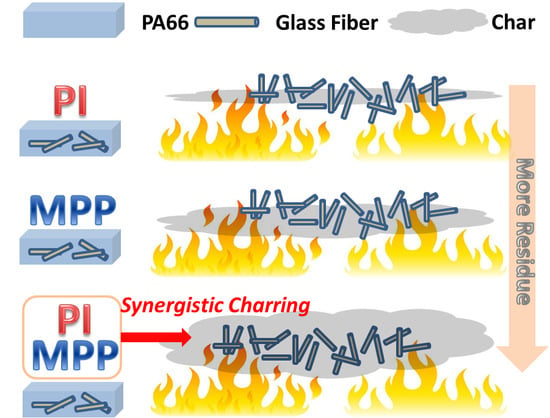
Synergistic Charring Flame-Retardant Behavior of Polyimide and Melamine Polyphosphate in Glass Fiber-Reinforced Polyamide 66
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Flame-Retardant PA66 Composites
2.3. Characterization
3. Results and Discussion
3.1. LOI and UL94 Tests
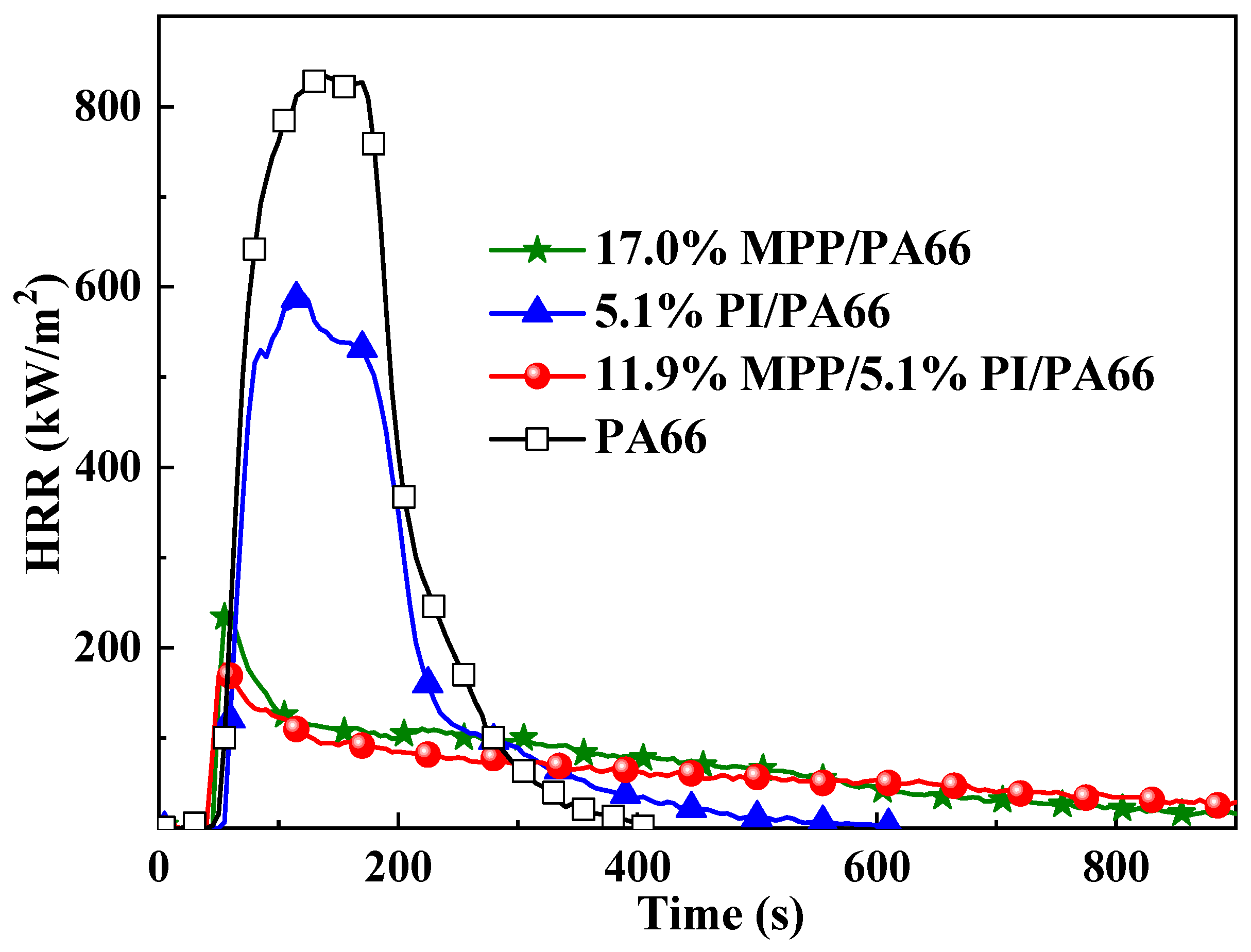
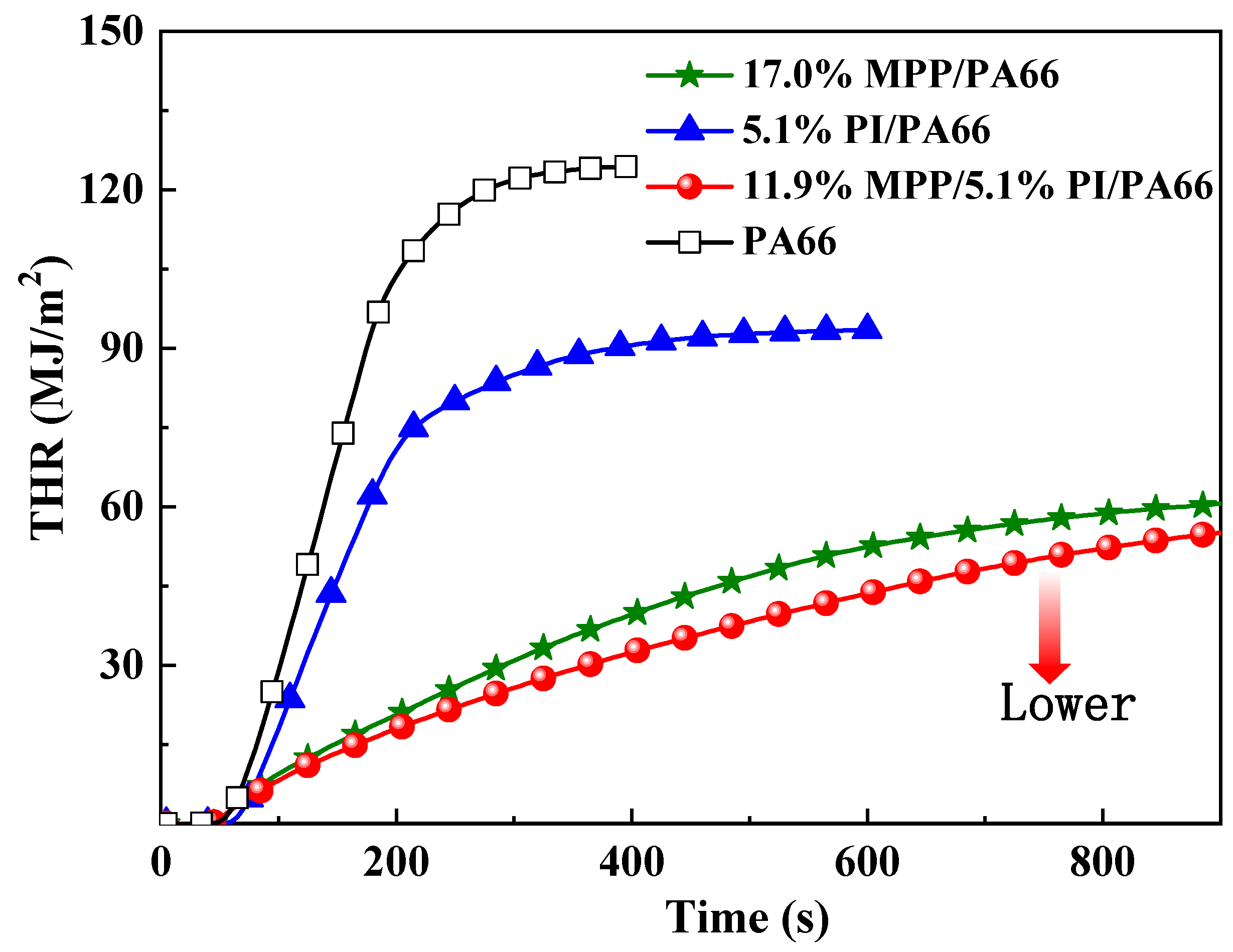
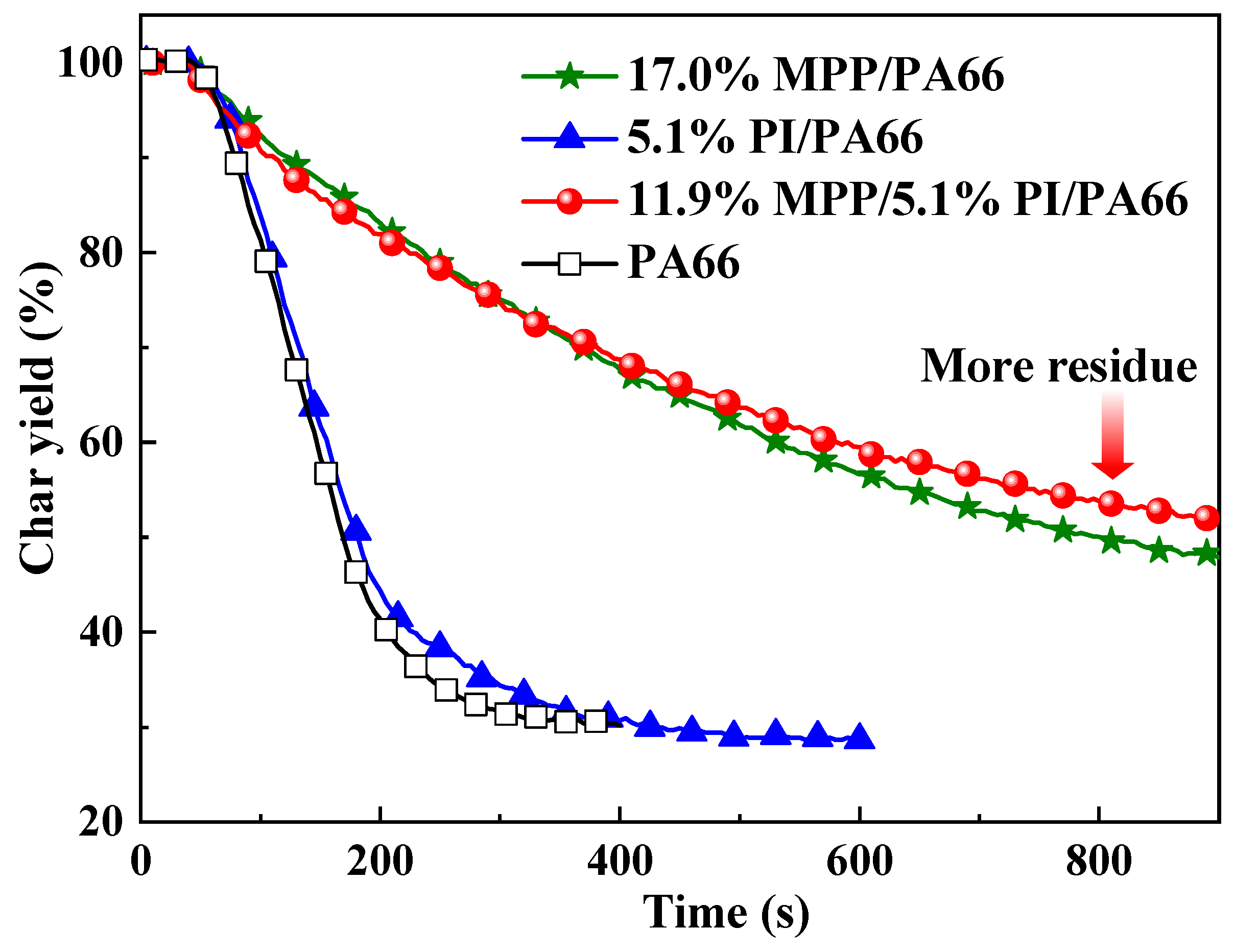
3.2. Cone Calorimeter Test
3.3. Thermal Properties of Flame-Retardant PA66 Composites
3.4. SEM/EDX Analysis of Residue
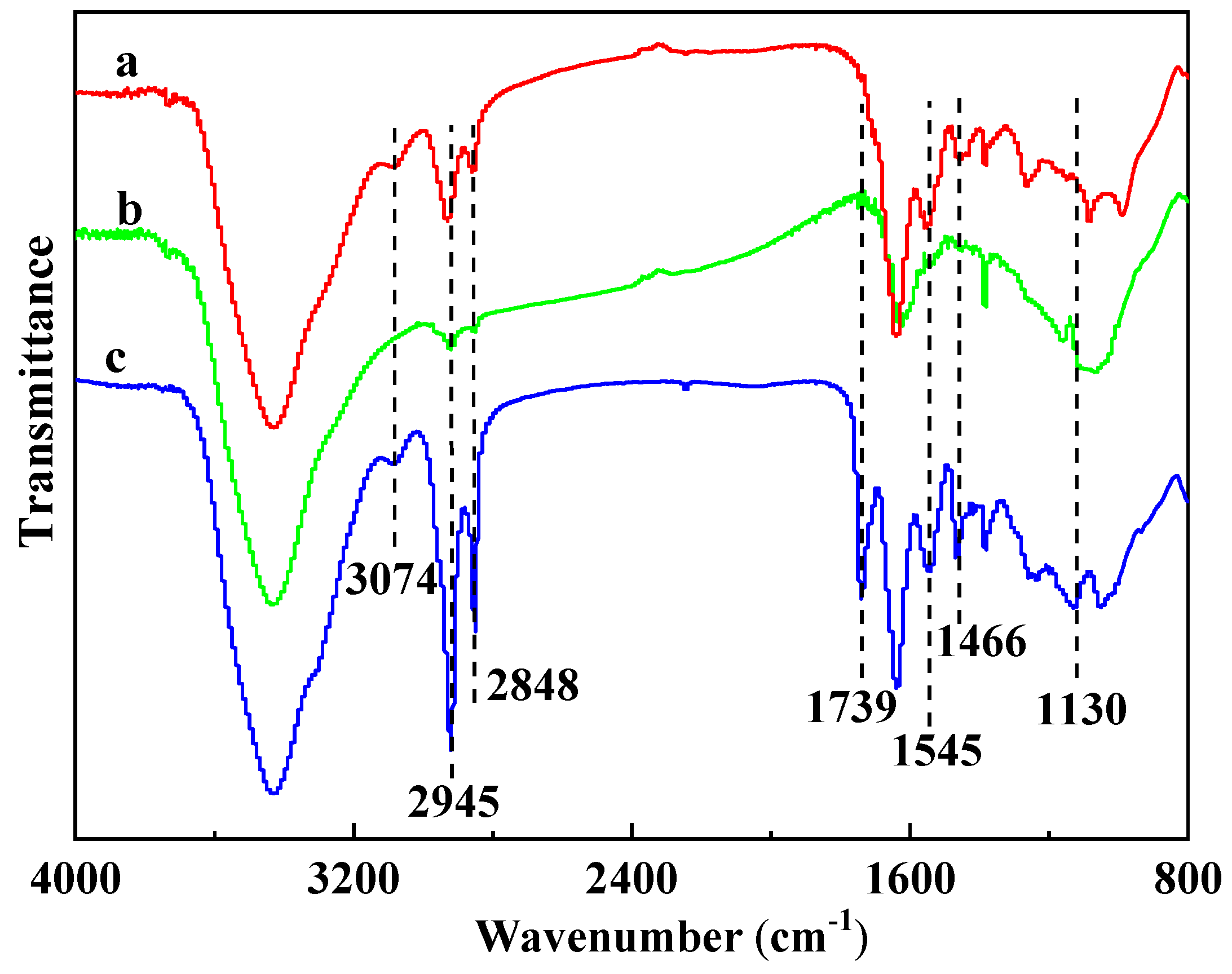
3.5. FTIR Analysis of Residues
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tamura, K.; Ohyama, S.; Umeyama, K.; Kitazawa, T.; Yamagishi, A. Preparation and properties of halogen-free flame retardant layered silicate-polyamide 66 nanocomposites. Appl. Clay Sci. 2016, 126, 107–112. [Google Scholar] [CrossRef]
- Lyu, W.Y.; Cui, Y.H.; Zhang, X.J.; Yuan, J.Y.; Zhang, W. Fire and thermal properties of PA 66 resin treated with poly-N-aniline-phenyl phosphamide as a flame retardant. Fire Mater. 2016, 41, 349–361. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Synthesis and thermal analytical screening of metal complexes as potential novel fire retardants in polyamide 6.6. Polym. Degrad. Stab. 2017, 144, 420–433. [Google Scholar] [CrossRef]
