pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of P(AA-co-NVF) and P(AA-co-VAm) Microgels
2.3. Characterization of Microgels
2.4. Fluid-Gel Transition
2.5. In Vitro Drug Release from the Hydrogels
3. Results and Discussions
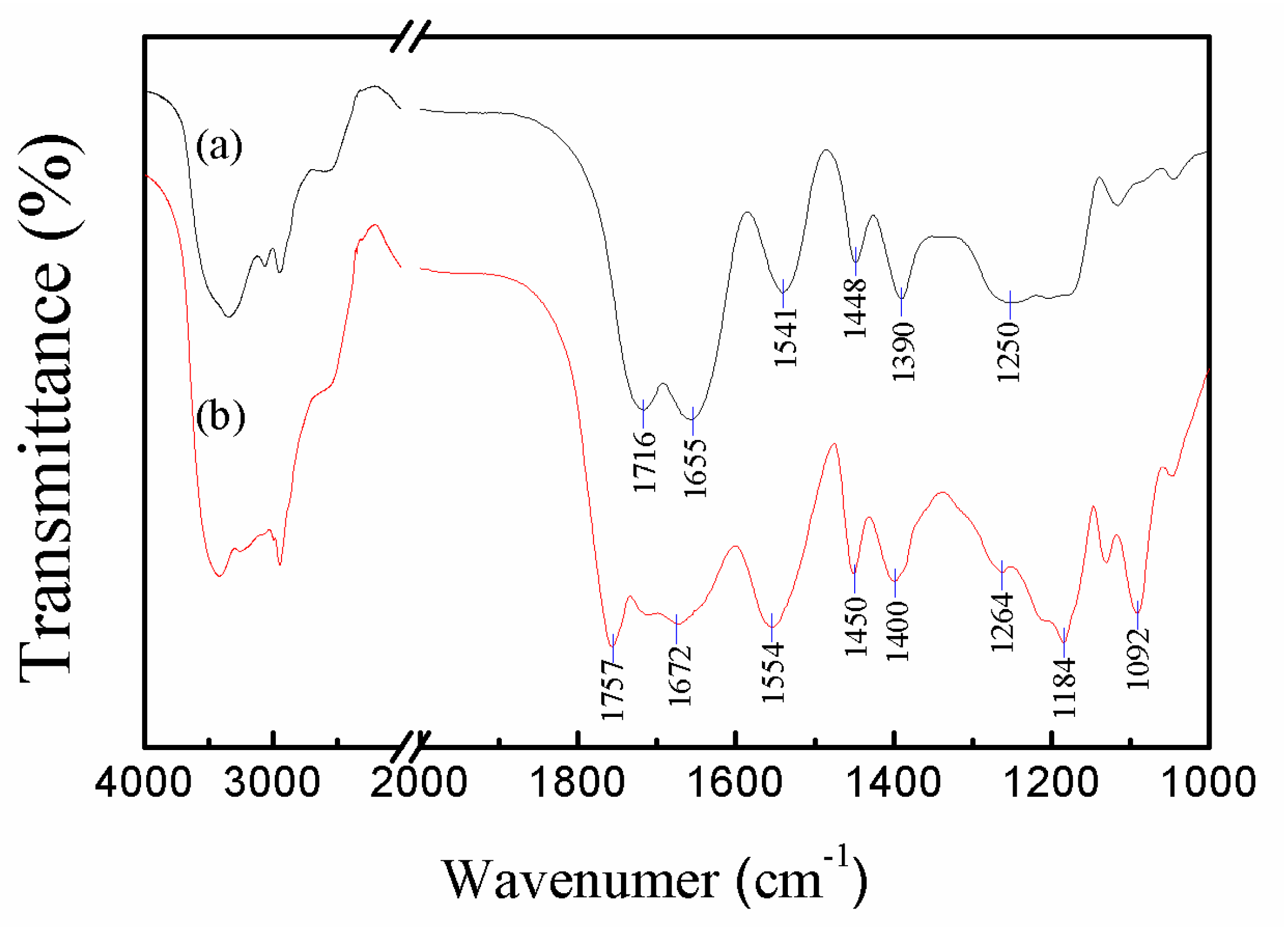
3.1. Preparation and Characterization of the Microgels
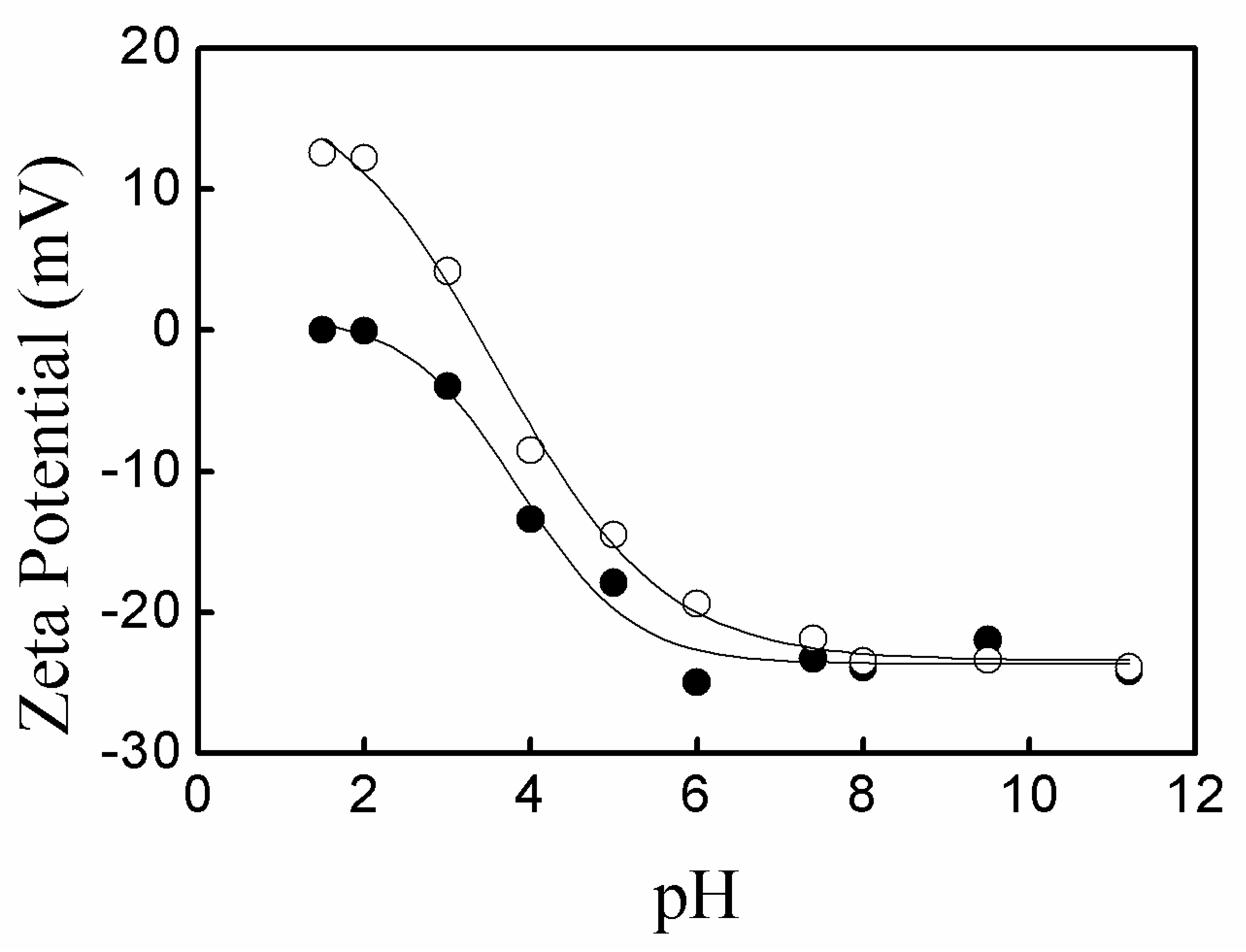
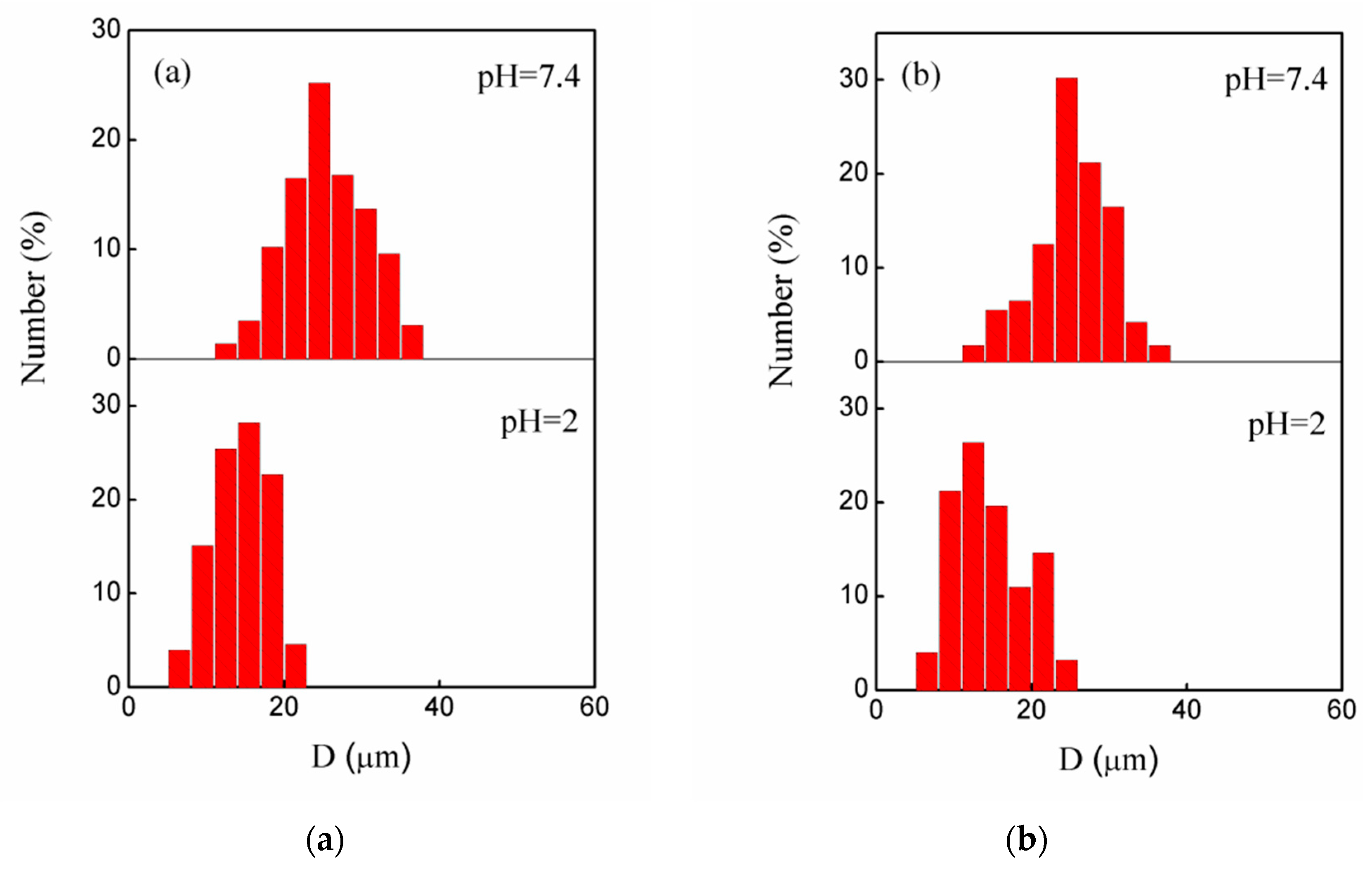
3.2. pH-Sensitivity of the Microgels
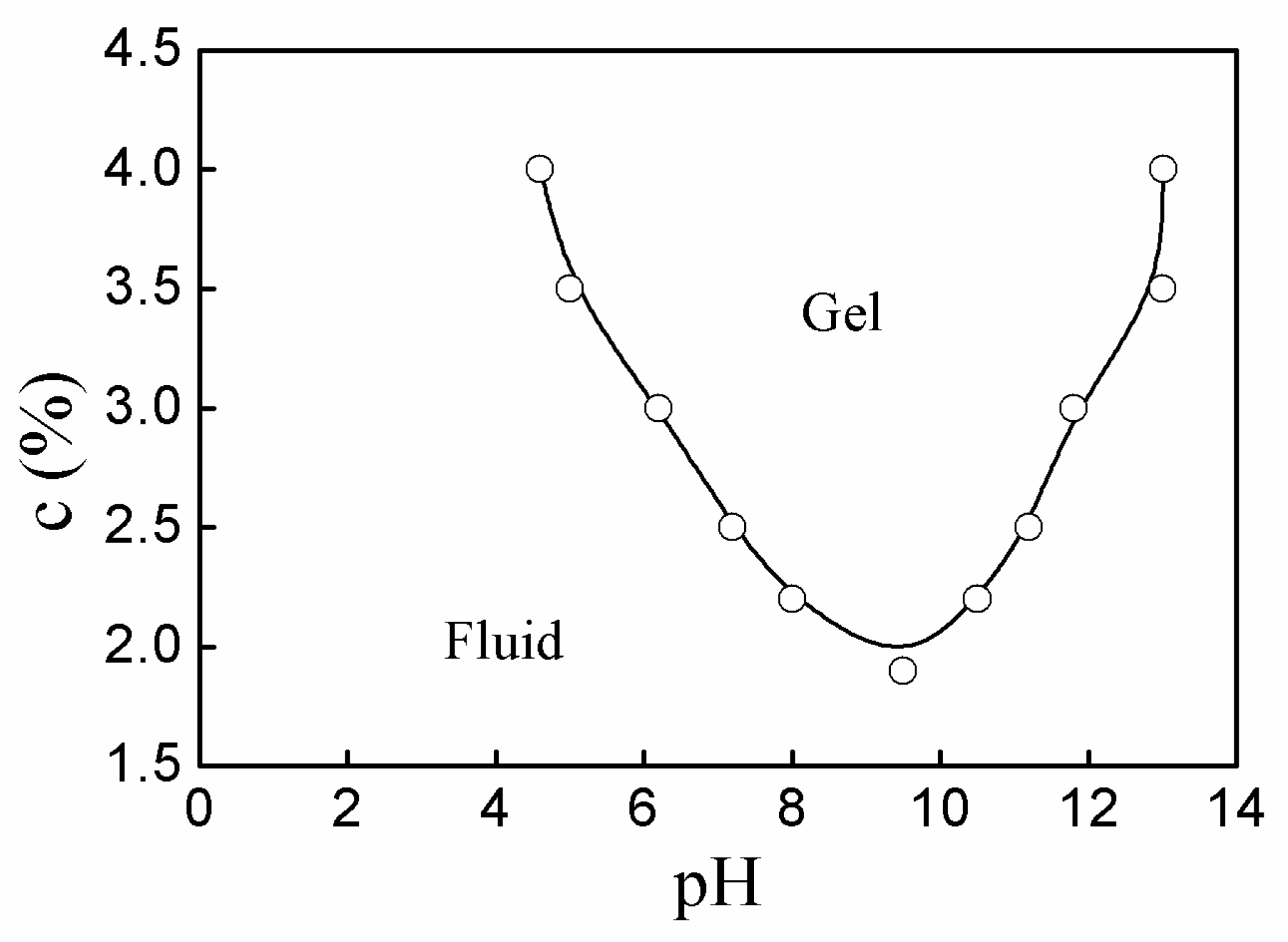
3.3. Fluid-Gel Transition of P(AA-co-VAm) Microgels
3.4. In Vitro Drug Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Lin, C.; Anseth, K. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Omar, Z.F.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Chen, X.; Wang, W.; Loh, X.J. Engineering bioresponsive hydrogels toward healthcare applications. Macromol. Chem. Phys. 2016, 217, 175–188. [Google Scholar] [CrossRef]
- Loh, X.J.; Karim, A.A.; Owh, C. Poly(glycerol sebacate) biomaterial: Synthesis and biomedical applications. J. Mater. Chem. B 2015, 3, 7641–7652. [Google Scholar] [CrossRef]
- Aucoin, H.; Wilson, A.N.; Wilson, A.M.; Ishihara, K.; Guiseppi-Elie, A. Release of potassium ion and calcium ion from phosphorylcholine group bearing hydrogels. Polymers 2013, 5, 1241–1257. [Google Scholar] [CrossRef]
