Investigation of Piezoelectricity and Resistivity of Surface Modified Barium Titanate Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
2.2.1. Surface Modification of BTO with Ethanol
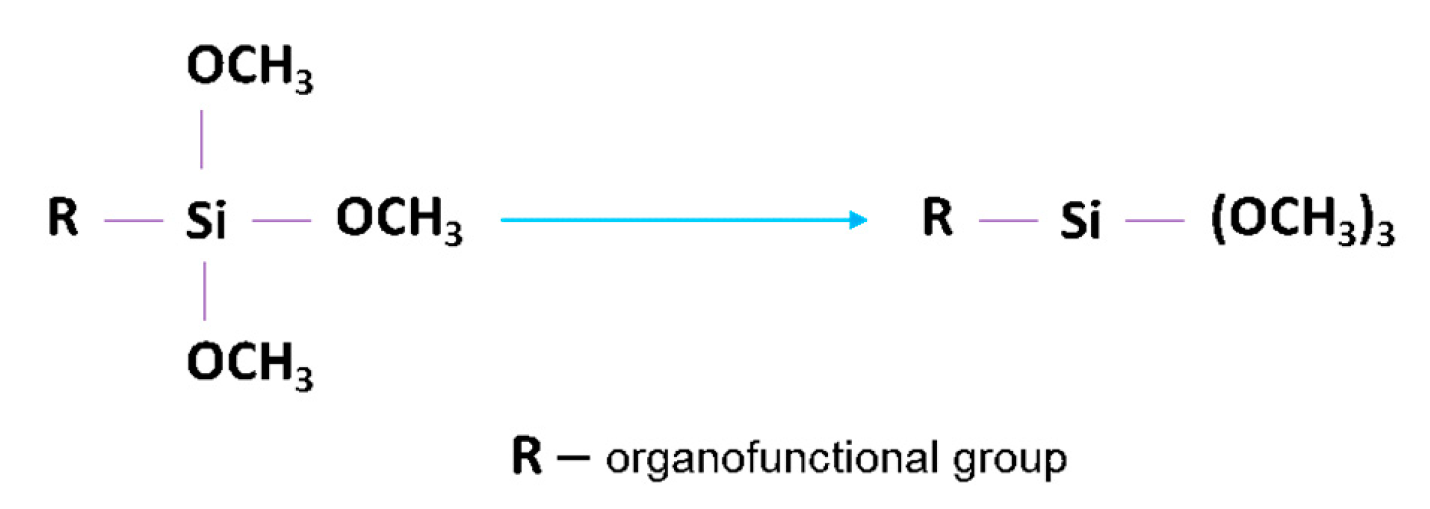
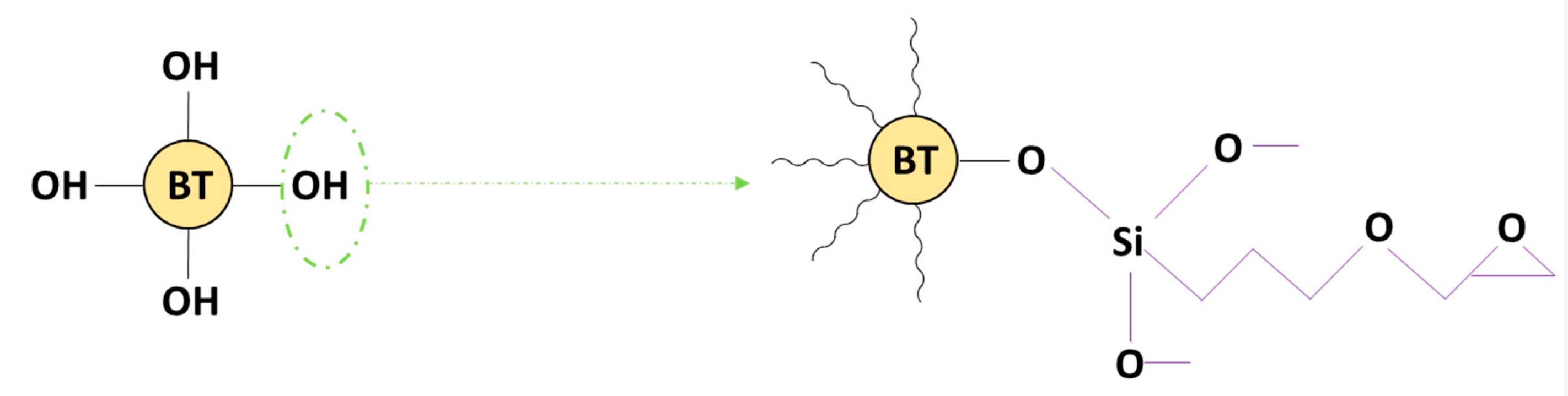
2.2.2. Surface Modification of BTO with Silane Coupling Agent
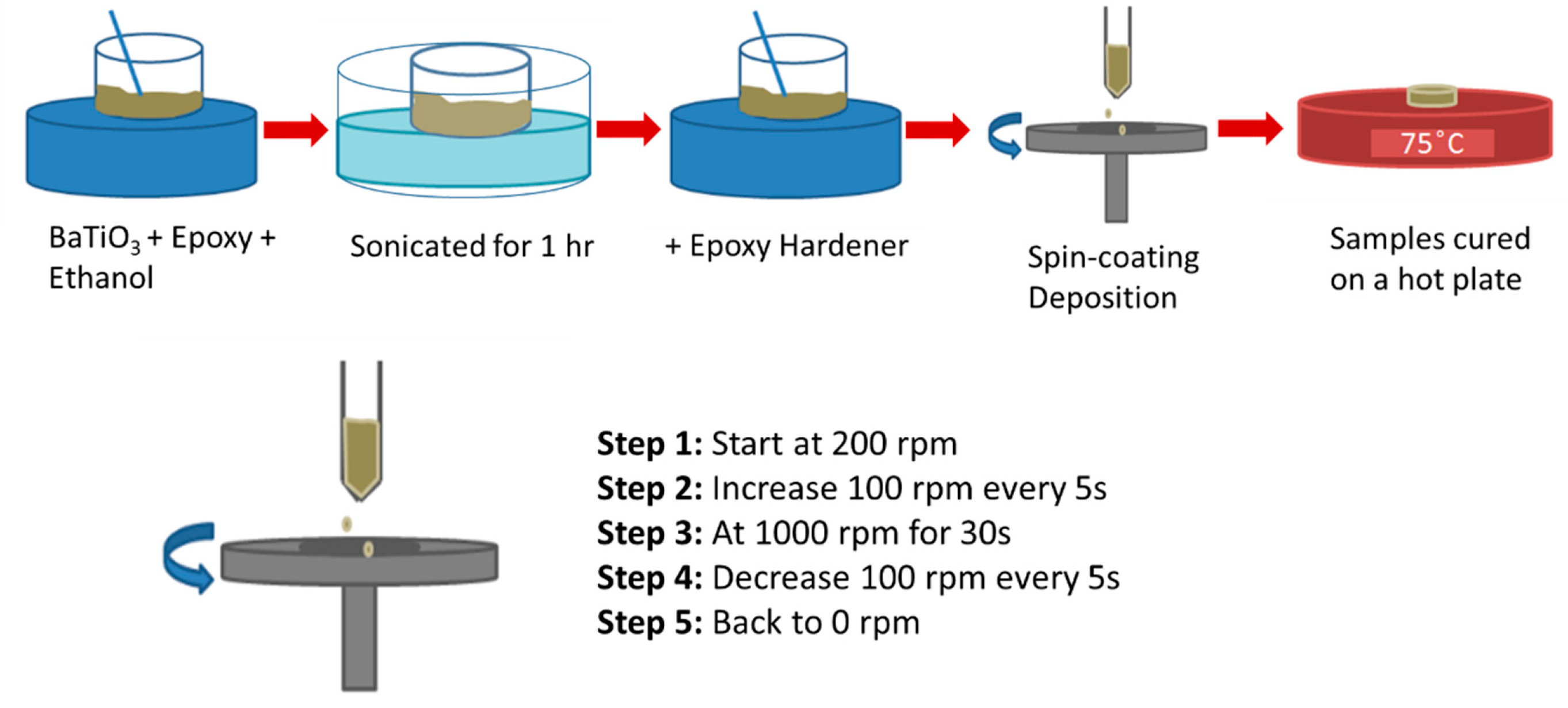
2.3. Sol Gel Synthesis and Film Deposition
2.4. Film Characterization
3. Results
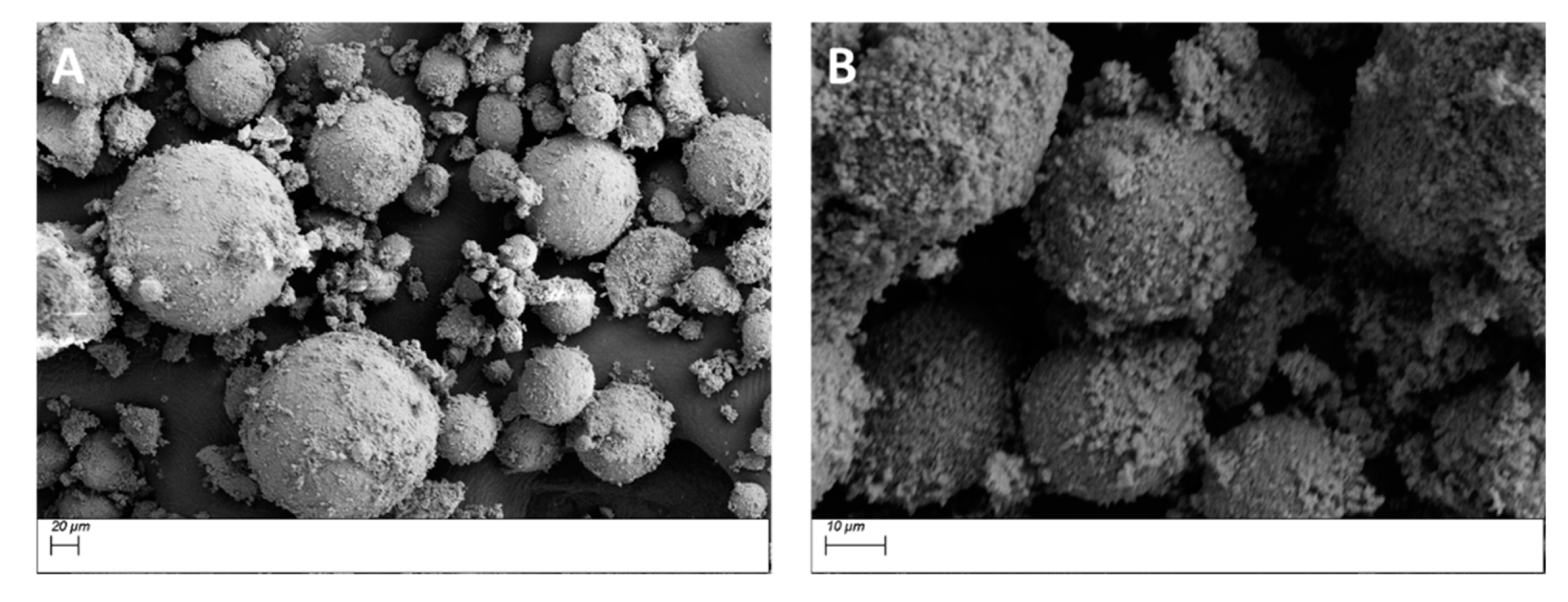
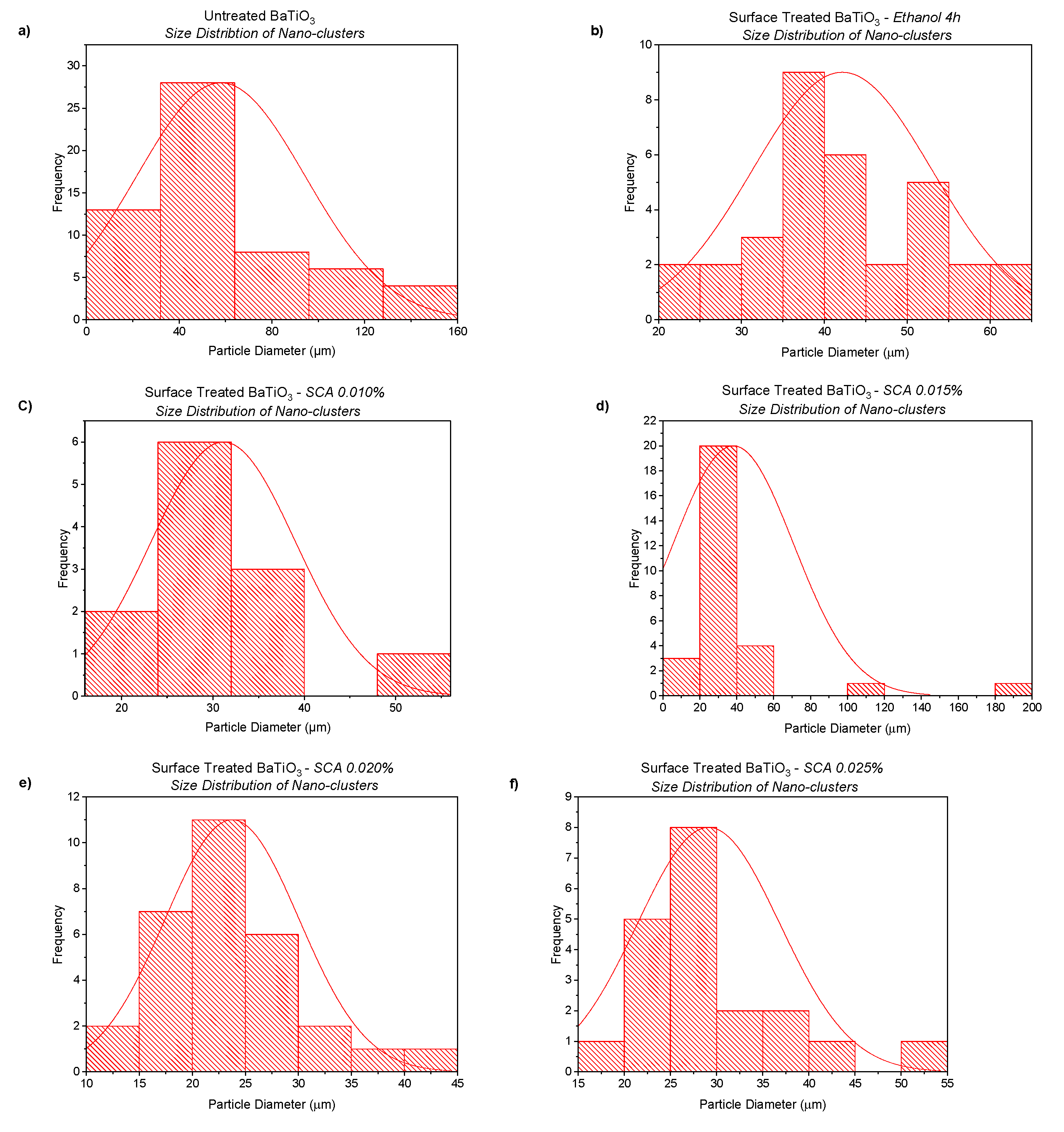
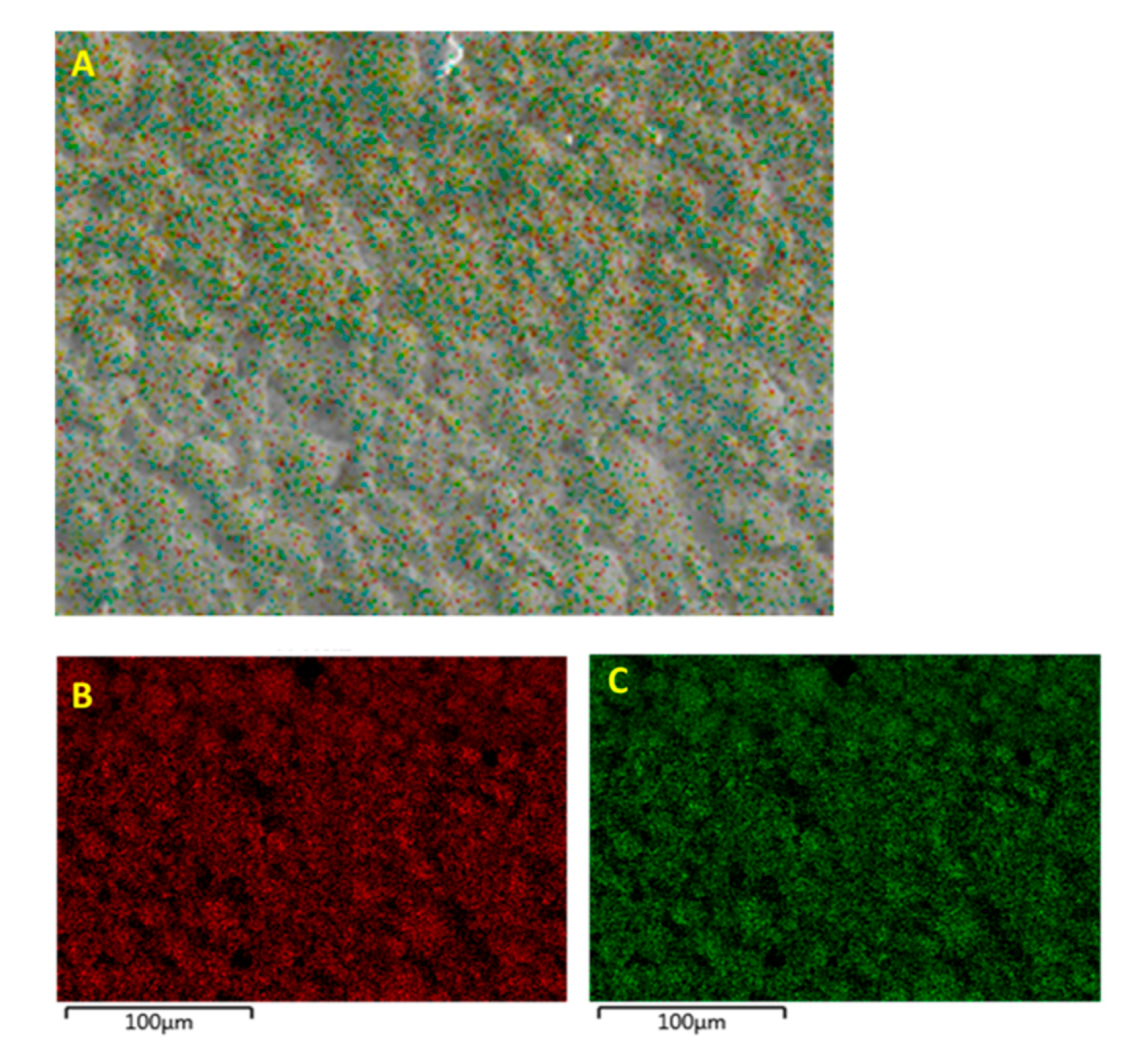
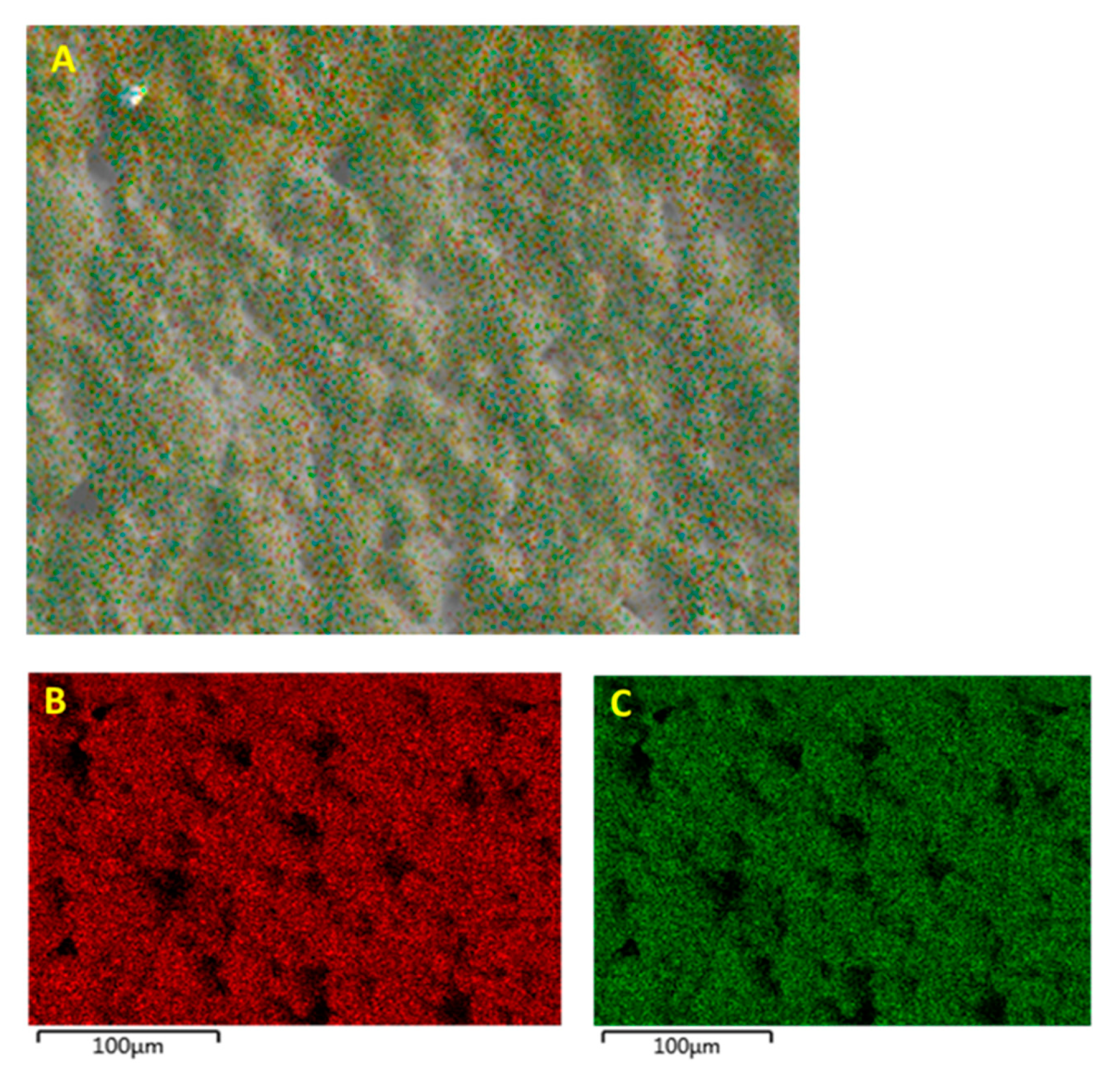
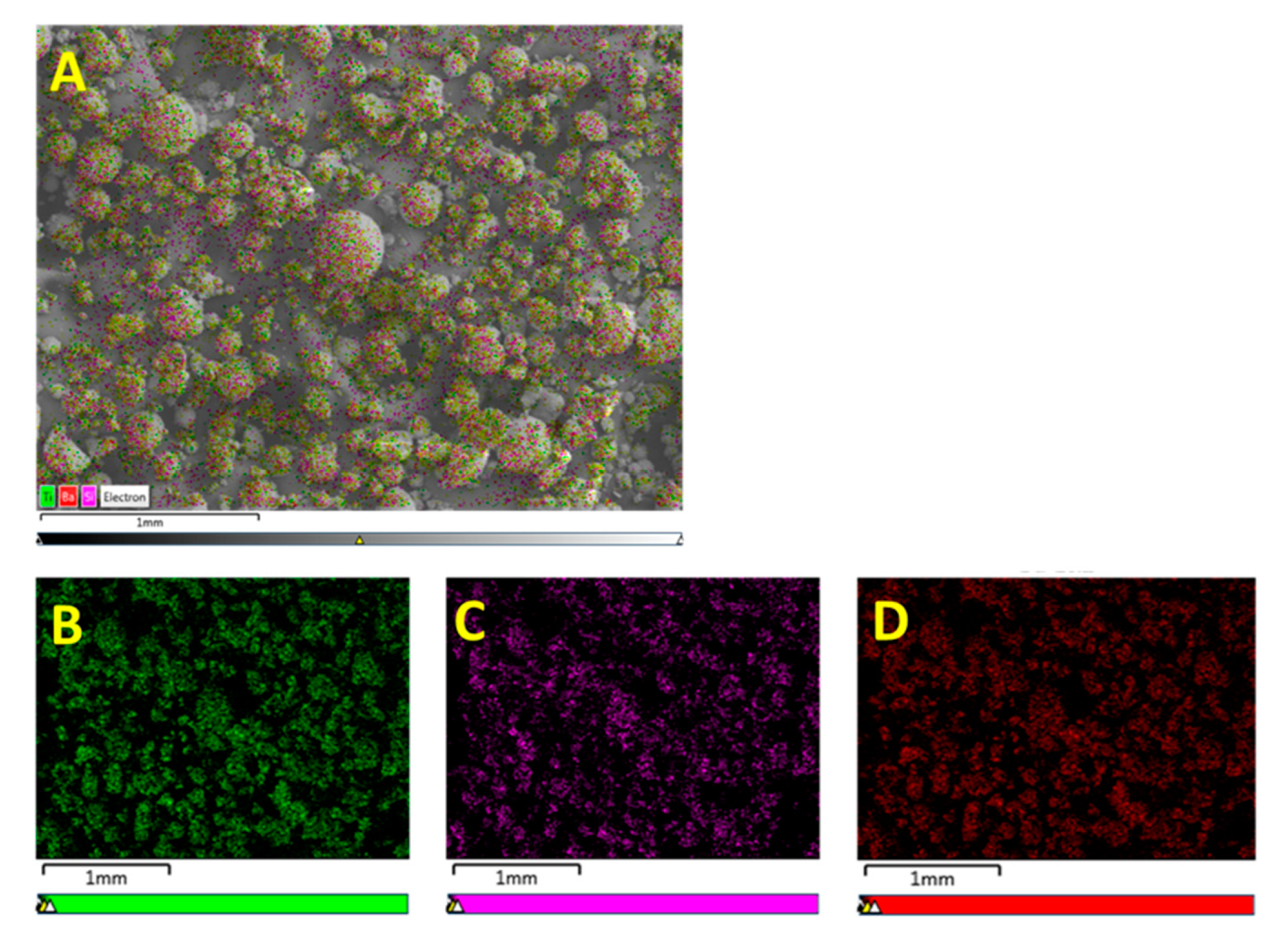
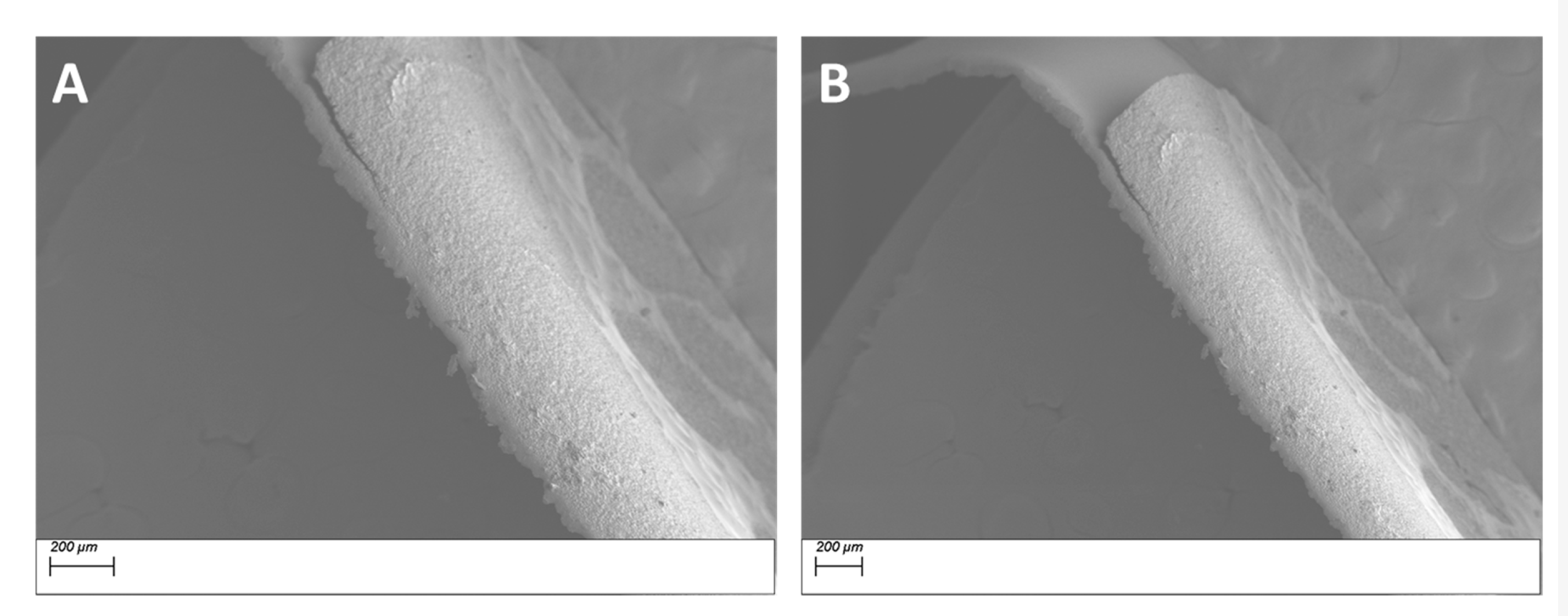
3.1. Morphological Characterization
3.1.1. Morphological Characterization—Surface Treatment with Ethanol—Datum
3.1.2. Morphological Characterization—Surface Treatment with Silane Coupling Agent (KH 560)
3.2. Piezoelectr Strain Characteristics
3.2.1. Piezoelectric Strain Coefficients—Surface Treatment with Ethanol—Datum
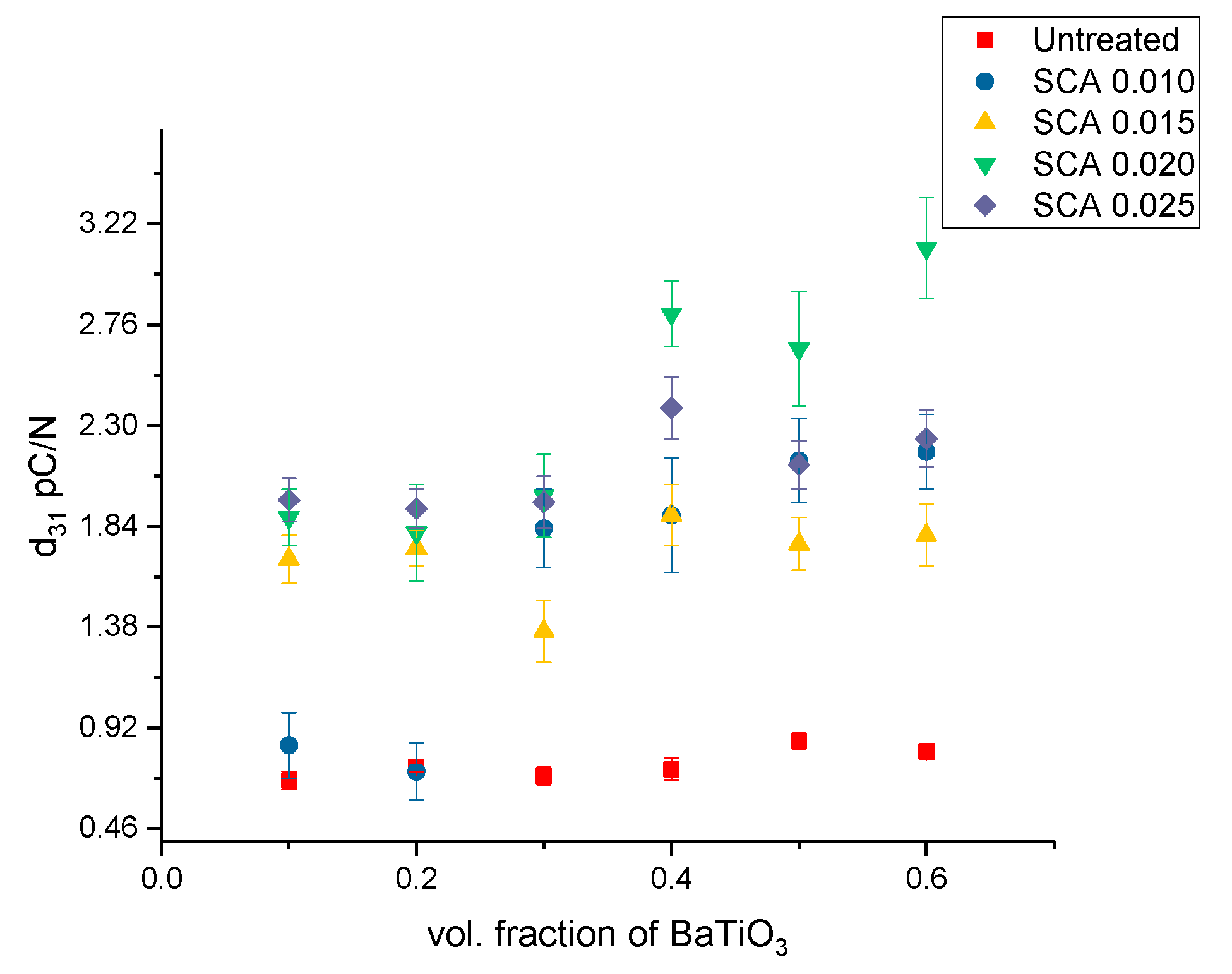
3.2.2. Piezoelectric Strain Coefficients—Surface Treatment with Silane Coupling Agent (KH 560)
3.3. Resistivity
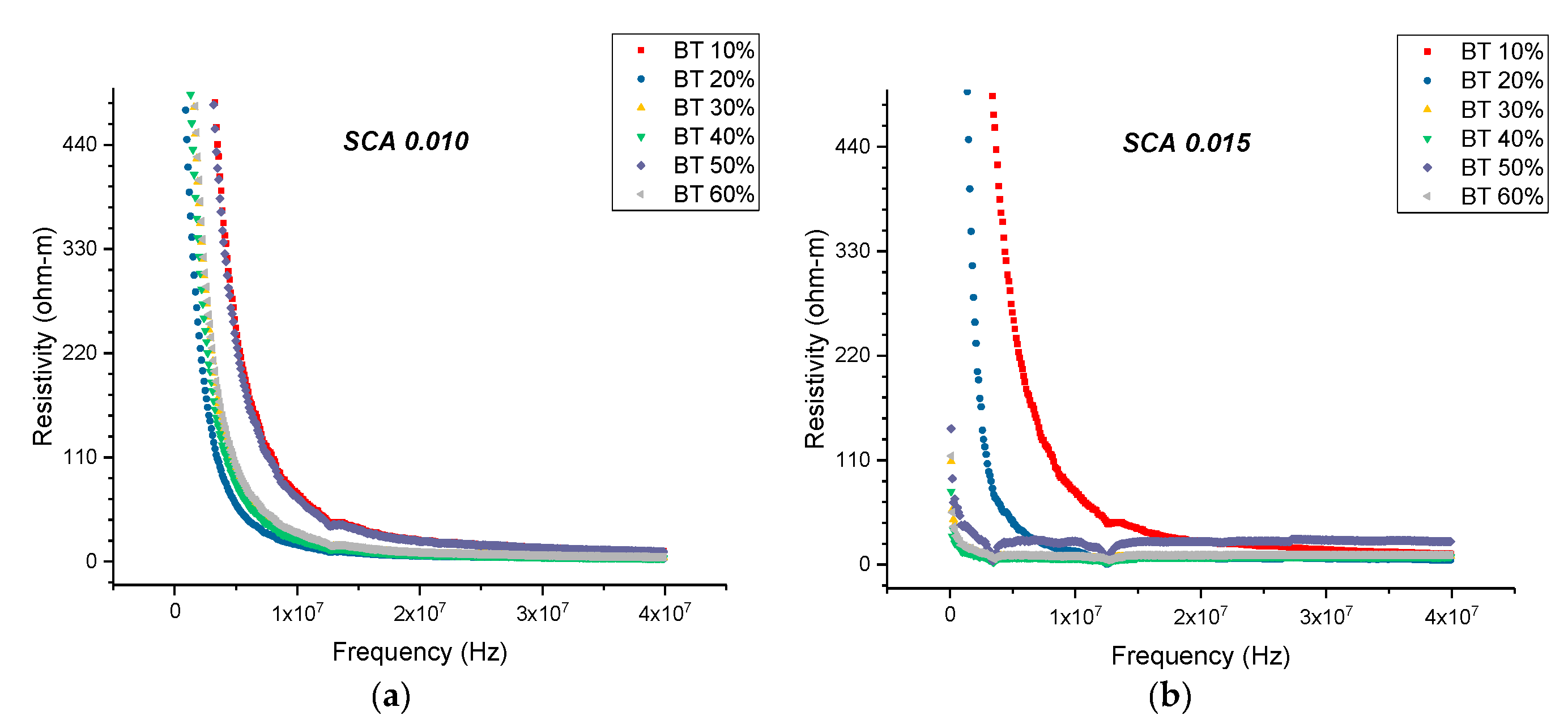
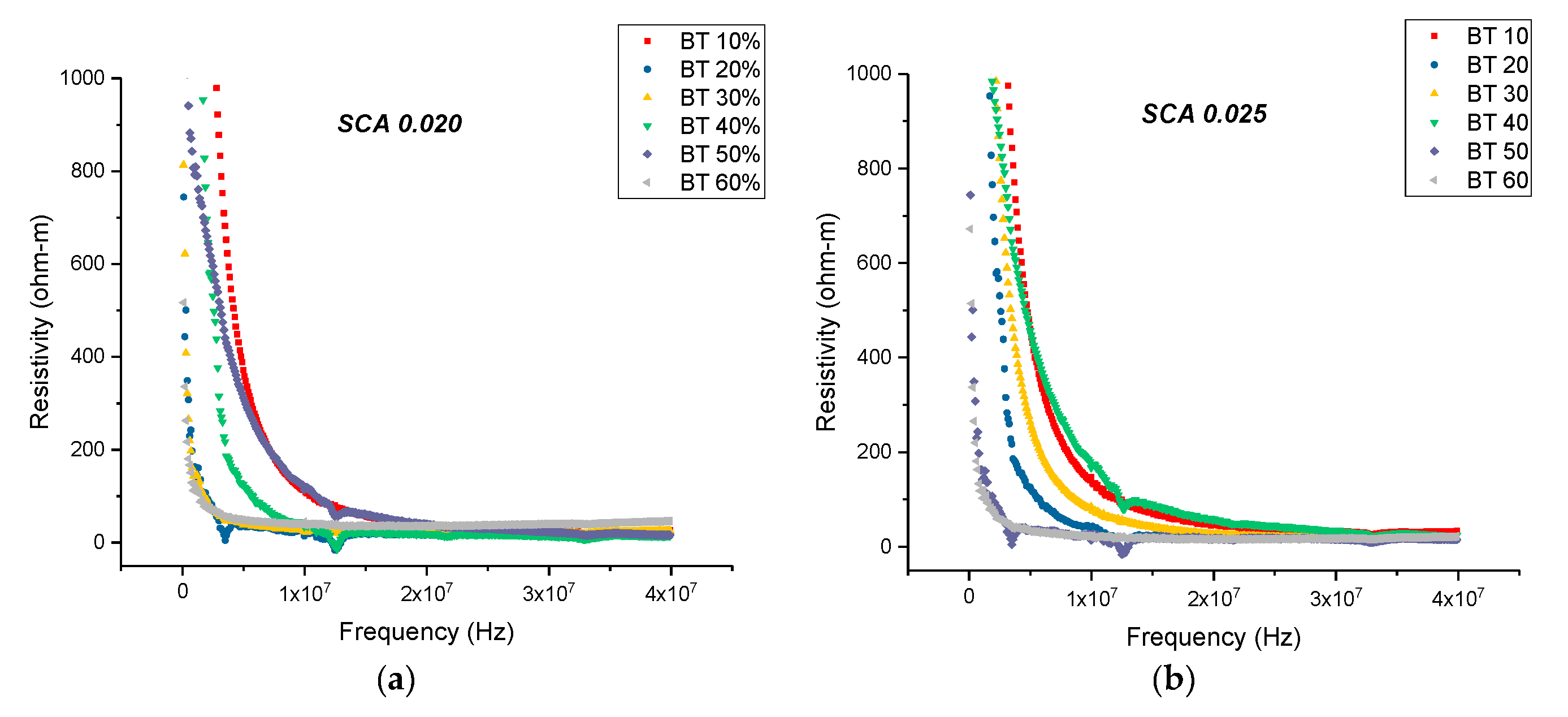
3.3.1. Resistivity—Surface Treatment with Ethanol—Datum
3.3.2. Resistivity—Surface Treatment with Ethanol—Datum
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giurgiutiu, V. Structural Health Monitoring: With Piezoelectric Wafer Active Sensors; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Giurgiutiu, V.; Zagrai, A.; Bao, J.J. Piezoelectric wafer embedded active sensors for aging aircraft structural health monitoring. Struct. Health Monit. 2002, 1, 41–61. [Google Scholar] [CrossRef]
- Park, G.; Cudney, H.H.; Inman, D.J. Impedance-based health monitoring of civil structural components. J. Infrastruct. Syst. 2000, 6, 153–160. [Google Scholar] [CrossRef]
- Staszewski, W.; Boller, C.; Tomlinson, G.R. Health Monitoring of Aerospace Structures: Smart Sensor Technologies and Signal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Cook-Chennault, K.A.; Thambi, N.; Sastry, A.M. Powering MEMS portable devices—A review of non-regenerative and regenerative power supply systems with special emphasis on piezoelectric energy harvesting systems. Smart Mater. Struct. 2008, 17, 043001. [Google Scholar] [CrossRef]
