Nanocomposite Furcellaran Films—the Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Graphene Oxide (GO)
2.2.2. Preparation of Carbon Dots (CQDs)
2.2.3. Preparation of Modified Maghemite (MAN)
2.2.4. Preparation of Nanocomposite Furcellaran Films
2.2.5. Fourier Transform Infrared Spectra (FTIR)
2.2.6. X-ray Diffraction (XRD)
2.2.7. Scanning Electron Microscopy (SEM)
2.2.8. Physical Properties
Thickness
Mechanical Properties
Water Content and Solubility
UV-Visible Absorption Spectra
The Color Measurement of Films
2.2.9. Biological Properties of Films
Antibacterial Properties of Films
Antioxidant Properties of Films
2.2.10. Oil Stability after UV Treatment
UV-B Treatment
PV, FFA, and MDA Content
2.3. Statistical Analysis
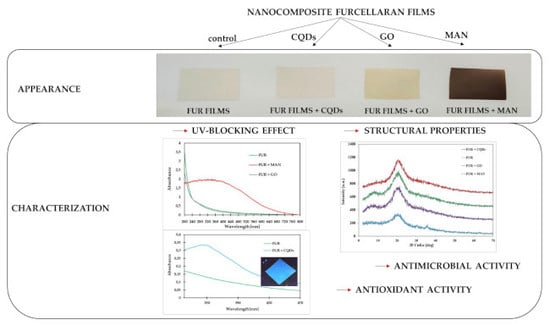
3. Results and Discussion
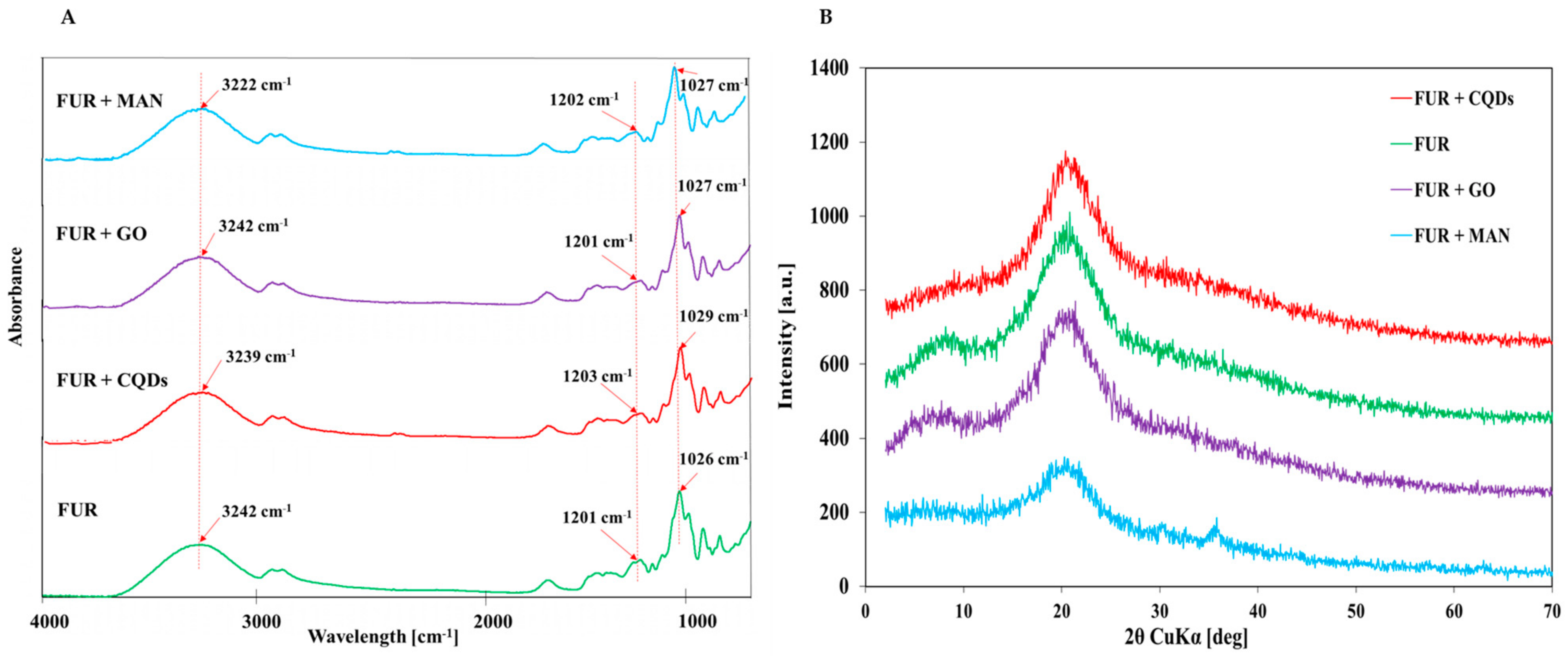
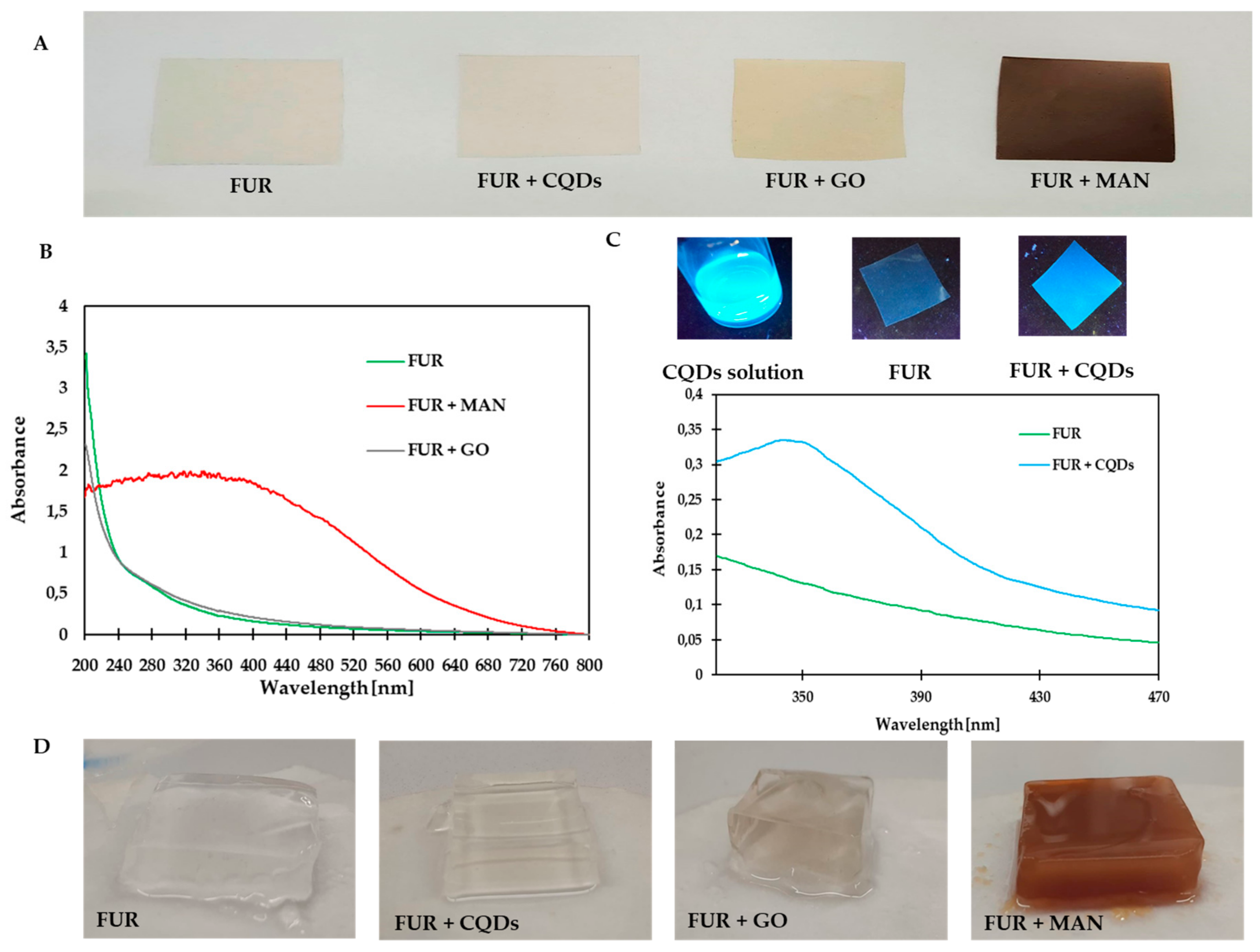
3.1. Structural and Physical Properties
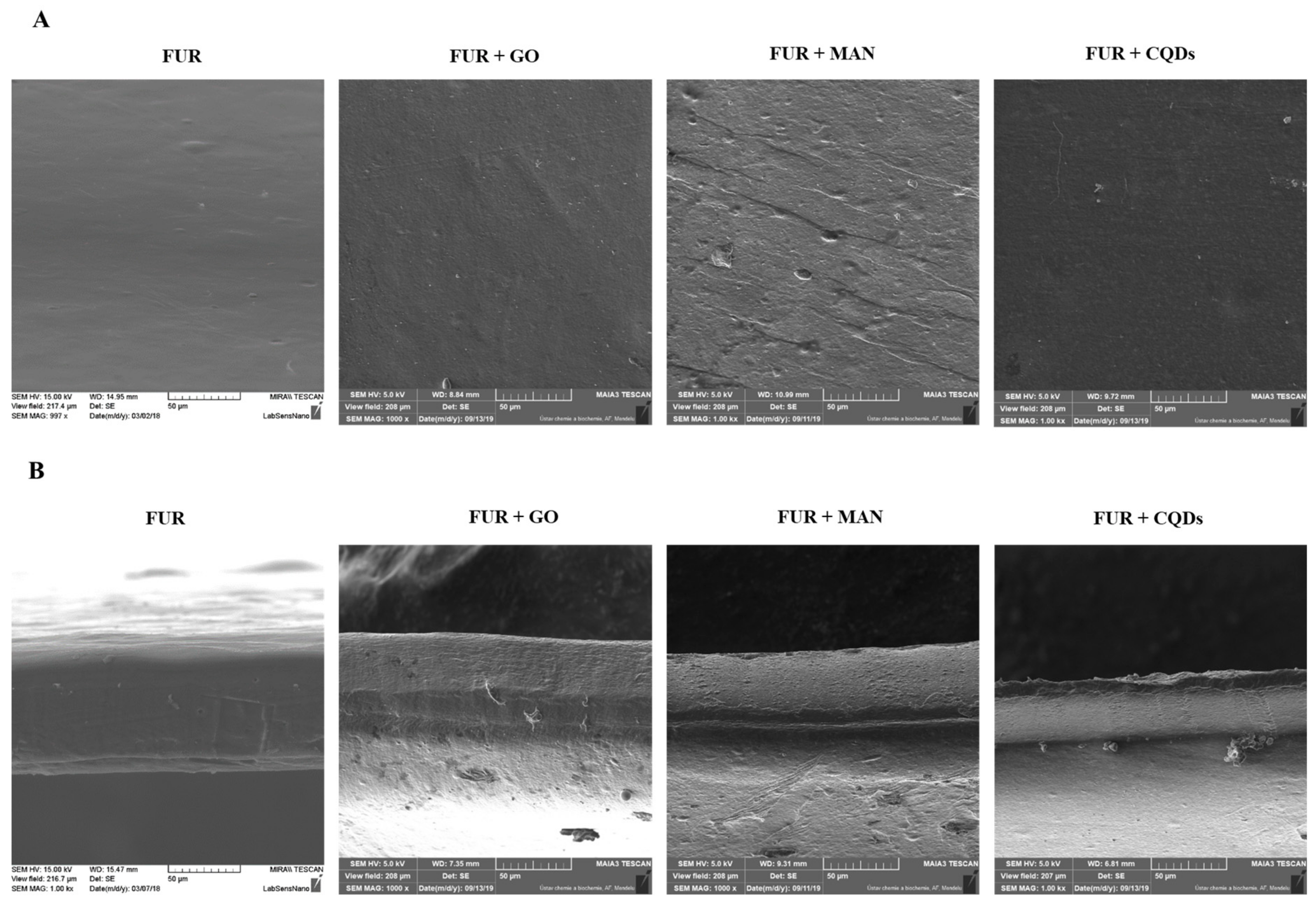
3.2. Morphology
3.3. Biological Properties
3.3.1. Antimicrobial Properties
3.3.2. Antioxidant Activity of Films
3.4. Oil Stability after UV Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Thai, T.L.A. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocoll. 2017, 71, 141–148. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Mostafa, T.B. Effect of γ-rays on carboxymethyl chitosan for use as antioxidant and preservative coating for peach fruit. Carbohydr. Polym. 2014, 104, 109–117. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Bindu, J.; Sivaraman, G.K.; Venkateshwarlu, G.; Ravishankar, C.N. Effect of chitosan based active packaging film on the keeping quality of chilled stored barracuda fish. J. Food Sci. Technol. 2016, 53, 685–693. [Google Scholar] [CrossRef]
- Shankar, S.; Kasapis, S.; Rhim, J.-W. Alginate-based nanocomposite films reinforced with halloysite nanotubes functionalized by alkali treatment and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1824–1832. [Google Scholar] [CrossRef]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W.; Kim, H.-Y. Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. Carbohydr. Polym. 2015, 117, 468–475. [Google Scholar] [CrossRef]
