A Selenone-Functionalized Polyhedral Oligomeric Silsesquioxane for Selective Detection and Adsorption of Hg2+ ions in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
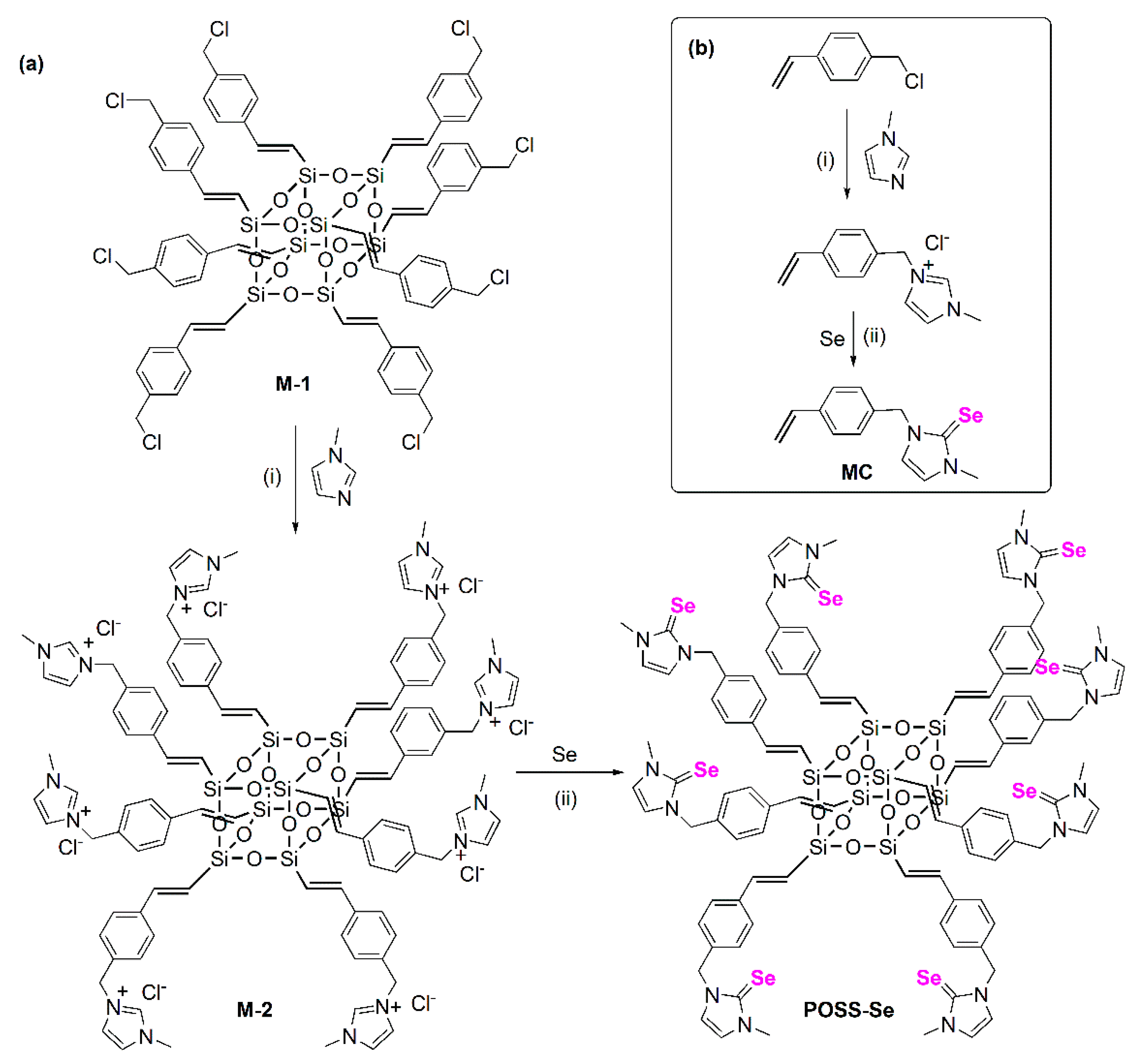
2.2. Synthesis of Octa((4-benzyl imidazolium chloride)ethenyl)silsesquioxane (M-2)
2.3. Synthesis of Octa((4-benzyl imidazoline-2-selenone)ethenyl)silsesquioxane (POSS-Se)
2.4. Detection of Hg2+ Ions by POSS-Se in Aqueous Solutions
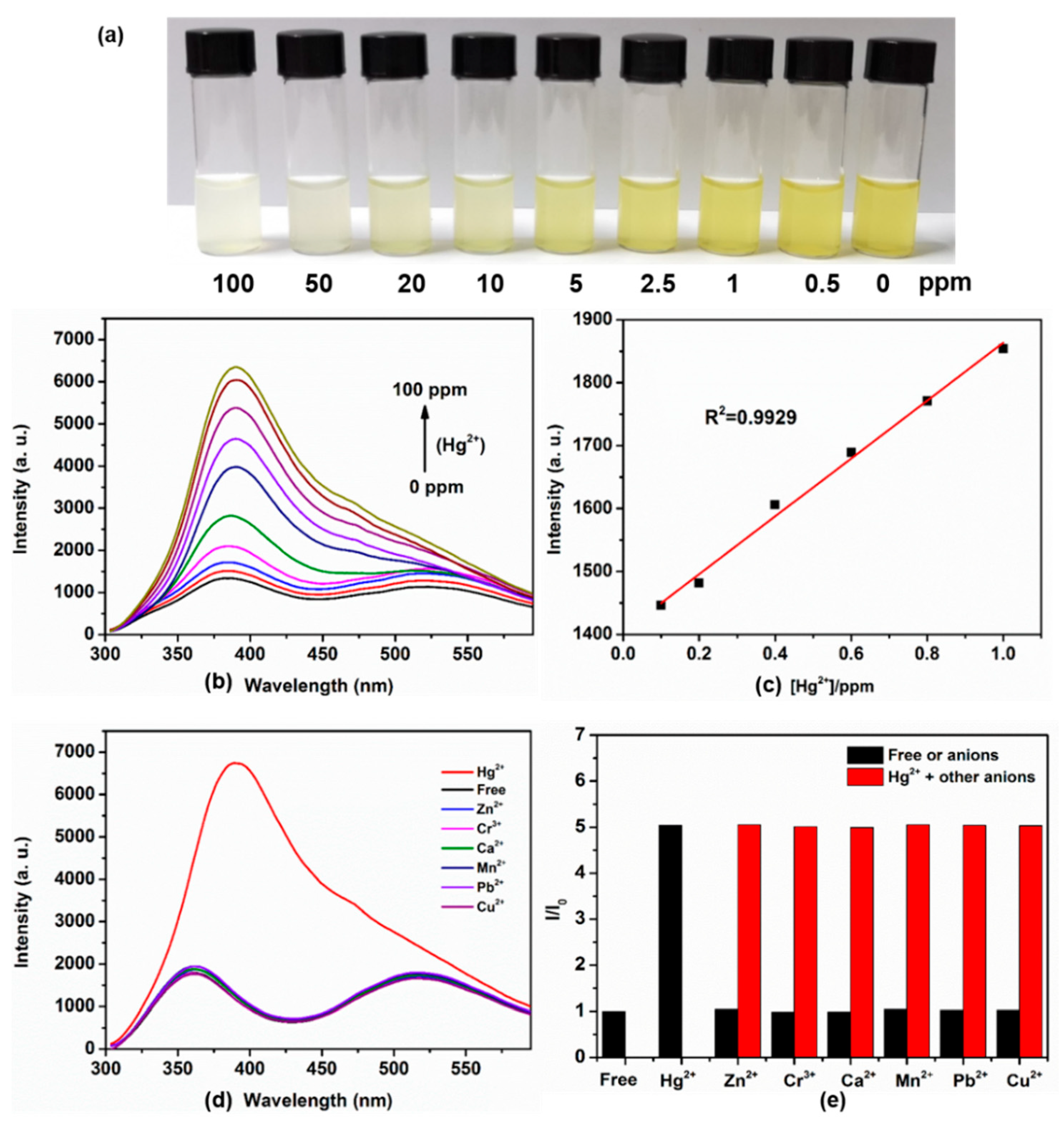
2.5. Calculating the Limit of Detection (LOD)
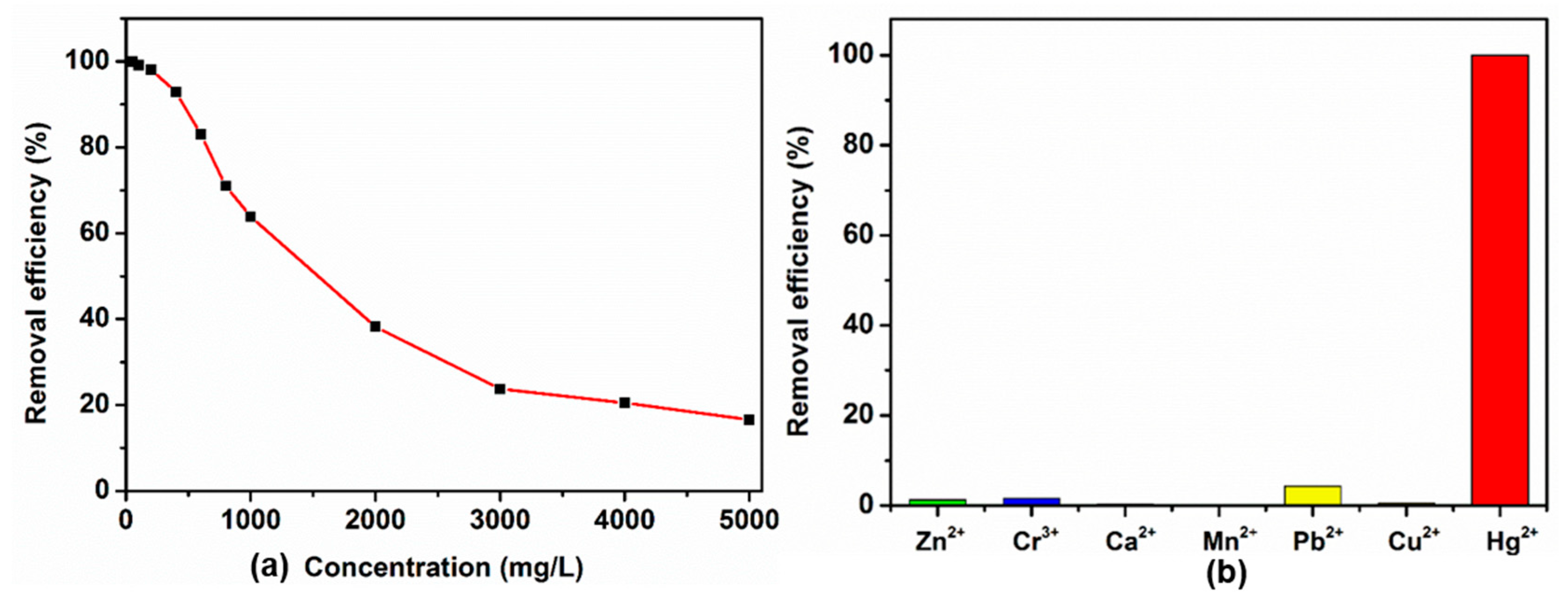
2.6. Adsorption of Hg2+ Ions by POSS-Se in Aqueous Solutions
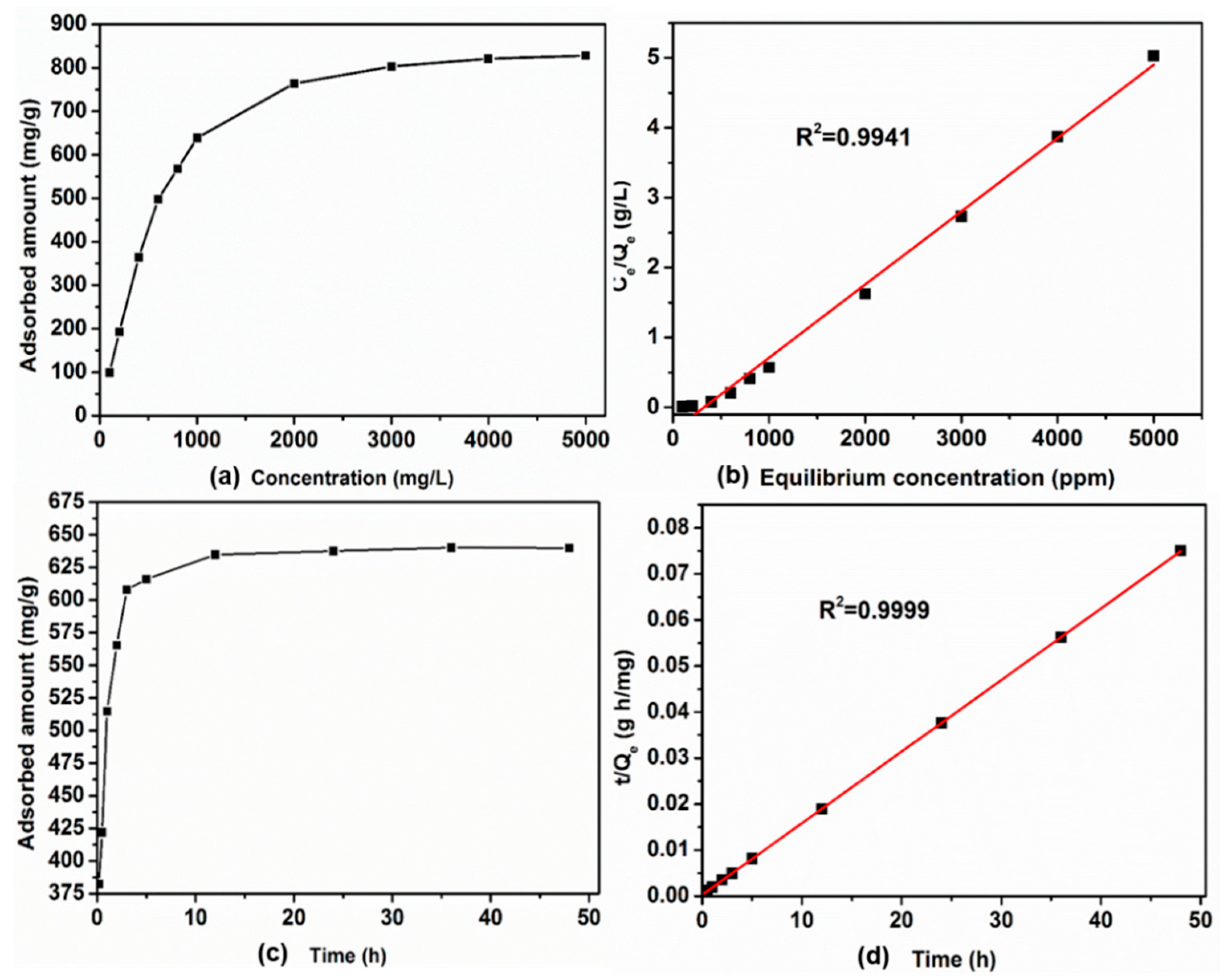
2.7. Adsorption Equilibrium and Kinetics
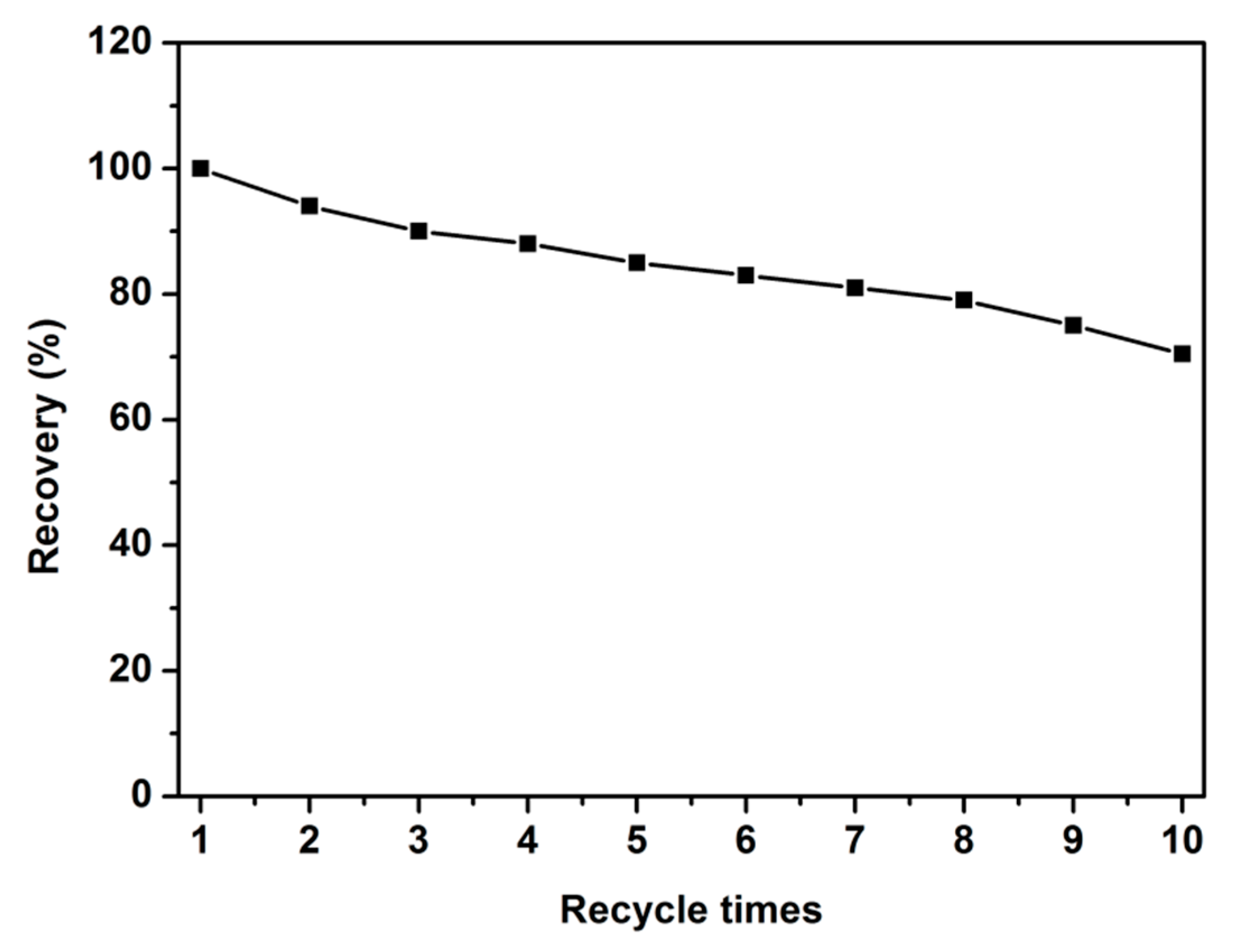
2.8. Recyclability of POSS-Se
3. Results and Discussion
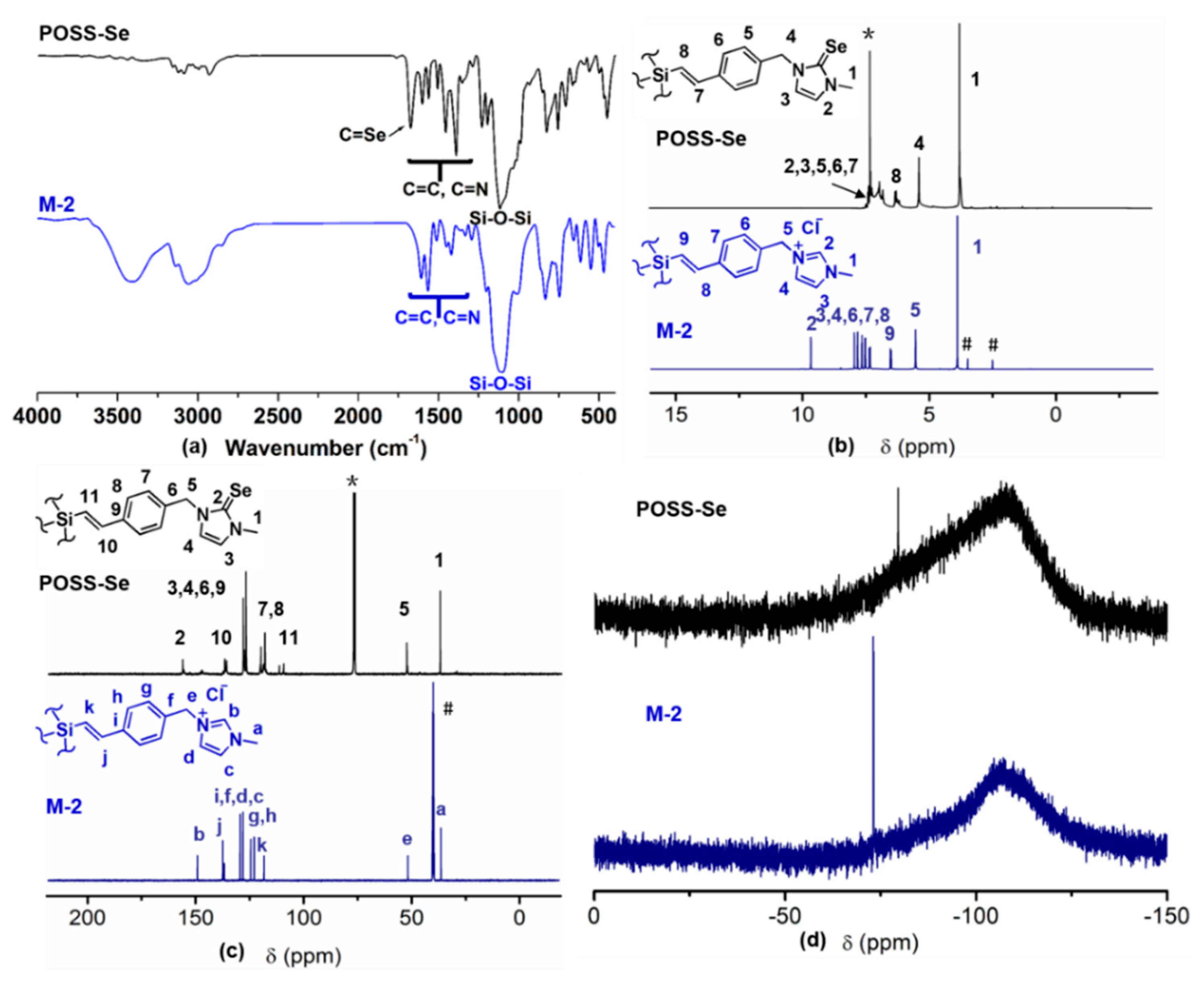
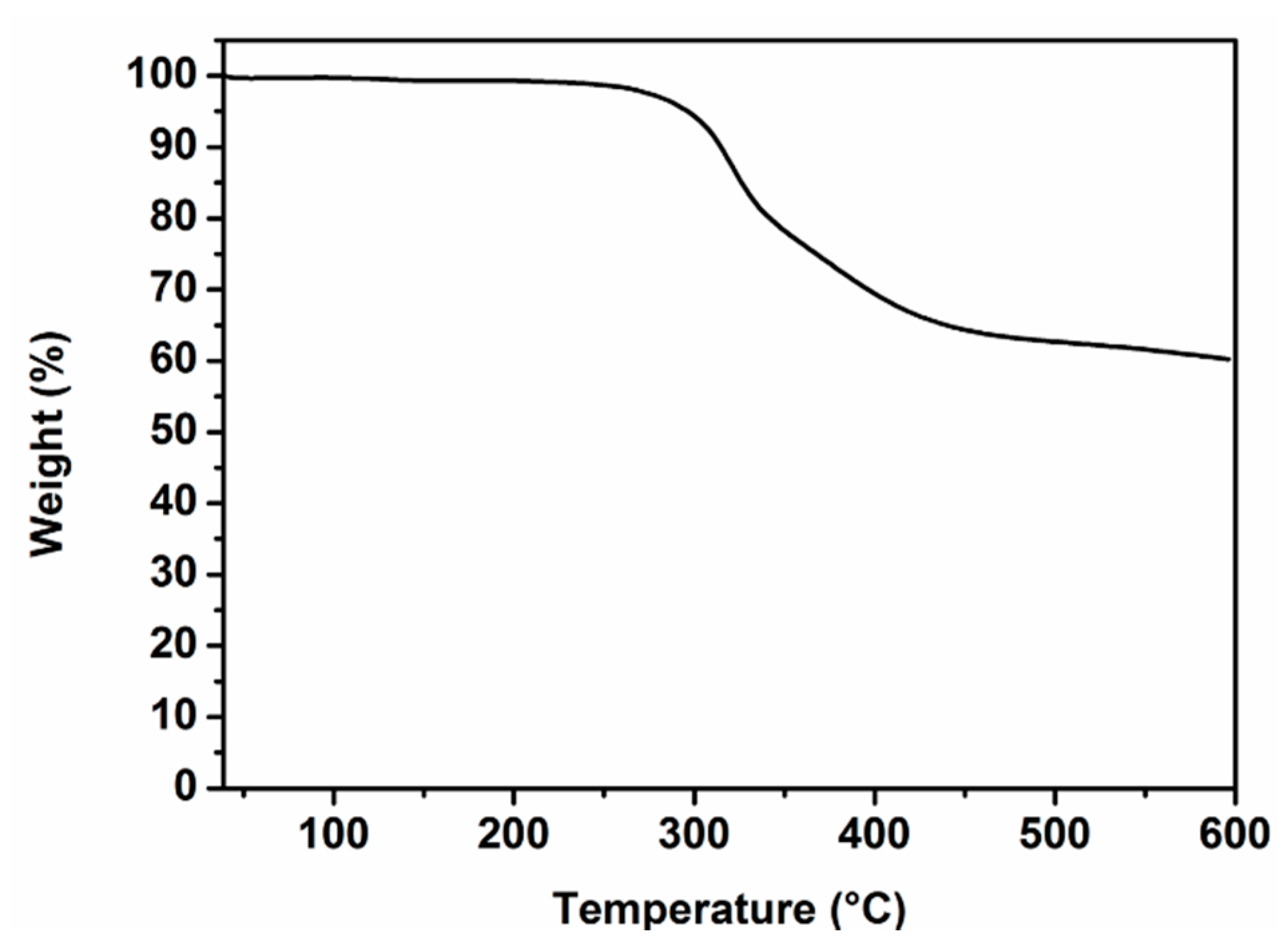
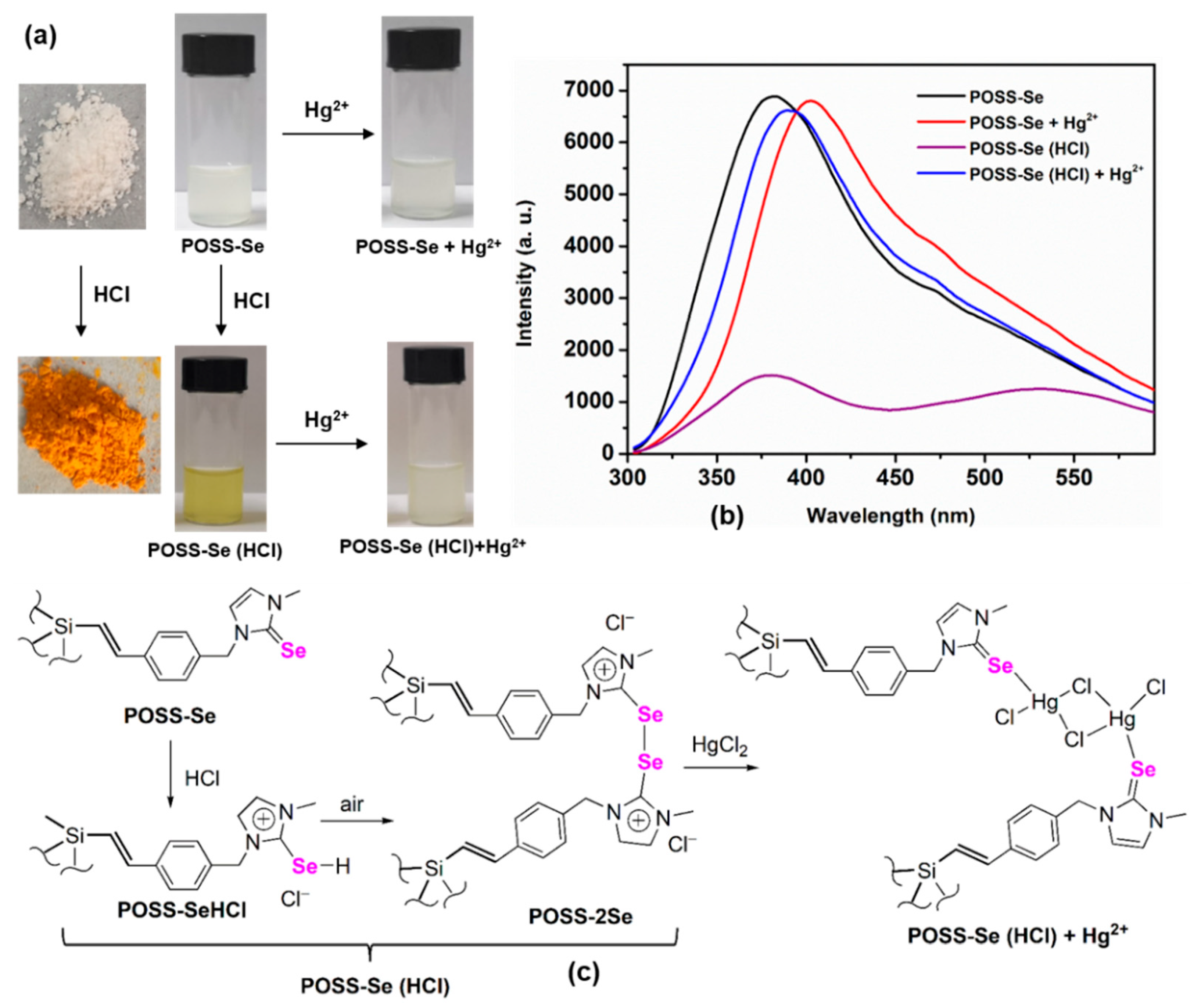
3.1. Synthesis and Characterization
3.2. Detection of Hg2+ Ions by POSS-Se
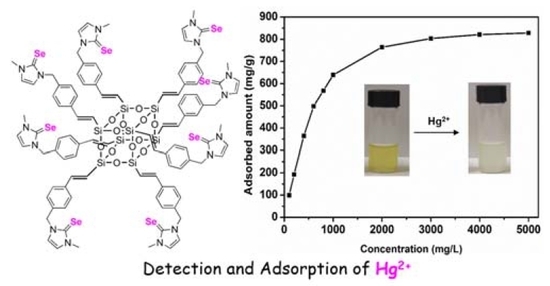
3.3. Adsorption of Hg2+ Ions by POSS-Se
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanaka, K.; Chujo, Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Abuosoaud, M.G.; Kuo, S.-W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2018, 11, 26. [Google Scholar]
- Chen, X.; Ludovic, D. Polyhedral Oligomeric Silsesquioxane (POSS) Nano-Composite Separation Membranes—A Review. Adv. Eng. Mater. 2018, 21, 1800667. [Google Scholar] [CrossRef]
- Chi, H.; Wang, M.; Xiao, Y.; Wang, F.; KS, J. Self-Assembly and Applications of Amphiphilic Hybrid POSS Copolymers. Molecules 2018, 23, 2481. [Google Scholar] [CrossRef]
- Dudziec, B.; Żak, P.; Marciniec, B. Synthetic Routes to Silsesquioxane-Based Systems as Photoactive Materials and Their Precursors. Polymers 2019, 11, 504. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- McCusker, C.; Carroll, J.B.; Rotello, V.M. Cationic polyhedral oligomeric silsesquioxane (POSS) units as carriers for drug delivery processes. Chem. Commun. 2005, 996–998. [Google Scholar] [CrossRef]