- Ryu, J.B.; Lyu, M.Y. A study on the mechanical property and 3D fiber distribution in injection molded glass fiber reinforced PA66. Int. Polym. Process. 2014, 29, 389–401. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Y.; Liu, Y.; Wang, Q.; Zhang, Z.J.; Wang, Z.Y. An efficient interfacial flame-resistance mode to prepare glass fiber reinforced and flame retarded polyamide 6 with high performance. J. Mater. Chem. A 2013, 1, 10228–10233. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2010, 21, 1–26. [Google Scholar] [CrossRef]
- Lu, H.D.; Song, L.; Hu, Y. A review on flame retardant technology in China. Part II: Flame retardant polymeric nanocomposites and coatings. Polym. Adv. Technol. 2011, 22, 379–394. [Google Scholar] [CrossRef]
- Li, L.P.; Li, B. Rheology, morphology and mechanical property relationship of non-halogen flame retarded glass fibre reinforced polyamide 66. Polym. Polym. Compos. 2011, 19, 603–609. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jäger, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6. Polym. Degrad. Stab. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Sun, J.; Gu, X.Y.; Zhang, S.; Coquelle, M.; Bourbigot, S.; Duquesne, S.; Casetta, M. Improving the flame retardancy of polyamide 6 by incorporating hexachlorocyclotriphosphazene modified MWNT. Polym. Adv. Technol. 2015, 25, 1099–1107. [Google Scholar] [CrossRef]
