Waste PET as a Reactant for Lanthanide MOF Synthesis and Application in Sensing of Picric Acid
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Instruments
2.3. Waste PET Hydrolysis
2.4. Synthesis of Lanthanide MOF
2.5. Photophysical Study
3. Results and Discussion
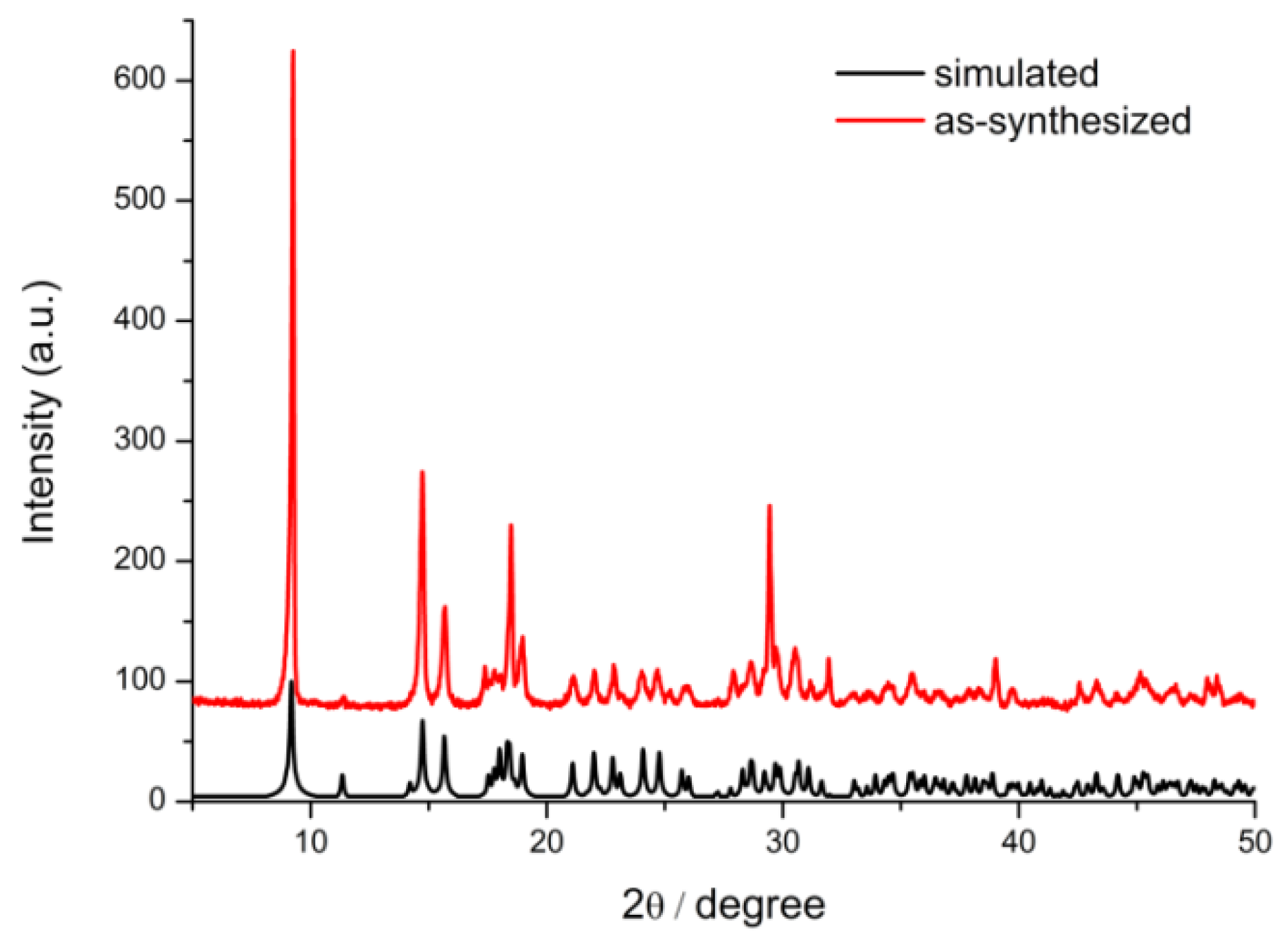
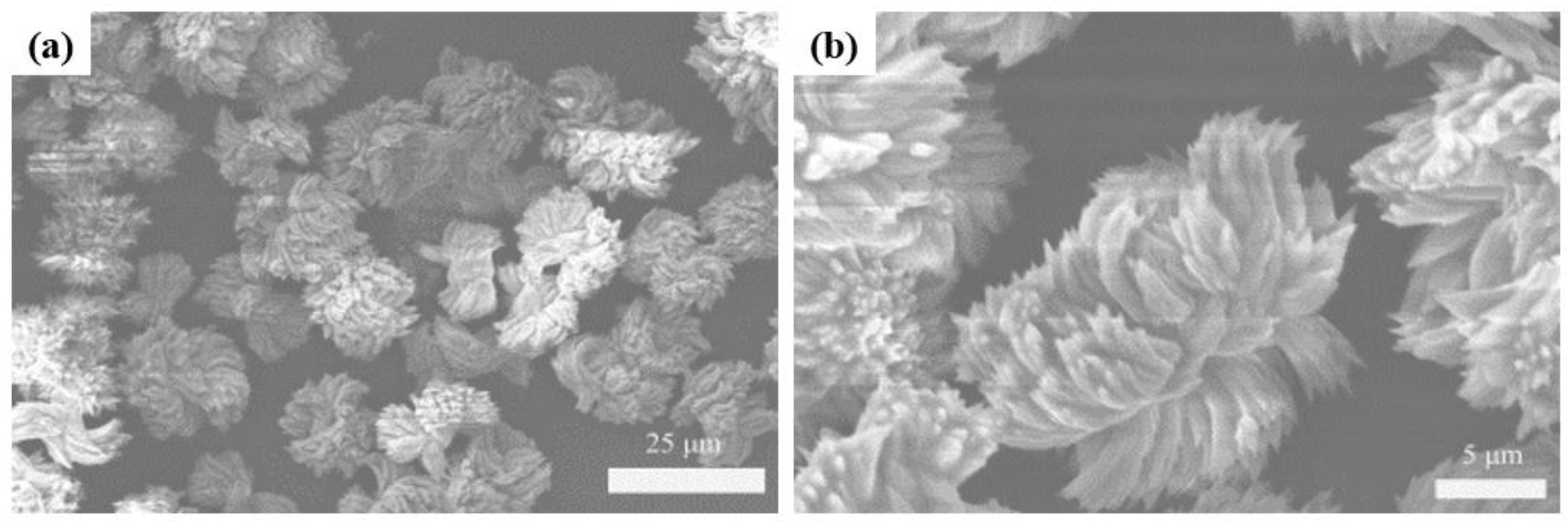
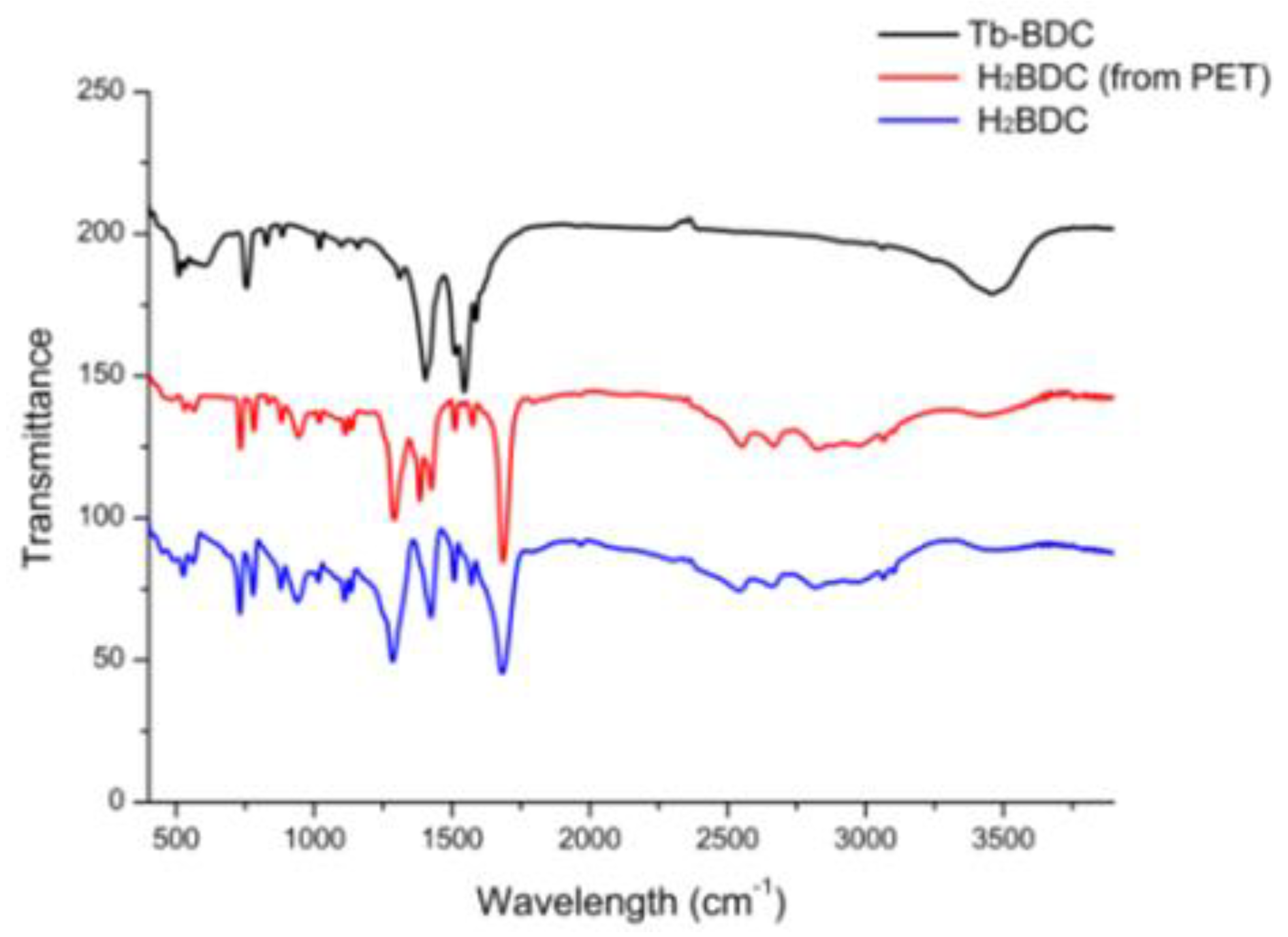
3.1. Characterization
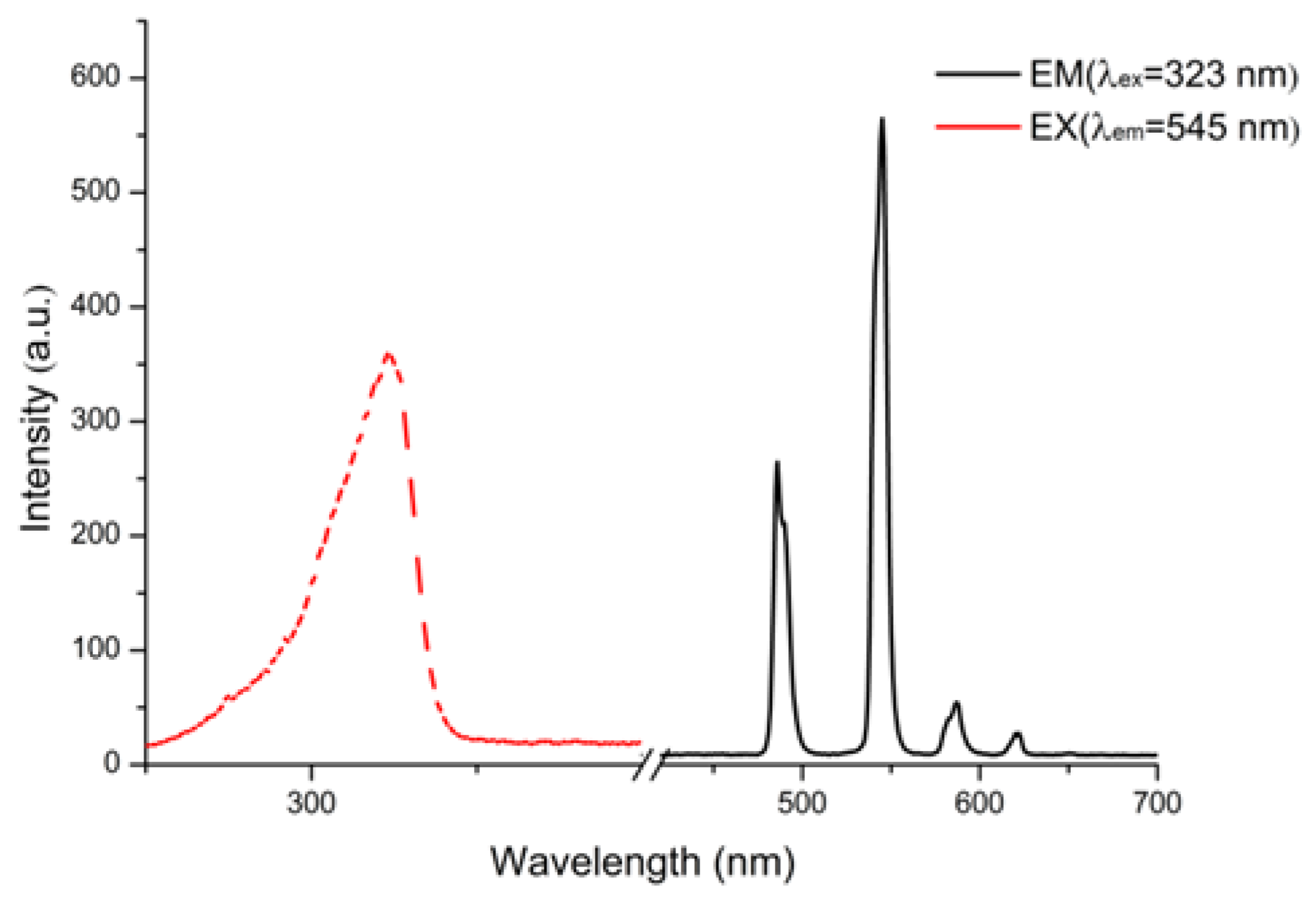
3.2. Photoluminescence Properties
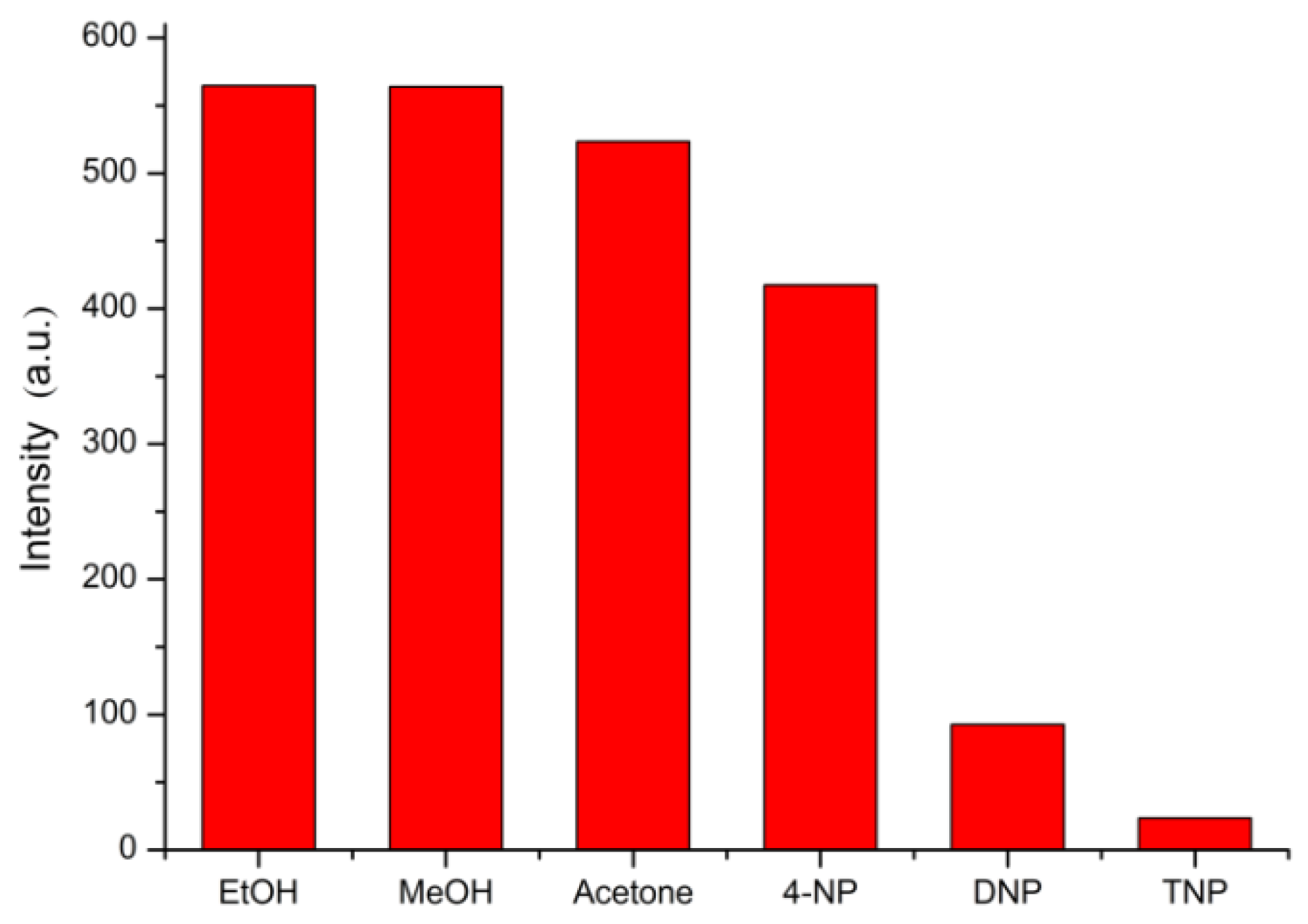
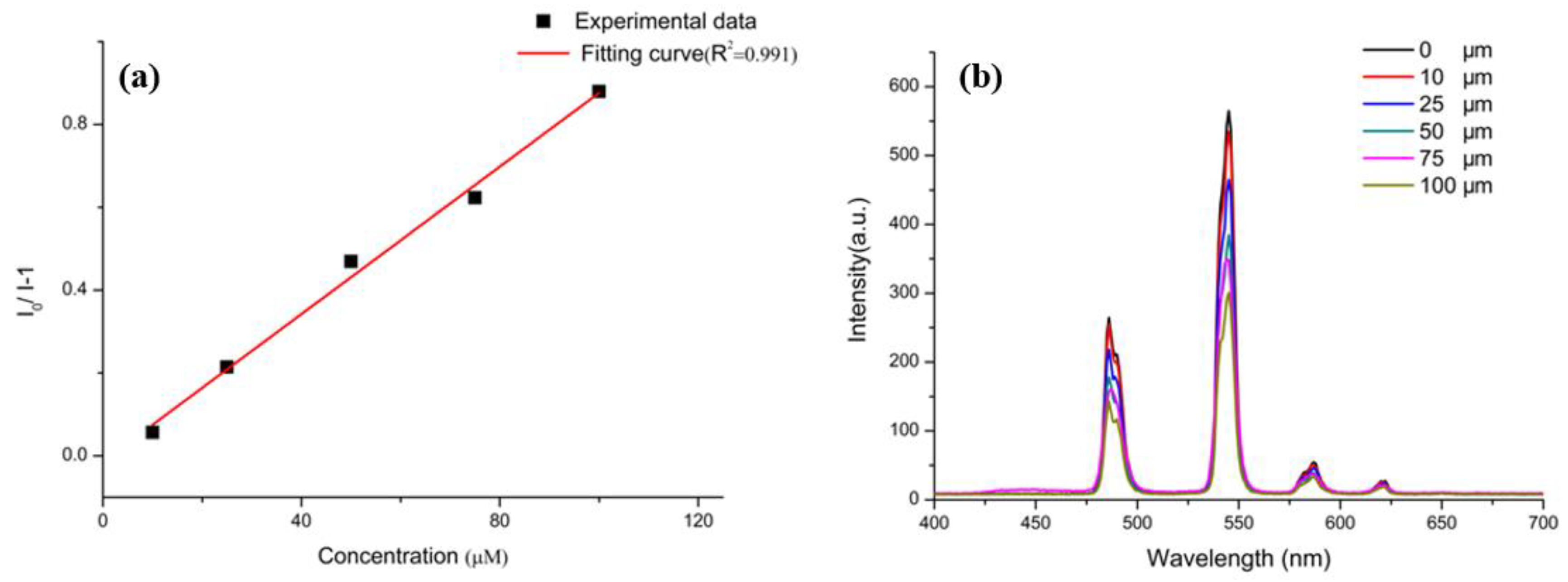
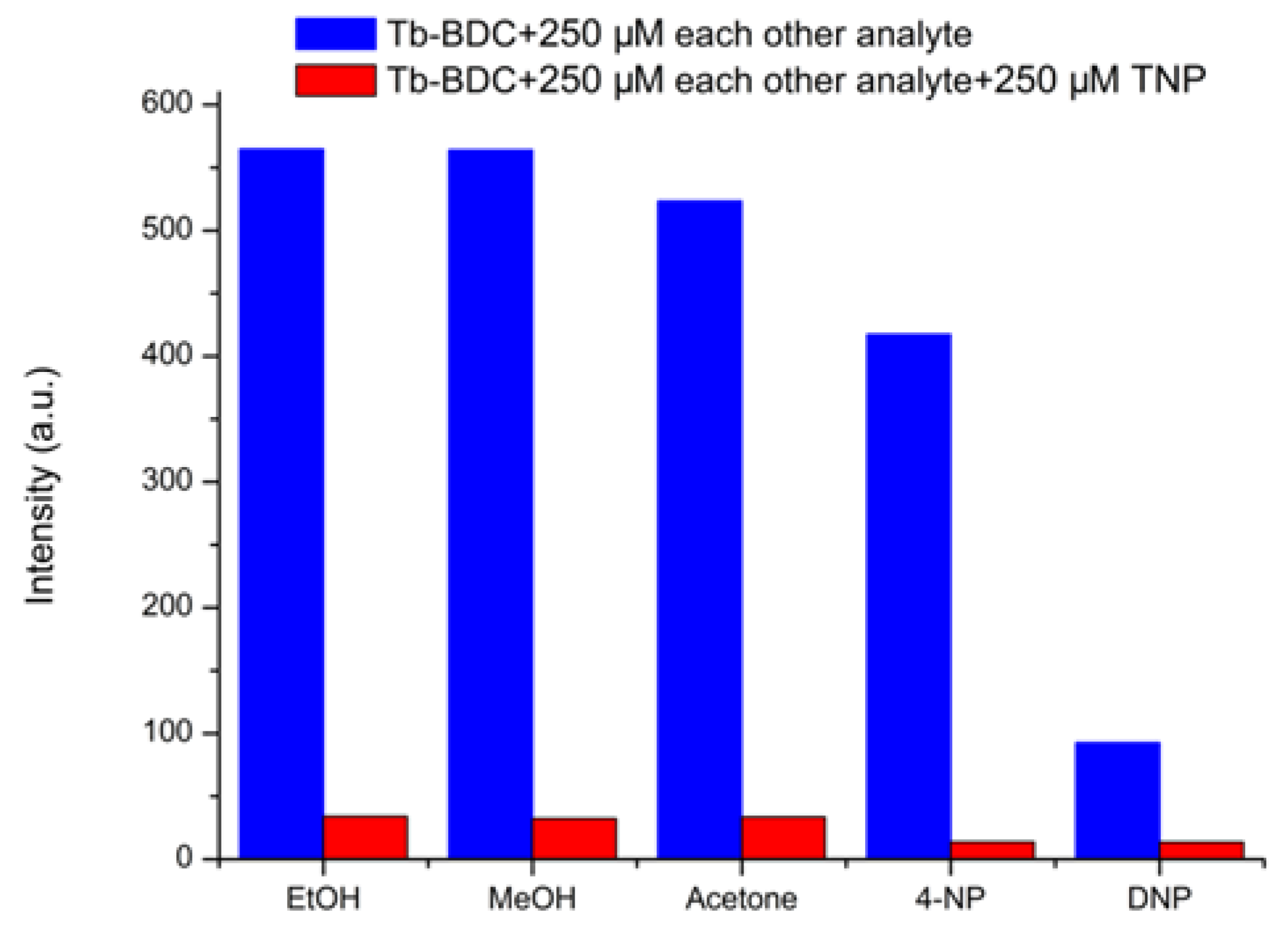
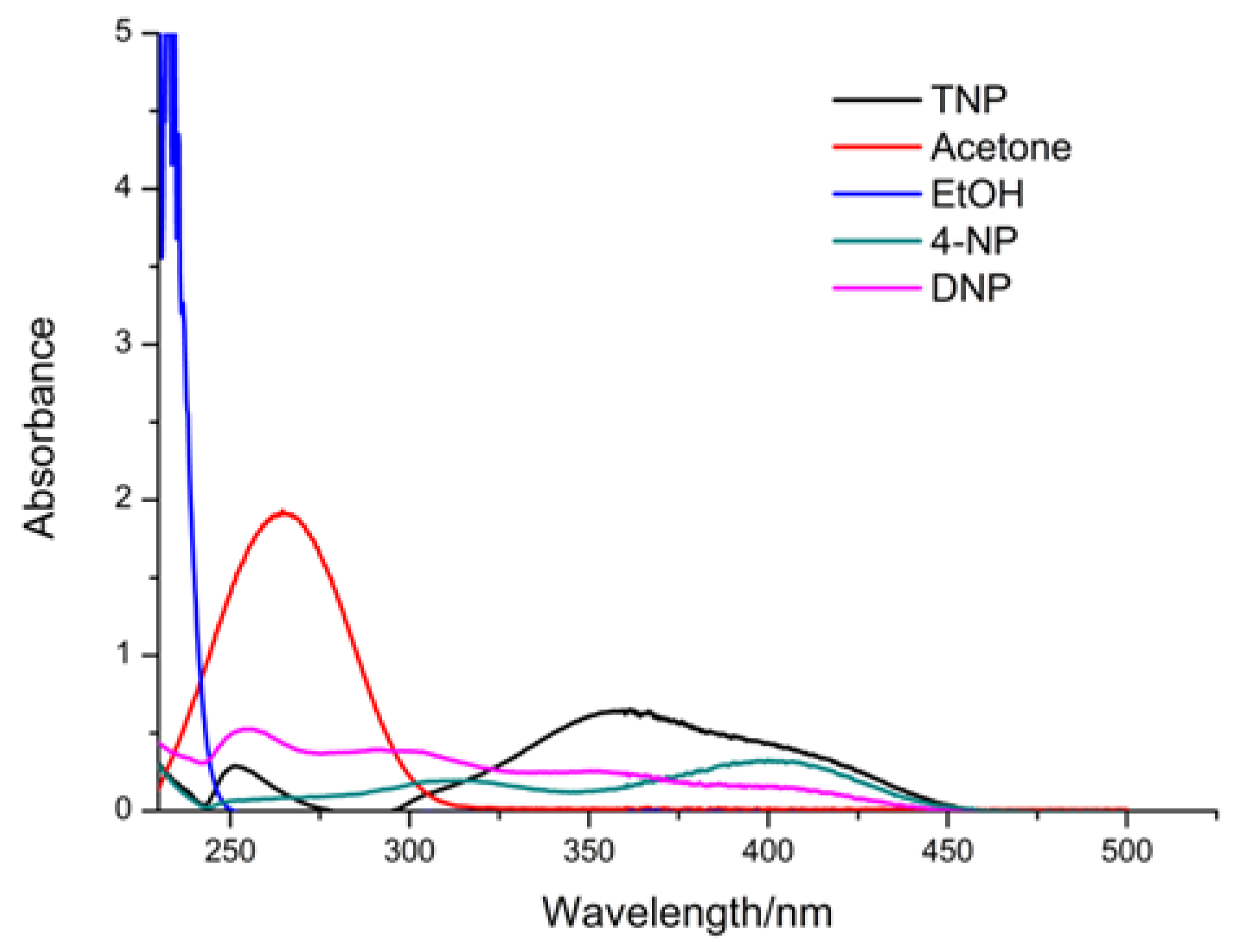
3.3. Selective Sensing for Picric Acid
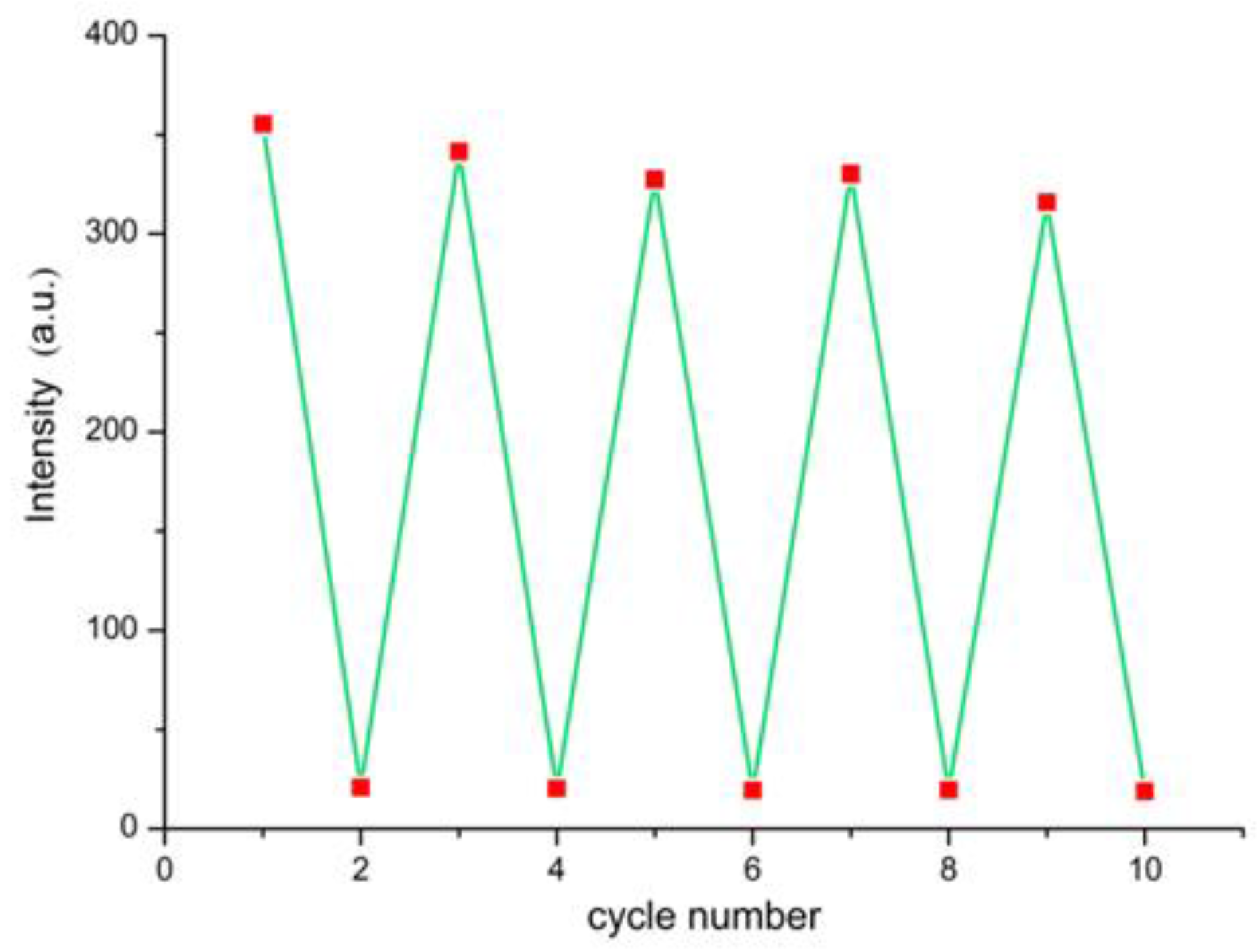
3.4. Luminescent Test Paper
3.5. Quenching Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murray, L.J.; Dinca, M.; Long, J.R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal–organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Lin, Y.N.; Liu, J.W.; Shi, W.; Yang, G.M. Rapid detection of the biomarkers for carcinoid tumors by a water stable luminescent lanthanide metal–organic framework sensor. Adv. Funct. Mater. 2018, 28, 1707169. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Cui, Y.J.; Qian, G.D.; Chen, B.L. Multifunctional lanthanide coordination polymers. Prog. Polym. Sci. 2015, 48, 40–84. [Google Scholar] [CrossRef]
