Facile Fabrication of Superhydrophobic Surface from Fluorinated POSS Acrylate Copolymer via One-Step Breath Figure Method and Its Anti-Corrosion Property
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
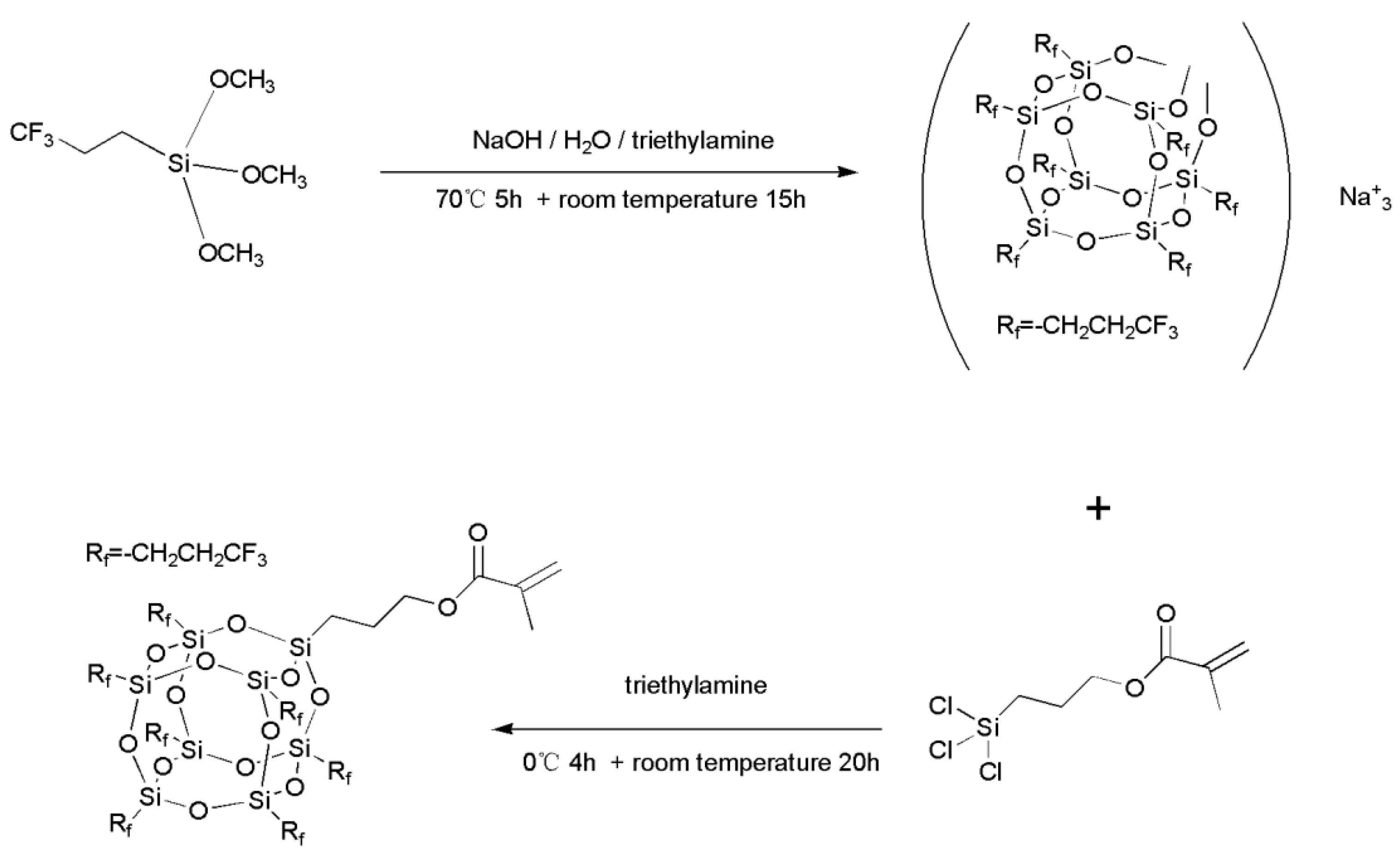
2.2. Synthesis of Methylacrylatepropyl Hepta(3,3,3-trifluoropropyl) POSS (7F-MAP-POSS)
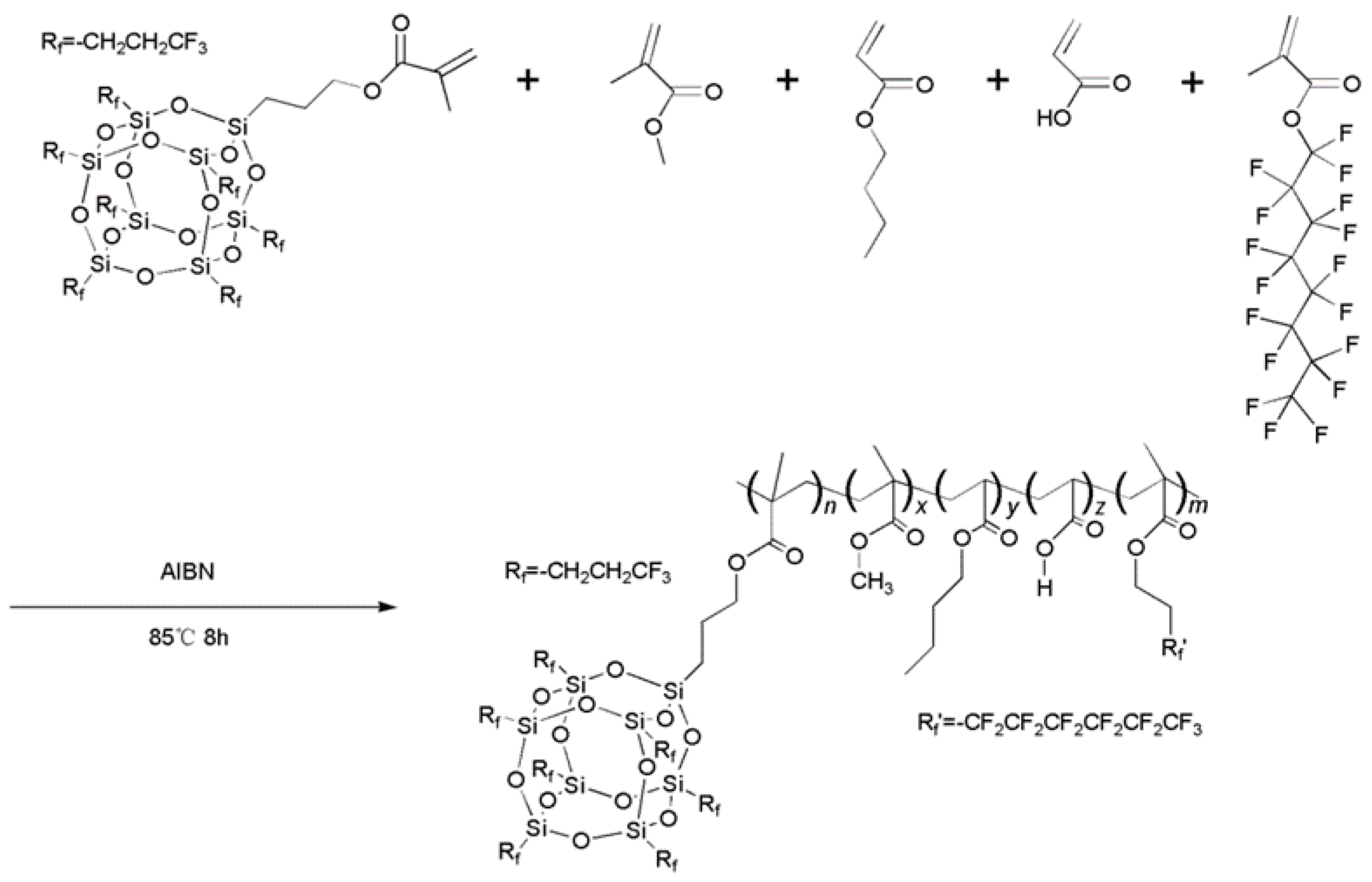
2.3. Synthesis of the 7F-MAP-POSS-Acrylate Copolymers
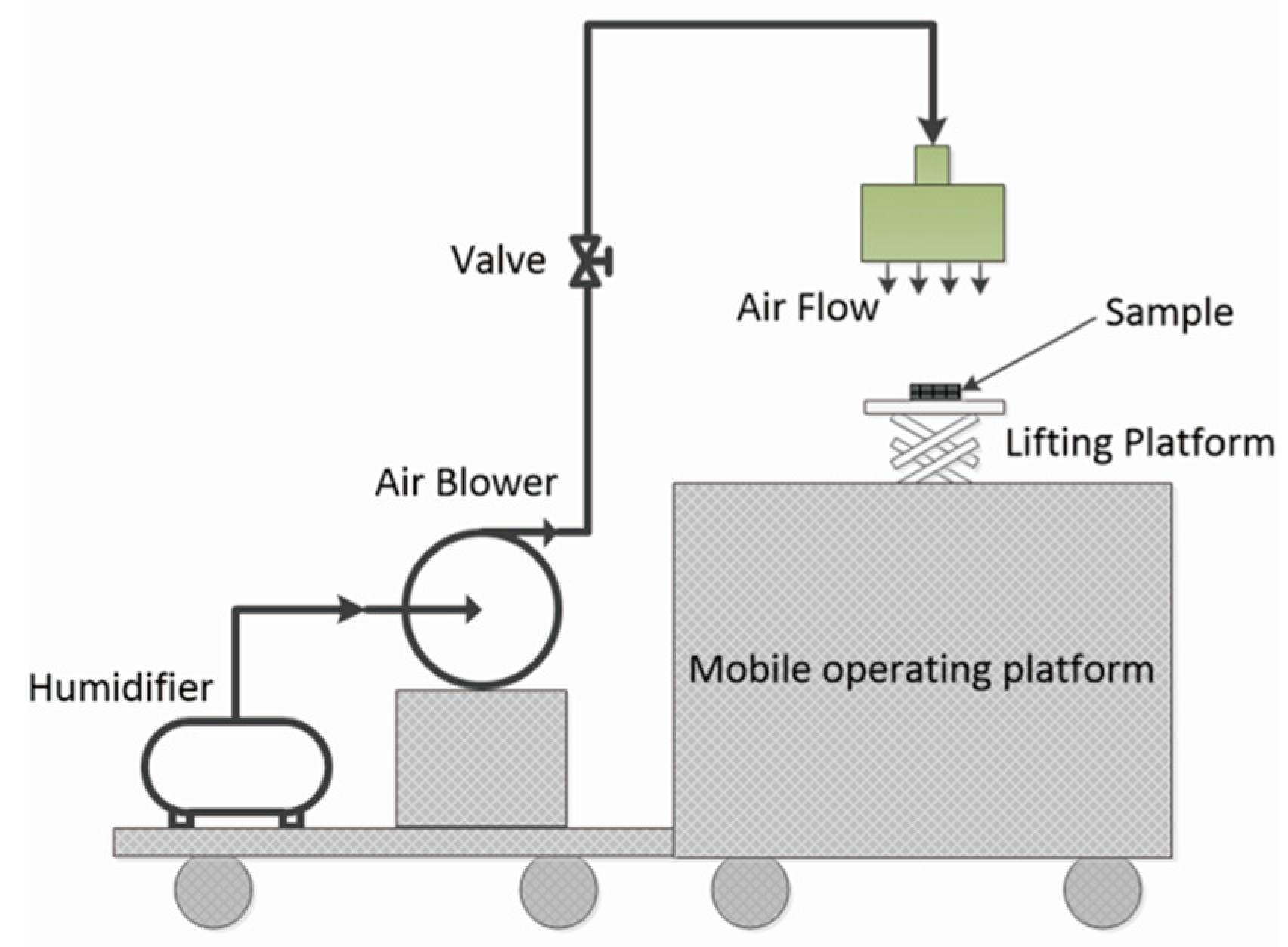
2.4. Preparation of the Superhydrophobic 7F-MAP-POSS-Acrylate Copolymer Coatings
2.5. Characterizations
2.6. Electrochemical Tests
3. Results and Discussion
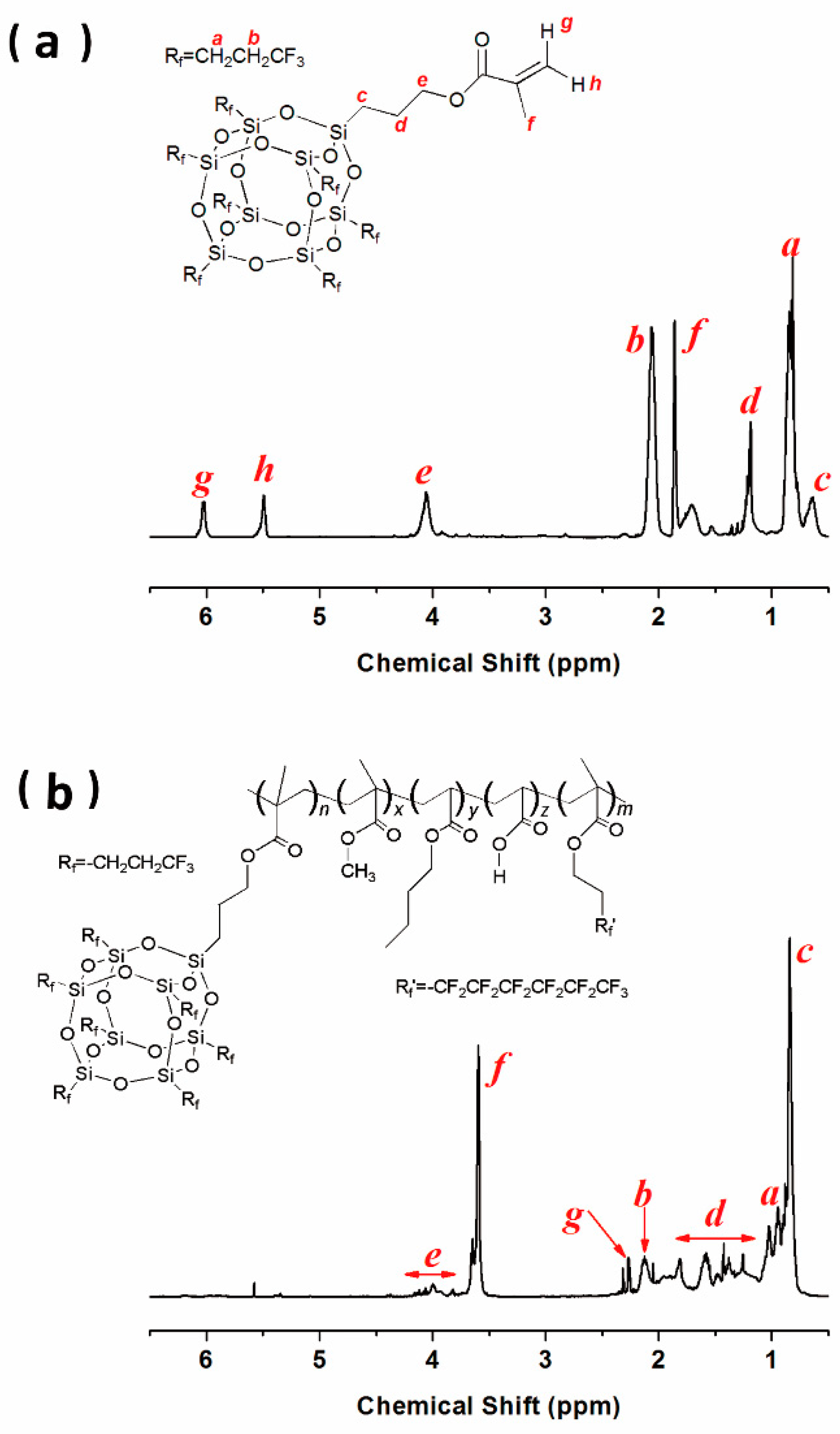
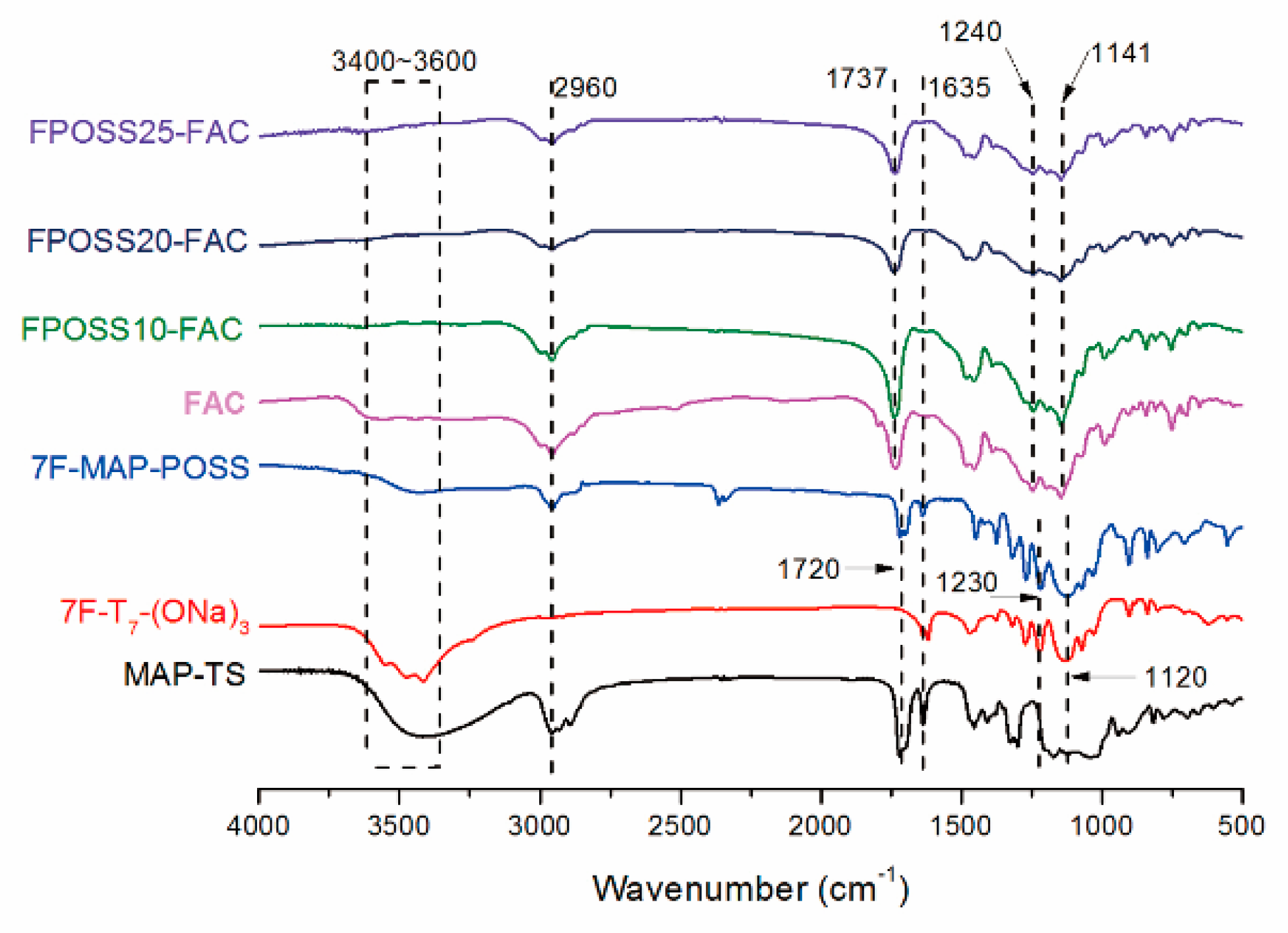
3.1. Chemical Characterization
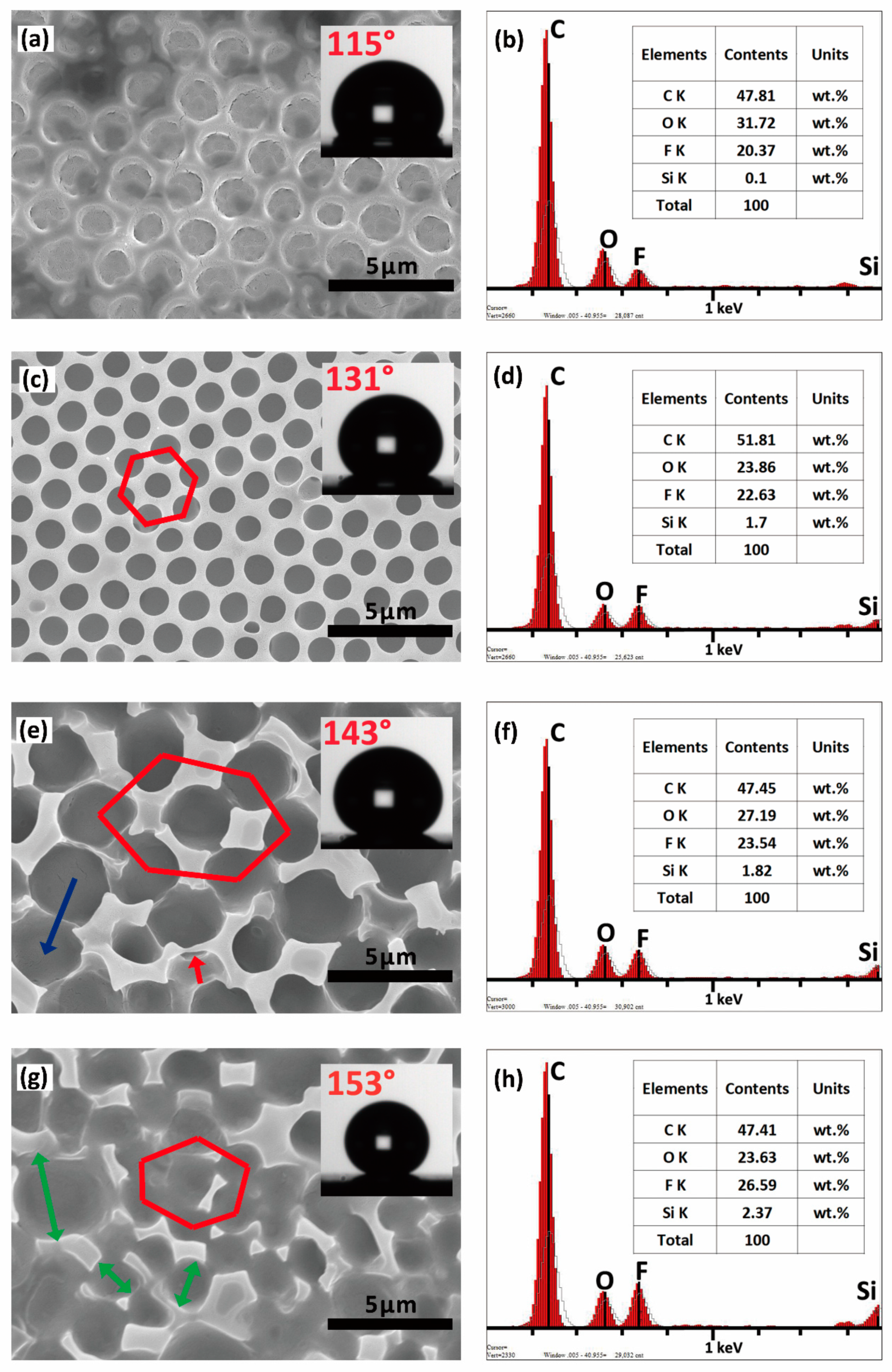
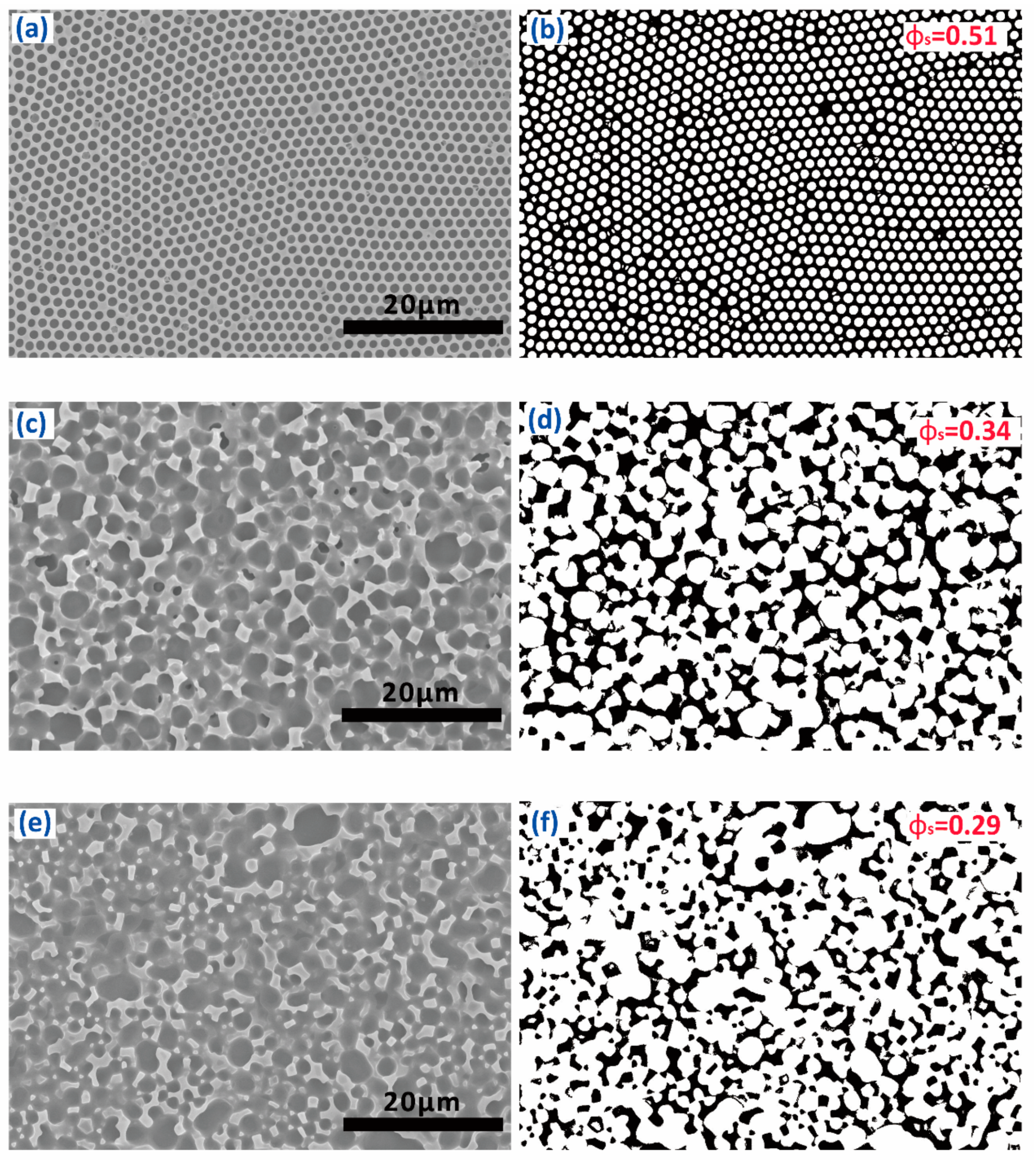
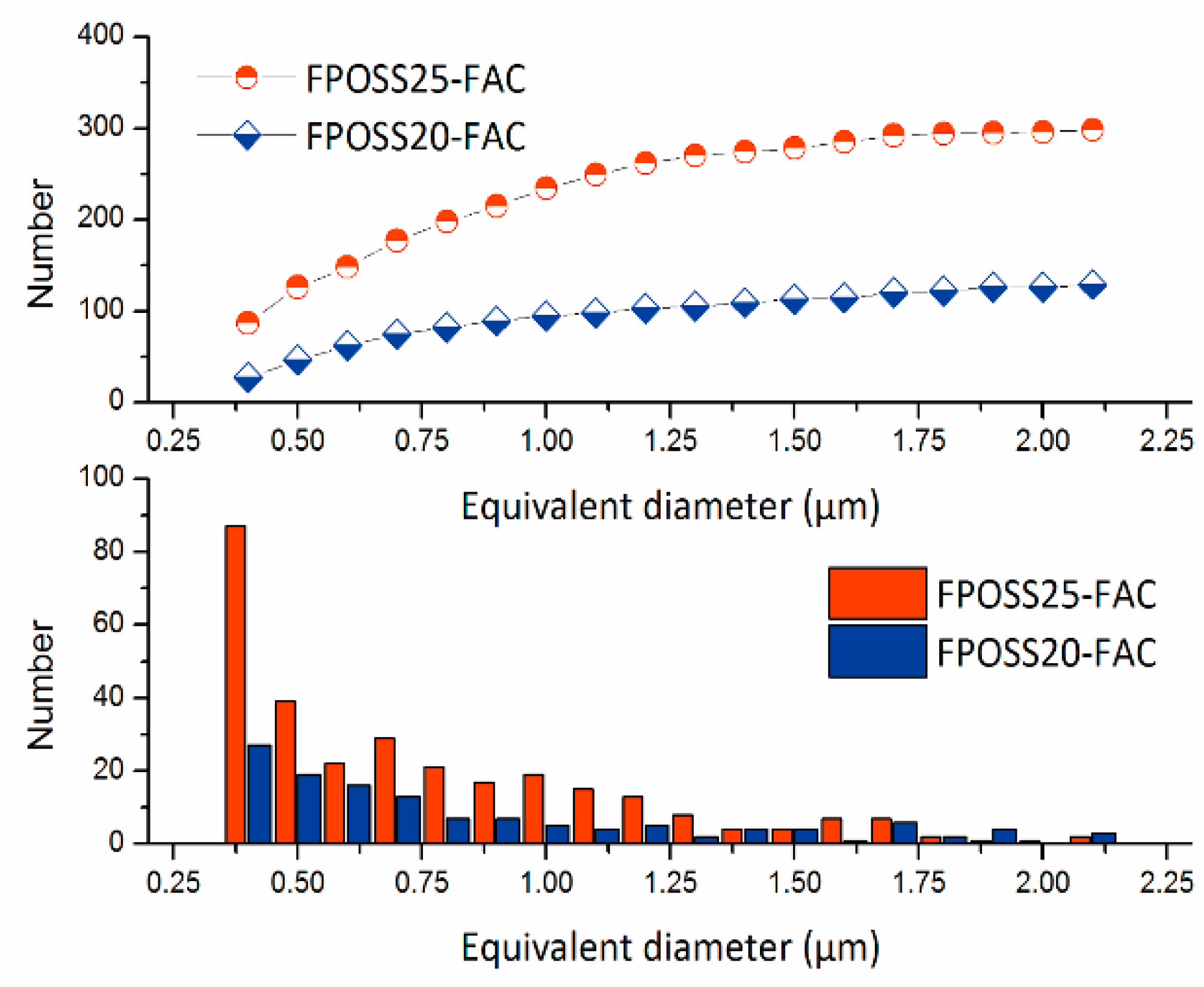
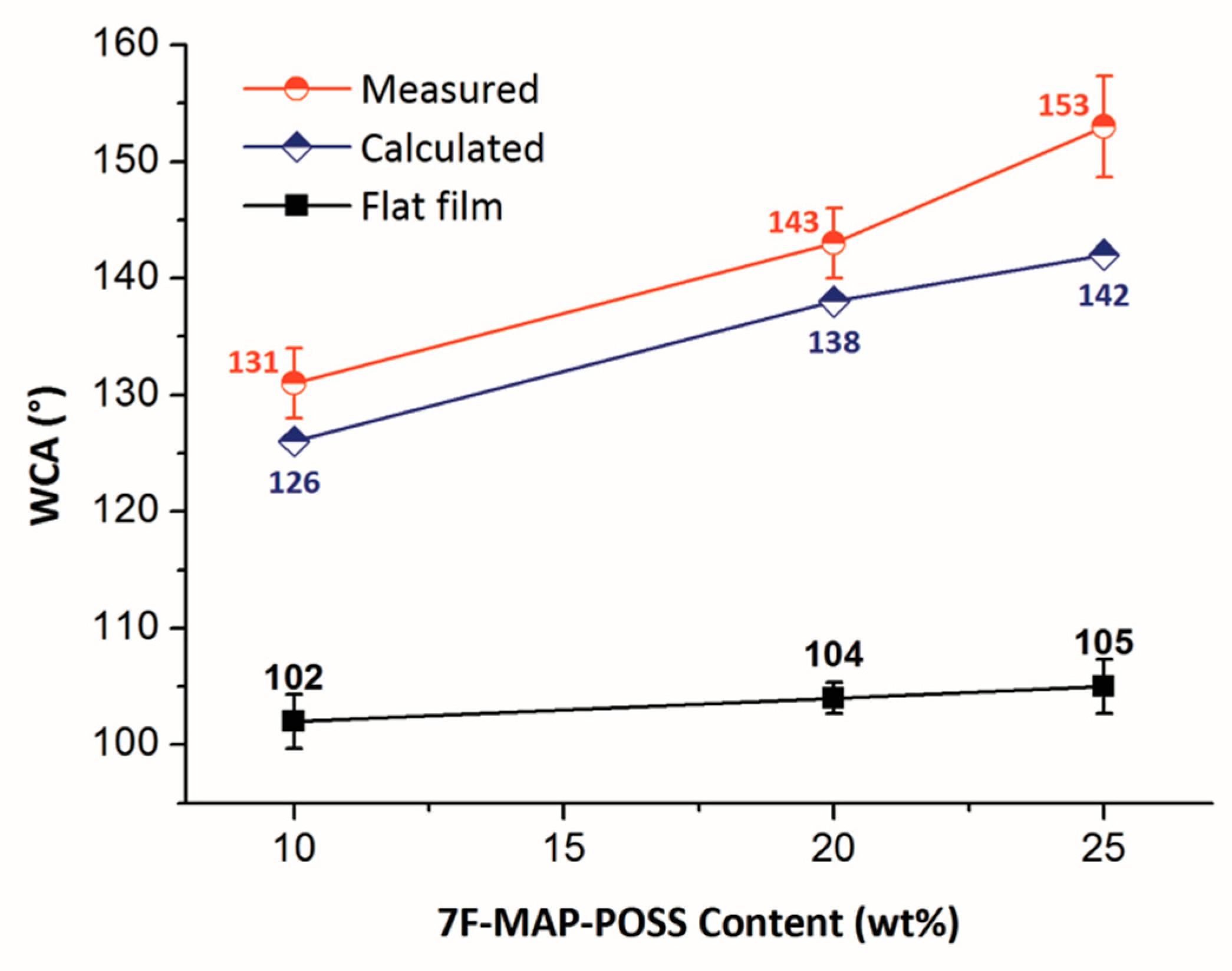
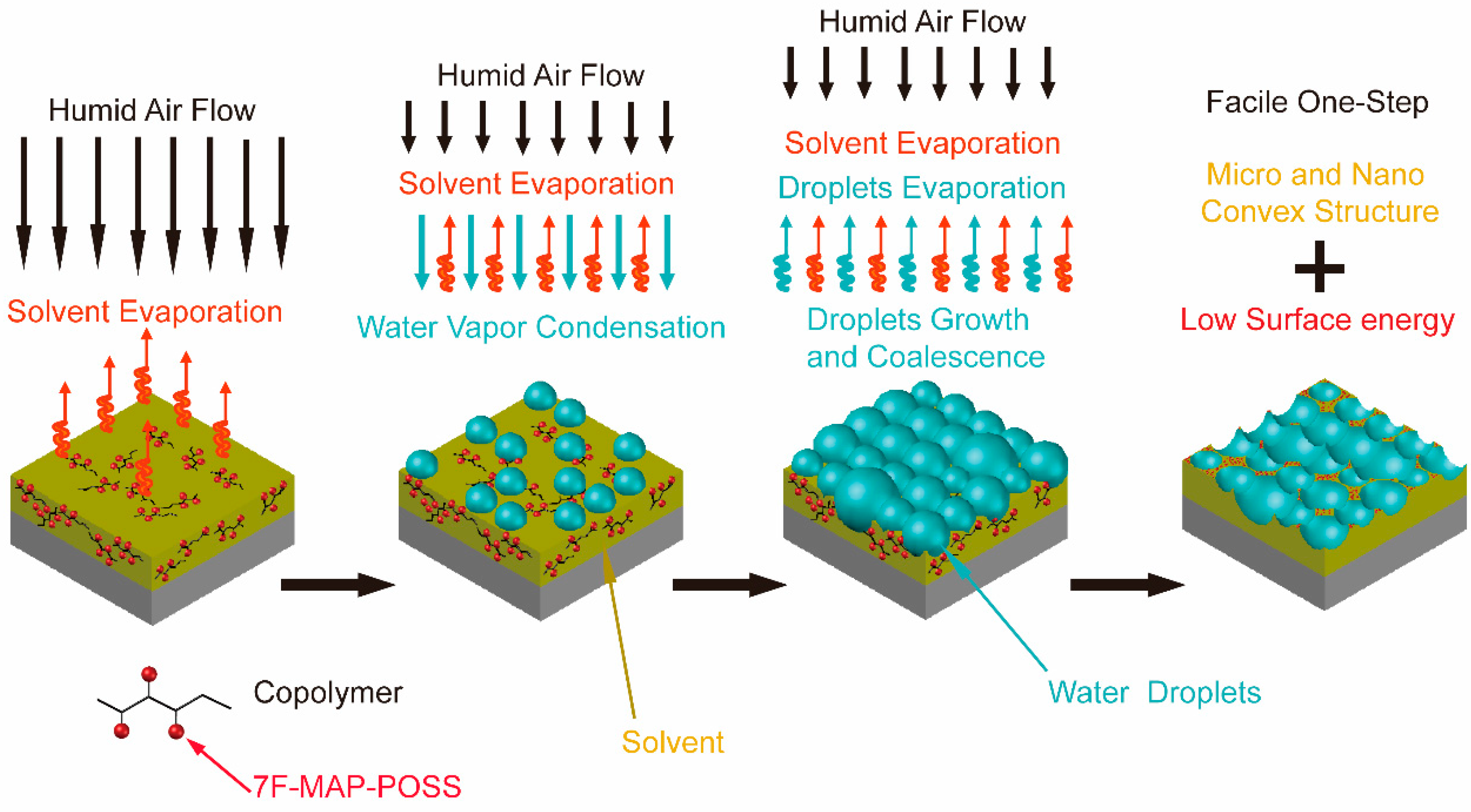
3.2. Morphology and Superhydrophobicity of the Superhydrophobic 7F-MAP-POSS-Acrylate Copolymer Coatings
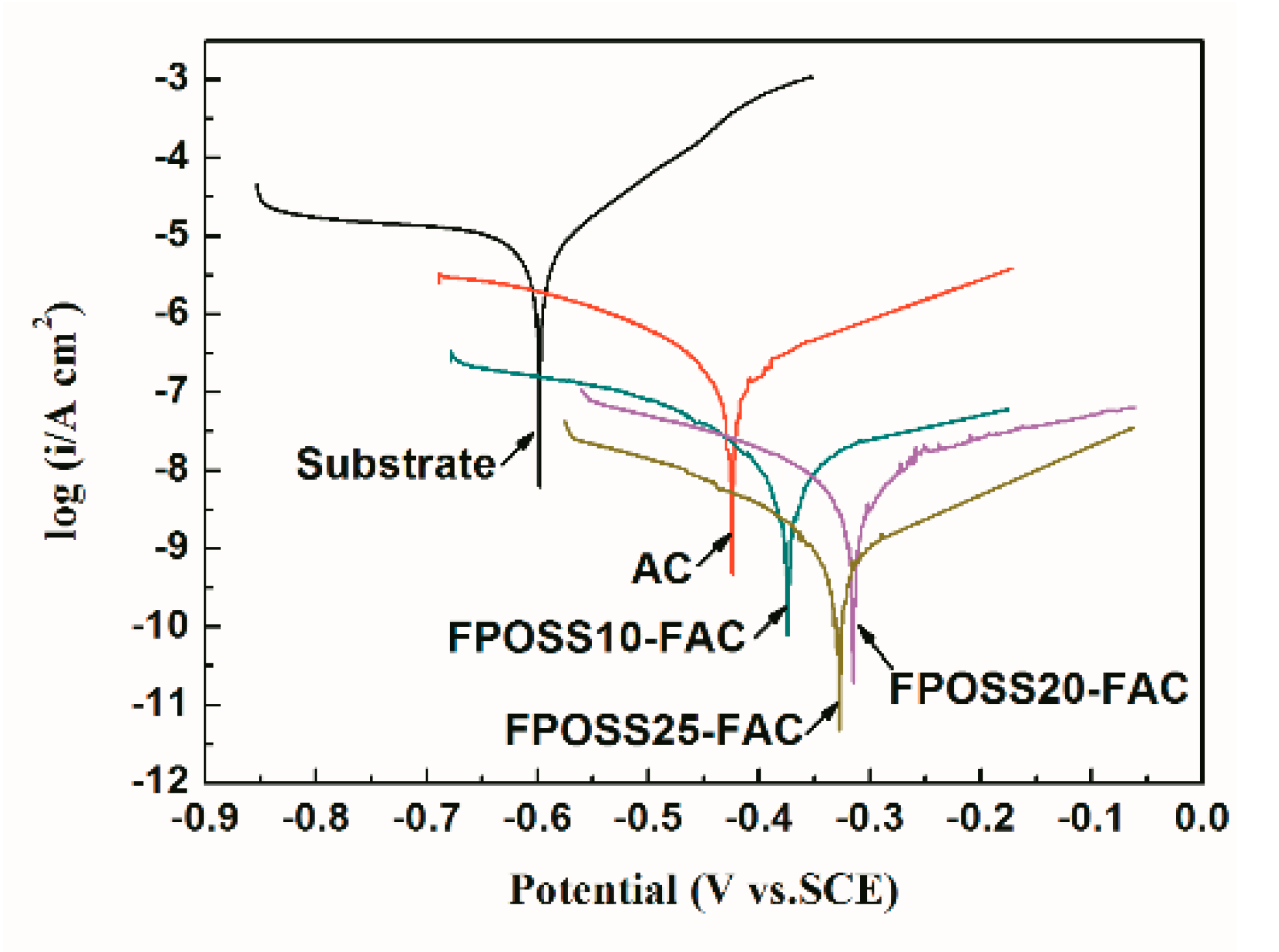
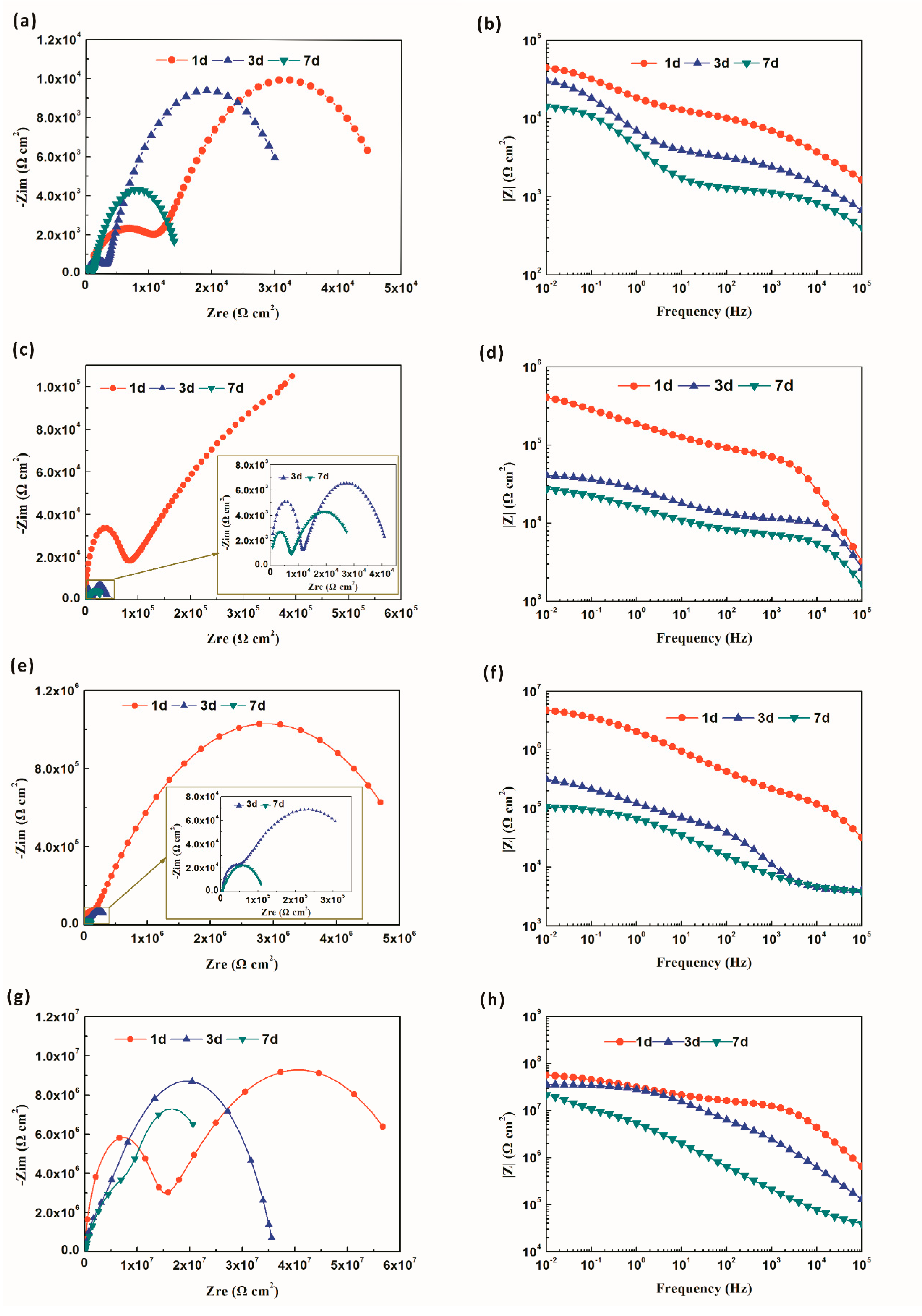
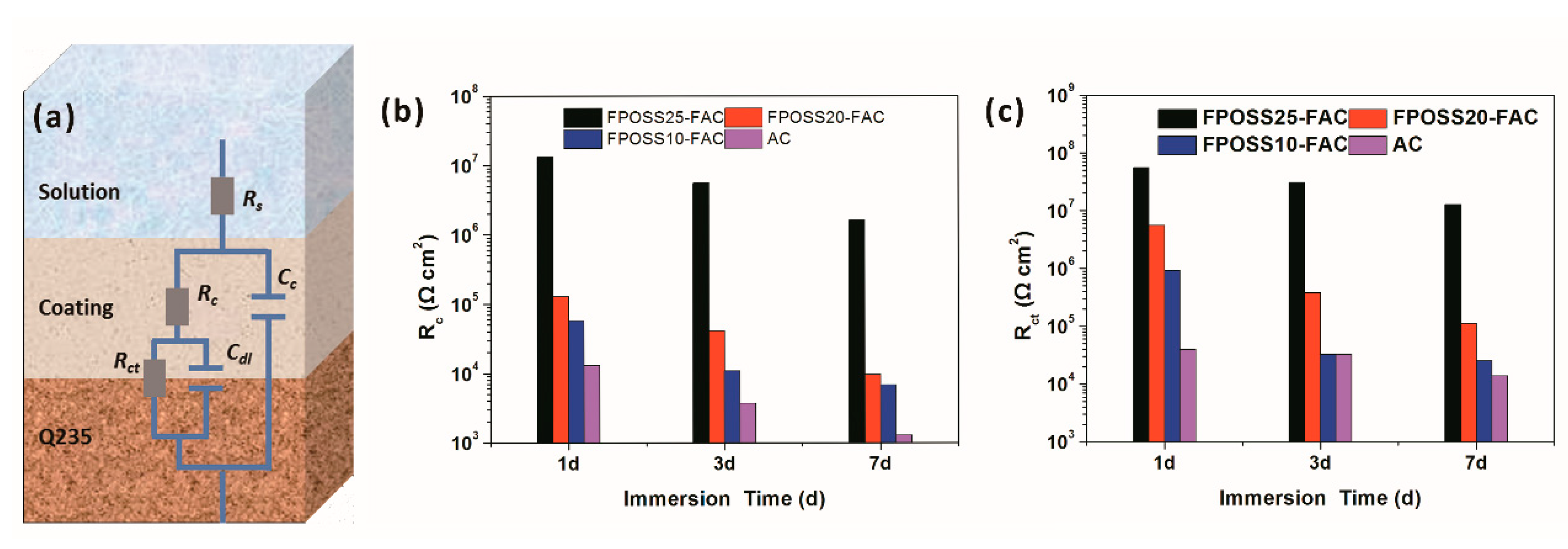
3.3. Anticorrosive Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: from natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Z.; Liu, W.; Zhang, D.; Zhao, H.; Wang, L.; Li, X. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J. 2018, 348, 940–951. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced corrosion resistance of superhydrophobic layered double hydroxide films with long-term stability on Al substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, Z. A mechanically robust transparent coating for anti-icing and self-cleaning applications. J. Mater. Chem. A 2018, 6, 16043–16052. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, Y.; Gong, J.; Gong, D.; Chi, L.; Lin, C.; Chen, Z. Transparent superhydrophobic/superhydrophilic TiO2-based coatings for self-cleaning and anti-fogging. J. Mater. Chem. 2012, 22, 7420. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; Niu, H.; Zhang, J.; Du, Y.; Lin, T. Dual-layer superamphiphobic/superhydrophobic-oleophilic nanofibrous membranes with unidirectional oil-transport ability and strengthened oil-water separation performance. Adv. Mater. Interfaces 2015, 2, 1400506. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J.; Ge, F.; Zhao, R.; Wang, C. Smart UV-curable fabric coatings with self-healing ability for durable self-cleaning and intelligent oil/water separation. Colloids Surf. Physicochem. Eng. Asp. 2019, 565, 86–96. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic coatings on cellulose-based materials: fabrication, properties, and applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Lafuma, A.; Quere, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Wen, Q.; Guo, Z. Recent advances in the fabrication of superhydrophobic surfaces. Chem. Lett. 2016, 45, 1134–1149. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Collings, D.; Philips, A.; Patel, S. Three-dimensionally ordered array of air bubbles in a polymer film. Science 2001, 292, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Hu, E.; Zhi, Y.; Shen, Q. Fabrication of highly ordered porous superhydrophobic polystyrene films by electric breath figure and surface chemical modification. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 294–299. [Google Scholar] [CrossRef]
