tg,wet(Max(tan(δ))) 1/°C |
|---|---|---|---|
| RTM6 | 215 | 1.02 | 218 |
| + 10% BA-DOPO | 208 | 1.02 | 205 |
| + 10% TAHHT-DOPO | 205 | 1.02 | 202 |
| + 10% TAHHT-DDPO | 202 | 1.03 | 198 |
| + 10% TAHHT-DOPO-DDPO | 204 | 1.02 | 197 |
| Sample | ILS (N·mm−2) | Relative ILSS (%) |
|---|---|---|
| RTM6 + CF | 67.8 ± 3.2 | 100 ± 5 |
| + 10% BA-DOPO | 65.4 ± 2.0 | 97 ± 3 |
| + 10% TAHHT-DOPO | 67.4 ± 1.8 | 99 ± 3 |
| + 10% TAHHT-DDPO | 71.0 ± 2.3 | 105 ± 4 |
| Sample | Tmax/°C | T1%/°C | T5%/°C | Residue/% |
|---|---|---|---|---|
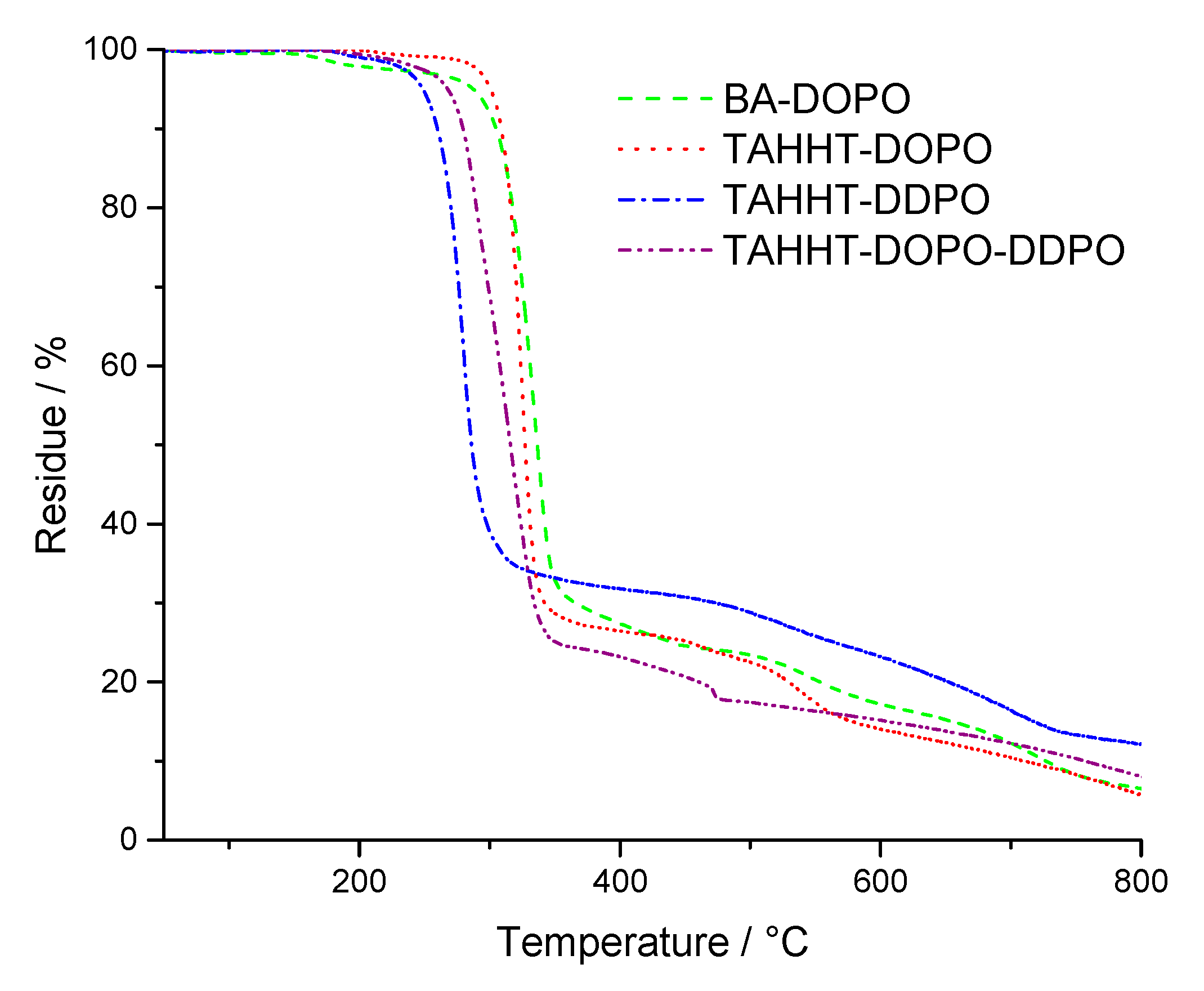
| RTM6, N2 | 379 | 265 | 341 | 10 (800 °C) |
| + 10% BA-DOPO | 373 | 211 | 314 | 15 (800 °C) |
| + 10% TAHHT-DOPO | 389 | 284 | 335 | 14 (800 °C) |
| + 10% TAHHT-DDPO | 362 | 170 | 293 | 19 (800 °C) |
| + 10% TAHHT-DOPO-DDPO | 371 | 216 | 301 | 17 (800 °C) |
| RTM6, air | I 369 | II 572 | 229 | 331 | 33 (492 °C) | 0 (800 °C) |
| + 10% BA-DOPO | I 384 | II 555 | 198 | 305 | 34 (488 °C) | 0 (800 °C) |
