Synthesis and Properties of Cyclopentyl Cardo-Type Polyimides Based on Dicyclopentadiene
Abstract
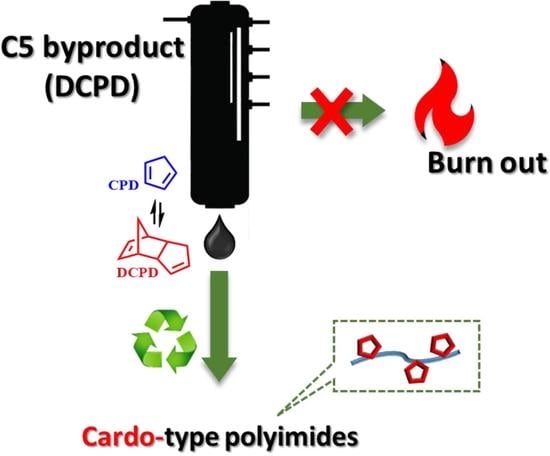
1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization Methods
2.3. Synthesis
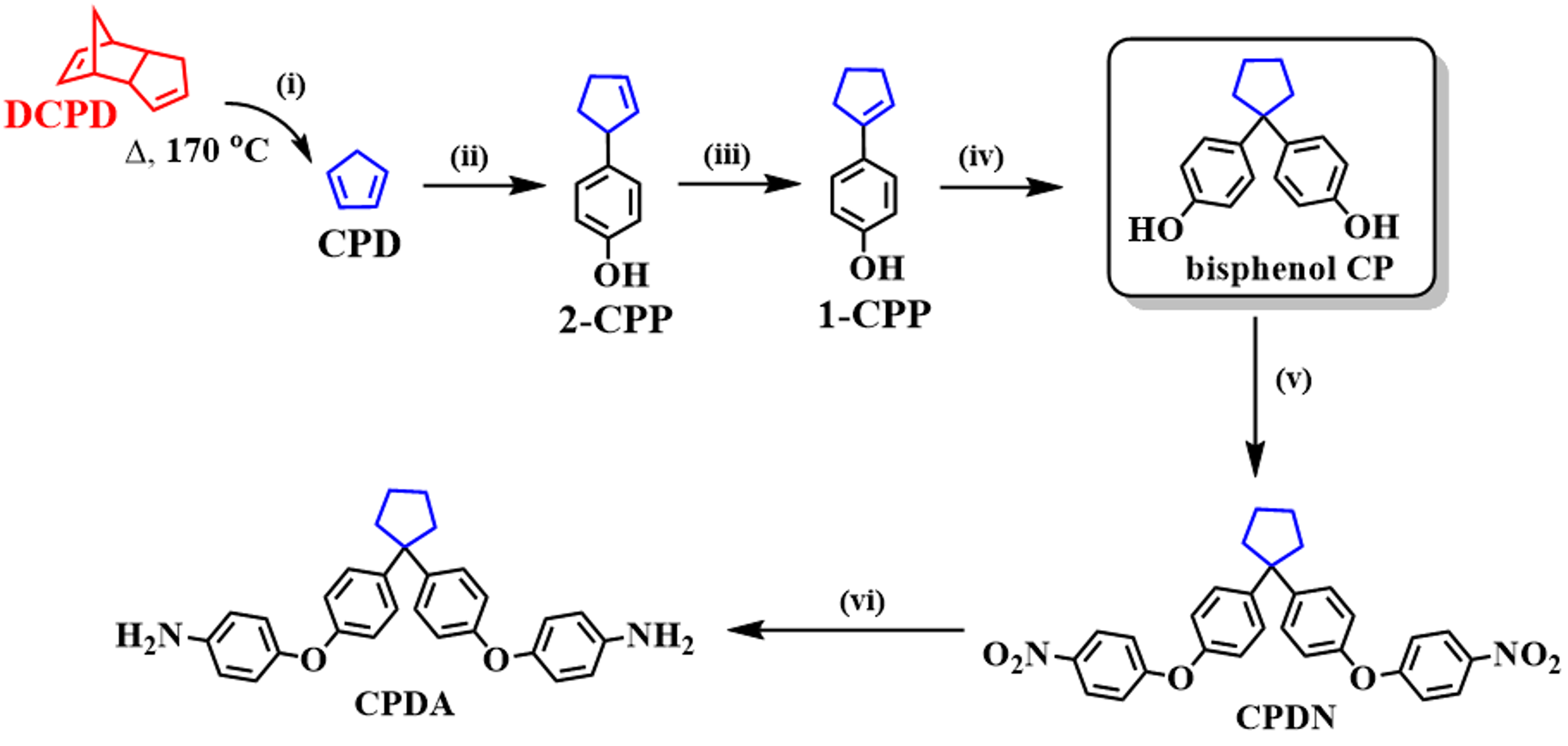
2.3.1. Cyclopentadiene (CPD) [1,35,36,37]
2.3.2. Cyclopent-2-enyl phenol (2-CPP) [1,35,36,37]
2.3.3. Cyclopentenyl phenol (1-CPP) [1,35,36,37]
2.3.4. 4,4′-(Cyclopentane-1,1-diyl)diphenol (bisphenol CP) [1,35,36,37]
2.3.5. General Synthetic Route of Dinitro Compound: 4,4′-(cyclopentane-1,1-diyl)bis((4-nitrophenoxy)benzene) (CPDN) [38,39]
2.3.6. General Synthetic Route of Diamine Compound: 4,4′-((cyclopentane-1,1-diylbis(4,1-phenylene))bis(oxy))dianiline (CPDA)
2.3.7. 4,4′-((Propane-2,2-diylbis(4,1-phenylene))bis(oxy))dianiline (BPAA) [40]
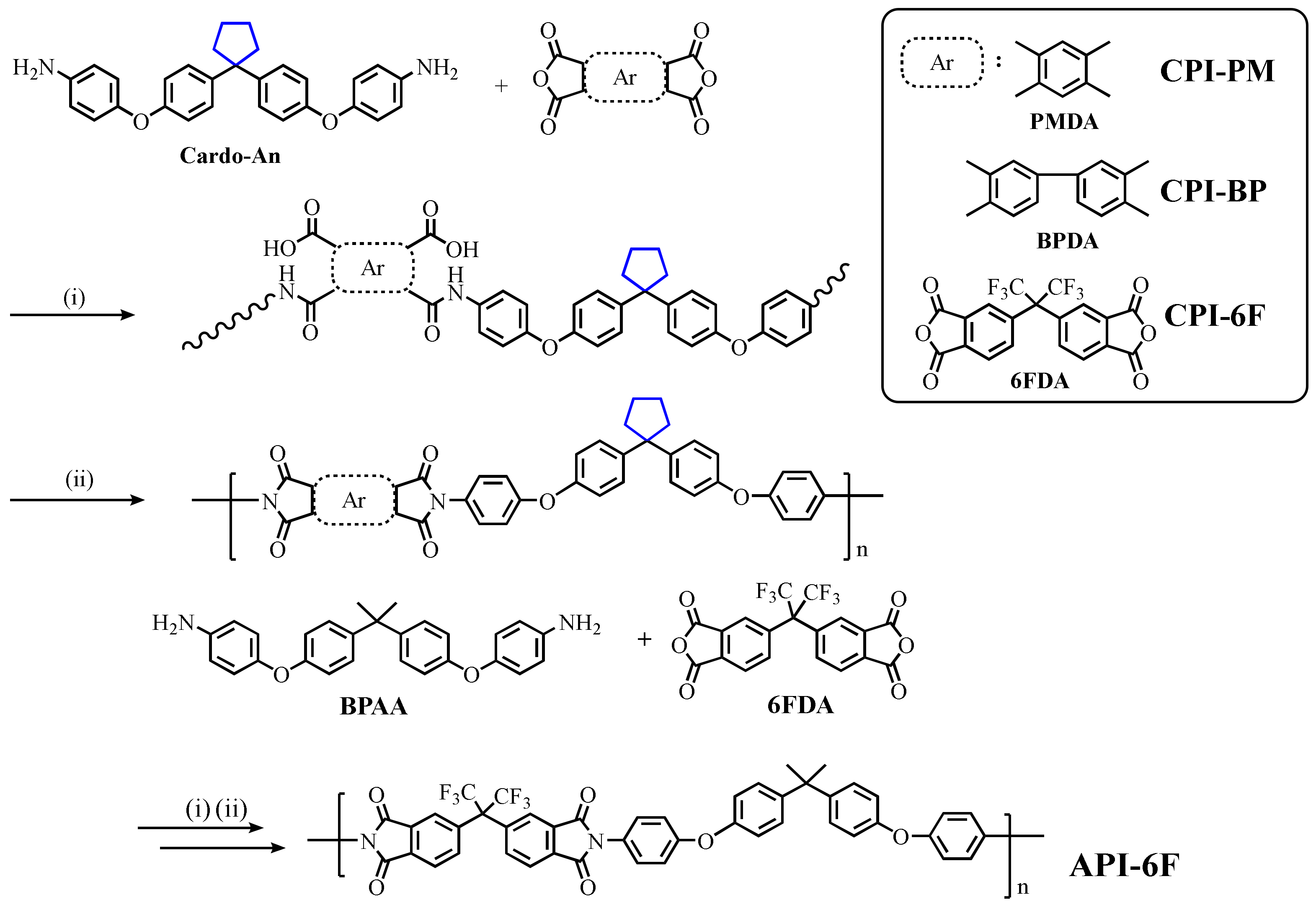
2.3.8. General Procedure for Synthesizing Polyimide
3. Results and Discussion
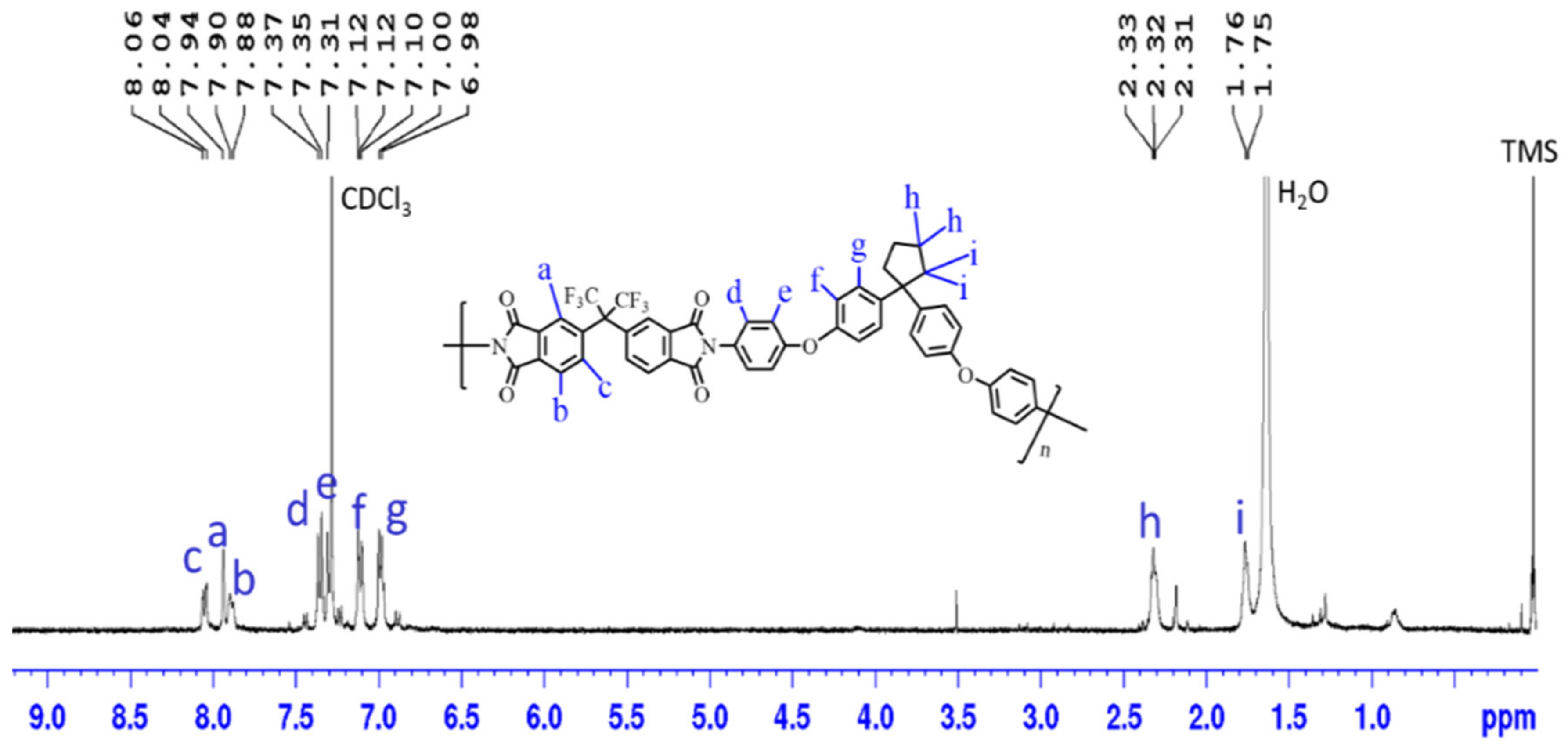
3.1. Synthesis and Characterization
3.2. Change of Average Interchain Distance (d-spacing)
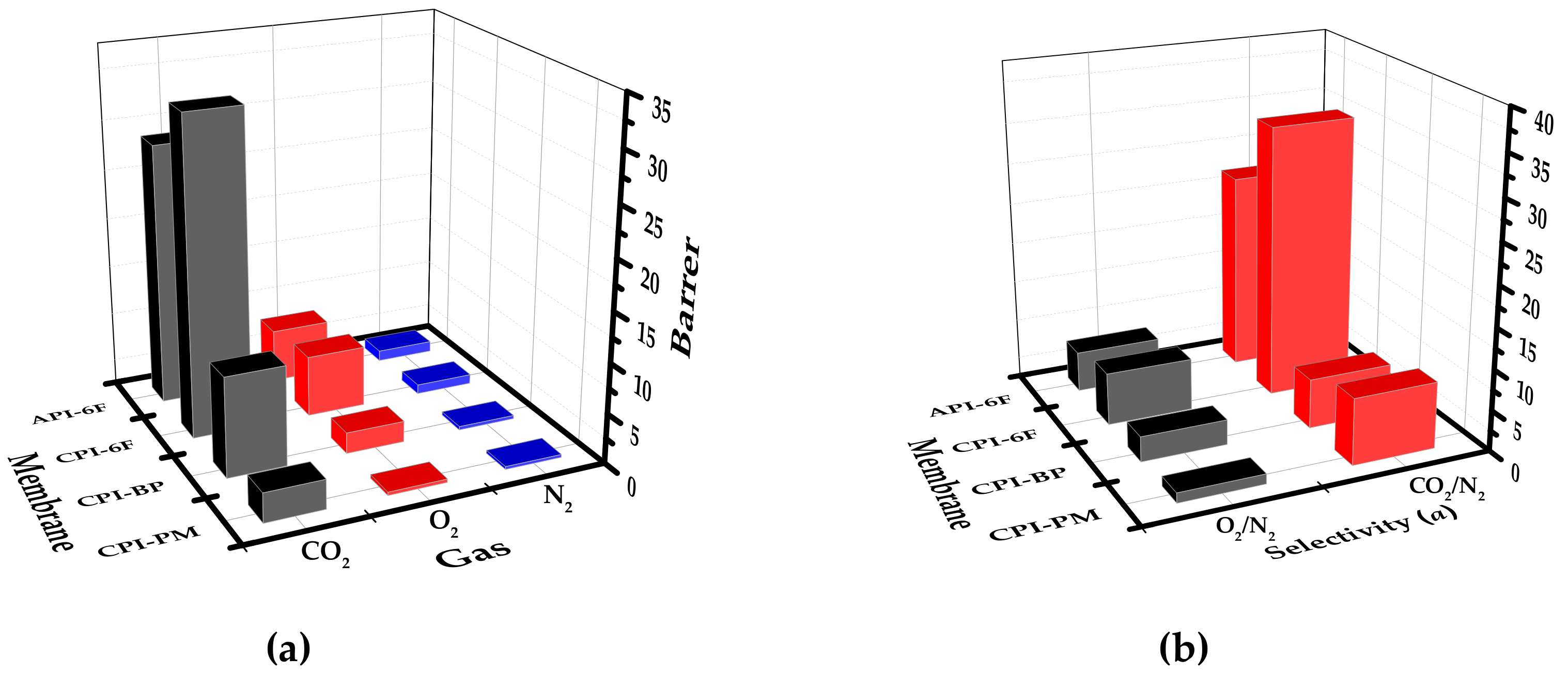
3.3. Gas Permeation Properties of Cardo-Type Polyimides
4. Conclusion and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, C.-H.; Lin, Y.-R.; Yeh, S.-C.; Huang, Y.-C.; Sun, K.-H.; Shih, Y.-F.; Su, W.-C.; Dai, C.-A.; Dai, S.A.; Jeng, R.-J. A Facile Synthetic Route to Ether Diols Derived from 1,1-cyclopentylenylbisphenol for Robust Cardo-Type Polyurethanes. Macromolecules 2019, 52, 959–967. [Google Scholar] [CrossRef]
- Saha, S.; Ginzburg, Y.; Rozenberg, I.; Iliashevsky, O.; Ben-Asuly, A.; Lemcoff, N.G. Cross-linked ROMP Polymers Based on Odourless Dicyclopentadiene Derivative. Polym. Chem. 2016, 7, 3071–3075. [Google Scholar] [CrossRef]