- He, H.-B.; Li, B.; Dong, J.-P.; Lei, Y.-Y.; Wang, T.-L.; Yu, Q.-W.; Feng, Y.-Q.; Sun, Y.-B. Mesostructured Nanomagnetic Polyhedral Oligomeric Silsesquioxanes (POSS) Incorporated with Dithiol Organic Anchors for Multiple Pollutants Capturing in Wastewater. ACS Appl. Mater. Interf. 2013, 5, 8058–8066. [Google Scholar] [CrossRef]
- Liu, H.; Sun, R.; Feng, S.; Wang, D.; Liu, H. Rapid synthesis of a silsesquioxane-based disulfide-linked polymer for selective removal of cationic dyes from aqueous solutions. Chem. Eng. J. 2019, 359, 436–445. [Google Scholar] [CrossRef]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Boonkitti, P.; Phuekphong, A.; Prigyai, N.; Kladsomboon, S.; Kiatkamjornwong, S. Anion identification using silsesquioxane cages. Chem. Sci. 2018, 9, 7753–7765. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Huo, X.; Lu, H.; Feng, S.; Wang, D.; Liu, H. Recyclable fluorescent paper sensor for visual detection of nitroaromatic explosives. Sens. Actuators Chem. 2018, 265, 476–487. [Google Scholar] [CrossRef]
- Zhou, H.; Chua, M.H.; Tan, H.R.; Lin, T.T.; Tang, B.Z.; Xu, J. Ionofluorochromic Nanoparticles Derived from Octapyrene-Modified Polyhedral Oligomeric Silsesquioxane Organic Frameworks for Fluoride-Ion Detection. ACS Appl. Nano Mater. 2019, 2, 470–478. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Han, D.; Sun, R.; Zhang, J.; Feng, S. New Polyhedral Oligomeric Silsesquioxanes-Based Fluorescent Ionic Liquids: Synthesis, Self-Assembly and Application in Sensors for Detecting Nitroaromatic Explosives. Polymers 2018, 10, 917. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.-Y.; Gao, G.-G.; Li, B.; Luo, P.; Kong, Y.-J.; Liu, H.; Zang, S.-Q. Smart Transformation of a Polyhedral Oligomeric Silsesquioxane Shell Controlled by Thiolate Silver (I) Nanocluster Core in Cluster@Clusters Dendrimers. Ang. Chem. Int. Ed. 2018, 57, 12775–12779. [Google Scholar] [CrossRef]