- Guo, Z.B.; Wang, C.L.; Li, J.; Yao, Q. Micro-intumescent flame retardant polyamide 6 based on cyclic phosphate grafting phenol formaldehyde. Polym. Adv. Technol. 2016, 27, 955–963. [Google Scholar] [CrossRef]
- Zhan, Z.S.; Li, B.; Xu, M.J.; Guo, Z.H. Synergistic effects of nano-silica on aluminum diethylphosphinate/polyamide 66 system for fire retardancy. High Perform. Polym. 2016, 28, 140–146. [Google Scholar] [CrossRef]
- Zhan, Z.S.; Li, B.; Xu, M.J. Synergistic effects of silicone resin on an aluminium diethylphosphinate flame retardant polyamide 6, 6 system. Sci. Adv. Mater. 2015, 7, 1830–1837. [Google Scholar] [CrossRef]
- Zhan, Z.S.; Li, B.; Xu, M.J. Synergistic effects of sepiolite on the flame retardant properties and thermal degradation behaviors of polyamide 66/aluminum diethylphosphinate composites. Polym. Degrad. Stab. 2015, 117, 66–74. [Google Scholar] [CrossRef]
- Yin, X.L.; Krifa, M.; Koo, J.H. Flame retardant polyamide 6/carbon nanotube nanofibers: Processing and characterization. J. Eng. Fiber Fabr. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Samyn, F.; Bourbigot, S. Thermal decomposition of flame retarded formulations PA6/aluminum phosphinate/melamine polyphosphate/organomodified clay: Interactions between the constituents? Polym. Degrad. Stab. 2011, 97, 2217–2230. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q. The investigation of melamine cyanurate-encapsulating magnesium hydroxide flame retarded polyamide-66. Polym. Polym. Compos. 2010, 18, 253–258. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q. Melamine cyanurate-microencapsulated red phosphorus flame retardant unreinforced and glass fiber reinforced polyamide 66. Polym. Degrad. Stab. 2006, 91, 3103–3109. [Google Scholar] [CrossRef]
- Cao, Y.F.; Qian, L.J.; Chen, Y.J.; Wang, Z. Synergistic flame retardant effect of phosphaphenanthrene derivative and aluminum diethylphosphinate in glass fiber reinforced polyamide 66. J. Appl. Polym. Sci. 2017, 134, 45126. [Google Scholar] [CrossRef]
- Sahyoun, J.; Bounor-Legare, V.; Ferry, L.; Sonnier, R.; Da Cruz-Boisson, F.; Melis, F.; Bonhomme, A.; Cassagnau, P. Synthesis of a new organophosphorous alkoxysilane precursor and its effect on the thermal and fire behavior of a PA66/PA6 copolymer. Eur. Polym. J. 2015, 66, 352–366. [Google Scholar] [CrossRef]
- Xie, M.C.; Zhang, S.M.; Ding, Y.F.; Wang, F.; Liu, P.; Tang, H.Y.; Wang, Y.T.; Yang, M.S. Synthesis of a heat-resistant DOPO derivative and its application as flame retardant in engineering plastics. J. Appl. Polym. Sci. 2017, 134, 44892. [Google Scholar] [CrossRef]
- Li, L.P.; Li, B.; Tang, F. Thermal Stability and Properties of Flame Retarded Glass Fiber Reinforced Polyamide 66 Composite. J. Reinf. Plast. Compos. 2008, 27, 277–285. [Google Scholar]