- Laos, K.; Brownsey, G.J.; Ring, S.G. Interactions between furcellaran and the globular proteins bovine serum albumin and β-lactoglobulin. Carbohydr. Polym. 2007, 67, 116–123. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The verification of intelligent properties of furcellaran films with plant extracts on the stored fresh Atlantic mackerel during storage at 2 °C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Zając, M.; Guzik, P.; Tkaczewska, J. The effect of furcellaran-gelatin edible coatings with green and pu-erh tea extracts on the microbiological, physicochemical and sensory changes of salmon sushi stored at 4 °C. Food Control 2019, 100, 83–91. [Google Scholar] [CrossRef]
- Jancikova, S.; Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Dordevic, D. Furcellaran/gelatin hydrolysate/rosemary extract composite films as active and intelligent packaging materials. Int. J. Biol. Macromol. 2019, 131, 19–28. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljevic, V.; Makarewicz, M. Development of furcellaran-gelatin films with Se-AgNPs as an active packaging system for extension of mini kiwi shelf life. Food Packag. Shelf Life 2019, 21, 100339. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and active furcellaran-gelatin films containing green or pu-erh tea extracts: Characterization, antioxidant and antimicrobial potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef]
- Namazi, H.; Belali, S. Starch-g-lactic acid/montmorillonite nanocomposite: Synthesis, characterization and controlled drug release study. Starch-Stärke 2016, 68, 177–187. [Google Scholar] [CrossRef]
- Barkhordari, S.; Yadollahi, M.; Namazi, H. pH sensitive nanocomposite hydrogel beads based on carboxymethyl cellulose/layered double hydroxide as drug delivery systems. J. Polym. Res. 2014, 21, 454. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films. Carbohydr. Polym. 2016, 147, 372–378. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Solid state photoluminescence thermoplastic starch film containing graphene quantum dots. Carbohydr. Polym. 2017, 176, 220–226. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, J.; Ma, J.; Yuan, G.; Sun, H. Effect of chitosan-carvacrol coating on the quality of Pacific white shrimp during iced storage as affected by caprylic acid. Int. J. Biol. Macromol. 2018, 106, 123–129. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Prodan, A.M.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Synthesis and characterization of polysaccharide-maghemite composite nanoparticles and their antibacterial properties. Nanoscale Res. Lett. 2012, 7, 576. [Google Scholar] [CrossRef]