- Chen, J.; Shan, J.; Xu, Y.; Su, P.; Tong, L.; Yuwen, L.; Weng, L.; Bao, B.; Wang, L. Polyhedral Oligomeric Silsesquioxane (POSS)-Based Cationic Conjugated Oligoelectrolyte/Porphyrin for Efficient Energy Transfer and Multiamplified Antimicrobial Activity. ACS Appl. Mater. Interfaces 2018, 10, 34455–34463. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Tang, B.Z.; Lo, K.K.-W. Luminescent Molecular Octopuses with a Polyhedral Oligomeric Silsesquioxane (POSS) Core and Iridium (III) Polypyridine Arms: Synthesis, Aggregation Induced Emission, Cellular Uptake, and Bioimaging Studies. Chem. Eur. J. 2019, 25, 10633–10641. [Google Scholar] [CrossRef]
- Han, M.; Li, W.; Chen, R.; Han, Y.; Liu, X.; Wang, T.; Guo, H.; Qiao, X. Amino acid and ionic liquid modified polyhedral oligomeric silsesquioxane-based hybrid monolithic column for high-efficiency capillary liquid chromatography. J. Chromatogr. A 2018, 1572, 82–89. [Google Scholar] [CrossRef]
- Lu, Q.; Fu, J.; Chen, L.; Shang, D.; Li, M.; Xu, Y.; Jia, R.; Yuan, S.; Shi, L. Polymeric polyhedral oligomeric silsesquioxane ionic liquids based solid polymer electrolytes for lithium ion batteries. J. Power Sour. 2019, 414, 31–40. [Google Scholar] [CrossRef]
- Prabu, R.; Peramaiah, K.; Palanisami, N.; Pescarmona, P.P.; Neppolian, B.; Shanmugan, S. Non-covalent polyhedral oligomeric silsesquioxane-polyoxometalates as inorganic-organic-inorganic hybrid materials for visible-light photocatalytic splitting of water. Inorg. Chem. Front. 2018, 5, 2678. [Google Scholar] [CrossRef]
- Calabrese, C.; Liotta, L.F.; Giacalone, F.; Gruttadauria, M.; Aprile, C. Supported Polyhedral Oligomeric Silsesquioxane-Based (POSS) Materials as Highly Active Organocatalysts for the Conversion of CO2. ChemCatChem 2019, 11, 560–567. [Google Scholar] [CrossRef]
- Lee, H.; Hong, S.H. Polyhedral oligomeric silsesquioxane-conjugated bis (diphenylphosphino) amine ligand for chromium (III) catalyzed ethylene trimerization and tetramerization. Appl. Catal. A Gen. 2018, 560, 21–27. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Z.; Xu, Y.; Zhang, S.; Gong, C.; Tang, Y.; Sun, D.; Wei, H.; Shen, C. Facile one-step fabrication of sulfonated polyhedral oligomeric silsesquioxane cross-linked poly (ether ether ketone) for proton exchange membranes. Polym. Chem. 2018, 9, 3624–3632. [Google Scholar] [CrossRef]