- Banerjee, S.; Du, W.; Wang, L.; Cook-Chennault, K.A. Fabrication of dome-shaped PZT-epoxy actuator using modified solvent and spin coating technique. J. Electroceram. 2013, 31, 148–158. [Google Scholar] [CrossRef]
- Buchanan, R.C.; Park, E.; Surana, R.; Tennakone, H.; Tennakone, K. High piezoelectric actuation response in graded Nd(2)O(3) and ZrO(2) doped BaTiO(3) structures. J. Electroceram. 2011, 26, 116–121. [Google Scholar] [CrossRef]
- Mehta, S.; McClarren, B.; Aijaz, A.; Chalaby, R.; Cook-Chennault, K.; Olabisi, R.M. The effect of low-magnitude, high-frequency vibration on poly(ethylene glycol)-microencapsulated mesenchymal stem cells. J. Tissue Eng. 2018, 9, 2041731418800101. [Google Scholar] [CrossRef]
- Ehterami, A.; Kazemi, M.; Nazari, B.; Saraeian, P.; Azami, M. Fabrication and characterization of highly porous barium titanate based scaffold coated by Gel/HA nanocomposite with high piezoelectric coefficient for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2018, 79, 195–202. [Google Scholar] [CrossRef]
- Erturk, A.; Inman, D.J. Piezoelectric Energy Harvesting; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Surmenev, R.A.; Orlova, T.; Chernozem, R.V.; Ivanova, A.A.; Bartasyte, A.; Mathur, S.; Surmeneva, M.A. Hybrid lead-free polymer-based nanocomposites with improved piezoelectric response for biomedical energy-harvesting applications: A review. Nano Energy 2019, 62, 475–506. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Tummala, R.R. Epoxy nanocomposite capacitors for application as MCM-L compatible integral passives. J. Electron. Packag. 2002, 124, 1241–1246. [Google Scholar] [CrossRef]
- Gonon, P.; Boudefel, A. Electrical properties of epoxy/silver nanocomposites. J. Appl. Phys. 2006, 99, 024308. [Google Scholar] [CrossRef]
- Li, L.; Takahashi, A.; Hao, J.; Kikuchi, R.; Hayakawa, T.; Tsurumi, T.A.; Kakimoto, M.A. Novel polymer-ceramic nanocomposite based on new concepts for embedded capacitor application (I). IEEE Trans. Compon. Packag. Technol. 2005, 28, 754–759. [Google Scholar] [CrossRef]
- Ogitani, S.; Bidstrup-Allen, S.A.; Kohl, P.A. Factors influencing the permittivity of polymer/ceramic composites for embedded capacitors. IEEE Trans. Adv. Packag. 2000, 23, 313–322. [Google Scholar] [CrossRef]