| + 10% TAHHT-DOPO | I 373 | II 546 | 212 | 318 | 34 (479 °C) | 0 (800 °C) |
| + 10% TAHHT-DDPO | I 360 | II 376 | III 537 | 198 | 293 | 52 (433 °C) | 1 (800 °C) |
| + 10% TAHHT-DOPO-DDPO | I 301 | II 368 | III 550 | 190 | 301 | 51 (454 °C) | 3 (800 °C) |
| Sample | tti/s | pHRR/kW·m−2 | THR·X−1/MJ·m−2 | MARHE/kW·m−2 | TSR·X−1/m2·m−2 | THR·ML−11/kW·m−2·g−1 | Residue/% | X | UL94-V * |
|---|---|---|---|---|---|---|---|---|---|
| RTM6 | 94 ± 1 | 1715 ± 58 | 104 ± 1 | 550 ± 6 | 5601 ± 31 | 2.25 | 4 ± 2 | 1 | NR |
| + 10% BA-DOPO | - | - | - | - | - | - | - | 1 | V1 |
| + 10% TAHHT-DOPO | 98 ± 12 | 1092 ± 54 | 89 ± 2 | 401 ± 22 | 6458 ± 51 | 1.85 | 2 ± 0 | 1 | V0 |
| + 10% TAHHT-DDPO | 81 ± 2 | 1419 ±8 | 85 ± 2 | 421 ± 3 | 3853 ± 17 | 2.19 | 3 ± 0 | 1 | V0 |
| + 10% TAHHT-DOPO-DDPO | 85 ± 1 | 1278 ± 30 | 85 ± 3 | 416 ± 8 | 5160 ± 123 | 1.97 | 2 ± 0 | 1 | V0 |
| RTM6 + CF | 31 ± 2 | 492 ± 19 | 72 ± 1 | 242 ± 5 | 3763 ± 165 | 2.51 | 59 ± 1 | 0.43 | V1 |
| + 10% BA-DOPO | 32 ± 5 | 384 ± 53 | 63 ± 3 | 203 ±16 | 5815 ± 600 | 2.05 | 56 ± 1 | 0.49 | V0 |