- Davidson, T.A.; Wagener, K.B.; Priddy, D.B. Polymerization of Dicyclopentadiene: A Tale of Two Mechanisms. Macromolecules 1996, 29, 786–788. [Google Scholar] [CrossRef]
- Monsaert, S.; Lozano Vila, A.; Drozdzak, R.; Van Der Voort, P.; Verpoort, F. Latent Olefin Metathesis Catalysts. Chem. Soc. Rev. 2009, 38, 3360–3372. [Google Scholar] [CrossRef]
- Robertson, I.D.; Pruitt, E.L.; Moore, J.S. Frontal Ring-Opening Metathesis Polymerization of Exo-Dicyclopentadiene for Low Catalyst Loadings. ACS Macro Lett. 2016, 5, 593–596. [Google Scholar] [CrossRef]
- Nuyken, O.; Pask, S. Ring-Opening Polymerization—An Introductory Review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- Yameen, B.; Rodriguez-Emmenegger, C.; Preuss, C.M.; Pop-Georgievski, O.; Verveniotis, E.; Trouillet, V.; Rezek, B.; Barner-Kowollik, C. A facile Avenue to Conductive Polymer Brushes via Cyclopentadiene–Maleimide Diels–Alder Ligation. Chem. Commun. 2013, 49, 8623–8625. [Google Scholar] [CrossRef]
- Huertas, D.; Florscher, M.; Dragojlovic, V. Solvent-free Diels–Alder Reactions of in Situ Generated Cyclopentadiene. Green Chem. 2009, 11, 91–95. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-Healing Polymers Based on Thermally Reversible Diels–Alder Chemistry. Polym. Chem. 2013, 4. [Google Scholar] [CrossRef]
- Meng, F.-Q.; Feng, X.-J.; Wang, W.-H.; Bao, M. Synthesis of 5-vinyl-2-norbornene through Diels–Alder Reaction of Cyclopentadiene with 1,3-butadiene in Supercritical Carbon Dioxide. Chin. Chem. Lett. 2017, 28, 900–904. [Google Scholar] [CrossRef]