- Toh, W.S.; Loh, X.J. Advances in hydrogel delivery systems for tissue regeneration. Mater. Sci. Eng. C 2014, 45, 690–697. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, D.Y.; Kwon, D.Y.; Kang, H.J.; Kim, J.H.; Min, B.H.; Kim, M.S. An injectable biodegradable temperature-responsive gel with an adjustable persistence window. Biomaterials 2012, 33, 2823–2834. [Google Scholar] [CrossRef]
- Hatefi, A.; Amsden, B. Biodegradable injectable in situ forming drug delivery systems. J. Controll. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Z.B.; Xue, M.Y.; Yang, J.; Tan, T.W. Detailed characterization of an injectable hyaluronic acid-polyaspartylhydrazide hydrogel for protein delivery. Carbohyd. Polym. 2011, 85, 717–725. [Google Scholar] [CrossRef]
- Tan, R.; She, Z.; Wang, M.; Fang, Z.; Liu, Y.; Feng, Q. Thermo-sensitive alginate-based injectable hydrogel for tissue engineering. Carbohyd. Polym. 2012, 87, 1515–1521. [Google Scholar] [CrossRef]
- Ji, D.Y.; Kuo, T.F.; Wu, H.D.; Yang, J.C.; Lee, S.Y. A novel injectable chitosan/polyglutamate polyelectrolyte complex hydrogel with hydroxyapatite for soft-tissue augmentation. Carbohyd. Polym. 2012, 89, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Chabrot, P.; Berrahmoune, S.; Coutu, J.; Soulez, G.; Lerouge, S. A new injectable radiopaque chitosan-based sclerosing embolizing hydrogel for endovascular therapies. Acta Biomater. 2012, 8, 2712–2721. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.P.; Sawhney, A.S.; Hubbell, J.A. Rapid photopolymerization of immunoprotective gels in contact with cells and tissue. J. Am. Chem. Soc. 1992, 114, 8311–8312. [Google Scholar] [CrossRef]
- Wang, G.; Cao, X.; Dong, H.; Zeng, L.; Yu, C.; Chen, X. A hyaluronic acid based injectable hydrogel formed via photo-crosslinking reaction and thermal-induced Diels-Alder reaction for cartilage tissue engineering. Polymers 2018, 10, 949. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- DeForest, C.A.; Polizzotti, B.D.; Anseth, K.S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 2009, 8, 659–664. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthesis and physicochemical characterization of endlinker poly(ethylene glycol)-co-peptide hydrogels formed by michael-type addition. Biomacromolecules 2003, 4, 713–722. [Google Scholar] [CrossRef]