- Li, X.; Tang, J.X.; Liu, H.; Gao, K.; Wu, J.; Hou, H.W. A highly sensitive and recyclable Ln-MOF luminescent sensor for the efficient detection of fe3+ and crvi anions. Chem. Asian J. 2019, 14, 3721–3727. [Google Scholar] [CrossRef]
- Maity, R.; Chakraborty, D.; Nandi, S.; Yadav, A.K.; Mullangi, D.; Vinod, C.P.; Vaidhyanathan, R. Aqueous-phase differentiation and speciation of Fe3+ and Fe2+ using water-stable photoluminescent lanthanide-based metal–organic framework. ACS Appl. Nano Mater. 2019, 28, 5169–5178. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, L.M.; Jin, C.Y.; Yan, B. Luminescence responsive composites of rare earth metal-organic frameworks covalently linking microsphere resin. Dyes Pigment. 2020, 173, 107883. [Google Scholar] [CrossRef]
- Wang, W.Z.; Gong, N.; Yin, H.; Zhang, B.; Guo, P.Y.; Liu, B.; Wang, Y.Y. Two stable terbium–organic frameworks based on predesigned functionalized ligands: Selective sensing of Fe3+ ions and C2H2/CH4 separation. Inorg. Chem. 2019, 58, 10295–10303. [Google Scholar] [CrossRef]