- Baur, C.; Apo, D.J.; Maurya, D.; Priya, S.; Voit, W. Advances in Piezoelectric Polymer Composites for Vibrational Energy Harvesting; American Chemical Society: Washington, DC, USA, 2014. [Google Scholar]
- Hori, M.; Aoki, T.; Ohira, Y.; Yano, S. New type of mechanical damping composites composed of piezoelectric ceramics, carbon black and epoxy resin. Compos. Part A-Appl. Sci. Manuf. 2001, 32, 287–290. [Google Scholar] [CrossRef]
- Sodano, H.A.; Inman, D.J.; Park, G. A review of power harvesting from vibration using piezoelectric materials. Shock Vib. Dig. 2004, 36, 197–206. [Google Scholar] [CrossRef]
- Kwon, J.; Seung, W.; Sharma, B.K.; Kim, S.-W.; Ahn, J.-H. A high performance PZT ribbon-based nanogenerator using graphene transparent electrodes. Energy Environ. Sci. 2012, 5, 8970–8975. [Google Scholar] [CrossRef]
- Cook-Chennault, K.A.; Thambi, N.; Bitetto, M.A.; Hameyie, E.B. Piezoelectric Energy Harvesting: A Green and Clean Alternative for Sustained Power Production. Bull. Sci. Technol. Soc. 2008, 28, 496–509. [Google Scholar] [CrossRef]
- Koripella, C.R. Mechanical behavior of ceramic capacitors. In Proceedings of the 1991 41st Electronic Components & Technology Conference, Atlanta, GA, USA, 11–16 May 1991; IEEE: Piscataway, NJ, USA, 1991; pp. 457–462. [Google Scholar]
- Freiman, S.W.; Pohanka, R.C. Review of mechanically related failures of ceramic capacitors and capacitor materials. J. Am. Ceram. Soc. 1989, 72, 2258–2263. [Google Scholar] [CrossRef]
- Hyun, J.-G.; Paik, K.-W.; Pak, J.S. Characterization of epoxy/BaTiO 3 composite embedded capacitors for high frequency behaviors. In Proceedings of the 2009 59th Electronic Components and Technology Conference, San Diego, CA, USA, 26–29 May 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 2046–2050. [Google Scholar]
- Liang, S.; Chong, S.R.; Giannelis, E.P. Barium titanate/epoxy composite dielectric materials for integrated thin film capacitors. In Proceedings of the 1998 48th Electronic Components and Technology Conference, Seattle, WA, USA, 25–28 May 1998; IEEE: Piscataway, NJ, USA, 1998; pp. 171–175. [Google Scholar]