- Chern, Y.-T. Low Dielectric Constant Polyimides Derived from Novel 1,6-Bis[4-(4-aminophenoxy)phenyl]diamantane. Macromolecules 1998, 31, 5837–5884. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Liaw, B.-Y.; Chung, C.-Y. Synthesis and Characterization of New Cardo Polyimides Prepared from 5,5-Bis[4-(4-aminophenoxy)phenyl]-4, 7-methanohexahydroindane. J. Polym. Sci. A Polym. Chem. 1999, 37, 2815–2821. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Liaw, B.-Y.; Yu, C.-W. Synthesis and Characterization of New Soluble Cardo Polyamide-imides Containing Cyclododecyl Groups. J. Polym. Sci. A Polym. Chem. 2000, 38, 2787–2793. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Chen, W.-H. Synthesis and Characterization of New Soluble Cardo Poly(amide–imide)s Derived from 2,2-Bis[4-(4-trimellitimidophenoxy)phenyl]norbornane. Polymer 2003, 44, 3865–3870. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Lai, J.; Fu, Z.; You, X. Comparative Investigations on the Effects of Pendent Trifluoromethyl Group to the Properties of the Polyimides Containing Diphenyl-Substituted Cyclopentyl Cardo-Structure. J. Fluor. Chem. 2014, 164, 27–37. [Google Scholar] [CrossRef]
- Klumpen, C.; Breunig, M.; Homburg, T.; Stock, N.; Senker, J. Microporous Organic Polyimides for CO2 and H2O Capture and Separation from CH4 and N2 Mixtures: Interplay Between Porosity and Chemical Function. Chem. Mater. 2016, 28, 5461–5470. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Yan, J.; Wang, Z. Microporous Polyimides with Functional Groups for the Adsorption of Carbon Dioxide and Organic Vapors. J. Mater. Chem. A 2016, 4, 11453–11461. [Google Scholar] [CrossRef]
- Neti, V.S.P.K.; Wang, J.; Deng, S.; Echegoyen, L. Synthesis of a Polyimide Porous Porphyrin Polymer for Selective CO2 Capture. J. Org. Chem. 2015, 2015, 281616. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Wang, T. Engineering a Hyperbranched Polyimide Membrane for Shape Memory and CO2 Capture. J. Mater. Chem. A 2017, 5, 13823–13833. [Google Scholar] [CrossRef]
