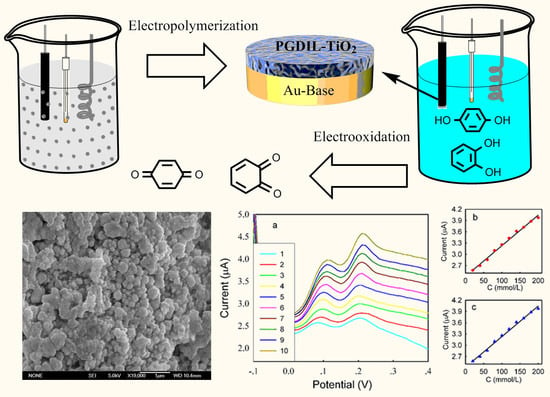
The Electrochemical Oxidation of Hydroquinone and Catechol through a Novel Poly-geminal Dicationic Ionic Liquid (PGDIL)–TiO2 Composite Film Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Main Reagents and Instrumentation
2.2. Synthesis of Poly-[C4(m-ABIM)2][PF6]2–TiO2 Composite Film
2.3. Electrochemical Performance Tests
3. Results and Discussion
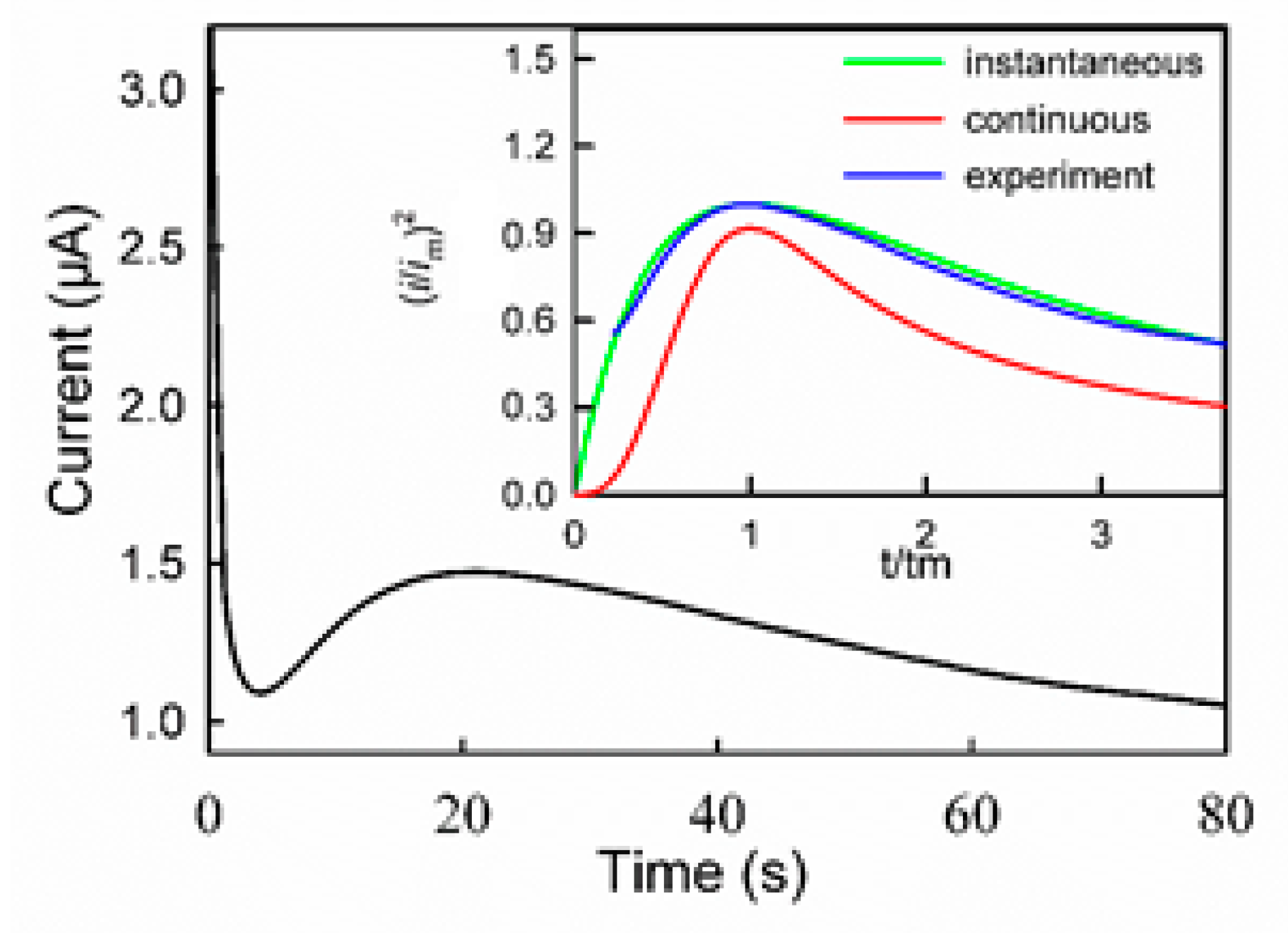
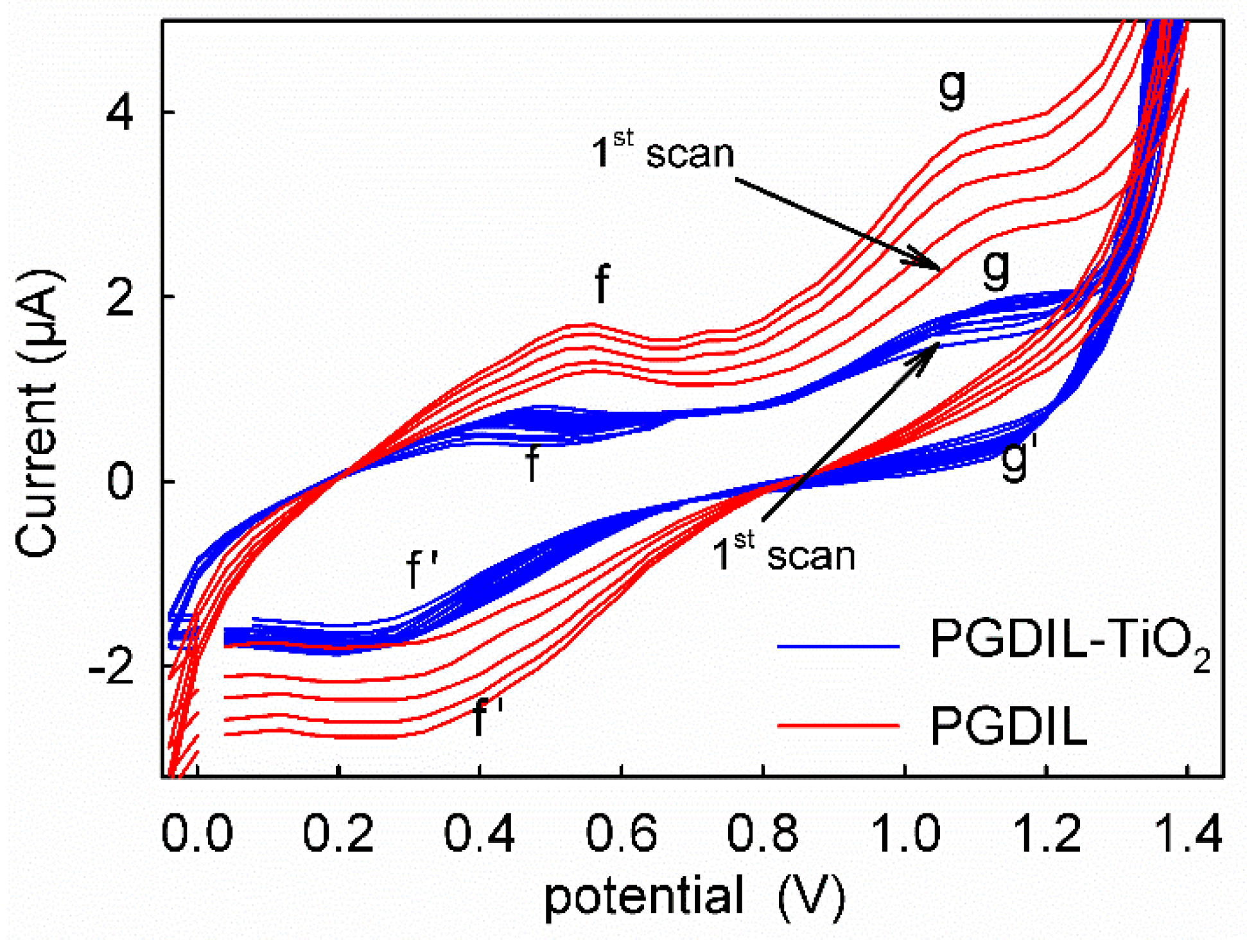
3.1. Polymerization Mechanism of PGDIL–TiO2 Electrode
3.2. SEM Characterization of PGDIL–TiO2 and PGDIL
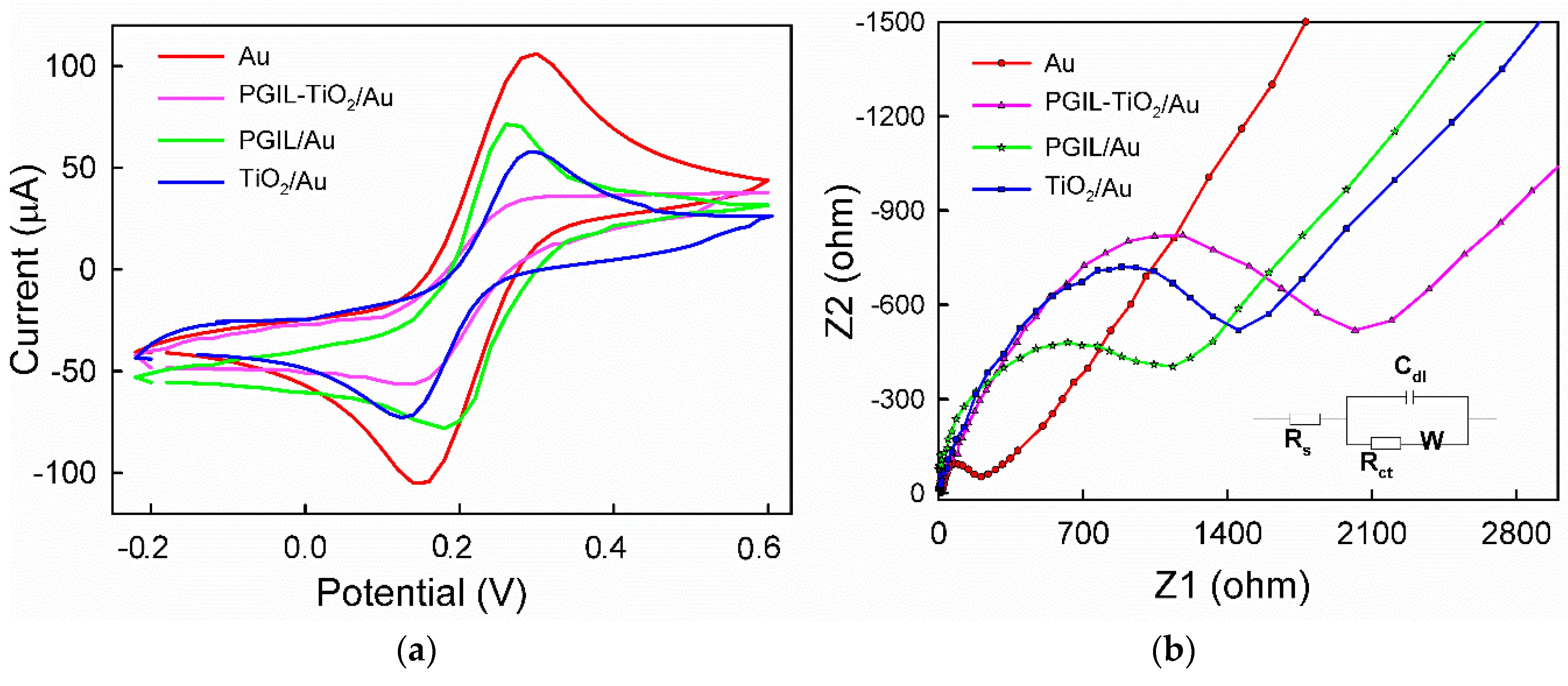
3.3. EIS Characterization of PGIL–TiO2/Au, TiO2/Au, and Bare Au Electrode
3.4. CVs of HQ and CC on Different Modified Electrodes
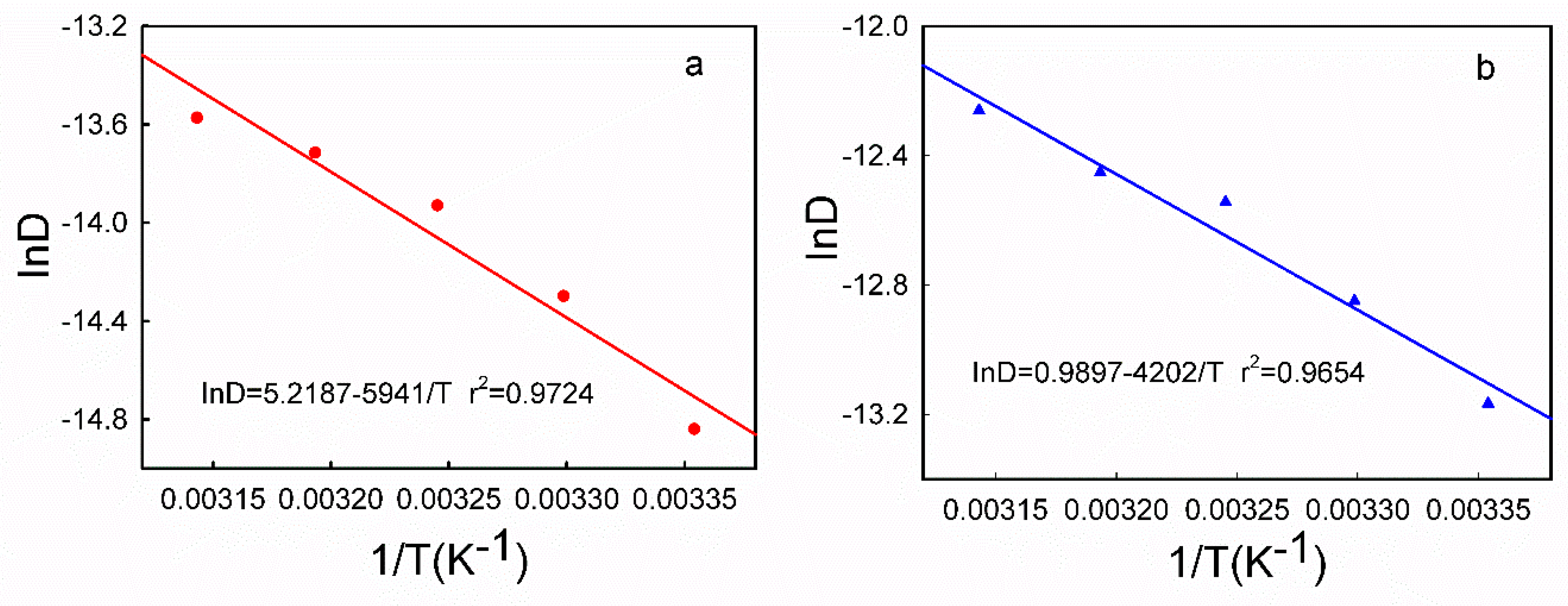
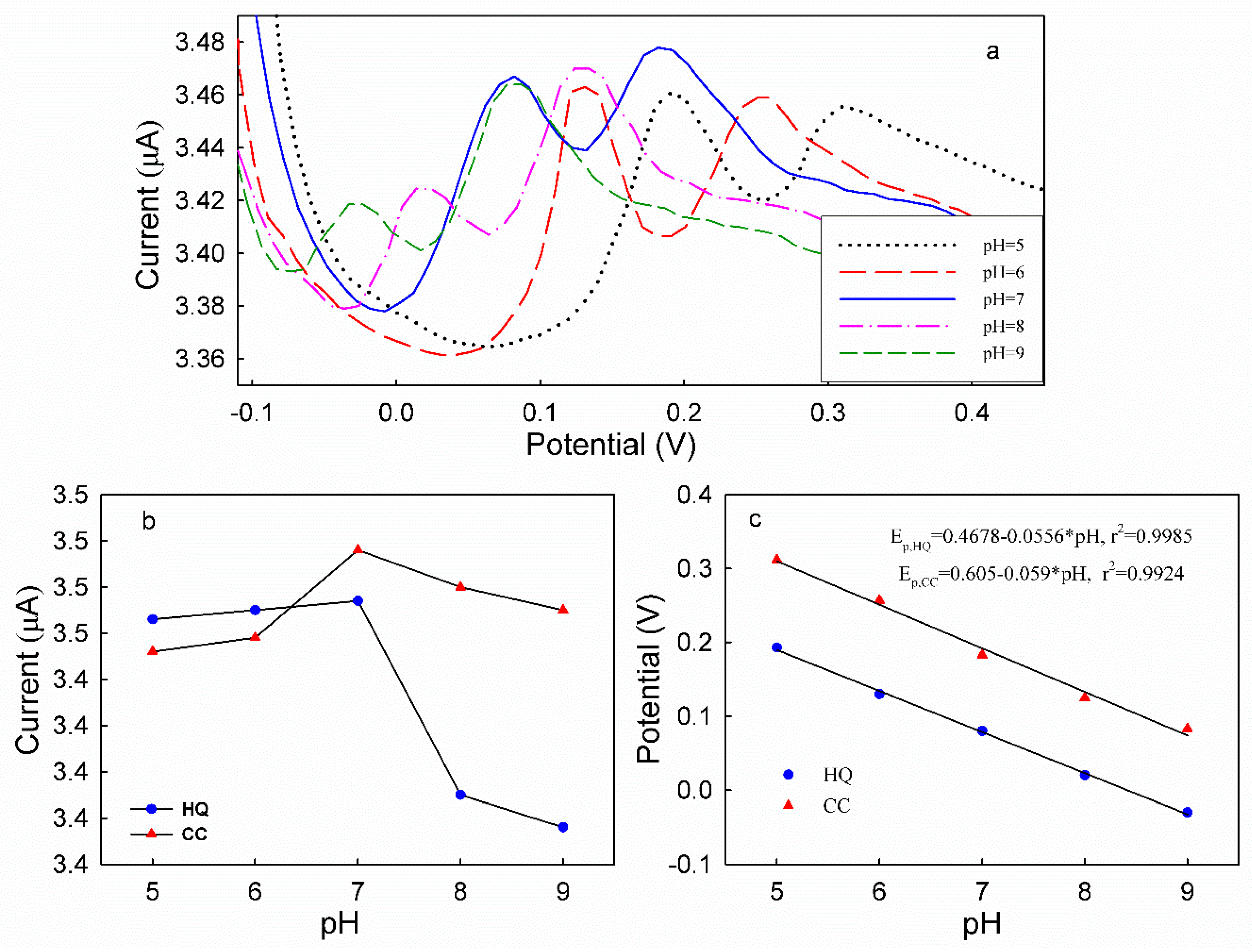
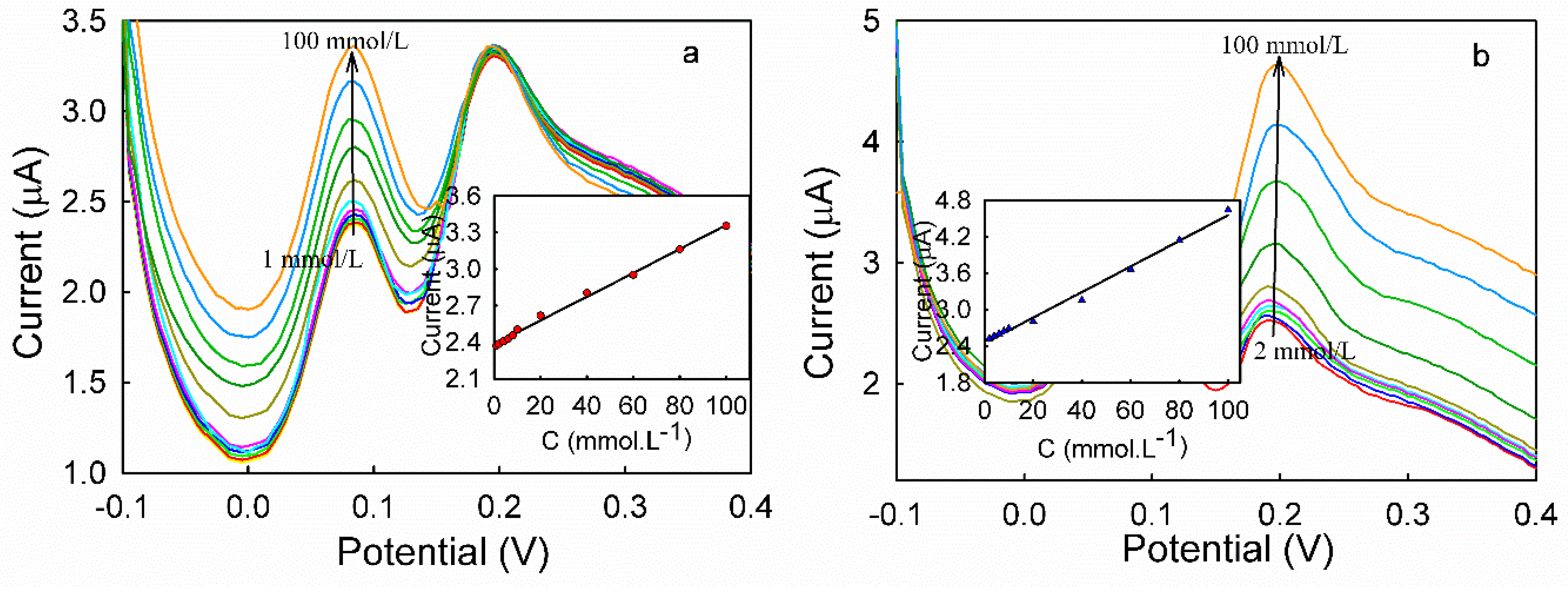
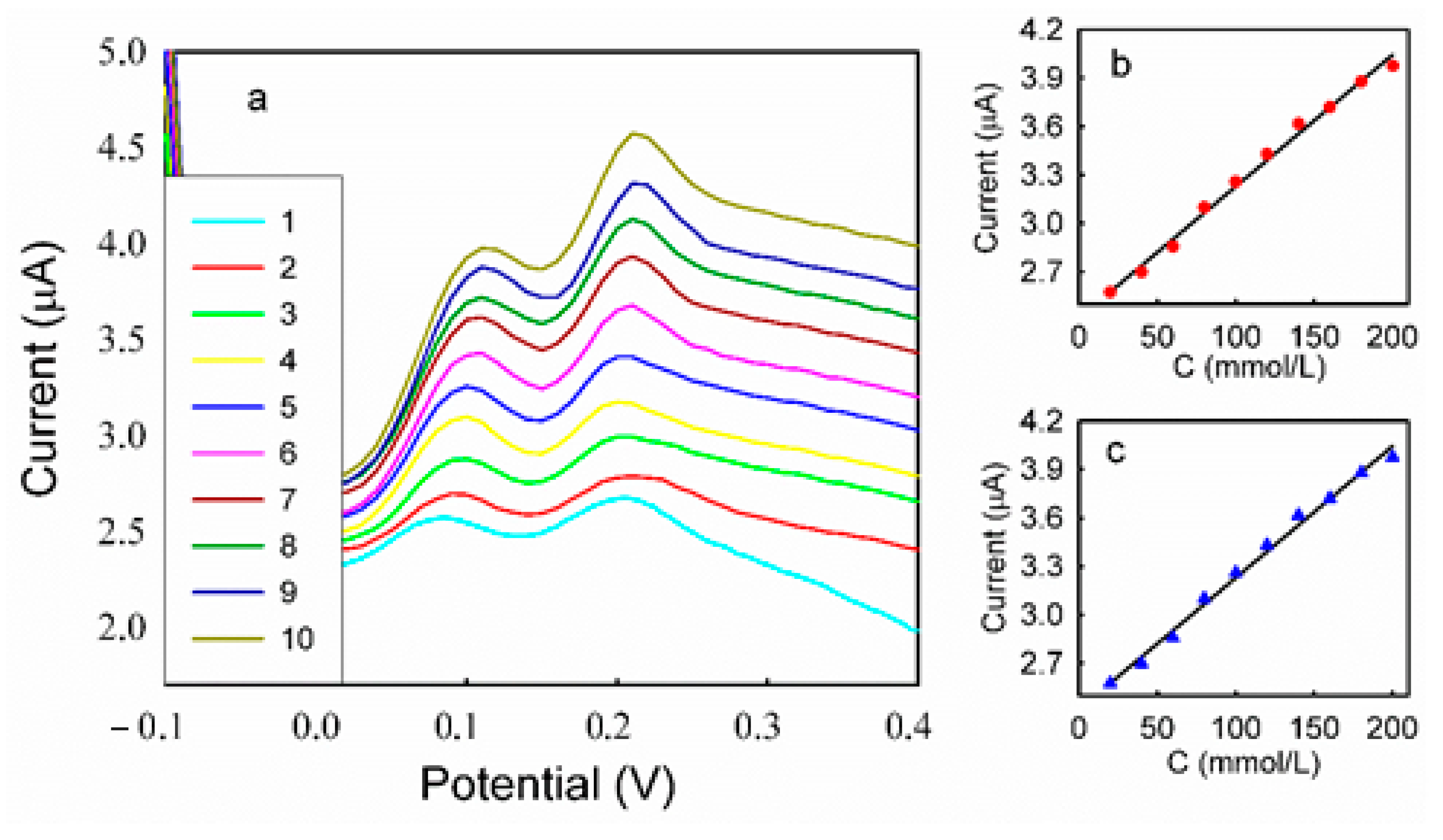
3.5. DPV Analyses of HQ and CC on Different Modified Electrodes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, C.; Dong, Y.; Liu, Y.; Zhao, X.; Yin, J. Enhanced stimuli-responsive electrorheological property of poly(ionic liquid) s-capsulated polyaniline particles. Polymers 2017, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Vollas, A.; Chouliaras, T.; Deimede, V.; Ioannides, T.; Kallitsis, J. New pyridinium type poly(Ionic Liquids) as membranes for CO2 separation. Polymers 2018, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H. Molten salt type polymer electrolytes. Electroehimiea Acta 2001, 46, 1407–1411. [Google Scholar] [CrossRef]
- Men, Y.; Kuzmicz, D.; Yuan, J. Poly(ionic liquid) colloidal particles. Curr. Opin. Colloid Interface Sci. 2014, 19, 76–83. [Google Scholar] [CrossRef]