- Ramesh, S.; Huang, C.; Liang, S.; Giannelis, E.P. Integrated thin film capacitors: Interfacial control and implications on fabrication and performance. In Proceedings of the 1999 49th Electronic Components and Technology Conference, San Diego, CA, USA, 1–4 June 1999; IEEE: Piscataway, NJ, USA, 1999; pp. 99–104. [Google Scholar]
- Pan, M.-J.; Randall, C.A. A brief introduction to ceramic capacitors. IEEE Electr. Insul. Mag. 2010, 26, 44–50. [Google Scholar] [CrossRef]
- Arbatti, M.; Shan, X.; Cheng, Z.Y. Ceramic–polymer composites with high dielectric constant. Adv. Mater. 2007, 19, 1369–1372. [Google Scholar] [CrossRef]
- Avila, H.; Ramajo, L.; Góes, M.; Reboredo, M.; Castro, M.; Parra, R. Dielectric behavior of epoxy/BaTiO3 composites using nanostructured ceramic fibers obtained by electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 505–510. [Google Scholar] [CrossRef]
- Bai, W.; Zheng, P.; Wen, F.; Zhang, J.; Chen, D.; Zhai, J.; Ji, Z. Lead-free BNT-based composite materials: Enhanced depolarization temperature and electromechanical behavior. Dalton Trans. 2017, 46, 15340–15353. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cheng, Z.-Y.; Bharti, V.; Xu, H.; Zhang, Q. High-dielectric-constant ceramic-powder polymer composites. Appl. Phys. Lett. 2000, 76, 3804–3806. [Google Scholar] [CrossRef]
- Sundar, U.; Cook-Chennault, K.A.; Banerjee, S.; Refour, E. Dielectric and piezoelectric properties of percolative three-phase piezoelectric polymer composites. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2016, 34, 041232. [Google Scholar] [CrossRef]
- Iijima, M.; Sato, N.; Lenggoro, I.W.; Kamiya, H. Surface modification of BaTiO 3 particles by silane coupling agents in different solvents and their effect on dielectric properties of BaTiO 3/epoxy composites. Colloids Surf. A Physicochem. Eng. Asp. 2009, 352, 88–93. [Google Scholar] [CrossRef]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Nan, C.-W.; Shen, Y.; Ma, J. Physical properties of composites near percolation. Annu. Rev. Mater. Res. 2010, 40, 131–151. [Google Scholar] [CrossRef]
- Banerjee, S. An Experimental and Theoretical Analysis of Two and Three Phase Epoxy Based Piezoelectric Composites; Rutgers University-Graduate School: New Brunswick, NJ, USA, 2013. [Google Scholar]
- Arlt, K.; Wegener, M. Piezoelectric PZT/PVDF-copolymer 0-3 composites: Aspects on film preparation and electrical poling. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1178–1184. [Google Scholar] [CrossRef]