- Xu, H.; Cao, W.; Zhang, X. Selenium-Containing Polymers: Promising Biomaterials for Controlled Release and Enzyme Mimics. Acc. Chem. Res. 2013, 46, 1647–1658. [Google Scholar] [CrossRef]
- Mukherjee, A.J.; Zade, S.S.; Singh, H.B.; Sunoj, R.B. Organoselenium Chemistry: Role of Intramolecular Interactions. Chem. Rev. 2010, 110, 4357–4416. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Nocentini, A.; Capperucci, A.; Ferraroni, M.; Gratteri, P.; Supuran, C.T. Selenols: A new class of carbonic anhydrase inhibitors. Chem. Commun. 2019, 55, 648–651. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Et Biophys. Acta-Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Yu, F.; Li, P.; Li, G.; Zhao, G.; Chu, T.; Han, K. A Near-IR Reversible Fluorescent Probe Modulated by Selenium for Monitoring Peroxynitrite and Imaging in Living Cells. J. Am. Chem. Soc. 2011, 133, 11030–11033. [Google Scholar] [CrossRef]
- Luo, J.; Cao, Q.; Cao, X.; Zhao, X. Selenide-catalyzed enantioselective synthesis of trifluoromethylthiolated tetrahydronaphthalenes by merging desymmetrization and trifluoromethylthiolation. Nat. Commun. 2018, 9, 527. [Google Scholar] [CrossRef]
- Choi, J.; Park, S.Y.; Yang, H.Y.; Kim, H.J.; Ihm, K.; Nam, J.H.; Ahn, J.R.; Son, S.U. Submicro-polymer particles bearing imidazoline-2-selenone: Dual mode adsorbents with color-sensing for halogens and mercury ions. Polym. Chem. 2011, 2, 2512–2517. [Google Scholar] [CrossRef]
- Panda, S.; Panda, A.; Zade, S.S. Organoselenium compounds as fluorescent probes. Coord. Chem. Rev. 2015, 300, 86–100. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Kubo, M.; Moteki, T.; Sugawara-Narutaki, A.; Shimojima, A.; Okubo, T. Porous Siloxane–Organic Hybrid with Ultrahigh Surface Area through Simultaneous Polymerization-Destruction of Functionalized Cubic Siloxane Cages. J. Am. Chem. Soc. 2011, 133, 13832–13835. [Google Scholar] [CrossRef] [PubMed]