- Khan, Z.M.S.H.; Rahman, R.S.; Shumaila; Islam, S.; Zulfequar, M. Hydrothermal treatment of red lentils for the synthesis of fluorescent carbon quantum dots and its application for sensing Fe3+. Opt. Mater. 2019, 91, 386–395. [Google Scholar] [CrossRef]
- Kosowska, K.; Domalik-Pyzik, P.; Nocuń, M.; Chłopek, J. Chitosan and graphene oxide/reduced graphene oxide hybrid nanocomposites—Evaluation of physicochemical properties. Mater. Chem. Phys. 2018, 216, 28–36. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Preparation and characterization of polyvinyl alcohol/β-cyclodextrin/GO-Ag nanocomposite with improved antibacterial and strength properties. Polym. Adv. Technol. 2019, 30, 447–456. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Xiao, L.; Lin, D.; Yang, Y.; Wang, H.; Yang, Y.; Wu, D.; Chen, H.; Zhang, Q.; et al. Physical properties and structural characterization of starch/polyvinyl alcohol/graphene oxide composite films. Int. J. Biol. Macromol. 2019, 123, 569–575. [Google Scholar] [CrossRef]
- Wang, H.; Chen, M.; Jin, C.; Niu, B.; Jiang, S.; Li, X.; Jiang, S. Antibacterial [2-(Methacryloyloxy) ethyl] Trimethylammonium Chloride Functionalized Reduced Graphene Oxide/Poly(ethylene-co-vinyl alcohol) Multilayer Barrier Film for Food Packaging. J. Agric. Food Chem. 2018, 66, 732–739. [Google Scholar] [CrossRef]
- Juita, J.; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Low temperature oxidation of linseed oil: A review. Fire Sci. Rev. 2012, 1, 3. [Google Scholar] [CrossRef]
- Parker, T.D.; Adams, D.A.; Zhou, K.; Harris, M.; Yu, L. Fatty Acid Composition and Oxidative Stability of Cold-pressed Edible Seed Oils. J. Food Sci. 2003, 68, 1240–1243. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Vlachova, J.; Tmejova, K.; Kopel, P.; Korabik, M.; Zitka, J.; Hynek, D.; Kynicky, J.; Adam, V.; Kizek, R. A 3D Microfluidic Chip for Electrochemical Detection of Hydrolysed Nucleic Bases by a Modified Glassy Carbon Electrode. Sensors 2015, 15, 2438–2452. [Google Scholar] [CrossRef]