| + 10% TAHHT-DOPO | 34 ± 1 | 476 ± 75 | 70 ± 3 | 234 ± 115 | 5070 ± 283 | 2.17 | 54 ± 1 | 0.52 | V0 |
| + 10% TAHHT-DDPO | 29 ± 4 | 388 ± 52 | 67 ± 4 | 218 ± 22 | 4277 ± 369 | 2.37 | 59 ± 1 | 0.50 | V0 |
| + 10% TAHHT-DOPO-DDPO | 37 ± 3 | 456 ± 58 | 69 ± 2 | 228 ± 13 | 5269 ± 136 | 2.21 | 55 ± 1 | 0.52 | V0 |
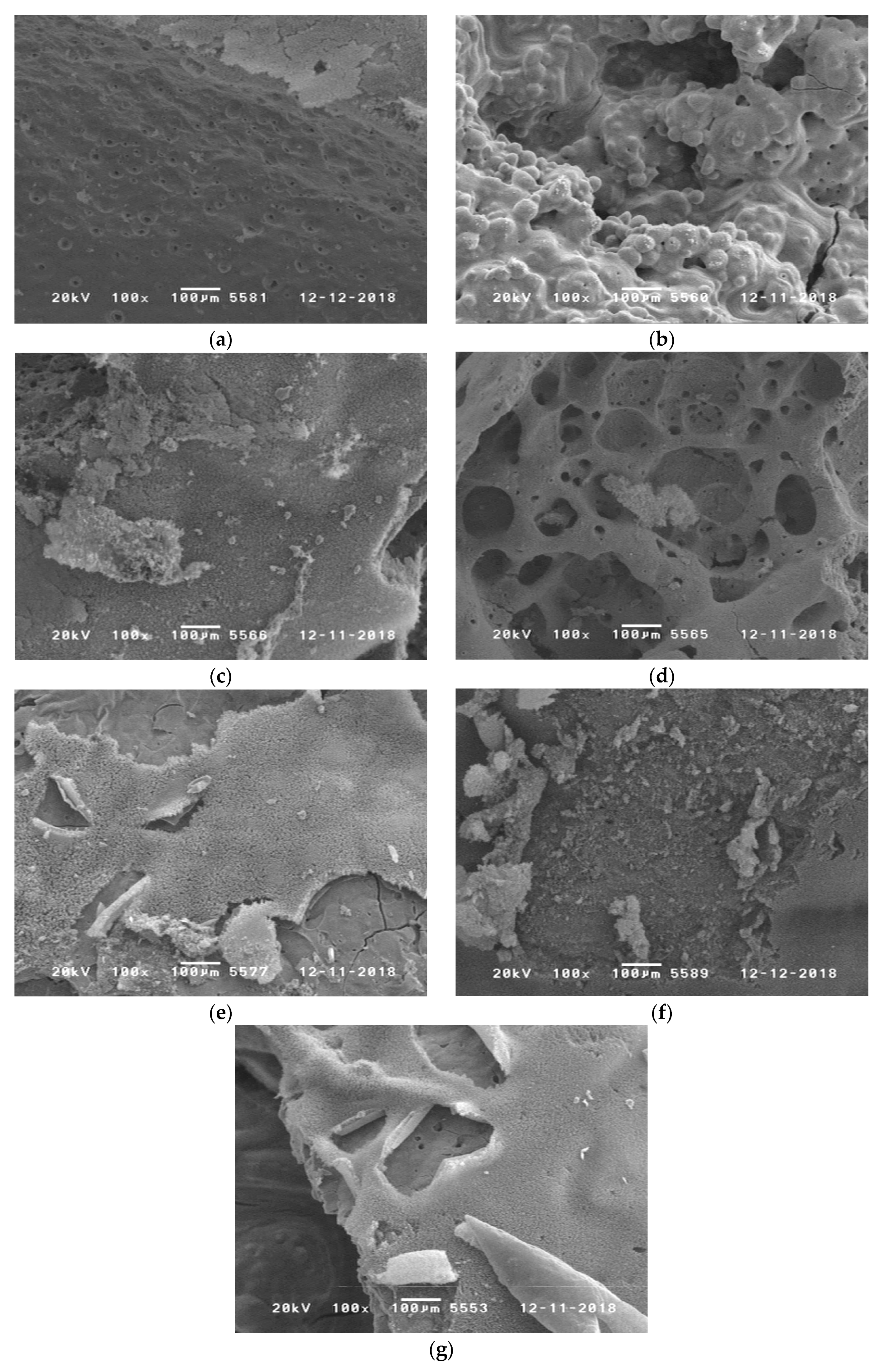
| Sample | Mean Diameter (µm) | Smallest Diameter (µm) |
|---|---|---|
| RTM6 + CF | 3.3 ± 0.7 | 2.1 |
| + 10% BA-DOPO | 5.2 ± 0.3 | 4.7 |
| + 10% TAHHT-DOPO | 5.3 ± 0.2 | 4.9 |
| + 10% TAHHT-DDPO | 6.3 ± 0.4 | 5.3 |
| + 10% TAHHT-DOPO-DDPO | 5.9 ± 0.3 | 5.3 |
| Sample | C on surface/% | N on surface/% | O on surface/% | P on surface/% |
|---|---|---|---|---|
| RTM6 + CF 1200 s at 60 kW·m−2 | 94.3 | 4.2 | 1.4 | not detected |
| + 10% TAHHT-DOPO 1200 s at 60 kW·m−2 | 91.9 | 4.6 | 2.8 | 0.7 |
| + 10% TAHHT-DDPO 1200 s at 60 kW·m−2 | 84.0 | 4.3 | 8.8 | 2.4 |
| + 10% TAHHT-DOPO-DDPO 1200 s at 60 kW·m−2 | 86.3 | 4.9 | 6.8 | 1.4 |