- Xu, L.; Lei, T.; Jing, B.; Zang, Y.; Miao, F.; Aoki, T.; Teraguchi, M.; Kaneko, T. Synthesis of Soluble Oligsiloxane-End-Capped Hyperbranched Polyazomethine and their Application to CO2/N2 Separation Membranes. Des. Monomers Polym. 2018, 21, 99–104. [Google Scholar] [CrossRef]
- Kurosawa, T.; Higashihara, T.; Ueda, M. Polyimide Memory: A Pithy Guideline for Future Applications. Polym. Chem. 2013, 4, 16–30. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced Polyimide Materials: Syntheses, Physical Properties and Applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Kim, Y.H.; Ka, J.-W.; Hong, S.-K.; Yi, M.H.; Jang, K.-S. A High-Temperature Resistant Polyimide Gate Inslulator Surface-Modified with a YOx Interlayer for High-Performance, Solution-Processed Li-Doped ZnO Thin-Film Transistors. J. Mater. Chem. C 2014, 2, 2191–2197. [Google Scholar] [CrossRef]
- Won, J.-M.; Suk, H.J.; Wee, D.; Kim, Y.H.; Ka, J.-W.; Kim, J.; Ahn, T.; Yi, M.H.; Jang, K.-S. Photo-Patternable Polyimide Gate Insulator with Fluorine Groups for Improving Performance of 2,7-Didecyl[1]benzothieno[3,2-b][1]benzothiopene (C10-BTBT) Thin-Film Transistors. Org. Electron. 2013, 14, 1777–1786. [Google Scholar] [CrossRef]
- Kim, S.; Ha, T.; Yoo, S.; Ka, J.W.; Kim, J.; Won, J.C.; Choi, D.H.; Jang, K.S.; Kim, Y.H. Metal-Oxide Assisted Surface Treatment of Polyimide Gate Insulators for High-Performance Organic Thin-Film Transistors. Phys. Chem. Chem. Phys. 2017, 19, 15521–15529. [Google Scholar] [CrossRef]
