Novel Bio-Based Pomelo Peel Flour/Polyethylene Glycol Composite Phase Change Material for Thermal Energy Storage
Abstract
1. Introduction
2. Materials and Methods
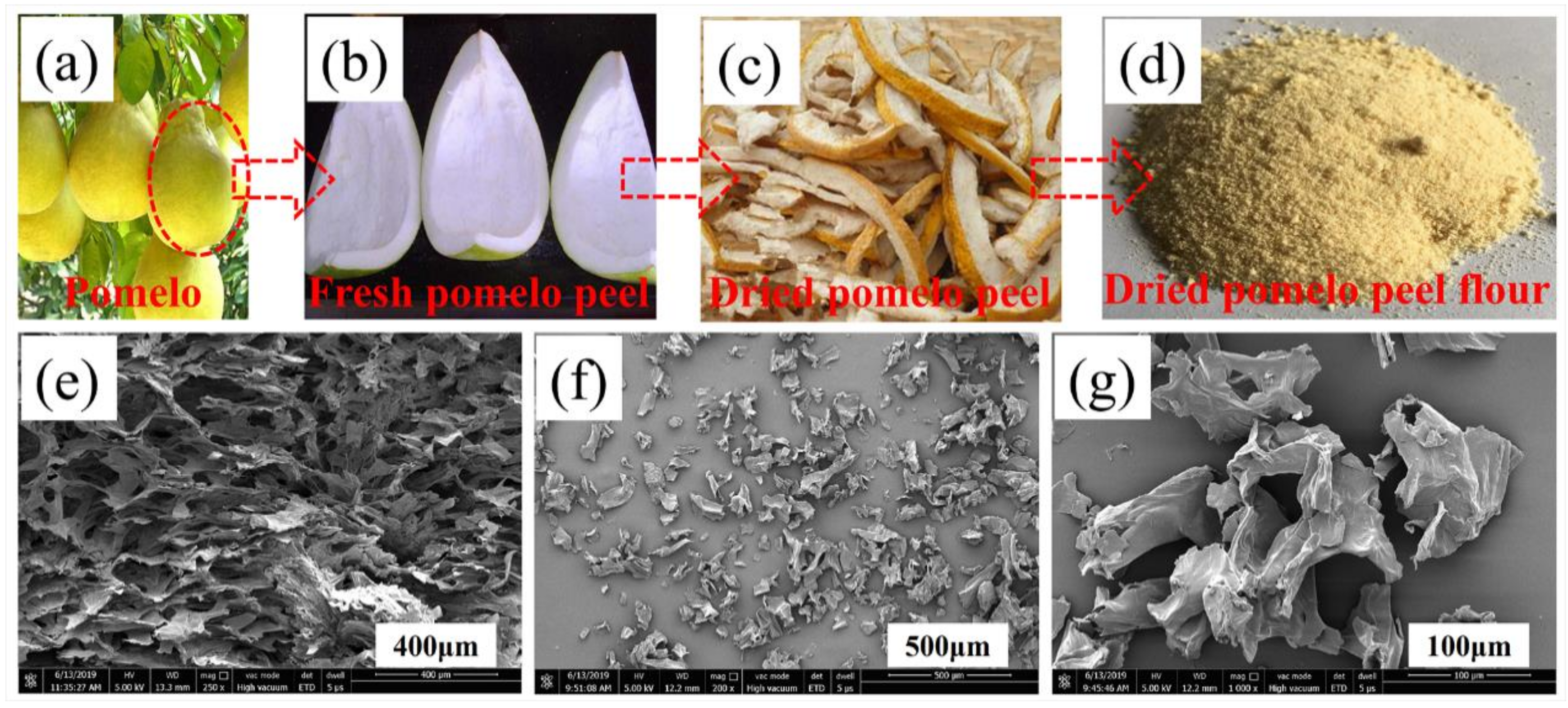
2.1. Raw Materials
2.2. Preparation Methods for Fs-CPCM-1 and Fs-CPCM-2
2.3. Analysis Methods
3. Results and Discussion
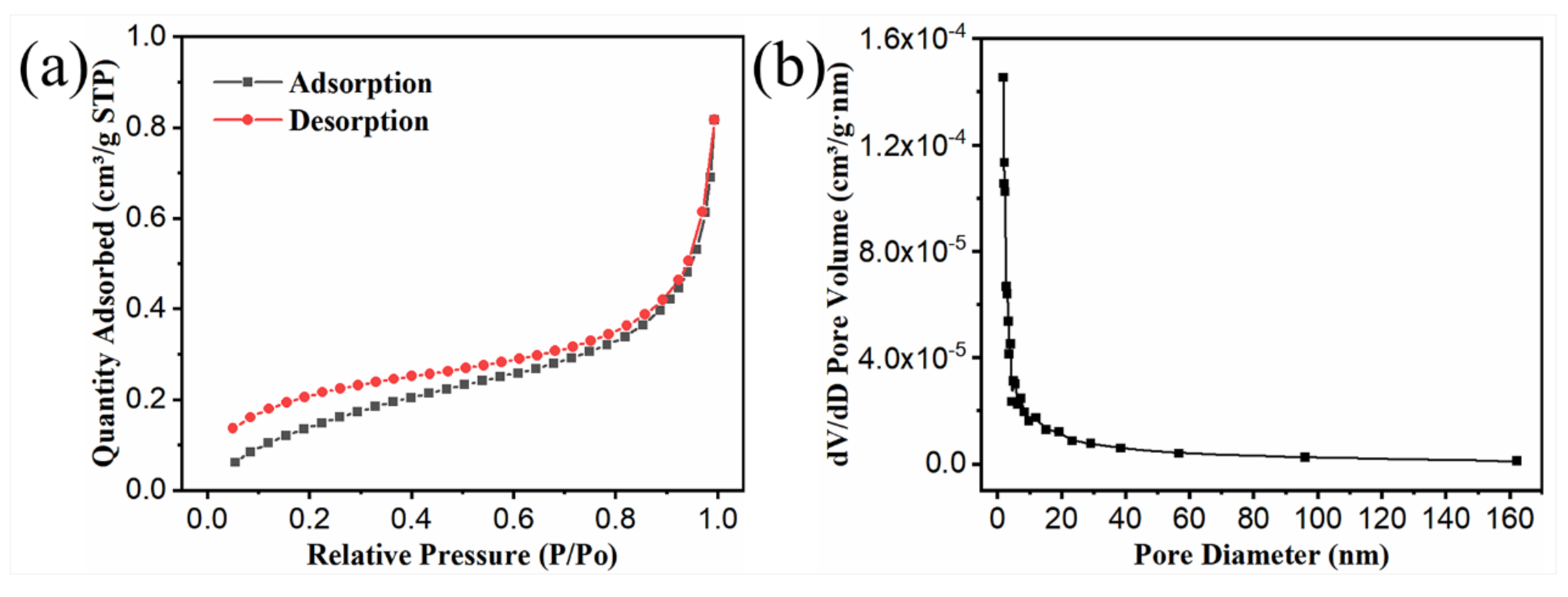
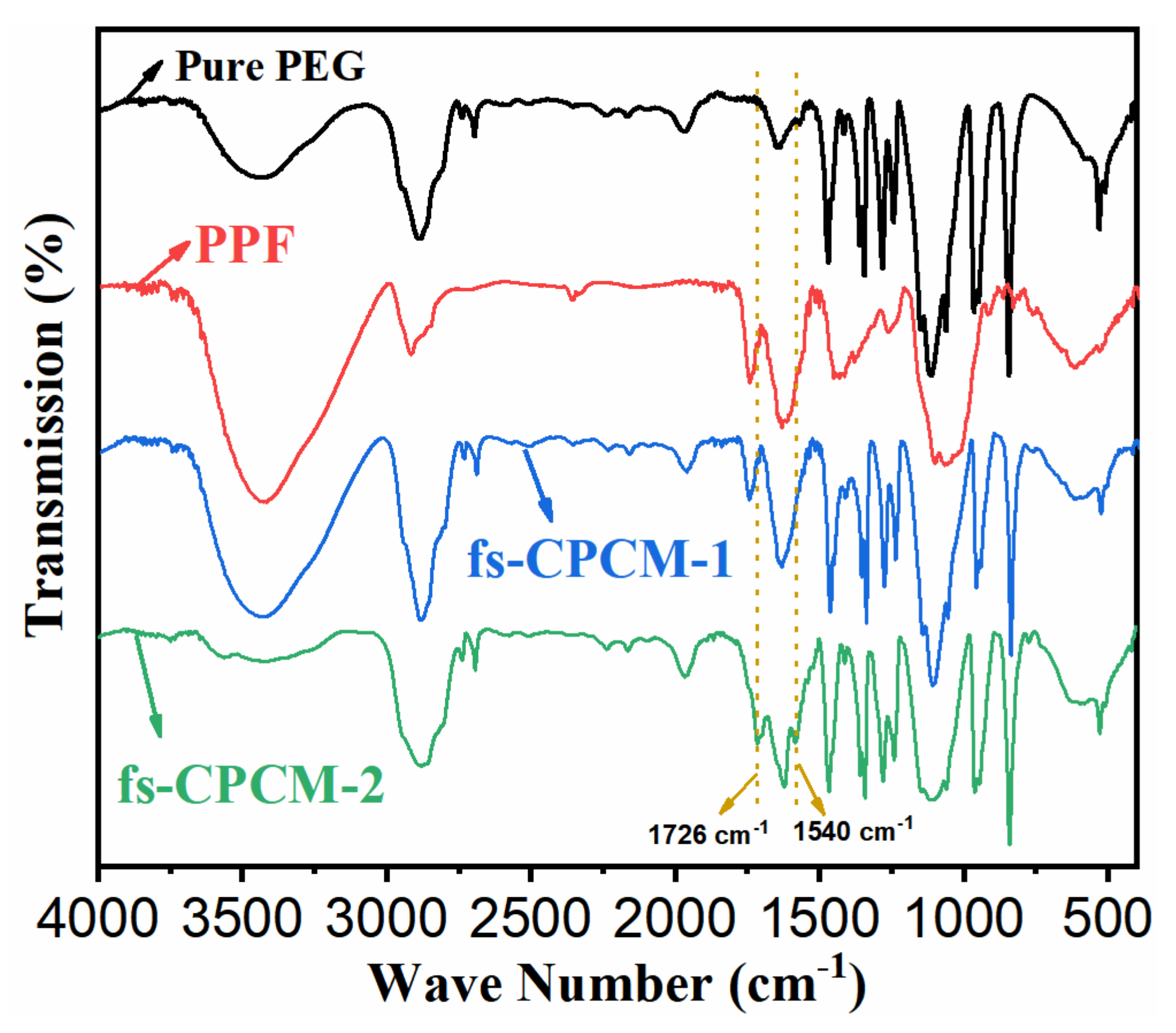
3.1. Morphology and Structure of PPF, Fs-CPCM-1, and Fs-CPCM-2
3.2. Crystalline Performances
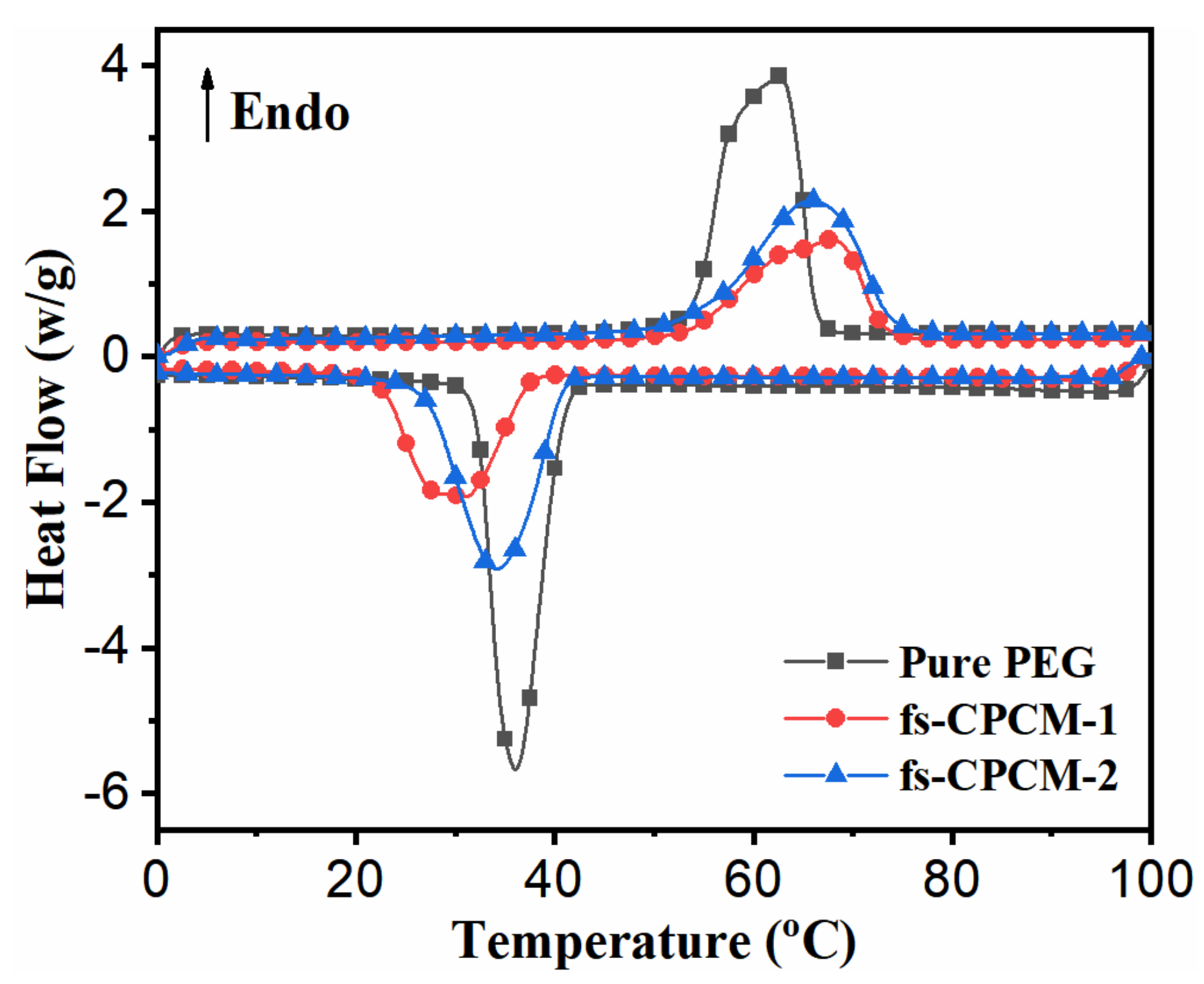
3.3. Phase Change Properties
3.4. Thermal Reliability and Reusability
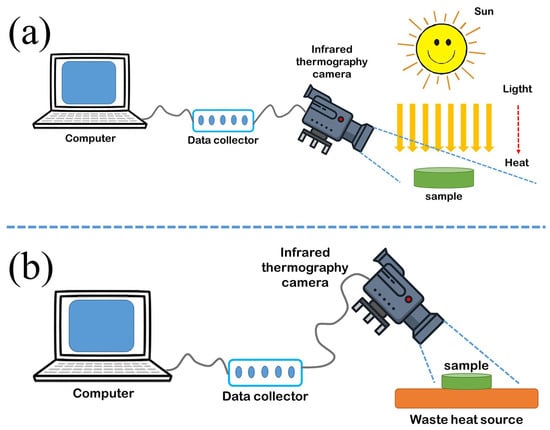
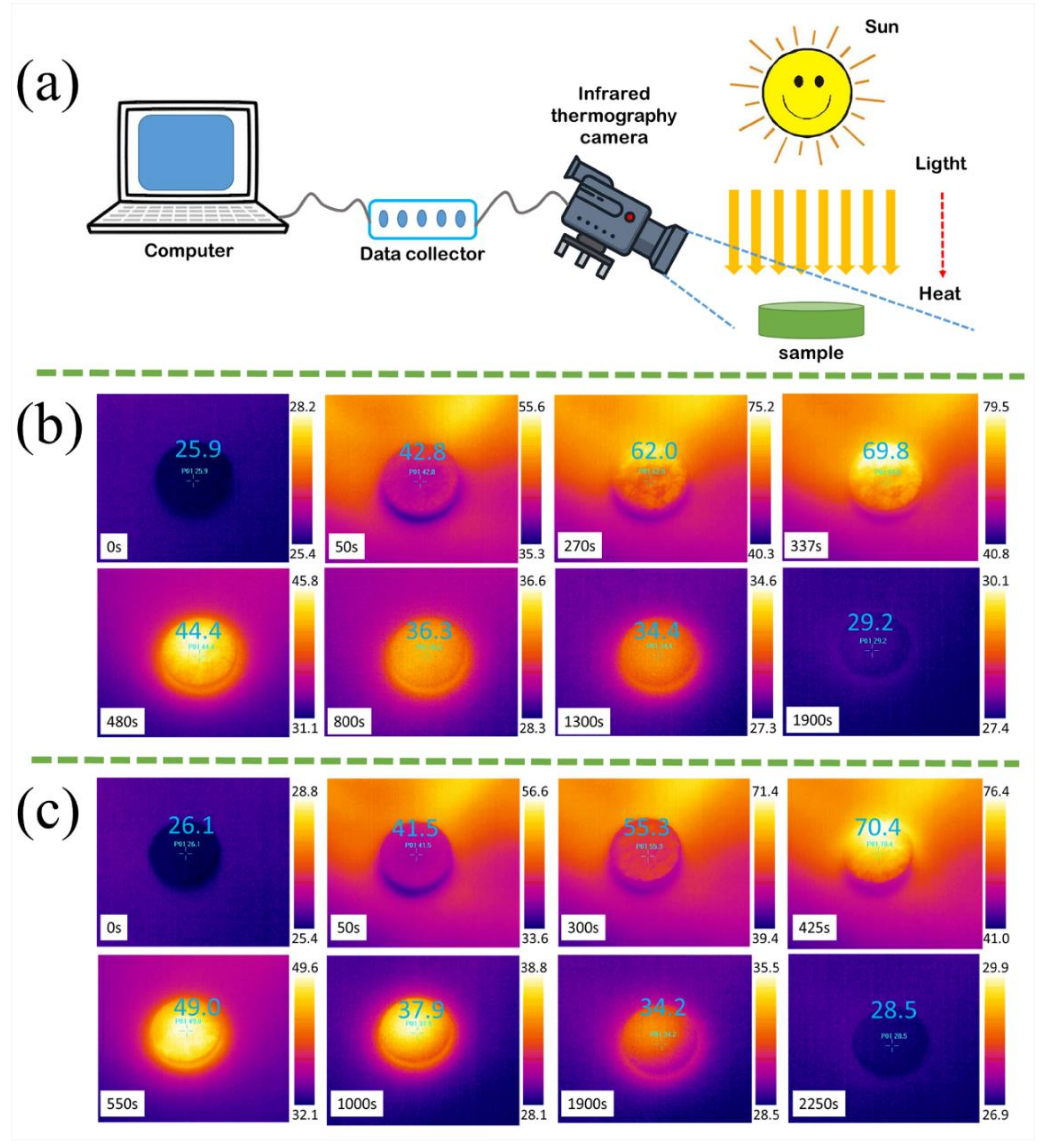
3.5. Light-to-Thermal Conversion and Storage
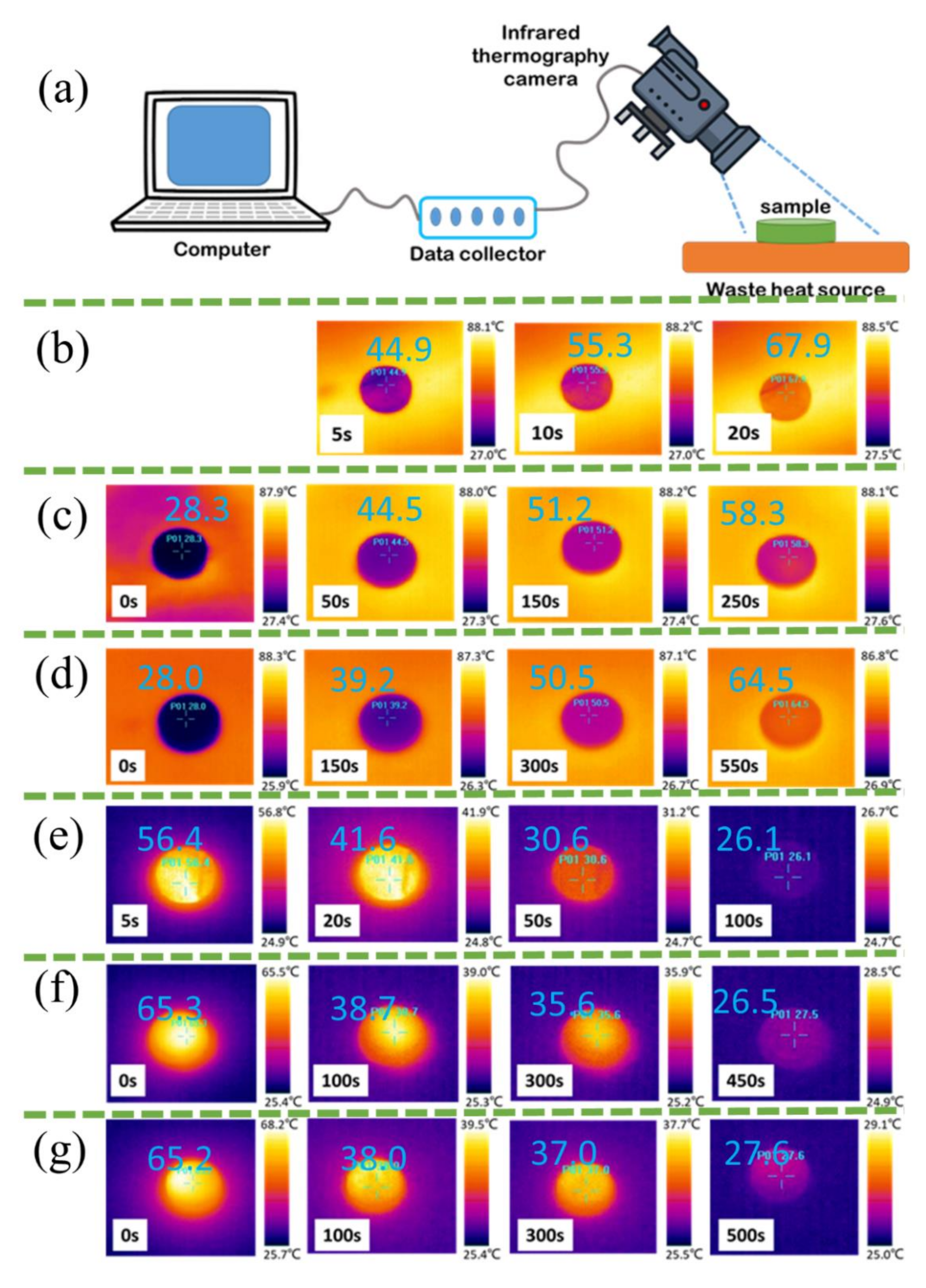
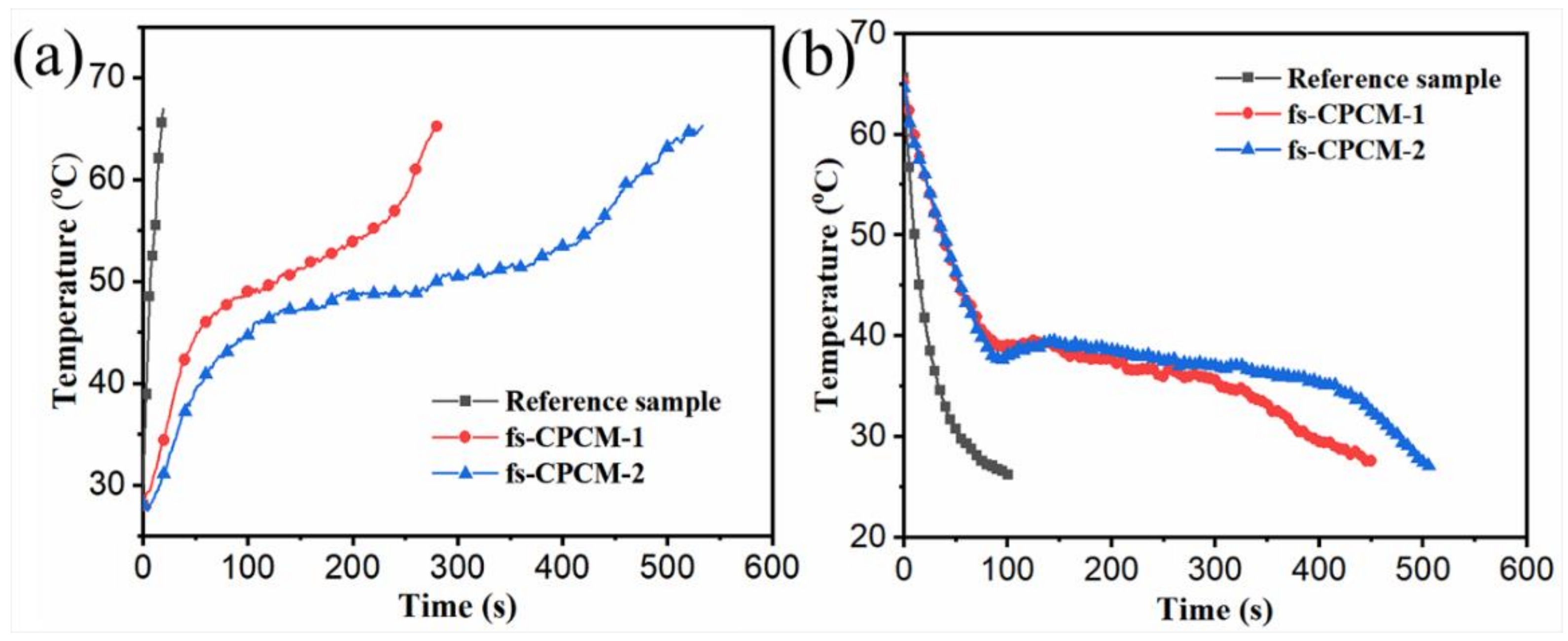
3.6. Waste Heat Recovery
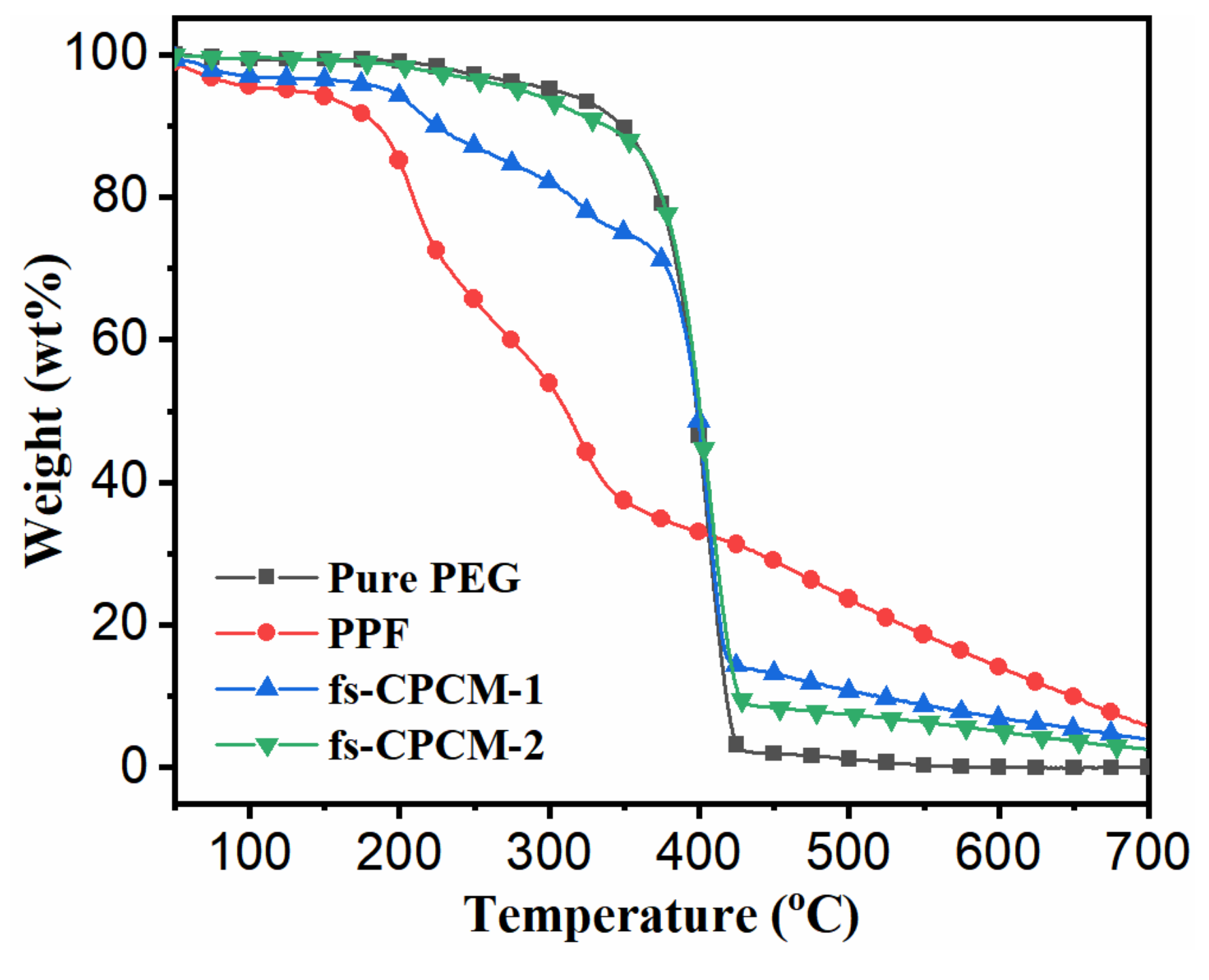
3.7. Thermal Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Zhang, J.; Li, X.; Wu, X. Preparation of hydrophobic lauric acid/SiO2 shape-stabilized phase change materials for thermal energy storage. J. Energy Storage 2019, 21, 611–617. [Google Scholar] [CrossRef]
- Yuan, M.; Ren, Y.; Xu, C.; Ye, F.; Du, X. Characterization and stability study of a form-stable erythritol/expanded graphite composite phase change material for thermal energy storage. Renew. Energy 2019, 136, 211–222. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Q.; Yuan, Y.; Zhang, Z.; Cao, X. A novel form-stable phase change composite with excellent thermal and electrical conductivities. Chem. Eng. J. 2018, 336, 342–351. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chen, R.-T.; Shao, Y.-W.; Qi, X.-D.; Yang, J.-H.; Wang, Y. Novel Flexible Phase Change Materials with Mussel-Inspired Modification of Melamine Foam for Simultaneous Light-actuated Shape Memory and Light-to-Thermal Energy Storage Capability. ACS Sustain. Chem. Eng. 2019, 7, 13532–13542. [Google Scholar] [CrossRef]
- Song, M.; Niu, F.; Mao, N.; Hu, Y.; Deng, S. Review on building energy performance improvement using phase change materials. Energy Build. 2018, 158, 776–793. [Google Scholar] [CrossRef]
- Pandey, A.K.; Hossain, M.S.; Tyagi, V.V.; Abd Rahim, N.; Selvaraj, J.A.L.; Sari, A. Novel approaches and recent developments on potential applications of phase change materials in solar energy. Renew. Sustain. Energy Rev. 2018, 82, 281–323. [Google Scholar] [CrossRef]
- Lizana, J.; Chacartegui, R.; Barrios-Padura, A.; Ortiz, C. Advanced low-carbon energy measures based on thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2018, 82, 3705–3749. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, C.; Wu, D.; Lin, Z. Influence factors of the evaporation rate of a solar steam generation system: A numerical study. Int. J. Heat Mass Transf. 2019, 128, 860–874. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Saidur, R.; Al-Sulaiman, F.A. A review for phase change materials (PCMs) in solar absorption refrigeration systems. Renew. Sustain. Energy Rev. 2017, 76, 105–137. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, X.; Fu, J.; Huan, C.; Qi, S.; Zhan, Y. Novel smart textile with phase change materials encapsulated core-sheath structure fabricated by coaxial electrospinning. Chem. Eng. J. 2019, 355, 532–549. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W. Shape-stabilized phase change materials based on porous supports for thermal energy storage applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Huang, P.; Huang, C.; Xu, F.; Zou, Y. Graphene-oxide-induced lamellar structures used to fabricate novel composite solid-solid phase change materials for thermal energy storage. Chem. Eng. J. 2019, 362, 909–920. [Google Scholar] [CrossRef]
- Niu, Z.; Yuan, W. Highly Efficient Thermo and Sunlight-Driven Energy Storage for Thermo-Electric Energy Harvesting Using Sustainable Nanocellulose-Derived Carbon Aerogels Embedded Phase Change Materials. ACS Sustain. Chem. Eng. 2019, 7, 17523–17534. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Sheng, D.; Lin, C.; Ji, F.; Dong, L. Polyurethane-based solid-solid phase change materials with in situ reduced graphene oxide for light-thermal energy conversion and storage. Chem. Eng. J. 2018, 338, 117–125. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.-S.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Xie, B.-H. Hybrid network structure of boron nitride and graphene oxide in shape-stabilized composite phase change materials with enhanced thermal conductivity and light-to-electric energy conversion capability. Sol. Energy Mater. Sol. Cells 2018, 174, 56–64. [Google Scholar] [CrossRef]
- Wang, F.; Fang, X.; Zhang, Z. Preparation of phase change material emulsions with good stability and little supercooling by using a mixed polymeric emulsifier for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 381–390. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.-S.; Bai, L.; Bao, R.-Y.; Liu, Z.; Xie, B.-H. Photodriven Shape-Stabilized Phase Change Materials with Optimized Thermal Conductivity by Tailoring the Microstructure of Hierarchically Ordered Hybrid Porous Scaffolds. ACS Sustain. Chem. Eng. 2018, 6, 6761–6770. [Google Scholar] [CrossRef]
- Zhou, Y.; Sheng, D.; Liu, X.; Lin, C.; Ji, F.; Dong, L. Synthesis and properties of crosslinking halloysite nanotubes/polyurethane-based solid-solid phase change materials. Sol. Energy Mater. Sol. Cells 2018, 174, 84–93. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Z.; Chen, K.; Huang, J.; Wang, T. Preparation and thermal properties of shape-stabilized polyethylene glycol/mesoporous silica composite phase change materials for thermal energy storage. Energy Storage 2019, 1, e45. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, Y.; Liu, Y.; Guo, H. Preparation and thermal properties of polyethylene glycol/expanded graphite as novel form-stable phase change material for indoor energy saving. Mater. Lett. 2018, 216, 220–233. [Google Scholar] [CrossRef]