- Holmes, R.; Yang, X.B.; Dunne, A.; Florea, L.; Wood, D.; Tronci, G. Thiol-ene photo-click collagen-PEG hydrogels: Impact of water-soluble photoinitiators on cell viability, gelation kinetics and rheological properties. Polymers 2017, 9, 226. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J. Control. Release 2000, 63, 155–163. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliver. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Hyun, H.; Park, S.H.; Kwon, D.Y.; Khang, G.; Lee, H.B.; Kim, M.S. Thermo-responsive injectable mpeg-polyester diblock copolymers for sustained drug release. Polymers 2014, 6, 2670–2683. [Google Scholar] [CrossRef]
- Chi, M.; Liu, C.; Shen, J.; Dong, Z.; Yang, Z.; Wang, L. Antibacterial Superabsorbent Polymers from Tara Gum Grafted Poly(Acrylic acid) Embedded Silver Particles. Polymers 2018, 10, 945. [Google Scholar] [CrossRef]
- Pelton, R. Polyvinylamine: A Tool for Engineering Interfaces. Langmuir 2014, 30, 15373–15382. [Google Scholar] [CrossRef] [PubMed]
- Nunamaker, E.A.; Purcell, E.K.; Kipke, D.R. In vivo stability and biocompatibility of implanted calcium alginate disks. J. Biomed. Mater. Res. A 2007, 83A, 1128–1137. [Google Scholar] [CrossRef]