- Wong, K.L.; Law, G.L.; Yang, Y.Y.; Wong, W.T. A Highly porous luminescent terbium-organic framework for reversible anion sensing. Adv. Mater. 2006, 18, 1051–1054. [Google Scholar] [CrossRef]
- Zhan, Z.Y.; Jia, Y.J.; Li, D.H.; Zhang, X.L.; Hu, M. A water-stable terbium-MOF sensor for the selective, sensitive, and recyclable detection of Al3+ and CO32− ions. Dalton Trans. 2019, 48, 15255–15262. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.S.; Yu, J.C.; Cui, Y.J.; Yang, Y.; Wang, Z.Y.; Yang, D.R.; Qian, G.D. Luminescent metal–organic framework films as highly sensitive and fast-response oxygen sensors. J. Am. Chem. Soc. 2014, 136, 5527–5530. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.H.; Tsoi, T.H.; Chan, C.F.; Dai, L.X.; Wu, Y.D.; Du, G.Y.; Zhu, L.Z.; Lee, C.S.; Wong, W.T.; Law, G.L.; et al. A smart “off–on” gate for the in situ detection of hydrogen sulphide with Cu(ii)-assisted europium emission. Chem. Sci. 2016, 7, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Konno, Y.; Nakanishi, T.; Kobayashi, A.; Kato, M.; Fushimi, K.; Hasegawa, Y. Chameleon luminophore for sensing temperatures: Control of metal-to-metal and energy back transfer in lanthanide coordination polymers. Angew. Chem. Int. Ed. 2013, 52, 6413–6416. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, D.; Lu, Y.; Sun, W.Y. Photoluminescent metal–organic frameworks and their application for sensing biomolecules. J. Mater. Chem. A 2019, 7, 22744–22767. [Google Scholar] [CrossRef]