- Lu, X.; Fang, C.; Sheng, X.; Zhang, L.; Qu, J. One-Step and Solvent-Free Synthesis of Polyethylene Glycol-Based Polyurethane As Solid–Solid Phase Change Materials for Solar Thermal Energy Storage. Ind. Eng. Chem. Res. 2019, 58, 3024–3032. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Z.; Yuan, Y.; Wang, Y.; Lei, J.; Zhou, C. Fabrication and characterization of fatty acid/wood-flour composites as novel form-stable phase change materials for thermal energy storage. Energy Build. 2018, 171, 88–99. [Google Scholar]
- Liu, Z.; Wei, H.; Tang, B.; Xu, S.; Zhang, S. Novel light-driven CF/PEG/SiO2 composite phase change materials with high thermal conductivity. Sol. Energy Mater. Sol. Cells 2018, 174, 538–544. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Dong, W.; Huang, X.; Gao, H.; Wang, J.; Yang, M. One-pot synthesis of light-driven polymeric composite phase change materials based on N-doped porous carbon for enhanced latent heat storage capacity and thermal conductivity. Sol. Energy Mater. Sol. Cells 2018, 179, 392–400. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Thermal Energy Storage Using Poly(ethylene glycol) Incorporated Hyperbranched Polyurethane as Solid–Solid Phase Change Material. Ind. Eng. Chem. Res. 2017, 56, 14401–14409. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, B.; Fu, X.; Yan, P.; Yuan, Y.; Zhou, C. Two components based polyethylene glycol/thermosetting solid-solid phase change material composites as novel form stable phase change materials for flexible thermal energy storage application. Sol. Energy Mater. Sol. Cells 2017, 170, 197–204. [Google Scholar] [CrossRef]
- Tang, B.; Wang, L.; Xu, Y.; Xiu, J.; Zhang, S. Hexadecanol/phase change polyurethane composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 144, 1–6. [Google Scholar] [CrossRef]
- Zhou, X.-M. Preparation and characterization of PEG/MDI/PVA copolymer as solid-solid phase change heat storage material. J. Appl. Polym. Sci. 2009, 113, 2041–2055. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, X.; Jiang, L.; Wu, B.; Wang, J.; Lei, J. Solvent-free synthesis and properties of novel solid–solid phase change materials with biodegradable castor oil for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 147, 177–184. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, W.; Cai, X. Solvent-free preparation and performance of novel xylitol based solid-solid phase change materials for thermal energy storage. Energy Build. 2018, 158, 37–42. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.; Wong, W.-Y.; Qu, J.-P. A novel bio-based polyurethane/wood powder composite as shape-stable phase change material with high relative enthalpy efficiency for solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 200, 109987. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Zhang, H.; Yu, X.; Ding, Y.; Yuan, Y. Functional phase change composites with highly efficient electrical to thermal energy conversion. Renew. Energy 2020, 145, 2629–2636. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, H.; Zheng, Q.; Ma, Z.; Gong, S. Flexible Infrared Responsive Multi-Walled Carbon Nanotube/Form-Stable Phase Change Material Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 21602–21609. [Google Scholar] [CrossRef]
- Lu, X.; Huang, H.; Zhang, X.; Lin, P.; Huang, J.; Sheng, X. Novel light-driven and electro-driven polyethylene glycol/two-dimensional MXene form-stable phase change material with enhanced thermal conductivity and electrical conductivity for thermal energy storage. Compos. Part B Eng. 2019, 177, 107372. [Google Scholar] [CrossRef]






| Samples | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pure PEG | 53.6 | 62.5 | 67.2 | 182.3 | 41.5 | 36.1 | 31.4 | 173.5 |
| fs-CPCM-1 | 52.7 | 67.1 | 72.6 | 88.9 | 38.6 | 30.2 | 22.5 | 85.1 |
| fs-CPCM-2 | 52.2 | 66.4 | 72.9 | 143.2 | 40.9 | 34.3 | 26.5 | 141.8 |
| fs-CPCMs | λ (%) | References | ||
|---|---|---|---|---|
| PEG/MDI/PVA | 168.4 | 72.8 | 43.2 | [29] |
| PEG/HDI/CO | 167.6 | 117.7 | 70.2 | [30] |
| PEG/MDI/Xylitol | 162.8 | 76.4 | 46.9 | [31] |
| PEG/HDIT | 157.0 | 136.8 | 87.1 | [22] |
| PEG/HDI/WP | 136.4 | 134.2 | 98.4 | [32] |
| PEG/CaCl/CNT | 152.5 | 128.6 | 84.3 | [33] |
| PEG/TTI | 169.5 | 105.1 | 62.0 | [34] |
