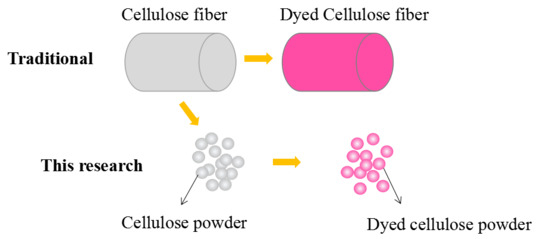
Dyeing and Characterization of Cellulose Powder Developed from Waste Cotton
Abstract
:1. Introduction
2. Experimental
2.1. Materials
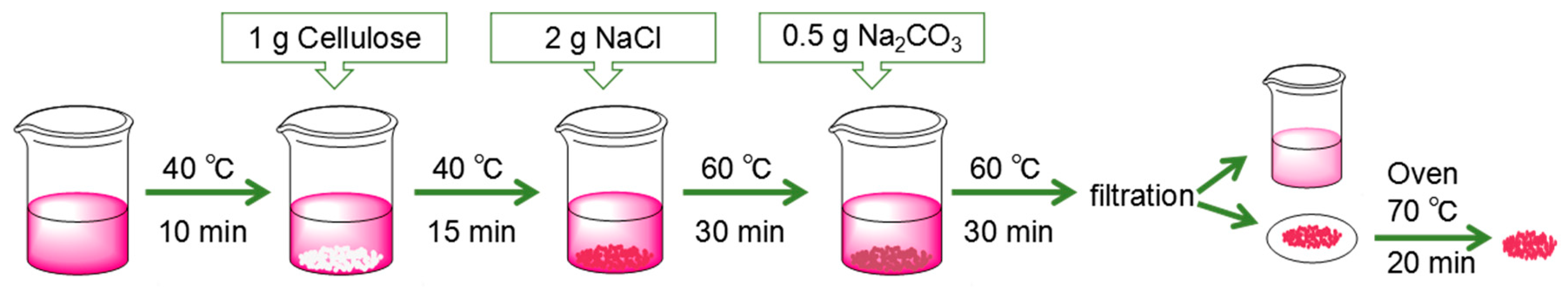
2.2. Milling Method of Cellulose Powder
2.3. Effect of Dye Process on Dyeing Properties of Cellulose Powder
2.4. Characterization of Cellulose Powder Before and After Dyeing
3. Results and Discussion
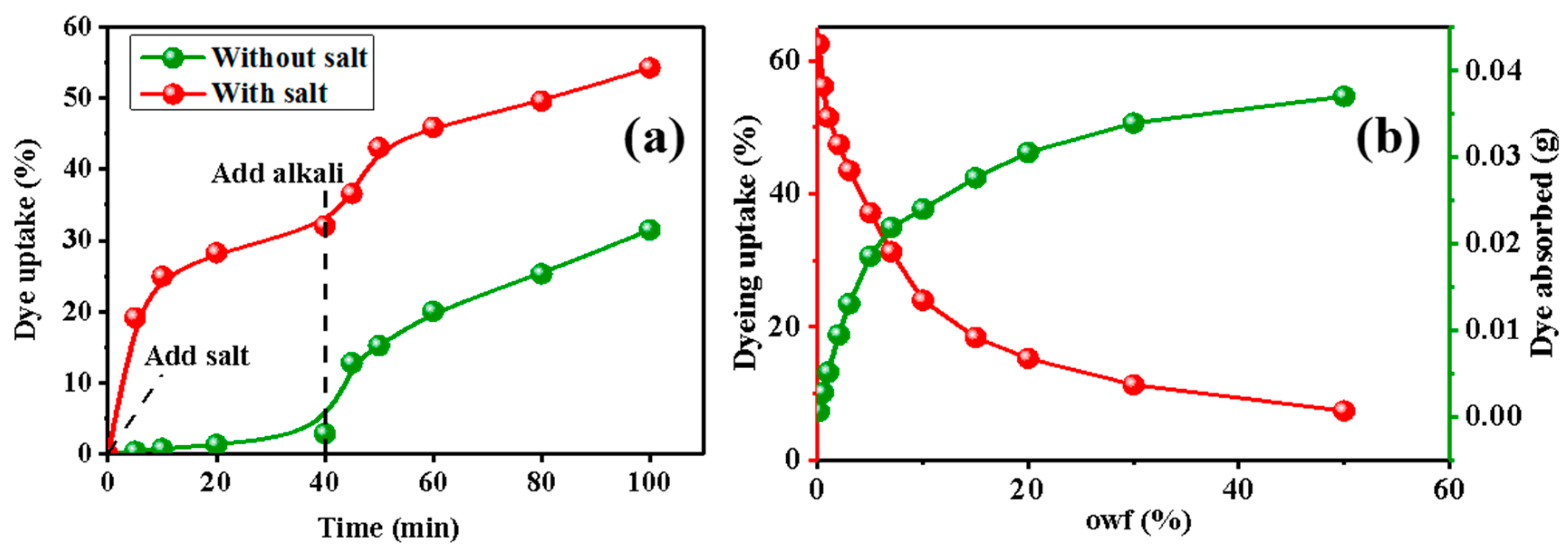
3.1. Influence of Bath Ratio and owf on Dyeing Properties of Cellulose Powder
3.2. The Influence of Salt on Dyeing Efficiency and the Effect of owf on Dye Adsorption
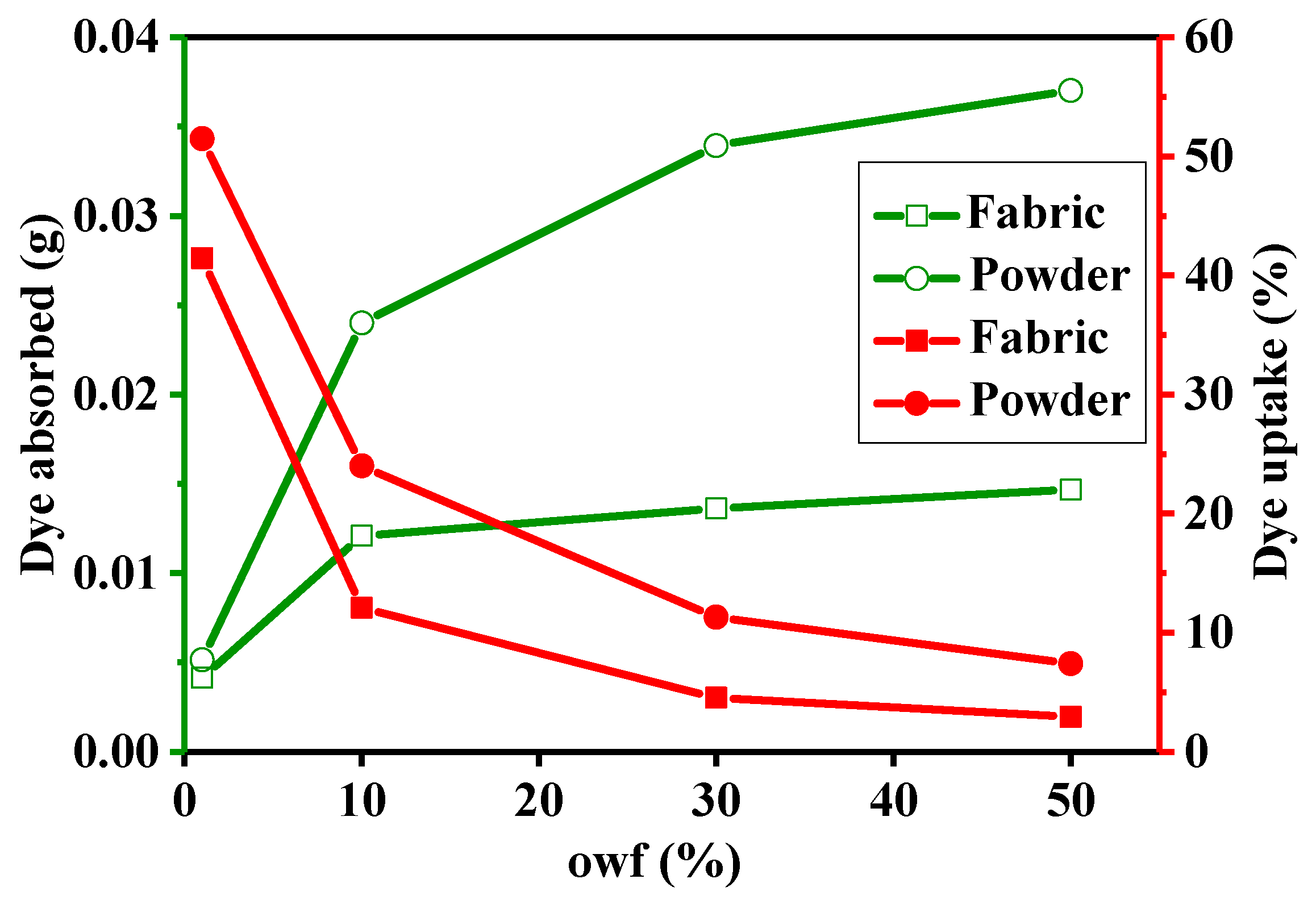
3.3. Dye Adsorption of Cotton Powder and Cotton Fabric with Different owf
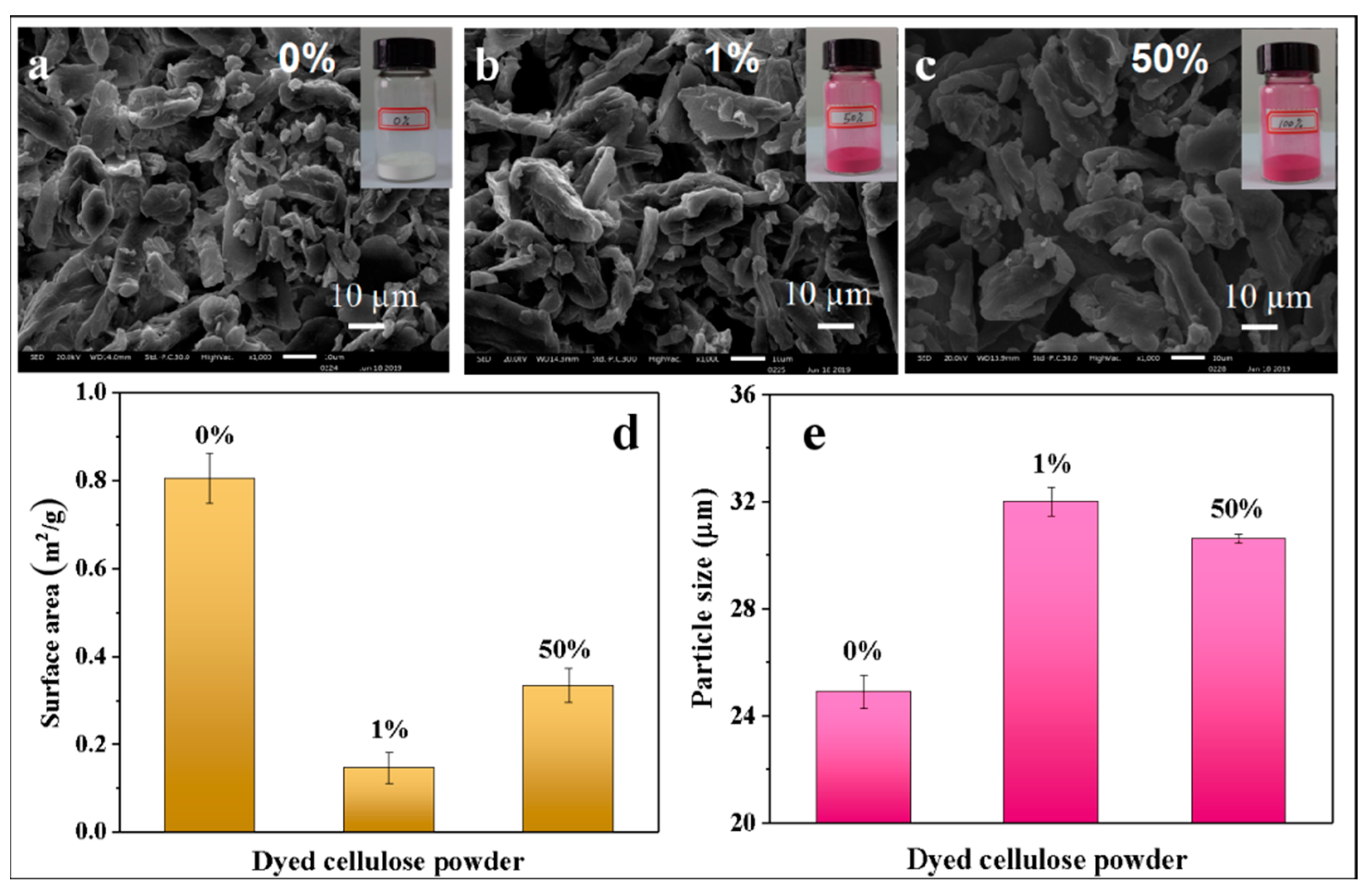
3.4. Powder Morphology and Particle Size
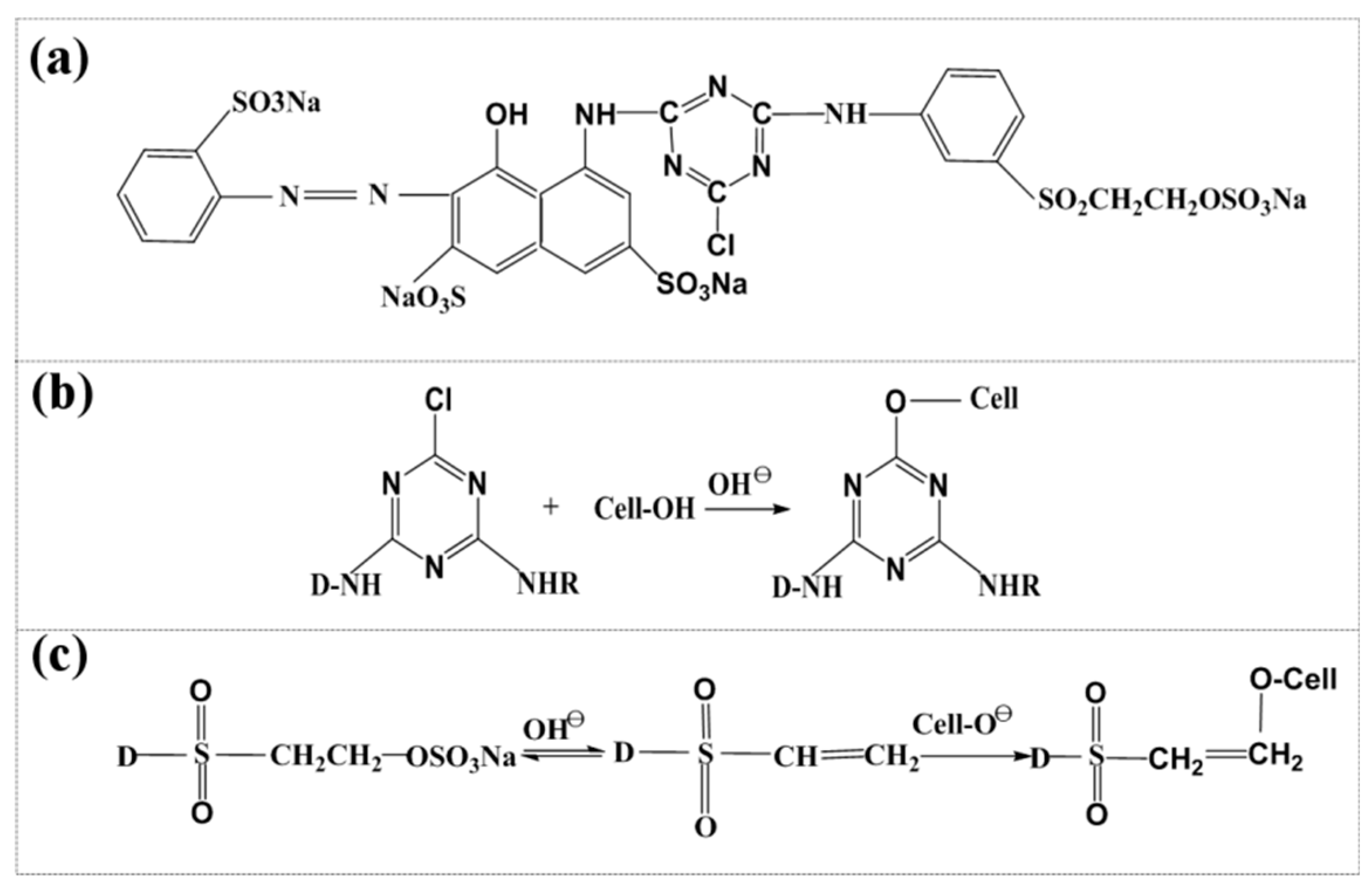
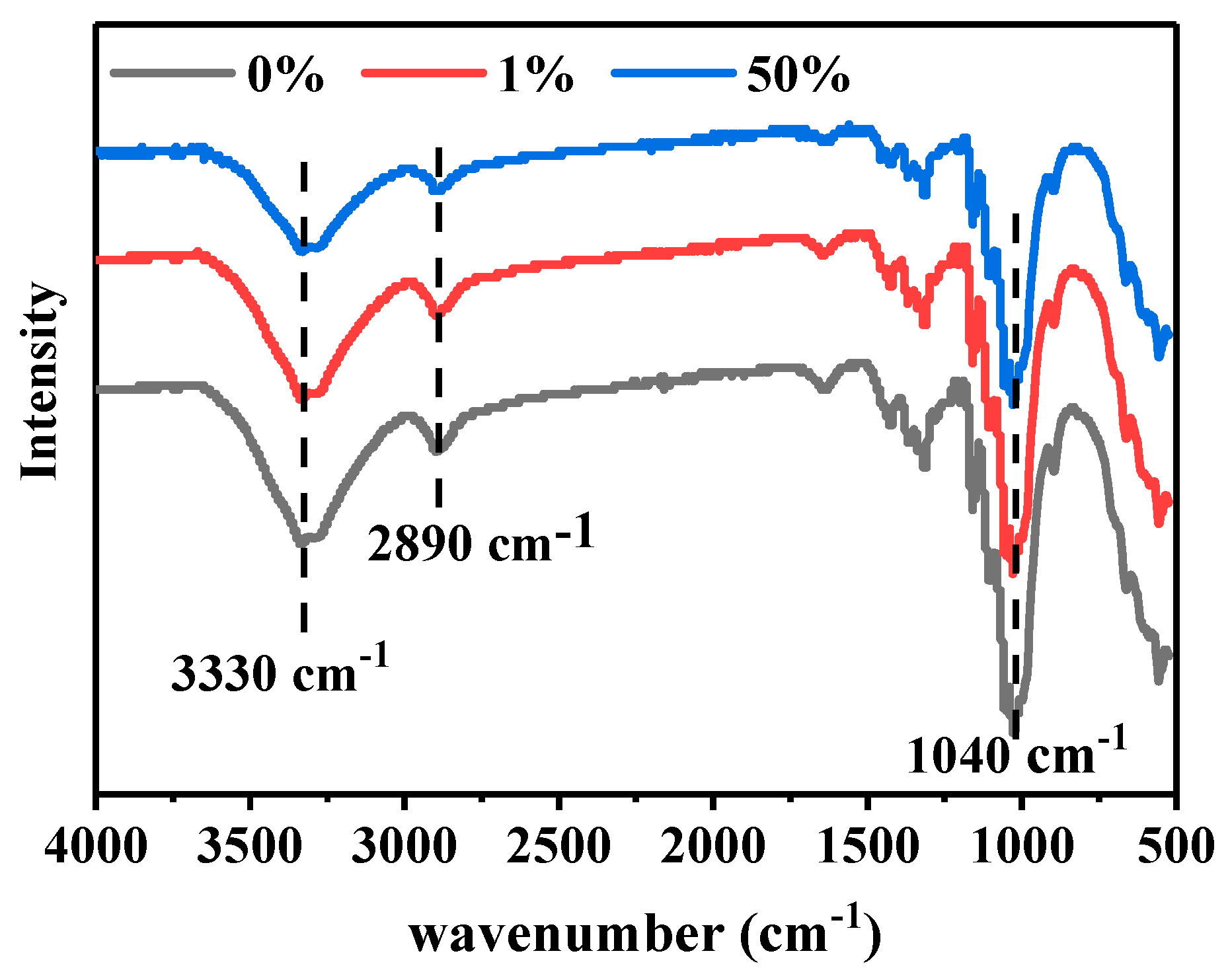
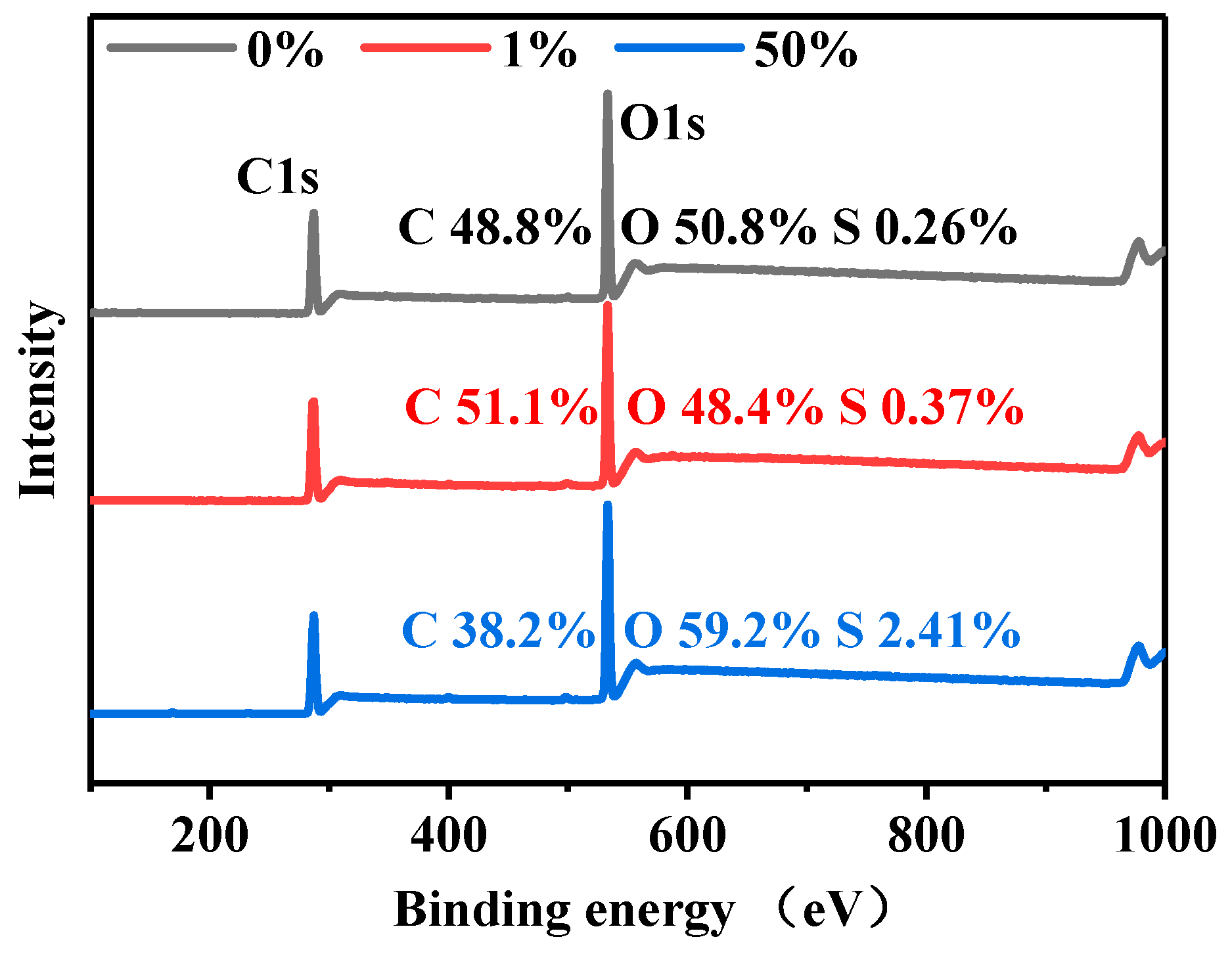
3.5. Composition Analysis of Dyed and Undyed Cellulose Powders