- Li, Z.; Ma, X.; Kong, Q.; Zang, D.; Guan, X.; Ren, X. Static and dynamic hydrophobic properties of honeycomb structured films via breath figure method. J. Phys. Chem. C 2016, 120, 18659–18664. [Google Scholar] [CrossRef]
- Yabu, H.; Tanaka, M.; Shimomura, M. Superhydrophobic and lipophobic properties of self-organized honeycomb and pincushion structures. Langmuir 2005, 21, 3235–3237. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lew, B.; Kim, S. Facile fabrication of super-hydrophobic nano-needle arrays via breath figures method. Nanoscale Res. Lett. 2011, 6, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.S.; Talbot, E.L.; Wood, T.J.; Bain, C.D.; Badyal, J.P. Superhydrophobic hierarchical honeycomb surfaces. Langmuir 2012, 28, 13712–13719. [Google Scholar] [CrossRef]
- Yabu, H.; Shimomura, M. Single-step fabrication of transparent superhydrophobic porous polymer films. Chem. Mater. 2005, 17, 5231–5234. [Google Scholar] [CrossRef]
- Han, Z.; Li, B.; Mu, Z.; Yang, M.; Niu, S.; Zhang, J.; Ren, L. Fabrication of the replica templated from butterfly wing scales with complex light trapping structures. Appl. Surf. Sci. 2015, 355, 290–297. [Google Scholar] [CrossRef]
- Guan, H.; Han, Z.; Cao, H.; Niu, S.; Qian, Z.; Ye, J.; Ren, L. Characterization of multi-scale morphology and superhydrophobicity of water bamboo leaves and biomimetic polydimethylsiloxane (PDMS) replicas. J. Bionic Eng. 2015, 12, 624–633. [Google Scholar] [CrossRef]