- He, D.; Li, F.; Xia, S.; Liu, F.; Xiong, Y.; Zhang, Q. Synthesis and characterization of a novel ionic liquid polymer: Poly(1-(3-aminobenzyl)-3-methylimidazolium chloride). Chin. J. Polym. Sci. 2014, 32, 163–168. [Google Scholar] [CrossRef]
- He, D.; Guo, Y.; Zhou, Z.; Xia, S.; Xie, X.; Yang, R. Electropolymerization of ionic liquid substituted polyphenylene as supercapacitors materials. Electrochem. Commun. 2009, 11, 1671–1674. [Google Scholar] [CrossRef]
- Masri, A.N.; MI, A.M.; Leveque, J. A review on dicationic ionic liquids: Classification and application. Ind. Eng. Manag. 2016, 5, 197–204. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Li, M.; Yang, B. Functional groups in geminal imidazolium ionic compounds and their influence on thermo-physical properties. J. M. Liq. 2018, 269, 738–745. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, R.F.; Ellern, A.; Armstrong, D.W. Structure and properties of high stability geminal dicationic ionic liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Adibi, M.; Seifkordi, A.A.; Fazli, Y. Separation of CO2/CH4 through alumina-supported geminal ionic liquid membranes. J. Membr. Sci. 2014, 455, 229–235. [Google Scholar] [CrossRef]
- Ding, Y.S.; Zha, M.; Zhang, J.; Wang, S.S. Synthesis, characterization and properties of geminal imidazolium ionic liquids. Colloids and Surfaces A: Physicochem. Eng. Aspects 2007, 298, 201–205. [Google Scholar] [CrossRef]
- Chinnappan, A.; Kim, H. Environmentally benign catalyst: Synthesis, characterization, and properties of pyridinium dicationic molten salts (ionic liquids) and use of application in esterification. Chem. Eng. J. 2012, 187, 283–288. [Google Scholar] [CrossRef]
- Cheng, J.; Xiang, C.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Sun, L.; Xu, F. Highly active nanoporous Co-B-TiO2 framework for hydrolysis of NaBH4. Ceram. Int. 2015, 41, 899–905. [Google Scholar] [CrossRef]