- He, Q.X.; Tang, L.; Fu, T.; Shi, Y.Q.; Wang, X.L.; Wang, Y.Z. Novel phosphorus-containing halogen-free ionic liquids: Effect of sulfonate anion size on physical properties, biocompatibility, and flame retardancy. RSC Adv. 2016, 6, 52485–52494. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Hou, Y.B.; Hu, Y. Construction of flame retardant coating on polyamide 6.6 via UV grafting of phosphorylated chitosan and sol-gel process of organo-silane. Carbohydr. Polym. 2017, 181, 833–840. [Google Scholar] [CrossRef]
- Majka, T.M.; Cokot, M.; Pielichowski, K. Studies on the thermal properties and flammability of polyamide 6 nanocomposites surface-modified via layer-by-layer deposition of chitosan and montmorillonite. J. Therm. Anal. Calorim. 2018, 131, 405–416. [Google Scholar] [CrossRef]
- Mohaddes, F.; Wang, L.J.; Shanks, R.A.; Fergusson, S.M. Elevation of charring level of polyamide-6,6 films via ionic introduction of phosphoric acid and boric acid esters. Green Chem. Lett. Rev. 2014, 7, 184–190. [Google Scholar] [CrossRef]
- Muller, P.; Morys, M.; Sut, A.; Jäger, C.; Illerhaus, B.; Schartel, B. Melamine poly(zinc phosphate) as flame retardant in epoxy resin: Decomposition pathways, molecular mechanisms and morphology of fire residues. Polym. Degrad. Stab. 2016, 130, 307–319. [Google Scholar] [CrossRef]