- Velempini, T.; Pillay, K. Sulphur functionalized materials for Hg (II) adsorption: A review. J. Environ. Chem. Eng. 2019, 7, Unsp 103350. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H. Nanomaterials for the adsorptive treatment of Hg (II) ions from water. Chem. Eng. J. 2019, 358, 264–282. [Google Scholar] [CrossRef]
- Tang, B.; Ding, B.Y.; Xu, K.H.; Tong, L.L. Use of Selenium to Detect Mercury in Water and Cells: An Enhancement of the Sensitivity and Specificity of a Seleno Fluorescent Probe. Chem. Eur. J. 2009, 15, 3147–3151. [Google Scholar] [CrossRef]
- Shi, W.; Sun, S.; Li, X.; Ma, H. Imaging Different Interactions of Mercury and Silver with Live Cells by a Designed Fluorescence Probe Rhodamine B Selenolactone. Inorg. Chem. 2010, 49, 1206–1210. [Google Scholar] [CrossRef]
- Choi, J.; Ko, J.H.; Jung, I.G.; Yang, H.Y.; Ko, K.C.; Lee, J.Y.; Lee, S.M.; Kim, H.J.; Nam, J.H.; Ahn, J.R.; et al. Reaction of Imidazoline-2-Selone with Acids and Its Use for Selective Coordination of Platinum Ions on Silica Surface. Chem. Mater. 2009, 21, 2571–2573. [Google Scholar] [CrossRef]
- Saleem, M.; Rafiq, M.; Hanif, M. Organic Material Based Fluorescent Sensor for Hg2+: A Brief Review on Recent Development. J. Fluoresc. 2017, 27, 31–58. [Google Scholar] [CrossRef]
- Egan, J.G.; Hynes, A.J.; Fruehwald, H.M.; Ebralidze, I.I.; King, S.D.; Esfahani, R.A.M.; Naumkin, F.Y.; Easton, E.B.; Zenkina, O.V. A novel material for the detection and removal of mercury (II) based on a 2,6-bis (2-thienyl) pyridine receptor. J. Mater. Chem. C 2019, 7, 10187–10195. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Dong, M.; Wang, Y.-W.; Chen, Y.-T.; Wang, H.-Z.; Su, C.-Y.; Wang, W. Thioether-Based Fluorescent Covalent Organic Framework for Selective Detection and Facile Removal of Mercury (II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Du, T.; Zhang, W.T.; Zhu, W.X.; Yang, C.Y.; Yue, T.L.; Sun, J.; Li, T.; Wang, J.L. NH2-MIL-53(Al) Metal-Organic Framework as the Smart Platform for Simultaneous High-Performance Detection and Removal of Hg2+. Inorg. Chem. 2019, 58, 12573–12581. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Q.; Li, C.; Liu, S.; Xia, K.S.; Han, B.; Zhou, C.G. Fabrication of Organic Probe Decorated Water-Soluble Polymer Chains on Natural Fibers for Selective Detection and Efficient Removal of Hg2+ Ions in Pure Aqueous Media. ACS Appl. Polym. Mater. 2019, 1, 2680–2691. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gohil, H.; Raval, I.; Chatterjee, S.; Paital, A.R. An Anthracene Excimer Fluorescence Probe on Mesoporous Silica for Dual Functions of Detection and Adsorption of Mercury (II) and Copper (II) with Biological In Vivo Applications. Small 2019, 15, 1804749. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Li, C.; Nabeel, F.; Huang, W.; Zhou, Y. Self-assembly of alternating copolymer vesicles for the highly selective, sensitive and visual detection and quantification of aqueous Hg2+. Chem. Eng. J. 2019, 358, 101–109. [Google Scholar] [CrossRef]
- Samb, I.; Bell, J.; Toullec, P.Y.; Michelet, V.; Leray, I. Fluorescent Phosphane Selenide As Efficient Mercury Chemodosimeter. Org. Lett. 2011, 13, 1182–1185. [Google Scholar] [CrossRef]
- Manna, B.; Raj, C.R. Nanostructured Sulfur-Doped Porous Reduced Graphene Oxide for the Ultrasensitive Electrochemical Detection and Efficient Removal of Hg(II). ACS Sustain. Chem. Eng. 2018, 6, 6175–6182. [Google Scholar] [CrossRef]
- Cheng, H.-R.; Qian, Y. Intramolecular fluorescence resonance energy transfer in a novel PDI–BODIPY dendritic structure: Synthesis, Hg2+ sensor and living cell imaging. Sens. Actuators Chem. 2015, 219, 57–64. [Google Scholar] [CrossRef]
- Abbas, K.; Znad, H.; Awual, M.R. A ligand anchored conjugate adsorbent for effective mercury(II) detection and removal from aqueous media. Chem. Eng. J. 2018, 334, 432–443. [Google Scholar] [CrossRef]
- Makam, P.; Shilpa, R.; Kandjani, A.E.; Periasamy, S.R.; Sabri, Y.M.; Madhu, C.; Bhargava, S.K.; Govindaraju, T. SERS and fluorescence-based ultrasensitive detection of mercury in water. Biosens. Bioelectron. 2018, 100, 556–564. [Google Scholar] [CrossRef]