| Sample | Tmax/°C | T1%/°C | T5%/°C | Residue/% |
|---|---|---|---|---|
| RTM6, N2 | 379 | 265 | 341 | 10 (800 °C) |
| + 30% Actilox | 376 | 301 | 343 | 29 (800 °C) |
| + 30% Actilox + 5% TAHHT-DDPO | 373 | 274 | 333 | 37 (800 °C) |
| + 30% Actilox + 5% TAHHT-DOPO | 379 | 304 | 342 | 34 (800 °C) |
| + 30% Disperal | 374 | 285 | 353 | 30 (800 °C) |
| + 30% Disperal + 5% TAHHT-DDPO | 380 | 277 | 349 | 46 (800 °C) |
| + 30% Disperal + 5% TAHHT-DOPO | 376 | 305 | 346 | 33 (800 °C) |
| RTM6, air | I 369 | II 572 | 229 | 331 | 33 (492 °C) | 0 (800 °C) |
| + 30% Actilox | I 366 | II 538 | 215 | 348 | 64 (458 °C) | 23 (800 °C) |
| + 30% Actilox + 5% TAHHT-DDPO | I 371 | II 394 | III 558 | 267 | 329 | 66 (453 °C) | 24 (800 °C) |
| + 30% Actilox + 5% TAHHT-DOPO | I 371 | II 409 | III 534 | 299 | 354 | 60 (456 °C) | 27 (800 °C) |
| + 30% Disperal | I 386 | II 543 | 267 | 350 | 60 (439 °C) | 25 (800 °C) |
| + 30% Disperal + 5% TAHHT-DDPO | I 383 | II 581 | 267 | 343 | 61 (474 °C) | 26 (800 °C) |