- Qiu, Y.; Wachtendorf, V.; Klack, P.; Qian, L.J.; Liu, Z.; Schartel, B. Improved flame retardancy by synergy between cyclotetrasiloxane and phosphaphenanthrene/triazine compounds in epoxy thermoset. Polym. Int. 2017, 66, 1883–1890. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Qiu, Y.; Dong, Y.P. Synergistic flame retardant effect and mechanisms of boron/phosphorus compounds on epoxy resins. Polym. Adv. Technol. 2018, 29, 641–648. [Google Scholar] [CrossRef]
- Gallo, E.; Fan, Z.; Schartel, B.; Greiner, A. Electrospun nanofiber mats coating-new route to flame retardancy. Polym. Adv. Technol. 2011, 22, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Hu, S.; Zhai, J.G.; Chen, T.; Mai, Y.Y. Thermal properties and combustion behaviors of flame-retarded glass fiber-reinforced polyamide 6 with piperazine pyrophosphate and aluminum hypophosphite. J. Therm. Anal. Calorim. 2016, 125, 175–185. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Liu, Y.; Wang, Q. Synergistic effect of melamine polyphosphate with macromolecular charring agent novolac in wollastonite filled PA66. J. Appl. Polym. Sci. 2010, 116, 45–49. [Google Scholar] [CrossRef]
- Xu, M.L.; Chen, Y.J.; Qian, L.J.; Wang, J.Y.; Tang, S. Component ratio effects of hyperbranched triazine compound and ammonium polyphosphate in flame retardant polypropylene composites. J. Appl. Polym. Sci. 2015, 131, 179–180. [Google Scholar] [CrossRef]