- Deleu, W.P.R.; Stassen, I.; Jonckheere, D.; Ameloot, R.; De Vos, D.E. Waste PET (bottles) as a resource or substrate for MOF synthesis. J. Mater. Chem. A 2016, 4, 9519–9525. [Google Scholar] [CrossRef]
- Ronkvist, A.M.; Xie, W.C.; Lu, W.H.; Gross, R.A. Cutinase-catalyzed hydrolysis of poly (ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Sun, Z.; Ling, Y.; Liu, S.G.; Yang, Y.Z.; Wang, X.H.; Fan, Y.Z.; Li, N.B.; Luo, H.Q. Metal–organic framework as a chemosensor based on luminescence properties for monitoring cetyltrimethylammonium bromide and its application in smartphones. Inorg. Chem. 2019, 58, 8388–8395. [Google Scholar] [CrossRef]
- Guo, X.D.; Zhu, G.S.; Sun, F.X.; Li, Z.Y.; Zhao, X.J.; Li, X.T.; Wang, H.C.; Qiu, S.L. Synthesis, structure, and luminescent properties of microporous lanthanide metal−organic frameworks with inorganic rod-shaped building units. Inorg. Chem. 2006, 45, 2581–2587. [Google Scholar] [CrossRef]
- Jurcic, M.; Peveler, W.J.; Savory, C.N.; Scanlon, D.O.; Kenyon, A.J.; Parkin, I.P. The vapour phase detection of explosive markers and derivatives using two fluorescent metal–organic frameworks. J. Mater. Chem. A 2015, 3, 6351–6359. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal–organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.B.; Fan, R.Q.; Lu, H.Y.; Wang, B.W.; Wu, J.K.; Wang, P.; Yang, Y.L. A dual-emitting Tb (iii) & Yb (iii)-functionalized coordination polymer: A “turn-on” sensor for N-methylformamide in urine and a “turn-off” sensor for methylglyoxal in serum. Dalton Trans. 2019, 48, 14408–14417. [Google Scholar] [PubMed]
- Wang, X.Y.; Yao, X.; Huang, Q.; Li, Y.X.; An, G.H.; Li, G.M. Triple-wavelength-region luminescence sensing based on a color-tunable emitting lanthanide metal organic framework. Anal. Chem. 2018, 9011, 6675–6682. [Google Scholar] [CrossRef]
- Kumari, S.; Joshi, S.; Cordova-Sintjago, T.C.; Pant, D.D.; Sakhuja, R. Highly sensitive fluorescent imidazolium-based sensors for nanomolar detection of explosive picric acid in aqueous medium. Sens. Actuators B Chem. 2016, 229, 599–608. [Google Scholar] [CrossRef]
- Pal, T.K.; Chatterjee, N.; Bharadwaj, P.K. Linker-induced structural diversity and photophysical property of MOFs for selective and sensitive detection of nitroaromatics. Inorg. Chem. 2016, 55, 1741–1747. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, H.; Chu, T.S.; Zhang, G.W.; Wang, Y.; Yang, Y.Y. The Co3O4 nano-anchors fixed Ln-MOF thin film as a highly efficient luminescent sensor for nitrofuran antibiotics. Chem. Eur. J. 2017, 23, 10293–10300. [Google Scholar] [CrossRef]
- Sun, G.T.; Tang, J.G.; Snow, C.D.; Li, Z.H.; Zhang, Y.; Wang, Y.; Belfiore, L.A. Drug Sensing Protein Crystals Doped with Luminescent Lanthanide Complexes. Cryst. Growth Des. 2019, 19, 5658–5664. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.G.; Ma, Y.; Li, L.C. Dual-functional recyclable luminescent sensors based on 2D lanthanide-based metal-organic frameworks for highly sensitive detection of Fe3+ and 2,4-dinitrophenol. Dyes Pigment. 2017, 146, 263–271. [Google Scholar] [CrossRef]
- Chen, D.M.; Sun, C.X.; Peng, Y.; Zhang, N.N.; Si, H.H.; Liu, C.S.; Du, M. Ratiometric fluorescence sensing and colorimetric decoding methanol by a bimetallic lanthanide-organic framework. Sens. Actuators B Chem. 2018, 265, 104–109. [Google Scholar] [CrossRef]
- Cui, R.X.; Wan, Y.Y.; Ji, G.F.; Liu, Z.L. A highly selective and sensitive fluorescent sensor based on Tb3+-functionalized MOFs to determine arginine in urine: A potential application for the diagnosis of cystinuria. Analyst 2019, 144, 5875–5881. [Google Scholar] [CrossRef] [PubMed]