- Kudr, J.; Richtera, L.; Nejdl, L.; Xhaxhiu, K.; Vitek, P.; Rutkay-Nedecky, B.; Hynek, D.; Kopel, P.; Adam, V.; Kizek, R. Improved Electrochemical Detection of Zinc Ions Using Electrode Modified with Electrochemically Reduced Graphene Oxide. Materials 2016, 9, 31. [Google Scholar] [CrossRef]
- Nejdl, L.; Kudr, J.; Cihalova, K.; Chudobova, D.; Zurek, M.; Zalud, L.; Kopecny, L.; Burian, F.; Ruttkay-Nedecky, B.; Krizkova, S.; et al. Remote-controlled robotic platform ORPHEUS as a new tool for detection of bacteria in the environment. Electrophoresis 2014, 35, 2333–2345. [Google Scholar] [CrossRef]
- Heger, Z.; Zitka, J.; Cernei, N.; Krizkova, S.; Sztalmachova, M.; Kopel, P.; Masarik, M.; Hodek, P.; Zitka, O.; Adam, V.; et al. 3D-printed biosensor with poly(dimethylsiloxane) reservoir for magnetic separation and quantum dots-based immunolabeling of metallothionein. Electrophoresis 2015, 36, 1256–1264. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crop. Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R. Antibacterial, antioxidant and optical properties of edible starch-chitosan composite film containing Thymus kotschyanus essential oil. Vet. Res. Forum 2012, 3, 167–173. [Google Scholar] [PubMed]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. Int. J. Biol. Macromol. 2017, 94, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Konieczka, P.; Rozbicka-Wieczorek, A.J.; Więsyk, E.; Smulikowska, S.; Czauderna, M. Improved derivatization of malondialdehyde with 2-thiobarbituric acid for evaluation of oxidative stress in selected tissues of chickens. J. Anim. Feed Sci. 2014, 23, 190–197. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P.; Balková, R.; Hynek, D.; Bytesnikova, Z.; Gagic, M.; Milosavljevic, V.; Adam, V. Intelligent and active composite films based on furcellaran: Structural characterization, antioxidant and antimicrobial activities. Food Packag. Shelf Life 2019, 22, 100405. [Google Scholar] [CrossRef]
- Rana, P.; Sharma, S.; Sharma, R.; Banerjee, K. Apple pectin supported superparamagnetic (γ-Fe2O3) maghemite nanoparticles with antimicrobial potency. Mater. Sci. Energy Technol. 2019, 2, 15–21. [Google Scholar] [CrossRef]