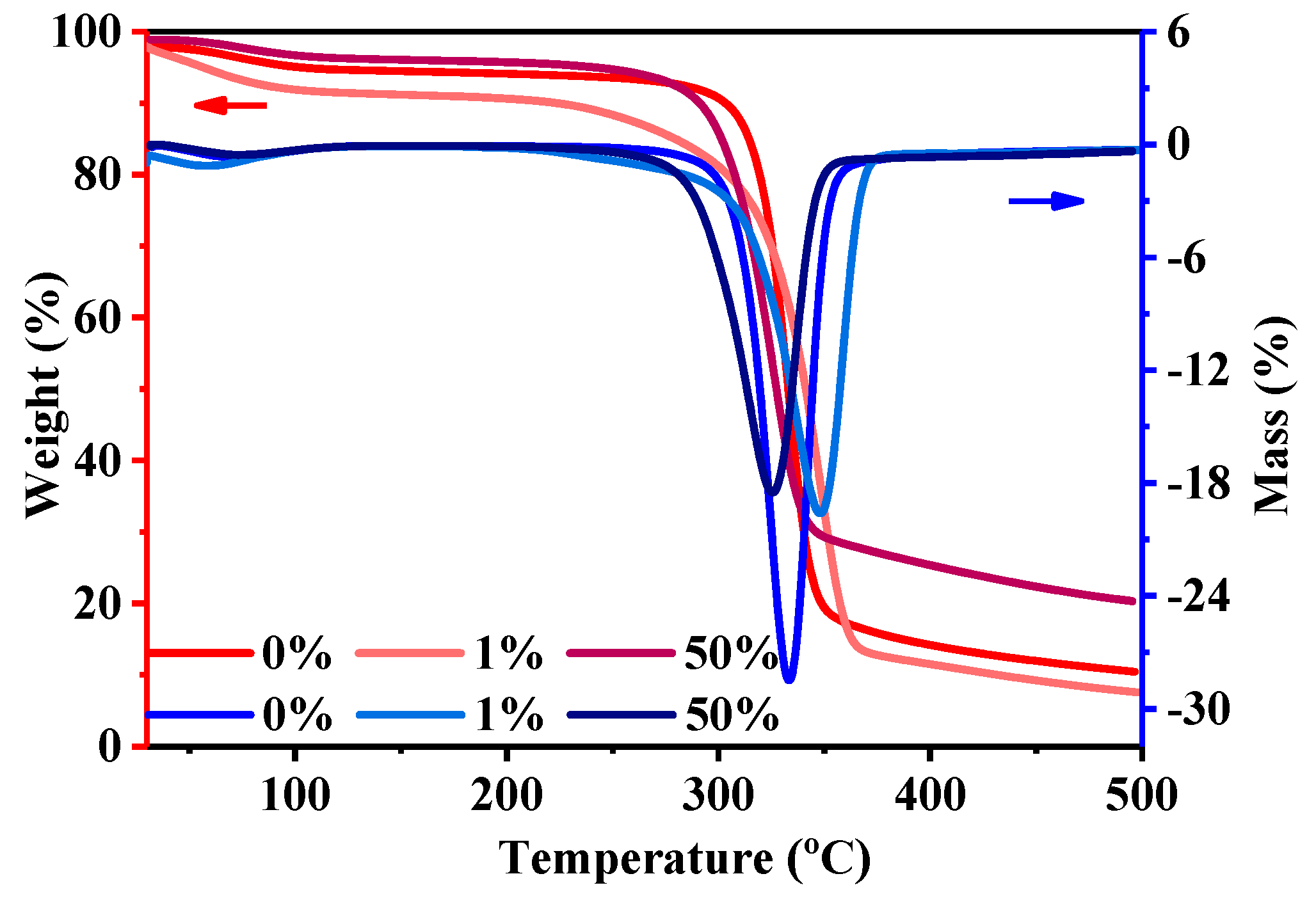
3.6. Thermal Gravity Analysis
3.7. DMA Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baydar, G.; Ciliz, N.; Mammadov, A. Life cycle assessment of cotton textile products in Turkey. Resour. Conserv. Recycl. 2015, 104, 213–223. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Liu, T.; Liu, T.; Zhang, J.; Liu, H. Eco-friendly post-consumer cotton waste recycling for regenerated cellulose fibers. Carbohydr. Polym. 2019, 206, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Sarwar, Z.; Jonuškienė, I.; Kliucininkas, L. A new strategy for using textile waste as a sustainable source of recovered cotton. Resour. Conserv. Recycl. 2019, 145, 359–369. [Google Scholar] [CrossRef]
- Yuen, C.W.M.; Cheng, Y.F.; Li, Y.; Hu, J.Y. Preparation and characterisation of nano-scale cotton powder. J. Text. Inst. 2009, 100, 165–172. [Google Scholar] [CrossRef]
- Haule, L.V.; Carr, C.M.; Rigout, M. Preparation and physical properties of regenerated cellulose fibres from cotton waste garments. J. Clean. Prod. 2016, 112, 4445–4451. [Google Scholar] [CrossRef]