- Zafer, C.; Ocakoglu, K.; Ozsoy, C.; Icli, S. Dicationic bis-imidazolium molten salts for efficient dye sensitized solar cells: Synthesis and photovoltaic properties. Electrochim. Acta 2009, 54, 5709–5714. [Google Scholar] [CrossRef]
- Li, X.J.; Bruce, D.W.; Shreeve, J.M. Dicationic imidazolium-based ionic liquids and ionic liquid crystals with variously positioned fluoro substituents. J. Mater. Chem. 2009, 19, 8232–8238. [Google Scholar] [CrossRef]
- Fatichi, A.Z.; Mello, M.G.; Caram, R.; Cremasco, A. Self-organized TiO2 nanotube layer on Ti–Nb–Zr alloys: Growth, characterization, and effect on corrosion behavior. J. Appl. Electrochem. 2019, in press. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Xiao, T.; Li, Z.; Wang, X. Preparation and properties of Co3O4-doped TiO2 nanotube array electrodes. J. Appl. Electrochem. 2019, 49, 305–314. [Google Scholar] [CrossRef]
- Bashiri, R.; Mohamed, N.M.; Kait, C.F.; Sufian, S. Optimization of hydrogen production over TiO2 supported copper and nickel oxides: Effect of photoelectrochemical features. J. Appl. Electrochem. 2019, 49, 27–38. [Google Scholar] [CrossRef]
- Kannan, R.; Kim, A.R.; Nahm, K.S.; Yoo, D.J. Manganese-titanium-oxide-hydroxide-supported palladium nanostructures–A facile electrocatalysts for the methanol, ethylene glycol and xylitol electrooxidation. Int. J. Hydrog. Energy 2016, 41, 6787–6797. [Google Scholar] [CrossRef]
- Guo, Y.; He, D.; Xia, S.; Xin, X.; Gao, X.; Zhang, Q. Preparation of a novel nanocomposite of polyaniline core decorated with anatase-TiO2 nanoparticles in ionic liquid/water microemulsion. J. Nanomater. 2011, 2012, 1–8. [Google Scholar] [CrossRef]