| PEG/PPF | 89.7 | 88.9 | 99.1 | Present study |
| PEG/HDI/PPF | 163.9 | 143.2 | 87.4 | Present study |
| Sample | ||||||
|---|---|---|---|---|---|---|
| fs-CPCM-1 | 88.9 | 87.6 | 1.5 | 85.1 | 84.7 | 0.5 |
| fs-CPCM-2 | 143.2 | 142.8 | 0.3 | 141.8 | 139.9 | 1.3 |
| Samples | |||||
|---|---|---|---|---|---|
| Pure PEG | 199.5 | 301.9 | 400.5 | − | − |
| PPF | 46.6 | 125.1 | 61.5 | 208.8 | 323.7 |
| fs-CPCM-1 | 60.1 | 192.3 | 400.1 | − | − |
| fs-CPCM-2 | 171.5 | 280.4 | 400.4 | − | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-C.; Kang, B.-h.; Sheng, X.; Lu, X. Novel Bio-Based Pomelo Peel Flour/Polyethylene Glycol Composite Phase Change Material for Thermal Energy Storage. Polymers 2019, 11, 2043. https://doi.org/10.3390/polym11122043
Zhang H-C, Kang B-h, Sheng X, Lu X. Novel Bio-Based Pomelo Peel Flour/Polyethylene Glycol Composite Phase Change Material for Thermal Energy Storage. Polymers. 2019; 11(12):2043. https://doi.org/10.3390/polym11122043
Chicago/Turabian StyleZhang, Hai-Chen, Ben-hao Kang, Xinxin Sheng, and Xiang Lu. 2019. "Novel Bio-Based Pomelo Peel Flour/Polyethylene Glycol Composite Phase Change Material for Thermal Energy Storage" Polymers 11, no. 12: 2043. https://doi.org/10.3390/polym11122043
APA StyleZhang, H.-C., Kang, B.-h., Sheng, X., & Lu, X. (2019). Novel Bio-Based Pomelo Peel Flour/Polyethylene Glycol Composite Phase Change Material for Thermal Energy Storage. Polymers, 11(12), 2043. https://doi.org/10.3390/polym11122043