- Haule, L.V.; Carr, C.M.; Rigout, M. Investigation into the removal of an easy-care crosslinking agent from cotton and the subsequent regeneration of lyocell-type fibres. Cellulose 2014, 21, 2147–2156. [Google Scholar] [CrossRef]
- Peña-Pichardo, P.; Martínez-Barrera, G.; Martínez-López, M.; Ureña-Núñez, F.; dos Reis, J.M.L. Recovery of cotton fibers from waste Blue-Jeans and its use in polyester concrete. Constr. Build. Mater. 2018, 177, 409–416. [Google Scholar] [CrossRef]
- Guan, Y.; Li, Y. Fabrication of cotton nano-powder and its textile application. Chin. Sci. Bull. 2008, 53, 3735–3740. [Google Scholar] [CrossRef]
- Xu, W.; Cui, W.; Li, W.; Guo, W. Development and characterizations of super-fine wool powder. Powder Technol. 2004, 140, 136–140. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Li, W.; Xu, W. Preparation and characterization of polypropylene/superfine down powder blend films. J. Thermoplast. Compos. Mater. 2012, 25, 75–88. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Li, W.; Peng, X.; Liu, X.; Wang, X.G. Characterization of superfine wool powder/poly(propylene) blend film. Macromol. Mater. Eng. 2007, 292, 674–680. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Huang, J.; Li, W.; Xu, W. Coating superfine down powder on polypropylene for the production of dyeable fibers. Fibers Polym. 2011, 12, 220. [Google Scholar] [CrossRef]
- Kazemimostaghim, M.; Rajkhowa, R.; Tsuzuki, T.; Wang, X. Production of submicron silk particles by milling. Powder Technol. 2013, 241, 230–235. [Google Scholar] [CrossRef]
- Khatri, A.; White, M.; Padhye, R. Effect of Dye Solution Ionic Strength on Dyeing of Cotton with Reactive Dyes. Fibers Polym. 2018, 19, 1266–1270. [Google Scholar] [CrossRef]
- Tang, A.Y.; Lee, C.H.; Wang, Y.M.; Kan, C.W. A study of PEG-based reverse micellar dyeing of cotton fabric: Reactive dyes with different reactive groups. Cellulose 2019, 26, 4159–4173. [Google Scholar] [CrossRef]