- Zhou, Z.; He, D.; Guo, Y.; Cui, Z.; Wang, M.; Li, G.; Yang, R. Fabrication of polyaniline-silver nanocomposites by chronopotentiometry in different ionic liquid microemulsion systems. Thin Solid Film. 2009, 517, 6767–6771. [Google Scholar] [CrossRef]
- Abdul-Manaf, N.A.; Yusoff, W.Y.W.; Demon, S.Z.N.; Shaari, N.A.; Shamshuddin, A.; Mohamed, N.S. Anodic and cathodic deposition of polyaniline films: A comparison between the two methods. Mater. Res. Express 2019, 6, 096453. [Google Scholar] [CrossRef]
- Wang, X.; Wei, H.; Liu, X.; Du, W.; Zhao, X.; Wang, X. Novel three-dimensional polyaniline nanothorns vertically grown on buckypaper as high-performance supercapacitor electrode. Nanotechnology 2019, 30, 325401. [Google Scholar] [CrossRef] [PubMed]
- Arukula, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Cumulative effect of bimetallic alloy, conductive polymer and graphene toward electrooxidation of methanol: An efficient anode catalyst for direct methanol fuel cells. J. Alloy. Compd. 2019, 771, 477–488. [Google Scholar] [CrossRef]
- Asiltürk, M.; Sayılkan, F.; Arpaç, E. Effect of Fe3+ ion doping to TiO2 on the photocatalytic degradation of Malachite Green dye under UV and vis-irradiation. J. Photochem. Photobiol. A: Chem. 2009, 203, 64–71. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Karuppannan, M.; Vinothkannan, M.; Ramachandran, K.; Kwon, O.J.; Yoo, D.J. Ultrafine Pt nanoparticles stabilized by MoS2/N-doped reduced graphene oxide as a durable electrocatalyst for alcohol oxidation and oxygen reduction reactions. ACS Appl. Mater. Interfaces 2019, 11, 12504–12515. [Google Scholar] [CrossRef]
- Wang, J.; Park, J.N.; Wei, X.Y.; Lee, C.W. Room-temperature heterogeneous hydroxylation of phenol with hydrogen peroxide over Fe2+, Co2+ ion-exchanged Naβ zeolite. Chem. Commun. 2003, 5, 628–629. [Google Scholar] [CrossRef]