- Reineke, T.M.; Eddaoudi, M.; Fehr, M.; Kelley, D.; Yaghi, O.M. From condensed lanthanide coordination solids to microporous frameworks having accessible metal sites. J. Am. Chem. Soc. 1999, 121, 1651–1657. [Google Scholar] [CrossRef]
- Yu, M.K.; Xie, Y.; Wang, X.Y.; Li, Y.X.; Li, G.M. Highly water-stable dye@Ln-MOFs for sensitive and selective detection toward antibiotics in water. ACS Appl. Mater. Interfaces 2019, 11, 1201–21210. [Google Scholar] [CrossRef] [PubMed]
- Kent, C.A.; Liu, D.; Meyer, T.J.; Lin, W.B. Amplified luminescence quenching of phosphorescent metal–organic frameworks. J. Am. Chem. Soc. 2012, 134, 3991–3994. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Lou, K.L.; Zeng, C.H.; Li, S.S.; Nie, Z.W.; Zhong, S. Hybrid membrane of agarose and lanthanide coordination polymer: A selective and sensitive Fe3+ sensor. Photochem. Photobiol. 2015, 91, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.L.; Dong, J.; Tang, M.H.; Jiang, X.L.; Jiao, Z.H.; Zhao, B. Triple-interpenetrated lanthanide-organic framework as dual wave bands self-calibrated pH luminescent probe. Anal. Chem. 2019, 918, 5455–5460. [Google Scholar] [CrossRef]
| NaOH (mol/L) | Crude Product BDC Yield (12 h) | Crude Product BDC Yield (24 h) | Tb2(BDC)3·(H2O)4 Yield (24 h) | BDC Yield (24 h) |