- Shu, D.; Fang, K.; Liu, X.; Cai, Y.; Zhang, X.; Zhang, J. Cleaner coloration of cotton fabric with reactive dyes using a pad-batch-steam dyeing process. J. Clean. Prod. 2019, 196, 935–942. [Google Scholar] [CrossRef]
- Irfan, M.; Zhang, H.; Syed, U.; Hou, A. Low liquor dyeing of cotton fabric with reactive dye by an eco-friendly technique. J. Clean. Prod. 2018, 197, 1480–1487. [Google Scholar] [CrossRef]
- Xia, L.; Wang, A.; Zhang, C.; Liu, Y.; Guo, H.; Ding, C.; Wang, Y.; Xu, W. Environmentally friendly dyeing of cotton in an ethanol-water mixture with excellent exhaustion. Green Chem. 2018, 20, 4473–4483. [Google Scholar] [CrossRef]
- Lee, C.H.; Tang, A.Y.L.; Wang, Y.; Kan, C.W. Effect of reverse micelle-encapsulated reactive dyes agglomeration in dyeing properties of cotton. Dyes Pigment. 2019, 161, 51–57. [Google Scholar] [CrossRef]
- Acharya, S.; Abidi, N.; Rajbhandari, R.; Meulewaeter, F. Chemical cationization of cotton fabric for improved dye uptake. Cellulose 2014, 21, 4693–4706. [Google Scholar] [CrossRef]