- Hong, Z.; Zhou, L.; Li, J.; Tang, J. A sensor based on graphitic mesoporous carbon/ionic liquids composite film for simultaneous determination of hydroquinone and catechol. Electrochim. Acta 2013, 109, 671–677. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Dong, Y.; Qu, J. Research progress of methods for detecting catechol and hydroquinone in water. Chem. Res. 2015, 26, 100–104. [Google Scholar] [CrossRef]
- Feng, X.; Gao, W.; Zhou, S.; Shi, H.; Huang, H.; Song, W. Discrimination and simultaneous determination of hydroquinone and catechol by tunable polymerization of imidazolium-based ionic liquid on multi-walled carbon nanotube surfaces. Anal. Chim. Acta 2013, 805, 36–44. [Google Scholar] [CrossRef]
- Kai, X.; Shen, Y.; Zhang, G.; Xie, J. Spectrophotometric simultaneous determination of pyrocatechol, resorcinol and hydroquinone by LM-BP neural network. Spectrosc. Spectr. Anal. 2005, 25, 2070–2072. [Google Scholar] [CrossRef]
- Penner, N.A.; Nesterenko, P.N. Simultaneous determination of dihydroxybenzenes, aminophenols and phenylenediamines in hair dyes by high-performance liquid chromatography on hypercross-linked polystyrene. Analyst 2000, 125, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, D.; Xie, A.; Qu, W.; Tang, Y.; Shang, J.; Zhu, R. Preparation and characterization of a novel poly-geminal dicationic ionic liquid (PGDIL). J. Mol. Liq. In press. [CrossRef]
- Ivanova, Y.A.; Ivanou, D.K.; Streltsov, E.A. Electrodeposition of Te onto monocrystalline n-and p-Si (1 0 0) wafers. Electrochim. Acta 2007, 52, 5213–5218. [Google Scholar] [CrossRef]
- Gunawardena, G.; Hills, G.; Montenegro, I.; Scharifker, B. Electrochemical nucleation: Part, I. General considerations. J. Electroanal. Chem. Interfacial Electrochem. 1982, 138, 225–239. [Google Scholar] [CrossRef]