- Pearton, S.J.; Ren, F.; Wang, Y.-L.; Chu, B.H.; Chen, K.H.; Chang, C.Y.; Lim, W.; Lin, J.; Norton, D.P. Recent advances in wide bandgap semiconductor biological and gas sensors. Prog. Mater. Sci. 2010, 55, 1–59. [Google Scholar] [CrossRef]
- Sas, I.; Gorga, R.E.; Joines, J.A.; Thoney, K.A. Literature review on superhydrophobic self-cleaning surfaces produced by electrospinning. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 824–845. [Google Scholar] [CrossRef]
- Kim, G.-M.; Lee, S.-M.; Knez, M.; Simon, P. Single phase ZnO submicrotubes as a replica of electrospun polymer fiber template by atomic layer deposition. Thin Solid Film. 2014, 562, 291–298. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M.; Lowery, J.L.; Fridrikh, S.V.; Rutledge, G.C. Electrospun poly(Styrene-block-dimethylsiloxane) block copolymer fibers exhibiting superhydrophobicity. Langmuir 2005, 21, 5549–5554. [Google Scholar] [CrossRef] [PubMed]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Roman, L.S.; Schreiner, W.H. Superhydrophobic electrosprayed PTFE. Surf. Coat. Technol. 2007, 202, 194–198. [Google Scholar] [CrossRef]
- Qiu, R.; Wang, P.; Zhang, D.; Wu, J. One-step preparation of hierarchical cobalt structure with inborn superhydrophobic effect. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 144–149. [Google Scholar] [CrossRef]
- Li, W.; Kang, Z. Fabrication of corrosion resistant superhydrophobic surface with self-cleaning property on magnesium alloy and its mechanical stability. Surf. Coat. Technol. 2014, 253, 205–213. [Google Scholar] [CrossRef]