- Corker, D.; Zhang, Q.; Whatmore, R.; Perrin, C. PZT ‘composite’ferroelectric thick films. J. Eur. Ceram. Soc. 2002, 22, 383–390. [Google Scholar] [CrossRef]
- Corkovic, S.; Whatmore, R.; Zhang, Q. Sol-gel fabrication of PZT thick films for MEMS. Integr. Ferroelectr. 2007, 88, 93–102. [Google Scholar] [CrossRef]
- Guan, X.C.; Zhang, Y.D.; Li, H.; Ou, J.P. PZT/PVDF composites doped with carbon nanotubes. Sensors Actuators A-Phys. 2013, 194, 228–231. [Google Scholar] [CrossRef]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free piezoceramics. Nature 2004, 432, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Urabe, K.; Banno, H. BaTiO3 particle-size dependence of ferroelectricity in BaTiO3/polymer composites. Jpn. J. Appl. Phys. 1993, 32, 4272. [Google Scholar] [CrossRef]
- Capsal, J.F.; Dantras, E.; Laffont, L.; Dandurand, J.; Lacabanne, C. Nanotexture influence of BaTiO3 particles on piezoelectric behaviour of PA 11/BaTiO3 nanocomposites. J. Non-Cryst. Solids 2010, 356, 629–634. [Google Scholar] [CrossRef]
- Defebvin, J.; Barrau, S.; Lyskawa, J.; Woisel, P.; Lefebvre, J.M. Influence of nitrodopamine-functionalized barium titanate content on the piezoelectric response of poly(vinylidene fluoride) based polymer-ceramic composites. Compos. Sci. Technol. 2017, 147, 16–21. (In English) [Google Scholar] [CrossRef]
- Li, R.; Zhao, Z.H.; Chen, Z.X.; Pei, J.Z. Novel BaTiO3/PVDF composites with enhanced electrical properties modified by calcined BaTiO3 ceramic powders. Mater. Express 2017, 7, 536–540. [Google Scholar] [CrossRef]
- Huang, X.; Xie, L.; Yang, K.; Wu, C.; Jiang, P.; Li, S.; Wu, S.; Tatsumi, K.; Tanaka, T. Role of interface in highly filled epoxy/BaTiO 3 nanocomposites. Part I-correlation between nanoparticle surface chemistry and nanocomposite dielectric property. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 467–479. [Google Scholar] [CrossRef]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The role of interparticle and external forces in nanoparticle assembly. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef]
- Lewis, J.A. Colloidal processing of ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame retardants in commercial use or development for polyolefins. J. Fire Sci. 2008, 26, 5–43. [Google Scholar] [CrossRef]