- Wang, H.; Hansen, M.B.; Lowik, D.W.; van Hest, J.C.; Li, Y.; Jansen, J.A.; Leeuwenburgh, S.C. Oppositely charged gelatin nanospheres as building blocks for injectable and biodegradable gels. Adv. Mater. 2011, 23, H119–H124. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Nostrum, C.F.; De Smedt, S.C.; Hennink, W.E. Degradation behavior of dextran hydrogels composed of positively and negatively charged microspheres. Biomaterials 2006, 27, 4141–4148. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Detamore, M.S.; Berkland, C. Biodegradable colloidal gels as moldable tissue engineering scaffolds. Adv. Mater. 2008, 20, 236–239. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Lu, Q.; Detamore, M.S.; Berkland, C. Injectable PLGA based colloidal gels for zero-order dexamethasone release in cranial defects. Biomaterials 2010, 31, 4980–4986. [Google Scholar] [CrossRef]
- Pafiti, K.S.; Philippou, Z.; Loizou, E.; Porcar, L.; Patrickios, C.S. End-Linked Poly[2-(dimethylamino)ethyl Methacrylate]Poly(methacrylic acid) Polyampholyte Conetworks: Synthesis by Sequential RAFT Polymerization and Swelling and SANS Characterization. Macromolecules 2011, 44, 5352–5362. [Google Scholar] [CrossRef]
- Bradley, M.; Vincent, B.; Burnett, G. Uptake and release of surfactants from polyampholyte microgel particles. Colloid. Polym. Sci. 2009, 287, 345–350. [Google Scholar] [CrossRef]
- Shi, L.; Khondee, S.; Linz, T.H.; Berkland, C. Poly(N-vinylformamide) nanogels capable of pH-sensitive protein release. Macromolecules 2008, 41, 6546–6554. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, S.; Hrymak, A.N.; Pelton, R.H. The nature of crosslinking in N-vinylformamide free-radical polymerization. Macromol. Rapid Commun. 2001, 22, 212–214. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Sun, P. pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release. Polymers 2019, 11, 285. https://doi.org/10.3390/polym11020285
Chen Y, Sun P. pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release. Polymers. 2019; 11(2):285. https://doi.org/10.3390/polym11020285
Chicago/Turabian StyleChen, Yanmin, and Peijian Sun. 2019. "pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release" Polymers 11, no. 2: 285. https://doi.org/10.3390/polym11020285
APA StyleChen, Y., & Sun, P. (2019). pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release. Polymers, 11(2), 285. https://doi.org/10.3390/polym11020285