- Weidlich, C.; Mangold, K.-M.; Jüttner, K. EQCM study of the ion exchange behaviour of polypyrrole with different counterions in different electrolytes. Electrochim. Acta 2005, 50, 1547–1552. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, S.; Xie, J.; Yang, Z.; Pang, P.; Gao, Y. Simultaneous electrochemical determination of catechol andhydroquinone based on grapheme-TiO2nanocomposite modifiedglassy carbon electrode. Sens. Actuators B 2014, 204, 102–108. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications, 2nd ed.; Shao, Y.; Zhu, G.; Dong, X.; Zhang, B., Translators; Chemical Industry Press: Beijing, China, 2005; pp. 124–125. ISBN 978-7-5025-96704-0. [Google Scholar]
- He, D.; Mho, S. Electrocatalytic reactions of phenolic compounds at ferric ion co-doped SnO2: Sb5+ electrodes. J. Electroanal. Chem. 2004, 568, 19–27. [Google Scholar] [CrossRef]
- Feng, X.; Shi, Y.; Hu, Z. Polyaniline/polysulfone composite film electrode for simultaneous determination of hydroquinone and catechol. Mater. Chem. Phys. 2011, 131, 72–76. [Google Scholar] [CrossRef]
- Lee, C.W.; Jin, S.H.; Jeong, H.M.; Chi, K.-W. Efficient oxidation of hydroquinone and alcohols by tailor-made solid polyaniline catalyst. Tetrahedron Lett. 2009, 50, 559–561. [Google Scholar] [CrossRef]
- Mao, J.; Zhao, B.; Zhou, J.; Zhang, L.; Yang, F.; Guo, X.; Zhang, Z.C. Identification and characteristics of catalytic quad-functions on Au/Anatase TiO2. ACS Catal. 2019, 9, 7900–7911. [Google Scholar] [CrossRef]