- Hornsby, P.R. Fire retardant fillers for polymers. Int. Mater. Rev. 2001, 46, 199–210. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Price, D.; Price, D. Fire Retardant Materials; Woodhead Publishing: Sawston, UK, 2001. [Google Scholar]
- Kim, S. Flame retardancy and smoke suppression of magnesium hydroxide filled polyethylene. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 936–944. [Google Scholar] [CrossRef]
- Ramesh, S.; Shutzberg, B.A.; Huang, C.; Gao, J.; Giannelis, E.P. Dielectric nanocomposites for integral thin film capacitors: Materials design, fabrication and integration issues. IEEE Trans. Adv. Packag. 2003, 26, 17–24. [Google Scholar] [CrossRef]
- Li, C.-C.; Chang, S.-J.; Lee, J.-T.; Liao, W.-S. Efficient hydroxylation of BaTiO 3 nanoparticles by using hydrogen peroxide. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 143–149. [Google Scholar] [CrossRef]
- Zhou, T.; Zha, J.-W.; Cui, R.-Y.; Fan, B.-H.; Yuan, J.-K.; Dang, Z.-M. Improving dielectric properties of BaTiO3/ferroelectric polymer composites by employing surface hydroxylated BaTiO3 nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, G.; Huang, X.; Bu, J.; Sun, X.; Jiang, P. Molecular structures of (3-aminopropyl)trialkoxysilane on hydroxylated barium titanate nanoparticle surfaces induced by different solvents and their effect on electrical properties of barium titanate based polymer nanocomposites. Appl. Surf. Sci. 2016, 364, 798–807. [Google Scholar] [CrossRef]
- Almadhoun, M.N.; Bhansali, U.S.; Alshareef, H.N. Nanocomposites of ferroelectric polymers with surface-hydroxylated BaTiO3 nanoparticles for energy storage applications. J. Mater. Chem. 2012, 22, 11196–11200. [Google Scholar] [CrossRef]
- Kim, P.; Doss, N.M.; Tillotson, J.P.; Hotchkiss, P.J.; Pan, M.J.; Marder, S.R.; Li, J.; Calame, J.P.; Perry, J.W. High energy density nanocomposites based on surface-modified BaTiO3 and a ferroelectric polymer. ACS Nano 2009, 3, 2581–2592. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, B.; Fan, W.; Hu, Y.; Shen, X. Effects of PE-g-DBM as a compatiblizer on mechanical properties and crystallization behaviors of magnesium hydroxide-based LLDPE blends. Polym. Degrad. Stab. 2002, 76, 123–128. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Fan, W.; Nie, W. Effects of surface modifiers on mechanical and rheological properties of halogen-free flame retarded polyethylene composites. Polym.-Plast. Technol. Eng. 2006, 45, 191–196. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Guo, S. Structure and properties of polypropylene composites filled with magnesium hydroxide. J. Appl. Polym. Sci. 2006, 102, 4943–4951. [Google Scholar] [CrossRef]
- Huang, H.; Tian, M.; Yang, J.; Li, H.; Liang, W.; Zhang, L.; Li, X. Stearic acid surface modifying Mg (OH) 2: Mechanism and its effect on properties of ethylene vinyl acetate/Mg (OH) 2 composites. J. Appl. Polym. Sci. 2008, 107, 3325–3331. [Google Scholar] [CrossRef]
- Liauw, C.M.; Dumitru, P.; Lees, G.C.; Clemens, M.L.; Rothon, R.N. Interfacial Modification of Polystyrene-block-polybutadiene-block-polystyrene/Magnesium Hydroxide Composites, 1. Macromol. Mater. Eng. 2003, 288, 412–420. [Google Scholar] [CrossRef]
- Schofield, W.; Hurst, S.; Lees, G.; Liauw, C.; Rothon, R. Influence of surface modification of magnesium hydroxide on the processing and mechanical properties of composites of magnesium hydroxide and an ethylene vinyl acetate copolymer. Compos. Interfaces 1997, 5, 515–528. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, D. Effect of coupling agents on the dielectric properties of aluminum particles reinforced epoxy resin composites. J. Compos. Mater. 2011, 45, 1981–1989. [Google Scholar] [CrossRef]
- Sasikala, T.; Raman, S.; Mohanan, P.; Pavithran, C.; Sebastian, M. Effect of silane coupling agent on the dielectric and thermal properties of DGEBA-forsterite composites. J. Polym. Res. 2011, 18, 811–819. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Yu, Y.-F.; Xu, H.-P.; Bai, J. Study on microstructure and dielectric property of the BaTiO 3/epoxy resin composites. Compos. Sci. Technol. 2008, 68, 171–177. [Google Scholar] [CrossRef]
- Epofix Cold-Setting Embedding Resin, EMS Catalog #1232. Available online: https://www.emsdiasum.com/microscopy/technical/datasheet/1232.aspx (accessed on 1 October 2019).
- Gao, J.H.; Xue, D.Z.; Liu, W.F.; Zhou, C.; Ren, X.B. Recent Progress on BaTiO3-Based Piezoelectric Ceramics for Actuator Applications. Actuators 2017, 6, 24. [Google Scholar] [CrossRef]
- Barium Titanate (IV) Nanopowder Specifications; Sigma-Aldrich: St.Louis, MO, USA, 2017.