- Niu, Y.; Qu, R.; Chen, H.; Mu, L.; Liu, X.; Wang, T.; Zhang, Y.; Sun, C. Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of Hg(II) from aqueous solution. J. Hazard. Mater. 2014, 278, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Ram, B.; Chauhan, G.S. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. Chem. Eng. J. 2018, 331, 587–596. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Xu, H.; Jiang, D. Stable Covalent Organic Frameworks for Exceptional Mercury Removal from Aqueous Solutions. J. Am. Chem. Soc. 2017, 139, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, N.; Zhang, Q.; Chen, S. Adsorption of Hg2+ on a novel chelating fiber prepared by preirradiation grafting and amination. J. Appl. Polym. Sci. 2009, 113, 3638–3645. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.-G.; Yuan, Y.-P.; Peng, F.-M.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.; Earl, L.D.; Abney, C.W.; Cheng, Y.; Wei, H.; Nguyen, N.; Wojtas, L.; Ma, S. Postsynthetically Modified Covalent Organic Frameworks for Efficient and Effective Mercury Removal. J. Am. Chem. Soc. 2017, 139, 2786–2793. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, X.; Zhang, G.; Hu, J.; Sheng, W.; Ma, Z.; Lu, J.; Liu, Z. Efficient removal and highly selective adsorption of Hg2+ by polydopamine nanospheres with total recycle capacity. Appl. Surf. Sci. 2014, 314, 166–173. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Wang, H.; Tong, Y.; Sheng, X.; Sun, Y.; Zhou, X.; Zhou, Q. Simultaneous enrichment and determination of cadmium and mercury ions using magnetic PAMAM dendrimers as the adsorbents for magnetic solid phase extraction coupled with high performance liquid chromatography. J. Hazard. Mater. 2020, 121658. [Google Scholar] [CrossRef]
| Samples | LOD/ppb | Adsorption Capacity/mg g−1 | Ref. |
|---|---|---|---|
| POSS-Se | 8.48 a | 952 | This work |
| Fe3O4@TiO2–L | 1.40 ppm | 15 b | [40] |
| Thiol-based fluorescent covalent organic framework (COF-LZU8) | 25.0 | - | [41] |
| SiO2@PBATPA | 37 | 482 | [44] |