| + 30% Disperal 40 + 5% TAHHT-DOPO | I 376 | II 404 | III 530 | 295 | 353 | 61 (445 °C) | 25 (800 °C) |
| Sample | tti/s | pHRR/kW·m−2 | THR/MJ·m−2 | MARHE/kW·m−2 | TSR/m2·m−2 | THR·ML−1/kW·m−2·g−1 | Residue/% | UL94-V * |
|---|---|---|---|---|---|---|---|---|
| RTM 6 | 94 ± 2 | 1715 ± 58 | 113 ± 2 | 550 ± 6 | 6305 ± 394 | 2.39 | 1.9 ± 1.4 | NR |
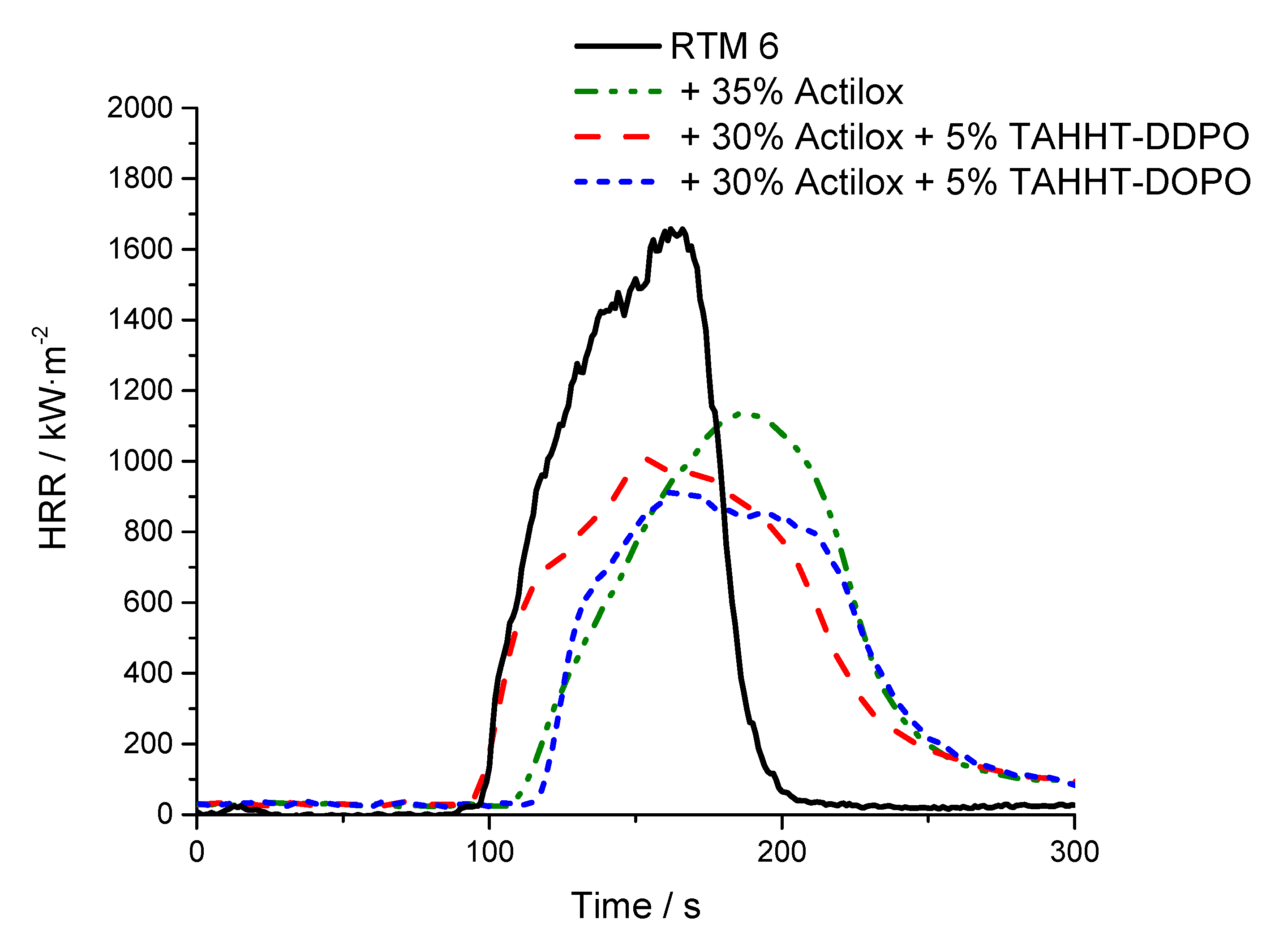
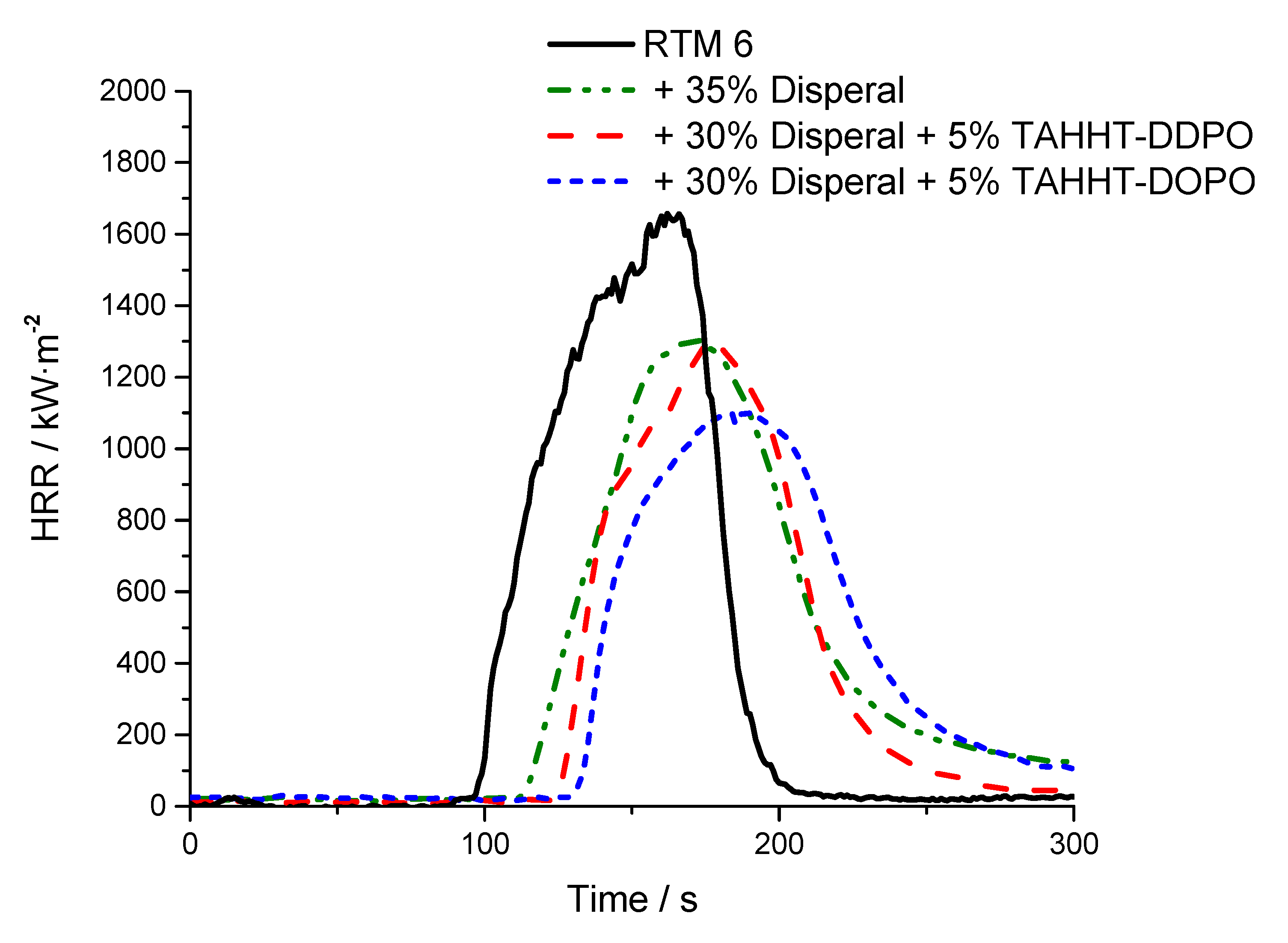
| + 35% Actilox B30 | 109 ± 12 | 1142 ± 14 | 107 ± 3 | 420 ± 13 | 5079 ± 94 | 2.38 | 5.7 ± 1.0 | NR |
| + 30% Actilox B30 + 5% TAHHT-DDPO | 96 ± 5 | 1017 ± 60 | 99 ± 2 | 410 ± 1 | 4868 ± 118 | 2.24 | 33.6 ± 0.5 | V0 |
| + 30% Actilox B30 + 5% TAHHT-DOPO | 110 ± 4 | 919 ± 20 | 91 ± 3 | 359 ± 11 | 5309 ± 148 | 1.99 | 32.5 ± 0.7 | NR |
| + 35% Disperal 40 | 95 ± 7 | 1330 ± 22 | 102 ± 3 | 454 ± 22 | 4239 ± 249 | 2.11 | 25.8 ± 6.4 | NR |
| + 30 % Disperal 40 + 5% TAHHT-DDPO | 108 ± 2 | 1289 ± 31 | 96 ± 3 | 423 ± 5 | 4511 ± 204 | 2.22 | 32.0 ± 0.7 | NR |