- Wan, W.; Nie, X.; Janik, M.J.; Song, C.; Guo, X. Adsorption, dissociation, and spillover of hydrogen over Au/TiO2 catalysts: The effects of cluster size and meta-support interaction from DFT. J. Phys. Chem. C 2018, 122, 17895–17916. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Zanello, P. Inorganic Electrochemistry: Theory, Practice and Application; The Royal Society of Chemistry: Cambridge, UK, 2003; p. 38. ISBN 0-85404-661-5. [Google Scholar]
- Rodríguez-Aragón, L.J.; López-Fidalgo, J. Optimal designs for the Arrhenius equation. Chemom. Intell. Lab. Syst. 2005, 77, 131–138. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, H.; Liu, D. Simultaneous Determination of Catechol and Hydroquinone Based on Gold Electrode Modified with Carbon Nanotubes-graphene Nanosheet Hybrid Films. Chin. J. Appl. Chem. 2014, 31, 983–989. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, C. Simultaneous determination of hydroquinone and catechol at a glassy carbon electrode modified with multiwall carbon nanotubes. Electroanalysis 2005, 17, 832–838. [Google Scholar] [CrossRef]
- Kumar, A.A.; Swamy, B.E.K.; Rani, T.S.; Ganesh, P.S.; Raj, Y.P. Voltammetric determination of catechol and hydroquinone at poly(murexide) modified glassy carbon electrode. Mater. Sci. Eng. C 2019, 98, 746–752. [Google Scholar] [CrossRef]

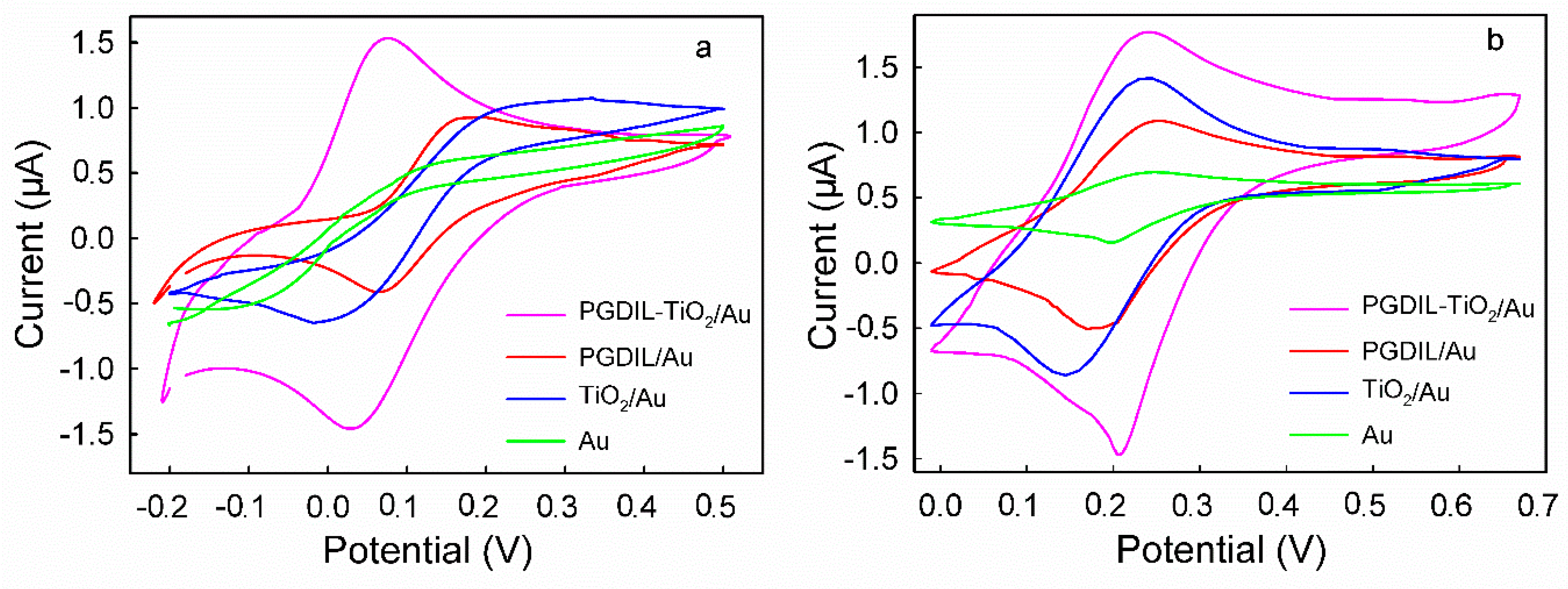
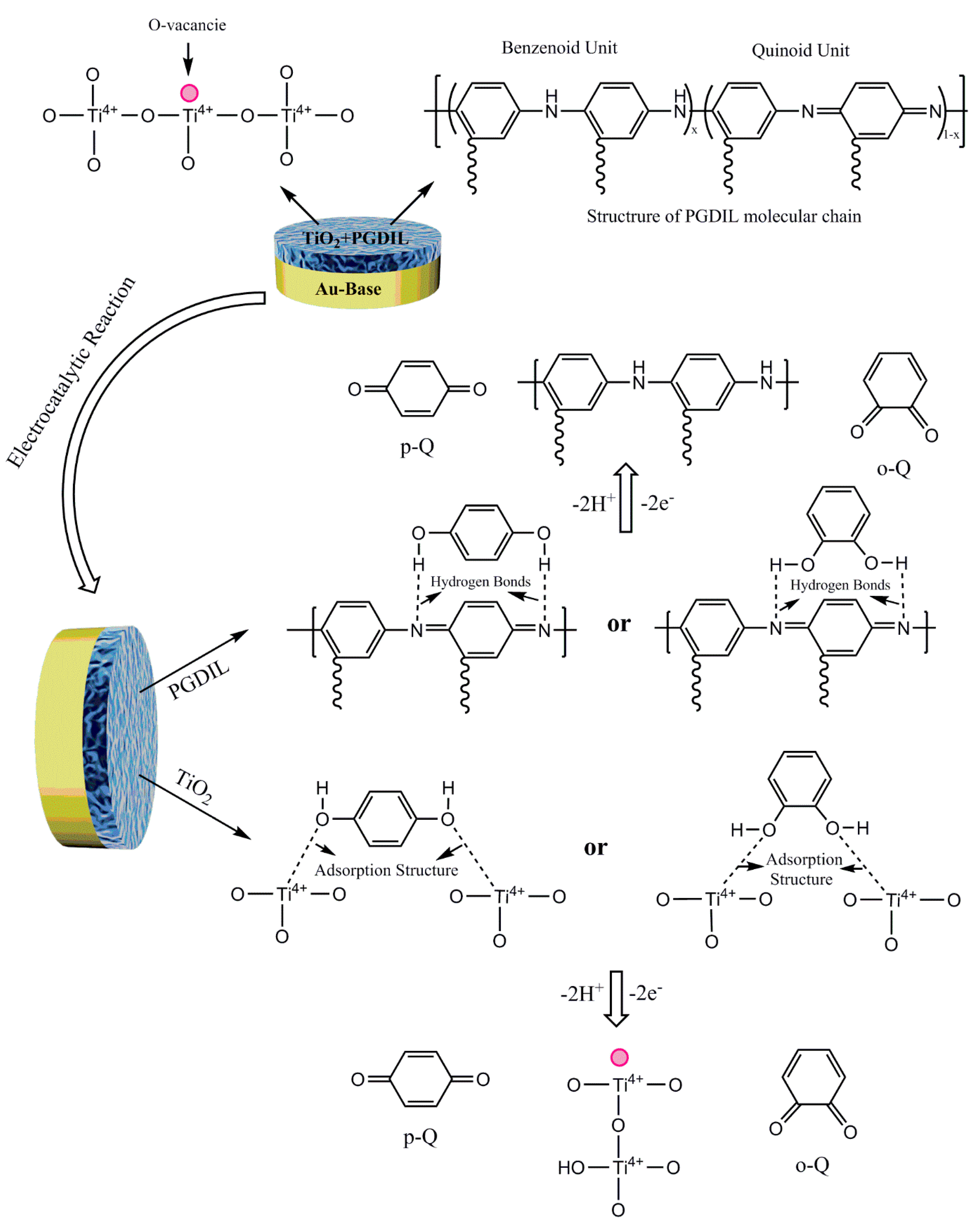
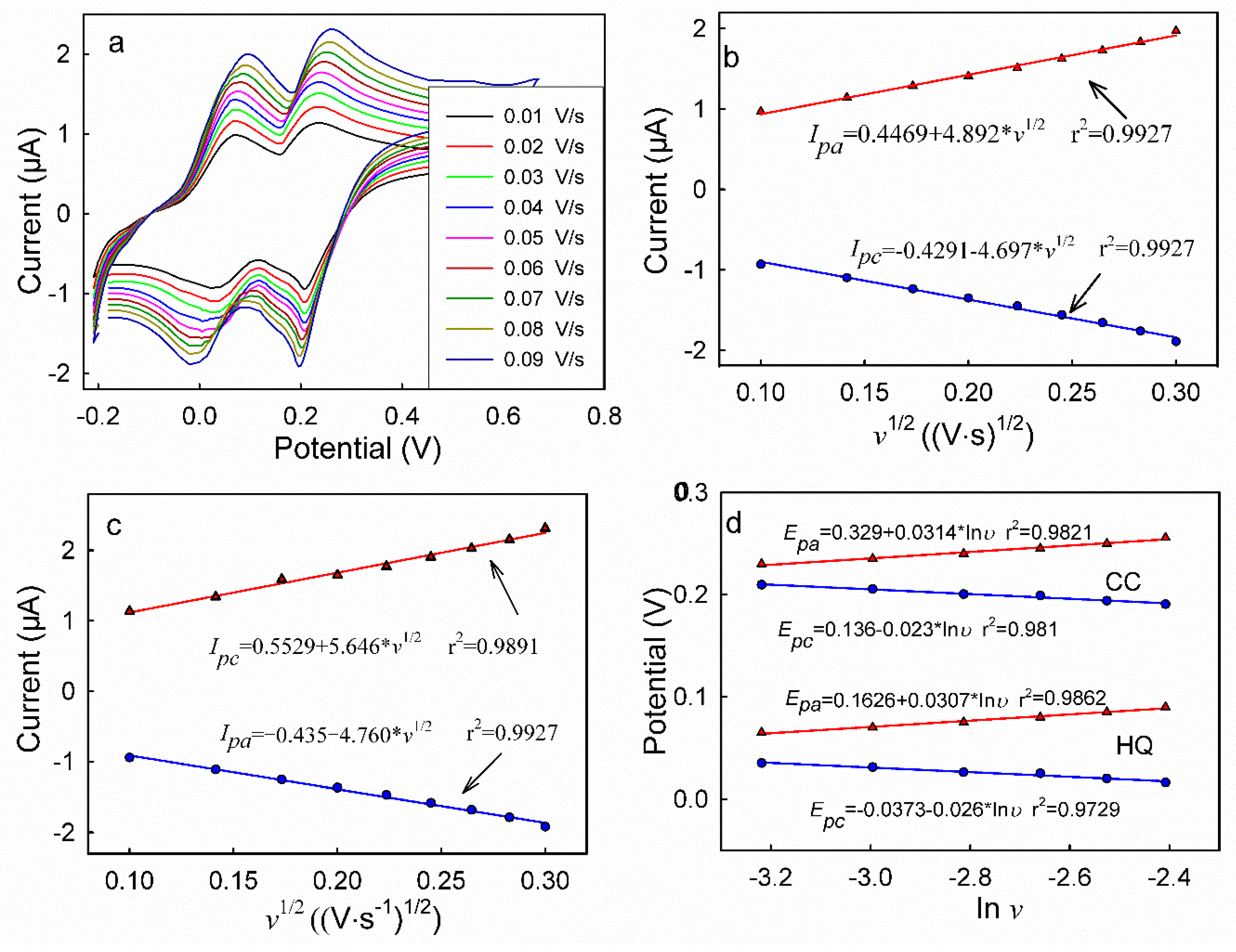


| Electrode | PGIL-TiO2/Au | PGIL/Au | TiO2/Au | Au | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E/V vs Ag/AgCl | Epa | Epc | ∆Ep | Epa | Epc | ∆Ep | Epa | Epc | ∆Ep | Epa | Epc | ∆Ep |
| HQ | 0.070 | 0.030 | 0.040 | 0.165 | 0.065 | 0.100 | >0.225 | −0.005 | >0.23 | >0.175 | <−0.05 | >0.23 |
| CC | 0.235 | 0.205 | 0.03 | 0.249 | 0.175 | 0.074 | 0.234 | 0.140 | 0.094 | 0.244 | 0.195 | 0.049 |
| HQ | T (°C) | 25 | 30 | 35 | 40 | 45 |
| k × 10−6 | 4.892 | 6.467 | 7.840 | 8.794 | 9.521 | |