- Su, C. A simple and cost-effective method for fabricating lotus-effect composite coatings. J. Coat. Technol. Res. 2009, 9, 135–141. [Google Scholar] [CrossRef]
- Bico, J.; Marzolin, C.; Quere, D. Pearl drops. Europhys. Lett. 1999, 47, 220–226. [Google Scholar] [CrossRef]
- Amirkhani, M.; Berger, N.; Abdelmohsen, M.; Zocholl, F.; Gonçalves, M.R.; Marti, O. The effect of different stabilizers on the formation of self-assembled porous film via the breath-figure technique. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1430–1436. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, Q.; Shi, G.; Shi, Y.; Chen, G. Formation of ordered microporous films with water as templates from poly(D,L-lactic-co-glycolic acid) solution. J. Appl. Polym. Sci. 2003, 90, 1846–1850. [Google Scholar] [CrossRef]
- Li, Z.; Kong, J.; Wang, F.; He, C. Polyhedral oligomeric silsesquioxanes (POSSs): an important building block for organic optoelectronic materials. J. Mater. Chem. C 2017, 5, 5283–5298. [Google Scholar] [CrossRef]
- Blanco, I. The rediscovery of POSS: a molecule rather than a filler. Polymers 2018, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Lichtenhan, J.D.; Pielichowski, K.; Blanco, I. POSS-based polymers. Polymers 2019, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fu, Y.; Li, Z.; Nan, N.; Zhu, Y.; Li, Y. Froth flotation giant surfactants. Polymer 2019, 162, 58–62. [Google Scholar] [CrossRef]
- Hong, Q.; Ma, X.; Li, Z.; Chen, F.; Zhang, Q. Tuning the surface hydrophobicity of honeycomb porous films fabricated by star-shaped POSS-fluorinated acrylates polymer via breath-figure-templated self-assembly. Mater. Design 2016, 96, 1–9. [Google Scholar] [CrossRef]