- You, Y.; Zhang, H.; Liu, Y.; Lei, B. Transparent sunlight conversion film based on carboxymethyl cellulose and carbon dots. Carbohydr. Polym. 2016, 151, 245–250. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Lizundia, E.; Vilas, J.L.; Sangroniz, A.; Etxeberria, A. Light and gas barrier properties of PLLA/metallic nanoparticles composite films. Eur. Polym. J. 2017, 91, 10–20. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, S.R.; He, Q.S.; Bazinet, L.; Udenigwe, C.C. Encapsulation of food protein hydrolysates and peptides: A review. RSC Adv. 2015, 5, 79270–79278. [Google Scholar] [CrossRef]
- Yamashita, S.; Sugita-Konishi, Y.; Shimizu, M. In vitro bacteriostatic effects of dietary polysaccharides. Food Sci. Technol. Res. 2001, 7, 262–264. [Google Scholar] [CrossRef]
- Raman, M.; Devi, V.; Doble, M. Biocompatible ι-carrageenan-γ-maghemite nanocomposite for biomedical applications–synthesis, characterization and in vitro anticancer efficacy. J. Nanobiotech. 2015, 13, 18. [Google Scholar] [CrossRef]
- Bing, W.; Sun, H.; Yan, Z.; Ren, J.; Qu, X. Programmed Bacteria Death Induced by Carbon Dots with Different Surface Charge. Small 2016, 12, 4713–4718. [Google Scholar] [CrossRef]
- Li, Y.-J.; Harroun, S.G.; Su, Y.-C.; Huang, C.-F.; Unnikrishnan, B.; Lin, H.-J.; Lin, C.-H.; Huang, C.-C. Synthesis of Self-Assembled Spermidine-Carbon Quantum Dots Effective against Multidrug-Resistant Bacteria. Adv. Healthc. Mater. 2016, 5, 2545–2554. [Google Scholar] [CrossRef]
- Laos, K.; Lõugas, T.; Mändmets, A.; Vokk, R. Encapsulation of β-carotene from sea buckthorn (Hippophaë rhamnoides L.) juice in furcellaran beads. Innov. Food Sci. Emerg. Technol. 2007, 8, 395–398. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljević, V.; Kucharek, M.; Makarewicz, M.; Adam, V. Development and characterisation of furcellaran-gelatin films containing SeNPs and AgNPs that have antimicrobial activity. Food Hydrocoll. 2018, 83, 9–16. [Google Scholar] [CrossRef]
- Gu, Y.S.; Regnier, L.; McClements, D.J. Influence of environmental stresses on stability of oil-in-water emulsions containing droplets stabilized by beta-lactoglobulin-iota-carrageenan membranes. J. Colloid Interface Sci. 2005, 286, 551–558. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Chu, C.; She, Y.; Jiang, S.; Zhai, L.; Jiang, S.; Li, X. Diffusion and Antibacterial Properties of Nisin-Loaded Chitosan/Poly (L-Lactic Acid) Towards Development of Active Food Packaging Film. Food Bioprocess Technol. 2015, 8, 1657–1667. [Google Scholar] [CrossRef]