- Plueddemann, E.P. Silane coupling agents. Addit. Plast. 1978, 1, 123–167. [Google Scholar]
- Zeng, X.; Yu, S.; Sun, R.; Du, R. Microstructure, thermal and dielectric properties of homogeneous bismaleimide-triazine/barium titanate nanocomposite films. Mater. Chem. Phys. 2011, 131, 387–392. [Google Scholar] [CrossRef]
- Paik, U.; Hackley, V.A.; Choi, S.-C.; Jung, Y.-G. The effect of electrostatic repulsive forces on the stability of BaTiO3 particles suspended in non-aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 1998, 135, 77–88. [Google Scholar] [CrossRef]
- Mikeska, K.R.; Cannon, W. Non-aqueous dispersion properties of pure barium titanate for tape casting. Colloids Surf. 1988, 29, 305–321. [Google Scholar] [CrossRef]
- Fowkes, F.M. Interface acid-base/charge-transfer properties. In Surface and Interfacial Aspects of Biomedical Polymers; Springer: Berlin/Heidelberg, Germany, 1985; pp. 337–372. [Google Scholar]
- Fowkes, F.M.; Mostafa, M.A. Acid-base interactions in polymer adsorption. Ind. Eng. Chem. Prod. Res. Dev. 1978, 17, 3–7. [Google Scholar] [CrossRef]
- Fan, L.; Rao, Y.; Tison, C.; Moon, K.; Pothukuchi, S.; Wong, C. Use of dispersant in high K polymer-ceramic nano-composite to improve manufacturability and performance of integral capacitors. In Proceedings of the 52nd Electronic Components and Technology Conference, San Diego, CA, USA, 28–31 May 2002; IEEE: Piscataway, NJ, USA, 2002; pp. 936–940. [Google Scholar]
- Rao, Y.; Takahashi, A.; Wong, C. Di-block copolymer surfactant study to optimize filler dispersion in high dielectric constant polymer-ceramic composite. Compos. Part A Appl. Sci. Manuf. 2003, 34, 1113–1116. [Google Scholar] [CrossRef]
- Cho, S.-D.; Lee, J.-Y.; Hyun, J.-G.; Paik, K.-W. Study on epoxy/BaTiO 3 composite embedded capacitor films (ECFs) for organic substrate applications. Mater. Sci. Eng. B 2004, 110, 233–239. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Wang, H.-Y.; Xu, H.-P. Influence of silane coupling agent on morphology and dielectric property in BaTiO3/polyvinylidene fluoride composites. Appl. Phys. Lett. 2006, 89, 112902. [Google Scholar] [CrossRef]
- Nhuapeng, W.; Tunkasiri, T. Properties of 0–3 Lead Zirconate Titanate–Polymer Composites Prepared in a Centrifuge. J. Am. Ceram. Soc. 2002, 85, 700–702. [Google Scholar] [CrossRef]
- Banno, H.; Ogura, K. Piezoelectric properties of 0-3 composite of polymer and ceramic powder mixture of PZT and PbTiO3. Jpn. J. Appl. Phys. 1991, 30, 2247. [Google Scholar] [CrossRef]
- Han, K.H.; Safari, A.; Riman, R.E. COLLOIDAL PROCESSING FOR IMPROVED PIEZOELECTRIC PROPERTIES OF FLEXIBLE 0-3 CERAMIC POLYMER COMPOSITES. J. Am. Ceram. Soc. 1991, 74, 1699–1702. [Google Scholar] [CrossRef]
- Lam, K.; Chan, H. Piezoelectric and pyroelectric properties of 65PMN-35PT/P (VDF-TrFE) 0–3 composites. Compos. Sci. Technol. 2005, 65, 1107–1111. [Google Scholar] [CrossRef]
- Jie, L.; Dongyan, T.; Ji, L. Preparation and Electric Properties of 0-3 Modified PZT/IPN Piezoelectric Composites. Rare Met. Mater. Eng. 2007, 36, 411. [Google Scholar]
- Poon, Y.M.; Ho, C.H.; Wong, Y.W.; Shin, F.G. Theoretical predictions on the effective piezoelectric coefficients of 0–3 PZT/Polymer composites. J. Mater. Sci. 2007, 42, 6011–6017. [Google Scholar] [CrossRef]
- Son, Y.; Kweon, S.; Kim, S.; Kim, Y.; Hong, T.; Lee, Y. Fabrication and electrical properties of PZT-PVDF 0–3 type composite film. Integr. Ferroelectr. 2007, 88, 44–50. [Google Scholar] [CrossRef]
- Topolov, V.Y.; Bisegna, P.; Bowen, C.R. Analysis of the piezoelectric performance of modern 0-3 type composites based on relaxor-ferroelectric single crystals. Ferroelectrics 2011, 413, 176–191. [Google Scholar] [CrossRef][Green Version]
- Rujijanagul, G.; Boonyakul, S.; Tunkasiri, T. Effect of the particle size of PZT on the microstructure and the piezoelectric properties of 0-3 PZT/polymer composites. J. Mater. Sci. Lett. 2001, 20, 1943–1945. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, J.; Wu, Y.; Wang, C.; Koval, V.; Shi, B.; Ye, H.; McKinnon, R.; Viola, G.; Yan, H. Unfolding grain size effects in barium titanate ferroelectric ceramics. Sci. Rep. 2015, 5, 9953. [Google Scholar] [CrossRef]
- Huang, X.; Xie, L.; Yang, K.; Wu, C.; Jiang, P.; Li, S.; Wu, S.; Tatsumi, K.; Tanaka, T. Role of interface in highly filled epoxy/BaTiO 3 nanocomposites. Part II-effect of nanoparticle surface chemistry on processing, thermal expansion, energy storage and breakdown strength of the nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 480–487. [Google Scholar] [CrossRef]









| Barium Titanate Nano-Powder | |
|---|---|
| Property | |
| Density (g/cm3) | 6.08 |
| Mean Diameter * | ~100 nm |
| Curie Point (°C) | 130 °C |
| Dielectric constant | 150 [69] |
| d33 (pC/N) | ~85.6 (crystal)/~191 (ceramic) |
| Property | |
|---|---|
| Dielectric Constant | 2.9–3.0 * |
| Dielectric dissipation | ~0.02–0.04 |
| Electromechanical coupling factor, k33 | -- |
| Piezoelectric strain coefficient, d33 | -- |
| Density | 1.16 g/cm3 |
| Electrical Resistivity | 0.15 @ 1 kHz |
| Property | |
|---|---|
| Density | 1.07 g/mL |
| Boiling point | 120 °C/2 mmHg(lit.) |
| KH-560 | Set 1 | Set 2 | Set 3 | Set 4 |
|---|---|---|---|---|
| Concentration | 0.01 | 0.015 | 0.020 | 0.025 |
| Sample Set. | Average Particle Diameter (Nano-Clusters) |
|---|---|
| Non-surface Treated BaTiO3 | ~60 µm |
| Ethanol Surface Treated (4 h) | ~40 µm |
| Silane Coupling Agent–0.010 | ~30 µm |
| Silane Coupling Agent–0.015 | ~<30 µm |
| Silane Coupling Agent–0.020 | ~25 µm |
| Silane Coupling Agent–0.025 | ~28 µm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundar, U.; Lao, Z.; Cook-Chennault, K. Investigation of Piezoelectricity and Resistivity of Surface Modified Barium Titanate Nanocomposites. Polymers 2019, 11, 2123. https://doi.org/10.3390/polym11122123
Sundar U, Lao Z, Cook-Chennault K. Investigation of Piezoelectricity and Resistivity of Surface Modified Barium Titanate Nanocomposites. Polymers. 2019; 11(12):2123. https://doi.org/10.3390/polym11122123
Chicago/Turabian StyleSundar, Udhay, Zichen Lao, and Kimberly Cook-Chennault. 2019. "Investigation of Piezoelectricity and Resistivity of Surface Modified Barium Titanate Nanocomposites" Polymers 11, no. 12: 2123. https://doi.org/10.3390/polym11122123
APA StyleSundar, U., Lao, Z., & Cook-Chennault, K. (2019). Investigation of Piezoelectricity and Resistivity of Surface Modified Barium Titanate Nanocomposites. Polymers, 11(12), 2123. https://doi.org/10.3390/polym11122123