- Ahn, T.; Choi, Y.; Jung, H.; Yi, M. Fully Aromatic Polyimide Gate Insulators with Low Temperature Processability for Pentacene Organic Thin-Film Transistors. Org. Electron. 2009, 10, 12–17. [Google Scholar] [CrossRef]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. A review of the latest development of polyimide based membranes for CO2 separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides Containing Aliphatic/Alicyclic Segments in the Main Chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Bermejo, L.A.; Alvarez, C.; Maya, E.M.; Garcia, C.; de la Campa, J.G.; Lozano, A.E. Synthesis, Characterization and Gas Separation Properties of Novel Polyimides Containing Cardo and Tert-butyl-m-terphenyl Moieties. Express Polym. Lett. 2018, 12, 479–489. [Google Scholar] [CrossRef]
- Yahaya, G.O.; Mokhtari, I.; Alghannam, A.A.; Choi, S.-H.; Maab, H.; Bahamdan, A.A. Cardo-Type Random Co-Polyimide Membranes for High Pressure Pure and Mixed Sour Gas Feed Separations. J. Membr. Sci. 2018, 550, 526–535. [Google Scholar] [CrossRef]
- Wiegand, J.R.; Smith, Z.P.; Liu, Q.; Patterson, C.T.; Freeman, B.D.; Guo, R. Synthesis and Characterization of Triptycene-based Polyimides with Tunable High Fractional Free volume for Gas Separation Membranes. J. Mater. Chem. A 2014, 2, 13309–13320. [Google Scholar] [CrossRef]
- Park, J.Y.; Paul, D.R. Correlation and Prediction of Gas Permeability in Glassy Polymer Membrane Materials via a Modified Free Volume Based Group Contribution Method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Lee, C.; Seo, J.; Shul, Y.; Han, H. Optical Properties of Polyimide Thin Films. Effect of Chemical Structure and Morphology. Polym. J. 2003, 35, 578–585. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Dai, S.A.; Lin, C.Y.; Rao, D.V.; Stuber, F.A.; Carleton, P.S.; Ulrich, H. Selective Indirect Oxidation of Phenol to Hydroquinone and Catechol. J. Org. Chem. 1985, 50, 1722–1725. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, H.; Zhang, L.; Ran, T.; Zhang, D. Breath Figure Patterns Prepared by Spraying Ultrasonic Atomized Water Droplets. In Proceedings of the 2016 IEEE 11th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, 17–20 April 2016. [Google Scholar]