| + 30% Disperal 40 + 5% TAHHT-DOPO | 117 ± 4 | 1065 ± 5 | 89 ± 4 | 372 ± 20 | 5261 ± 16 | 1.94 | 32.6 ± 1.1 | NR |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiner, L.; Kukla, P.; Eibl, S.; Döring, M. Phosphorus Containing Polyacrylamides as Flame Retardants for Epoxy-Based Composites in Aviation. Polymers 2019, 11, 284. https://doi.org/10.3390/polym11020284
Greiner L, Kukla P, Eibl S, Döring M. Phosphorus Containing Polyacrylamides as Flame Retardants for Epoxy-Based Composites in Aviation. Polymers. 2019; 11(2):284. https://doi.org/10.3390/polym11020284
Chicago/Turabian StyleGreiner, Lara, Philipp Kukla, Sebastian Eibl, and Manfred Döring. 2019. "Phosphorus Containing Polyacrylamides as Flame Retardants for Epoxy-Based Composites in Aviation" Polymers 11, no. 2: 284. https://doi.org/10.3390/polym11020284
APA StyleGreiner, L., Kukla, P., Eibl, S., & Döring, M. (2019). Phosphorus Containing Polyacrylamides as Flame Retardants for Epoxy-Based Composites in Aviation. Polymers, 11(2), 284. https://doi.org/10.3390/polym11020284