| r | 0.9964 | 0.9982 | 0.9992 | 0.9987 | 0.9984 | |
| D × 10−7 (cm2/s) | 3.591 | 6.171 | 8.923 | 11.05 | 12.75 | |
| CC | k × 10−6 | 5.646 | 6.674 | 7.245 | 8.272 | 9.172 |
| r | 0.9945 | 0.9964 | 0.9955 | 0.9986 | 0.9990 | |
| D × 10−6 (cm2/s) | 1.913 | 2.629 | 3.569 | 3.910 | 4.731 |
| Binary Mixture | Concentration of HQ (mmol/L) | Concentration of CC (mmol/L) |
|---|---|---|
| 1 | 0.02 | 0.01 |
| 2 | 0.04 | 0.02 |
| 3 | 0.06 | 0.03 |
| 4 | 0.08 | 0.04 |
| 5 | 0.10 | 0.05 |
| 6 | 0.12 | 0.06 |
| 7 | 0.14 | 0.07 |
| 8 | 0.16 | 0.08 |
| 9 | 0.18 | 0.09 |
| 10 | 0.20 | 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; He, D.; Xie, A.; Qu, W.; Tang, Y.; Zhou, L.; Zhu, R. The Electrochemical Oxidation of Hydroquinone and Catechol through a Novel Poly-geminal Dicationic Ionic Liquid (PGDIL)–TiO2 Composite Film Electrode. Polymers 2019, 11, 1907. https://doi.org/10.3390/polym11111907
Guo Y, He D, Xie A, Qu W, Tang Y, Zhou L, Zhu R. The Electrochemical Oxidation of Hydroquinone and Catechol through a Novel Poly-geminal Dicationic Ionic Liquid (PGDIL)–TiO2 Composite Film Electrode. Polymers. 2019; 11(11):1907. https://doi.org/10.3390/polym11111907
Chicago/Turabian StyleGuo, Yanni, Deliang He, Aomei Xie, Wei Qu, Yining Tang, Lei Zhou, and Rilong Zhu. 2019. "The Electrochemical Oxidation of Hydroquinone and Catechol through a Novel Poly-geminal Dicationic Ionic Liquid (PGDIL)–TiO2 Composite Film Electrode" Polymers 11, no. 11: 1907. https://doi.org/10.3390/polym11111907
APA StyleGuo, Y., He, D., Xie, A., Qu, W., Tang, Y., Zhou, L., & Zhu, R. (2019). The Electrochemical Oxidation of Hydroquinone and Catechol through a Novel Poly-geminal Dicationic Ionic Liquid (PGDIL)–TiO2 Composite Film Electrode. Polymers, 11(11), 1907. https://doi.org/10.3390/polym11111907