- Heng, L.; Meng, X.; Wang, B.; Jiang, L. Bioinspired Design of Honeycomb Structure Interfaces with Controllable Water Adhesion. Langmuir 2013, 29, 9491–9498. [Google Scholar] [CrossRef]
- Leu, T.-S.; Wang, C.-S. Synthesis and Properties of Polyimides Cotaining Bisphenol Unit and Flexible Ether Linkage. J. Appl. Polym. Sci. 2003, 87, 945–952. [Google Scholar] [CrossRef]
- Yang, C.-P.; Su, Y.-Y.; Hsiao, F.-Z. Synthesis and Properties of Organosoluble Polyimides Based on 1,1-bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]cyclohexane. Polymer 2004, 45, 7529–7538. [Google Scholar] [CrossRef]
- Wang, D.H.; Wie, J.J.; Lee, K.M.; White, T.J.; Tan, L.-S. Impact of Backbone Rigidity on the Photomechanical Response of Glassy, Azobenzene-Functionalized Polyimides. Macromolecules 2014, 47, 659–667. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hu, C.-C.; Lin, L.-K.; Lai, J.-Y.; Liu, Y.-L. Liberation of Small Molecules in Polyimide Membrane Formation: An Effect on Gas Separation Properties. J. Membr. Sci. 2016, 499, 20–27. [Google Scholar] [CrossRef]
- Bei, R.; Qian, C.; Zhang, Y.; Chi, Z.; Liu, S.; Chen, X.; Xu, J.; Aldred, M.P. Intrinsic Low Dielectric Constant Polyimides: Relationship Between Molecular Structure and Dielectric Properties. J. Mater. Chem. C 2017, 5, 12807–12815. [Google Scholar] [CrossRef]