| NH2-MIL-53(Al) | 30.13 b | 153.85 | [42] |
| Salicylaldehyde-containing polymeric quaternary ammonium chains onto the natural fibers (MA−SAPQA@NFs) | 16.72 b | 218.21 | [43] |
| Vesicular chemical sensor | 10.64 b | [45] | |
| PDI–BODIPY dendrimer | 4.02 b | - | [48] |
| Mesoporous conjugated adsorbent | 3.69 | 172.61b | [49] |
| Rhodamine B selenolactone | 4.62 | - | [37] |
| fluorescent phosphane selenide | 0.18 | - | [46] |
| Sulfur-doped porous reduced graphene oxide | 0.1 | 829.27 | [47] |
| Histidine conjugated perylene diimide bolaamphiphile | 0.01 ppq | - | [50] |
| SiO2-G0-SA∼SiO2-G2.0-SA | - | 363.07 b | [51] |
| Polymer particles containing imidazoline selenones | - | 110 | [31] |
| Cellulose and thiol-based adsorbent | - | 404.95 | [52] |
| TAPB-BMTTPA-COF | - | 734 | [53] |
| Chelating fiber ACHF | - | 785.2 | [54] |
| Thiol-functionalized [Cu3(BTC)2]n | - | 714.29 | [55] |
| COF-S-SH | - | 1350 | [56] |
| Polydopamine nanospheres | - | 1861.72 | [57] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Chen, Z.; Feng, S.; Wang, D.; Liu, H. A Selenone-Functionalized Polyhedral Oligomeric Silsesquioxane for Selective Detection and Adsorption of Hg2+ ions in Aqueous Solutions. Polymers 2019, 11, 2084. https://doi.org/10.3390/polym11122084
Liu H, Chen Z, Feng S, Wang D, Liu H. A Selenone-Functionalized Polyhedral Oligomeric Silsesquioxane for Selective Detection and Adsorption of Hg2+ ions in Aqueous Solutions. Polymers. 2019; 11(12):2084. https://doi.org/10.3390/polym11122084
Chicago/Turabian StyleLiu, Hailong, Zixu Chen, Shengyu Feng, Dengxu Wang, and Hongzhi Liu. 2019. "A Selenone-Functionalized Polyhedral Oligomeric Silsesquioxane for Selective Detection and Adsorption of Hg2+ ions in Aqueous Solutions" Polymers 11, no. 12: 2084. https://doi.org/10.3390/polym11122084
APA StyleLiu, H., Chen, Z., Feng, S., Wang, D., & Liu, H. (2019). A Selenone-Functionalized Polyhedral Oligomeric Silsesquioxane for Selective Detection and Adsorption of Hg2+ ions in Aqueous Solutions. Polymers, 11(12), 2084. https://doi.org/10.3390/polym11122084