- Lewis, D.M. The chemistry of reactive dyes and their application processes. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 303–364. [Google Scholar]
- Chakraborty, J.N. (Ed.) Dyeing with reactive dye. In Fundamentals and Practices in Colouration of Textiles; Woodhead Publishing: New Delhi, India, 2014; pp. 61–76. [Google Scholar]
- Burkinshaw, S.M.; Kabambe, O. Attempts to reduce water and chemical usage in the removal of bifunctional reactive dyes from cotton: Part 2 bis (vinyl sulfone), aminochlorotriazine/vinyl sulfone and bis (aminochlorotriazine/vinyl sulfone) dyes. Dye. Pigment. 2011, 88, 220–229. [Google Scholar] [CrossRef]
- Chakraborty, J.N. (Ed.) Dyeing with Reactive Dye. In Fundamentals and Practices in Colouration of Textiles; Woodhead Publishing: New Delhi, India, 2010; pp. 57–75. [Google Scholar]
- Chakraborty, J.N. (Ed.) Introduction to Colouration of Textiles. In Fundamentals and Practices in Colouration of Textiles; Woodhead Publishing: New Delhi, India, 2014; pp. 1–11. [Google Scholar]
- Burkinshaw, S.M.; Paraskevas, M. The dyeing of silk: Part 3 the application and wash-off of modified vinyl sulfone dyes. Dyes Pigment. 2011, 88, 212–219. [Google Scholar] [CrossRef]
- Biswas, S.; Huang, X.; Badger, W.R.; Nantz, M.H. Nucleophilic cationization reagents. Tetrahedron Lett. 2010, 51, 1727–1729. [Google Scholar] [CrossRef] [PubMed]
- Venudharan, V.; Chandrappa, A.K.; Biligiri, K.P.; Kaloush, K.E. Predictive models for storage modulus and loss modulus of asphalt mixtures. J. Mater. Civ. Eng. 2016, 28, 1943–5533. [Google Scholar] [CrossRef]
- Bhudolia, S.K.; Perrotey, P.; Joshi, S.C. Enhanced vibration damping and dynamic mechanical characteristics of composites with novel pseudo-thermoset matrix system. Compos. Struct. 2017, 179, 502–513. [Google Scholar] [CrossRef]
- Sever, K.; Tavman, I.H.; Seki, Y.; Turgut, A.; Omastova, M.; Ozdemir, I. Electrical and mechanical properties of expanded graphite/high density polyethylene nanocomposites. Compos. Part. B Eng. 2013, 53, 226–233. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, L.; Guo, H.; Xiao, Z.; Jia, Z.; Yang, H.; Sheng, D.; Pan, H.; Xu, W.; Wang, Y. Dyeing and Characterization of Cellulose Powder Developed from Waste Cotton. Polymers 2019, 11, 1982. https://doi.org/10.3390/polym11121982
Gan L, Guo H, Xiao Z, Jia Z, Yang H, Sheng D, Pan H, Xu W, Wang Y. Dyeing and Characterization of Cellulose Powder Developed from Waste Cotton. Polymers. 2019; 11(12):1982. https://doi.org/10.3390/polym11121982
Chicago/Turabian StyleGan, Linli, Heng Guo, Zhiheng Xiao, Zhiwei Jia, Han Yang, Dan Sheng, Heng Pan, Weilin Xu, and Yunli Wang. 2019. "Dyeing and Characterization of Cellulose Powder Developed from Waste Cotton" Polymers 11, no. 12: 1982. https://doi.org/10.3390/polym11121982
APA StyleGan, L., Guo, H., Xiao, Z., Jia, Z., Yang, H., Sheng, D., Pan, H., Xu, W., & Wang, Y. (2019). Dyeing and Characterization of Cellulose Powder Developed from Waste Cotton. Polymers, 11(12), 1982. https://doi.org/10.3390/polym11121982