- Farhoudian, S.; Yadollahi, M.; Namazi, H. Facile synthesis of antibacterial chitosan/CuO bio-nanocomposite hydrogel beads. Int. J. Biol. Macromol. 2016, 82, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Benjakul, S.; Martínez-Alvarez, O.; Rawdkuen, S. Fish skin gelatin hydrolysates produced by visceral peptidase and bovine trypsin: Bioactivity and stability. Food Chem. 2017, 215, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Hastak, V.; Bandi, S.; Kashyap, S.; Singh, S.; Luqman, S.; Lodhe, M.; Peshwe, D.; Srivastav, A.K. Antioxidant efficacy of chitosan/graphene functionalized superparamagnetic iron oxide nanoparticles. J. Mater. Sci. Mater. Med. 2018, 29, 154. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, Z.; Owens, A.C.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar] [CrossRef]
- Christensen, I.L.; Sun, Y.-P.; Juzenas, P. Carbon dots as antioxidants and prooxidants. J. Biomed. Nanotechnol. 2011, 7, 667–676. [Google Scholar] [CrossRef]
- Li, D.; Na, X.; Wang, H.; Xie, Y.; Cong, S.; Song, Y.; Xu, X.; Zhu, B.-W.; Tan, M. Fluorescent Carbon Dots Derived from Maillard Reaction Products: Their Properties, Biodistribution, Cytotoxicity, and Antioxidant Activity. J. Agric. Food Chem. 2018, 66, 1569–1575. [Google Scholar] [CrossRef]
- Suzuki, A.; Ulfiati, R.; Masuko, M. Evaluation of antioxidants in rapeseed oils for railway application. Tribol. Int. 2009, 42, 987–994. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Oomah, B.D.; Busson, M.; Godfrey, D.V.; Drover, J.C.G. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- Wang, H.; Zu, G.; Yang, L.; Zu, Y.-G.; Wang, H.; Zhang, Z.-H.; Zhang, Y.; Zhang, L.; Wang, H.-Z. Effects of Heat and Ultraviolet Radiation on the Oxidative Stability of Pine Nut Oil Supplemented with Carnosic Acid. J. Agric. Food Chem. 2011, 59, 13018–13025. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Bopitiya, D.; Madhujith, T. A comparative study on stability of different types of coconut (Cocos nucifera) oil against autoxidation and photo-oxidation. Afr. J. Food Sci. 2018, 12, 216–229. [Google Scholar]