- Qiang, X.; Ma, X.; Li, Z.; Hou, X. Synthesis of star-shaped polyhedral oligomeric silsesquioxane (POSS) fluorinated acrylates for hydrophobic honeycomb porous film application. Colloid Polym. Sci. 2014, 292, 1531–1544. [Google Scholar] [CrossRef]
- Koh, K.; Sugiyama, S.; Morinaga, T.; Ohno, K.; Tsujii, Y.; Fukuda, T.; Yamahiro, M.; Iijima, T.; Oikawa, H.; Watanabe, K.; et al. Precision Synthesis of a Fluorinated Polyhedral Oligomeric Silsesquioxane-Terminated Polymer and Surface Characterization of Its Blend Film with Poly(methyl methacrylate). Macromolecules 2005, 38, 1264–1270. [Google Scholar] [CrossRef]
- Zeng, K.; Wang, L.; Zheng, S. Rapid Deswelling and Reswelling Response of Poly(N-isopropylacrylamide) Hydrogels via Formation of Interpenetrating Polymer Networks with Polyhedral Oligomeric Silsesquioxane-Capped Poly(ethylene oxide) Amphiphilic Telechelics. J. Phys. Chem. B 2009, 113, 11831–11840. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickis, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wang, L.; Zheng, S.; Qian, X. Self-assembly behavior of hepta(3,3,3-trifluoropropyl) polyhedral oligomeric silsesquioxane-capped poly(ɛ-caprolactone) in epoxy resin: Nanostructures and surface properties. Polymer 2009, 50, 685–695. [Google Scholar] [CrossRef]
- Bolognesi, A.; Mercogliano, C.; Yunus, S. Self-organization of polystyrenes into ordered microstructured films and their replication by soft lithography. Langmuir 2005, 21, 3480–3485. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D. Permeability to water and water vapour of textiles and other fibrous materials. Discuss. Faraday Soc. 1948, 3, 239–243. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, H.; Wang, C.; Zhang, D.; Chen, H.; Liu, W. Design of novel superhydrophobic aniline trimer modified siliceous material and its application for steel protection. Appl. Surf. Sci. 2018, 457, 752–763. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Y.; Mao, H.; Lu, X.; Zhang, W.; Wei, Y. Synthesis of parent aniline tetramer and pentamer and redox properties. Mater. Lett. 2005, 59, 2446–2450. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Liu, Z.; Zhang, D.; Li, X.; Terryn, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Chen, J.; Liu, S.; Zhang, G.; Zhao, H.; Wang, L.; Xue, Q. Anticorrosive performance of waterborne epoxy coatings containing water-dispersible hexagonal boron nitride (h-BN) nanosheets. Appl. Surf. Sci. 2017, 397, 77–86. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Du, C.W.; Cheng, Y.F. Local additional potential model for effect of strain rate on SCC of pipeline steel in an acidic soil solution. Corros. Sci. 2009, 51, 2863–2871. [Google Scholar] [CrossRef]
- Sun, F.; Ren, S.; Li, Z.; Liu, Z.; Li, X.; Du, C. Comparative study on the stress corrosion cracking of X70 pipeline steel in simulated shallow and deep sea environments. Mater. Sci. Eng. A 2017, 685, 145–153. [Google Scholar] [CrossRef]



| Sample | FPOSS (wt %) | Mn (g/mol) | Mw (g/mol) | PDI (Mw/Mn) |
|---|---|---|---|---|
| FAC | 0 | 14,293 | 28,215 | 1.974 |
| FPOSS10-FAC | 10 | 15,435 | 30,669 | 1.987 |
| FPOSS20-FAC | 20 | 16,106 | 31,378 | 1.945 |