- Coleman, M.R.; Koros, W.J. Isomeric Polyimides Based on Fluorinated Dianhydrides and Diamines for Gas Separation Applications. J. Membr. Sci. 1990, 50, 285–297. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Chung, T.-S.; Tong, Y.W. Highly Permeable Chemically Modified PIM-1/Matrimid Membranes for Green Hydrogen Purification. J. Mater. Chem. A 2013, 1, 13914–13925. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Hoang, V.-T.; Chen, X.Y.; Rodrigue, D.; Kaliaguine, S. Crosslinked MOF-polymer to Enhance Gas Separation of Mixed Matrix Membranes. J. Membr. Sci. 2016, 520, 941–950. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing Mixed-matrix Membranes for CO2/CH4 and CO2/N2 Binary Gas Mixture Separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kanehashi, S. Polymer of Intrinsic Microporosity (PIM-1) Membranes Treated with Supercritical CO2. Membranes 2019, 9, 41. [Google Scholar] [CrossRef]
- Cecopierigomez, M.; Palaciosalquisira, J.; Dominguez, J. On the Limits of Gas Separation in CO2/CH4, N2/CH4 and CO2/N2 Binary Mixtures Using Polyimide Membranes. J. Membr. Sci. 2007, 293, 53–65. [Google Scholar] [CrossRef]


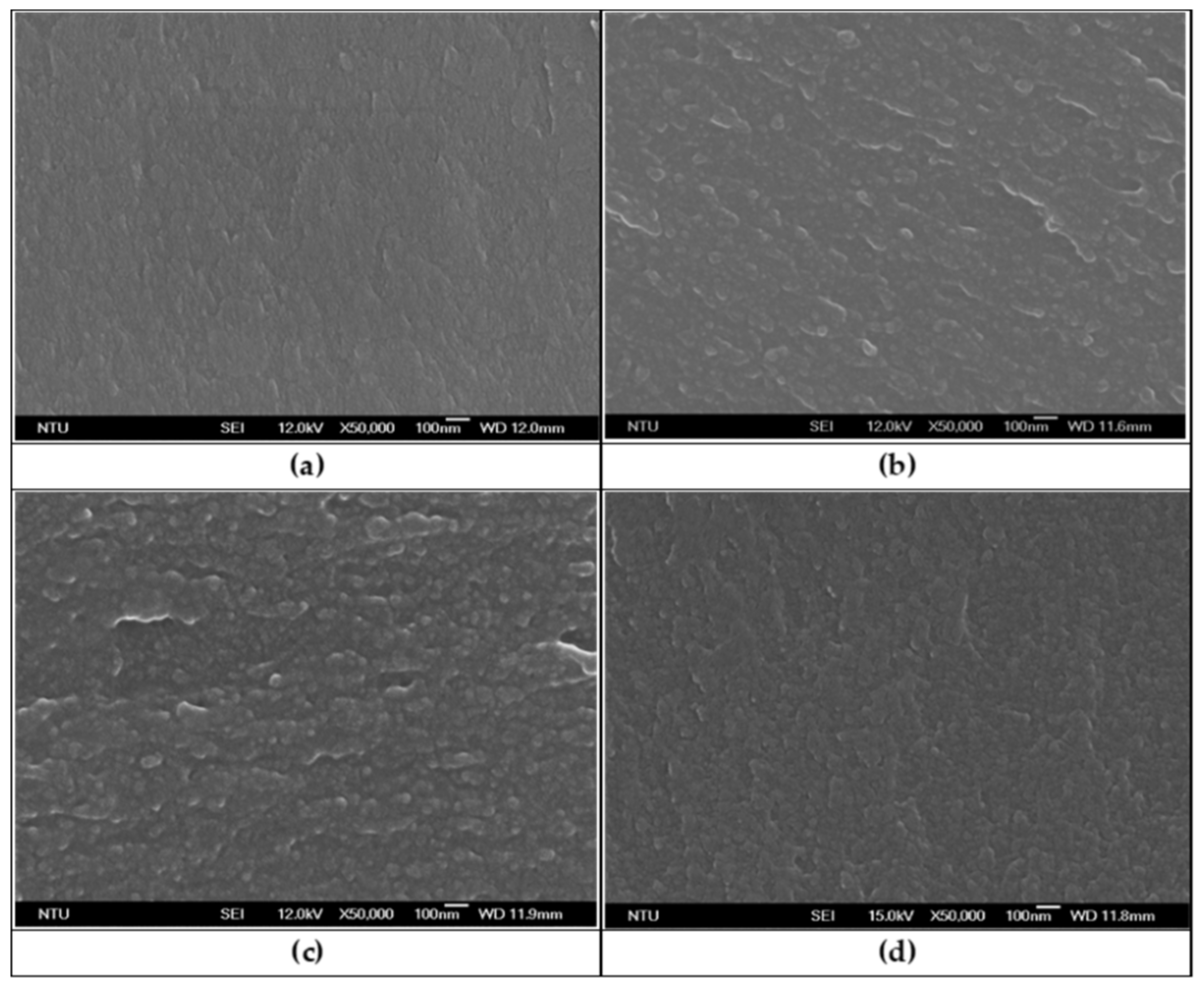
| Sample | Solubility * | Inherent Viscosity ηinh (dL/g) | ||||
|---|---|---|---|---|---|---|
| NMP | DMF | DMAc | THF | CHCl3 | ||
| CPI-PM | -- | -- | -- | -- | -- | 0.99 |
| CPI-BP | +h | -- | -- | -- | -- | 1.27 |
| CPI-6F | +h | +h | Δ | Δ | +h | 0.85 |
| API-6F | +h | +h | Δ | Δ | -- | 0.90 |
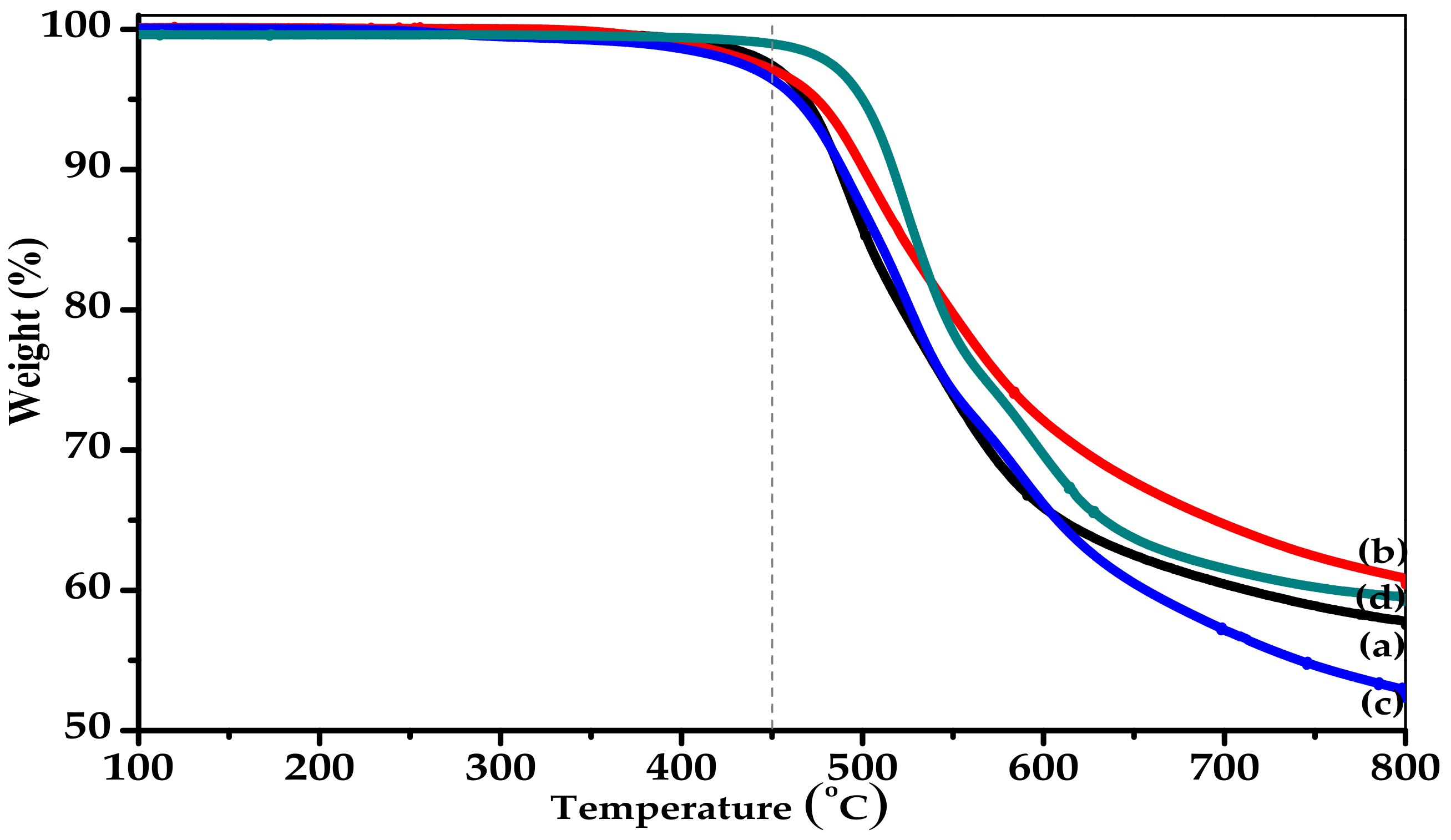
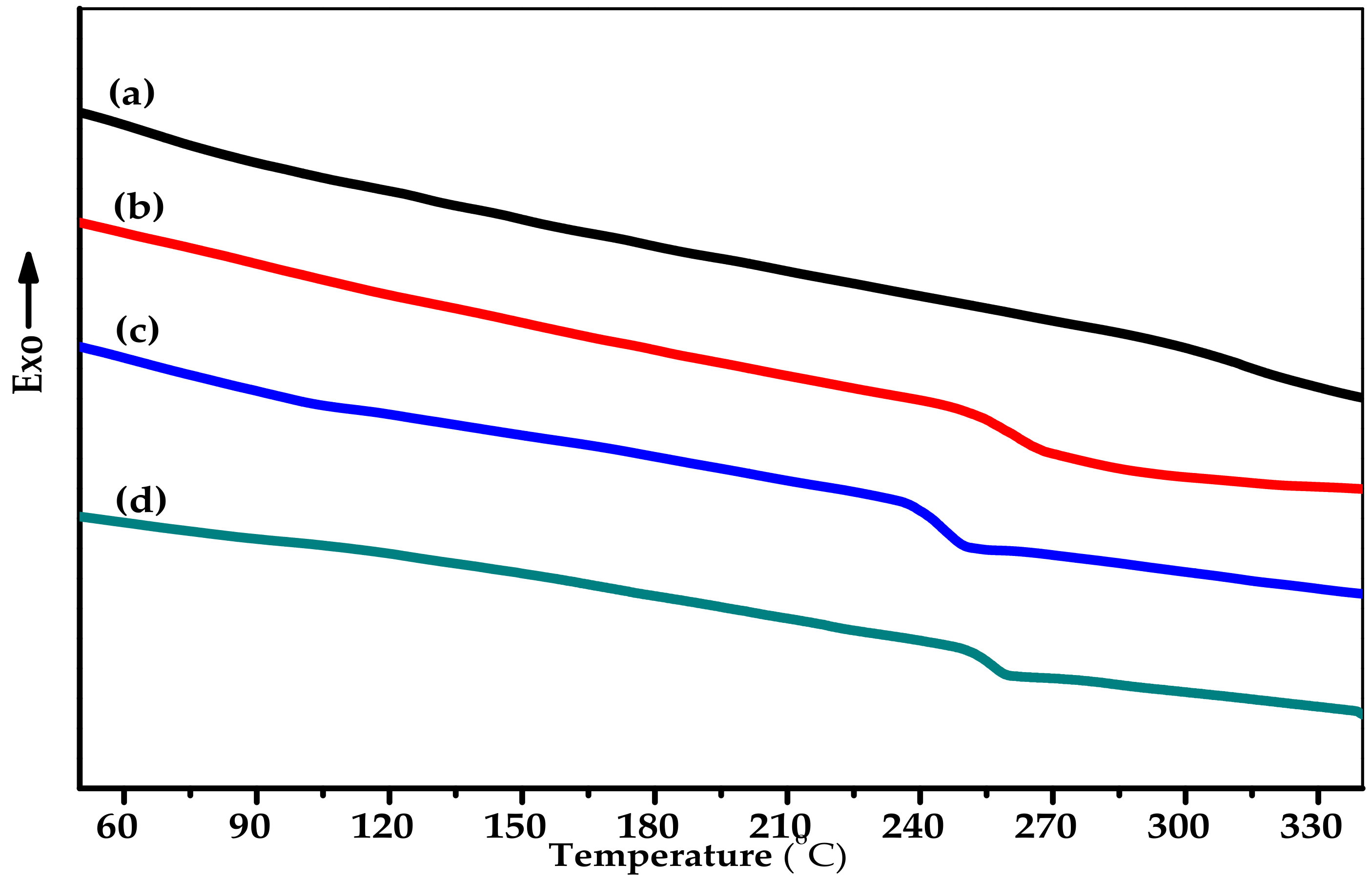
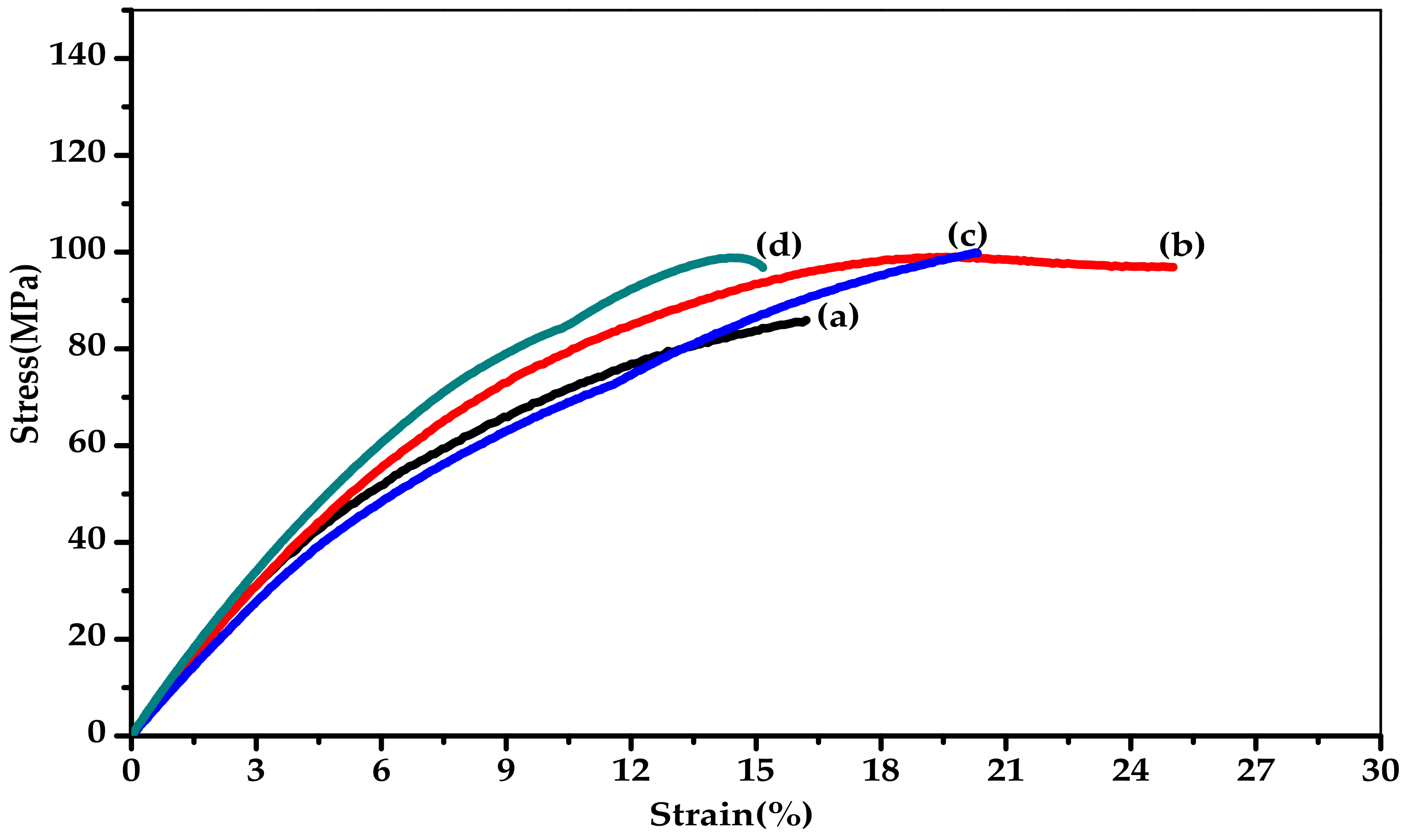
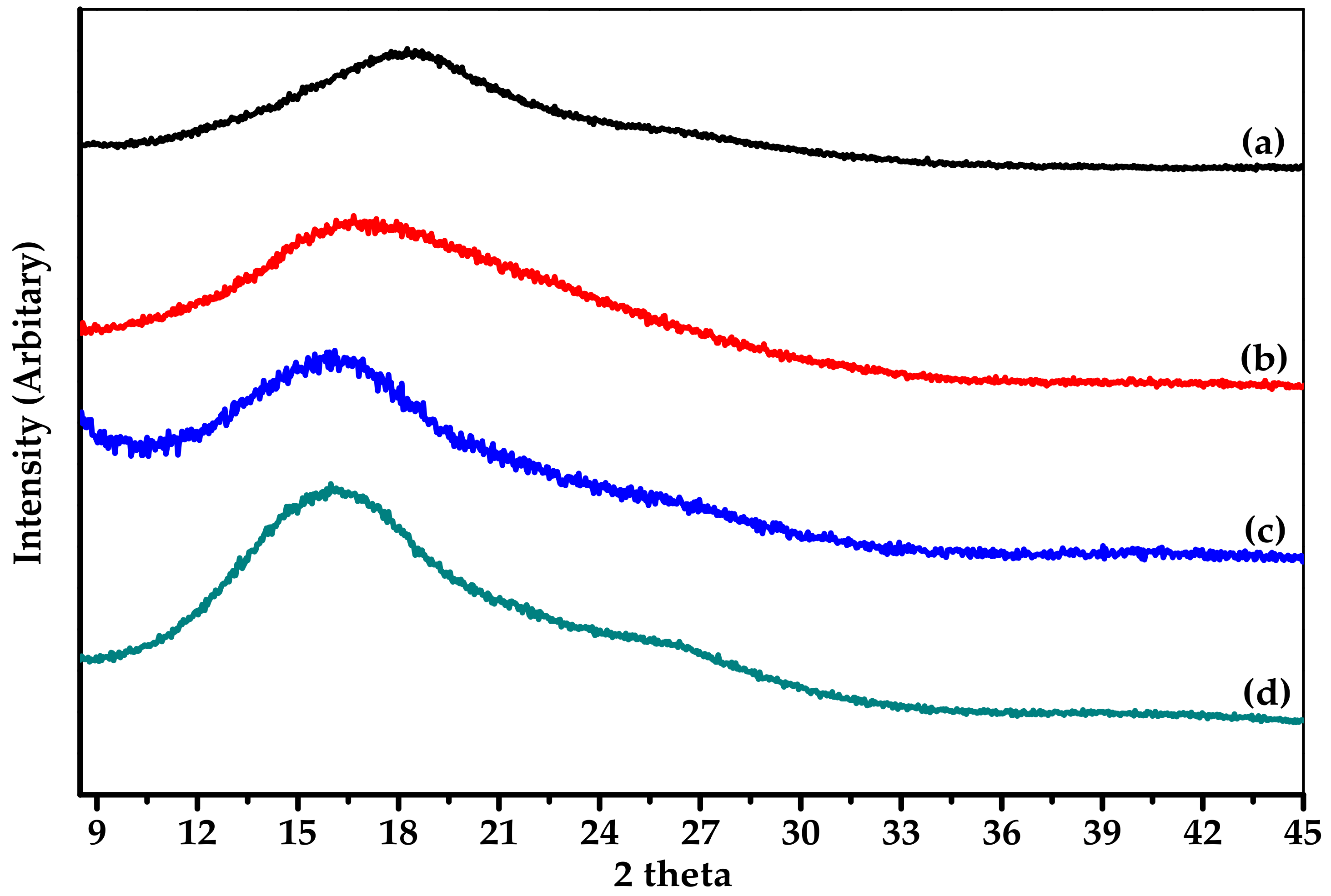
| Sample | Thermal Properties a,b | Mechanical Properties c | WAXD Pattern d | ||||
|---|---|---|---|---|---|---|---|
| Tg (°C) | Td5 (°C) | Tensile Strength at Break (MPa) | Elongation at Break (%) | Modulus (MPa) | 2θ (°) | d-spacing (Å) | |
| CPI-PM | 313 | 467 | 85.0 ± 0.4 | 15.7 ± 0.5 | 972 ± 50 | 18.50 | 4.80 |