|---|---|---|---|---|
| 0 | 0 g | 0 g | 0 g | 0 |
| 0.5 | 0.02 g | 0.07 g | 0.054 g | 0.031 g |
| 1 | 0.03 g | 0.1 g | 0.088 g | 0.053 g |
| 2 | 0.05 g | 0.1 g | 0.105 g | 0.062 g |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Chen, S.; Nie, S.; Luo, J.; Lin, S.; Wang, Y.; Yang, H. Waste PET as a Reactant for Lanthanide MOF Synthesis and Application in Sensing of Picric Acid. Polymers 2019, 11, 2015. https://doi.org/10.3390/polym11122015
Zhang F, Chen S, Nie S, Luo J, Lin S, Wang Y, Yang H. Waste PET as a Reactant for Lanthanide MOF Synthesis and Application in Sensing of Picric Acid. Polymers. 2019; 11(12):2015. https://doi.org/10.3390/polym11122015
Chicago/Turabian StyleZhang, Feng, Shuyi Chen, Shengqiang Nie, Jun Luo, Shaomin Lin, Yi Wang, and Huan Yang. 2019. "Waste PET as a Reactant for Lanthanide MOF Synthesis and Application in Sensing of Picric Acid" Polymers 11, no. 12: 2015. https://doi.org/10.3390/polym11122015
APA StyleZhang, F., Chen, S., Nie, S., Luo, J., Lin, S., Wang, Y., & Yang, H. (2019). Waste PET as a Reactant for Lanthanide MOF Synthesis and Application in Sensing of Picric Acid. Polymers, 11(12), 2015. https://doi.org/10.3390/polym11122015