- Haila, K.; Heinonen, M. Action of β-Carotene on Purified Rapeseed Oil During Light Storage. LWT Food Sci. Technol. 1994, 27, 573–577. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Huang, F.; Liu, C.; Wang, H. Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J. Food Compos. Anal. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Soman, R.; Raman, M. HACCP system—Hazard analysis and assessment, based on ISO 22000:2005 methodology. Food Control 2016, 69, 191–195. [Google Scholar] [CrossRef]
- Kourtis, L.K.; Arvanitoyannis, I.S. Implementation of hazard analysis critical control point (HACCP) system to the alcoholic beverages industry. Food Rev. Int. 2001, 17, 1–44. [Google Scholar] [CrossRef]
- Aranda, A.; Zabalza, I.; Scarpellini, S. Economic and environmental analysis of the wine bottle production in Spain by means of life cycle assessment. Int. J. Agric. Resour. Gov. Ecol. 2005, 4, 178–191. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Rodrigues, R.; Flynn, D. Packaging Influences on Olive Oil Quality: A Review of the Literature; UC Davis Olive Center: Davis, CA, USA, 2014. [Google Scholar]
- Piscopo, A.; Poiana, M. Packaging and storage of olive oil. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; IntechOpen: London, UK, 2012; pp. 201–222. [Google Scholar]
- Moyano, M.J.; Heredia, F.J.; Meléndez-Martínez, A.J. The Color of Olive Oils: The Pigments and Their Likely Health Benefits and Visual and Instrumental Methods of Analysis. Compr. Rev. Food Sci. Food Saf. 2010, 9, 278–291. [Google Scholar] [CrossRef]
| Type of Films * | L * | a * | b * | ΔE |
|---|---|---|---|---|
| FUR | 91.09 c ± 0.98 | −0.49 a ± 0.04 | 5.73 a ± 0.13 | |
| FUR + CQDs | 91.76 c ± 0.37 | −0.58 a ± 0.03 | 5.66 a ± 0.18 | 0.67 |
| FUR + MAN | 25.86 a ± 1.21 | 11.33 c ± 1.42 | 8.62 b ± 1.17 | 66.37 |
| FUR + GO | 82.62 b ± 0.75 | 0.50 b ± 0.08 | 11.06 c ± 0.59 | 10.06 |
| Type of Films * | Thickness [nm] | Tensile Strength [MPa] | Elongation at Break [%] | Water Content [%] | Solubility [%] |
|---|---|---|---|---|---|
| FUR | 0.08 a ± 0.00 | 13.69 a ± 0.61 | 72.40 b ± 1.13 | 26.1 a ± 1.2 | 91.8 c ± 2.2 |
| FUR + CQDs | 0.08 a ± 0.00 | 13.71 a ± 0.69 | 72.26 ab ± 1.26 | 22.0 a ± 0.8 | 63.1 b ± 4.7 |
| FUR + GO | 0.08 a ± 0.00 | 18.66 b ± 0.05 | 71.00 ab ± 2.66 | 24.3 a ± 1.8 | 62.9 b ± 3.4 |
| FUR + MAN | 0.08 a ± 0.00 | 18.63 b ± 0.01 | 69.55 a ± 1.24 | 22.0 a ± 3.9 | 32.4 a ± 2.7 |
| Type of Film | S. aureus | E. coli | S. enteritidis |
|---|---|---|---|
| FUR | - | - | + |
| FUR + CQDs | + | + | + |
| FUR + GO | - | - | + |