| CPI-BP | 262 | 461 | 96.7 ± 1.1 | 25.4 ± 1.5 | 949 ± 10 | 16.66 | 5.32 |
| CPI-6F | 245 | 464 | 99.4 ± 0.2 | 20.0 ± 0.3 | 913 ± 15 | 16.10 | 5.51 |
| API-6F | 255 | 474 | 95.8 ± 1.7 | 14.2 ± 1.0 | 1039 ± 45 | 16.15 | 5.46 |
| Sample | Permeability (Barrer) | Selectivity (αA/B) | Vfa | FFV (%) b | |||
|---|---|---|---|---|---|---|---|
| CO2 | O2 | N2 | O2/N2 | CO2/N2 | |||
| CPI-PM | 2.94 | 0.36 | 0.29 | 1.24 | 8.16 | 63.6 | 12.7 |
| CPI-BP | 10.00 | 2.10 | 0.32 | 3.09 | 6.09 | 89.5 | 15.3 |
| CPI-6F | 31.77 | 6.10 | 0.95 | 6.44 | 33.44 | 126.7 | 18.9 |
| API-6F | 26.28 | 5.33 | 1.07 | 4.98 | 24.30 | 116.5 | 18.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, S.-C.; Lee, J.-Y.; Hsieh, C.-T.; Huang, Y.-C.; Wang, K.-S.; Wu, C.-H.; Hu, C.-C.; Chiang, S.-C.; Jeng, R.-J. Synthesis and Properties of Cyclopentyl Cardo-Type Polyimides Based on Dicyclopentadiene. Polymers 2019, 11, 2029. https://doi.org/10.3390/polym11122029
Yeh S-C, Lee J-Y, Hsieh C-T, Huang Y-C, Wang K-S, Wu C-H, Hu C-C, Chiang S-C, Jeng R-J. Synthesis and Properties of Cyclopentyl Cardo-Type Polyimides Based on Dicyclopentadiene. Polymers. 2019; 11(12):2029. https://doi.org/10.3390/polym11122029
Chicago/Turabian StyleYeh, Shih-Chieh, Jen-Yu Lee, Chung-Ta Hsieh, Ya-Chin Huang, Kuan-Syun Wang, Chien-Hsin Wu, Chien-Chieh Hu, Shu-Chen Chiang, and Ru-Jong Jeng. 2019. "Synthesis and Properties of Cyclopentyl Cardo-Type Polyimides Based on Dicyclopentadiene" Polymers 11, no. 12: 2029. https://doi.org/10.3390/polym11122029
APA StyleYeh, S.-C., Lee, J.-Y., Hsieh, C.-T., Huang, Y.-C., Wang, K.-S., Wu, C.-H., Hu, C.-C., Chiang, S.-C., & Jeng, R.-J. (2019). Synthesis and Properties of Cyclopentyl Cardo-Type Polyimides Based on Dicyclopentadiene. Polymers, 11(12), 2029. https://doi.org/10.3390/polym11122029