| FPOSS25-FAC | 25 | 15,640 | 29,415 | 1.881 |
| Sample | Ecorr (V) | icorr (A cm−2) | IE% |
|---|---|---|---|
| Substrate | –0.598 | 1.25 × 10−5 | - |
| AC | –0.424 | 4.43 × 10−7 | 96.44 |
| FPOSS10-FAC | –0.371 | 7.20 × 10−8 | 99.42 |
| FPOSS20-FAC | –0.318 | 2.11 × 10−8 | 99.83 |
| FPOSS25-FAC | –0.320 | 2.01 × 10−9 | 99.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhang, X.; Wang, D.; Cheng, J.; Pang, X.; Qu, W.; Li, C.; Li, S. Facile Fabrication of Superhydrophobic Surface from Fluorinated POSS Acrylate Copolymer via One-Step Breath Figure Method and Its Anti-Corrosion Property. Polymers 2019, 11, 1953. https://doi.org/10.3390/polym11121953
Liu M, Zhang X, Wang D, Cheng J, Pang X, Qu W, Li C, Li S. Facile Fabrication of Superhydrophobic Surface from Fluorinated POSS Acrylate Copolymer via One-Step Breath Figure Method and Its Anti-Corrosion Property. Polymers. 2019; 11(12):1953. https://doi.org/10.3390/polym11121953
Chicago/Turabian StyleLiu, Meng, Xiaochen Zhang, Dong Wang, Jiaji Cheng, Xiujiang Pang, Wenjuan Qu, Chunxu Li, and Shaoxiang Li. 2019. "Facile Fabrication of Superhydrophobic Surface from Fluorinated POSS Acrylate Copolymer via One-Step Breath Figure Method and Its Anti-Corrosion Property" Polymers 11, no. 12: 1953. https://doi.org/10.3390/polym11121953
APA StyleLiu, M., Zhang, X., Wang, D., Cheng, J., Pang, X., Qu, W., Li, C., & Li, S. (2019). Facile Fabrication of Superhydrophobic Surface from Fluorinated POSS Acrylate Copolymer via One-Step Breath Figure Method and Its Anti-Corrosion Property. Polymers, 11(12), 1953. https://doi.org/10.3390/polym11121953