| FUR + MAN | - | - | - |
| Type of Films * | FRAP [mM/L] | DPPH [%] | Chelating Activity [%] |
|---|---|---|---|
| FUR | 0.008 b ± 0.000 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| FUR + MAN | 0.010 c ± 0.001 | 8.90 b ± 0.46 | 0.00 a ± 0.00 |
| FUR + CQDs | 0.002 a ± 0.000 | 10.30 bc ± 1.07 | 18.63 b ± 1.30 |
| FUR + GO | 0.005 b ± 0.001 | 11.69 c ± 1.76 | 0.00 a ± 0.00 |
| Type of Sample * | PV [meq O2/kg] | FFA [mg KOH/g] | MDA [mmol/L] | Carotenoids [mg/kg] |
|---|---|---|---|---|
| Untreated | 3.52 a ± 0.17 | 0.65 a ± 0.03 | 0.018 a ± 0.006 | 583.9 f ± 1.1 |
| UV-Control | 5.14 b ± 0.46 | 0.78 a ± 0.19 | 0.035 b ± 0.009 | 568.2 d ± 0.2 |
| T | 3.77 a ± 0.36 | 0.76 a ± 0.03 | 0.061 d ± 0.015 | 551.8 b ± 0.7 |
| FUR + MAN | 3.57 a ± 0.01 | 0.74 a ± 0.03 | 0.040 bc ± 0.005 | 525.7 a ± 0.9 |
| FUR + GO | 4.75 b ± 0.35 | 0.71 a ± 0.04 | 0.048 bcd ± 0.000 | 572.9 e ± 0.2 |
| FUR + CQDs | 5.03 b ± 0.42 | 0.71 a ± 0.04 | 0.055 cd ± 0.001 | 563.0 c ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamróz, E.; Kopel, P.; Tkaczewska, J.; Dordevic, D.; Jancikova, S.; Kulawik, P.; Milosavljevic, V.; Dolezelikova, K.; Smerkova, K.; Svec, P.; et al. Nanocomposite Furcellaran Films—the Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation. Polymers 2019, 11, 2046. https://doi.org/10.3390/polym11122046
Jamróz E, Kopel P, Tkaczewska J, Dordevic D, Jancikova S, Kulawik P, Milosavljevic V, Dolezelikova K, Smerkova K, Svec P, et al. Nanocomposite Furcellaran Films—the Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation. Polymers. 2019; 11(12):2046. https://doi.org/10.3390/polym11122046
Chicago/Turabian StyleJamróz, Ewelina, Pavel Kopel, Joanna Tkaczewska, Dani Dordevic, Simona Jancikova, Piotr Kulawik, Vedran Milosavljevic, Kristyna Dolezelikova, Kristyna Smerkova, Pavel Svec, and et al. 2019. "Nanocomposite Furcellaran Films—the Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation" Polymers 11, no. 12: 2046. https://doi.org/10.3390/polym11122046
APA StyleJamróz, E., Kopel, P., Tkaczewska, J., Dordevic, D., Jancikova, S., Kulawik, P., Milosavljevic, V., Dolezelikova, K., Smerkova, K., Svec, P., & Adam, V. (2019). Nanocomposite Furcellaran Films—the Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation. Polymers, 11(12), 2046. https://doi.org/10.3390/polym11122046