- Sturm, H.; Schartel, B.; Weiß, A.; Braun, U. SEM/EDX: Advanced investigation of structured fire residues and residue formation. Polym. Test. 2012, 31, 606–619. [Google Scholar] [CrossRef]
- Cardell, C.; Guerra, I. An overview of emerging hyphenated SEM-EDX and Raman spectroscopy systems: Applications in life, environmental and materials sciences. TrAC Trends Anal. Chem. 2016, 77, 156–166. [Google Scholar] [CrossRef]
- Feng, H.S.; Qiu, Y.; Qian, L.J.; Chen, Y.J.; Xu, B.; Xin, F. Flame inhibition and charring effect of aromatic polyimide and aluminum diethylphosphinate in polyamide 6. Polymers 2019, 11, 74. [Google Scholar] [CrossRef]



| Samples | PA66 (wt %) | Glass Fiber (wt %) | MPP (wt %) | PI (wt %) | Antioxidant (wt %) |
|---|---|---|---|---|---|
| PA66 | 69.4 | 30 | - | - | 0.6 |
| 17.0%MPP /PA66 | 52.4 | 30 | 17.0 | - | 0.6 |
| 5.1%PI/PA66 | 64.3 | 30 | - | 5.1 | 0.6 |
| 11.9%MPP/5.1%PI/PA66 | 52.4 | 30 | 11.9 | 5.1 | 0.6 |
| 10.2%MPP/6.8%PI/PA66 | 52.4 | 30 | 10.2 | 6.8 | 0.6 |
| Samples | LOI (%) | UL94 | |||
|---|---|---|---|---|---|
| av-t1(s) | av-t2(s) | Dripping | Rating | ||
| PA66 | 23.6 | 185.0 | - | Yes | No rating |
| 17.0%MPP/PA66 | 31.7 | 3.6 | 8.8 | No | V-1 |
| 5.1%PI/PA66 | 26.3 | 24.6 a | - | Yes | No rating |
| 11.9%MPP/5.1%PI/PA66 | 33.9 | 2.2 | 3.7 | No | V-0 |
| 10.2%MPP/6.8%PI/PA66 | 33.7 | 8.7 | 14.4 | No | V-1 |
| Samples | pk-HRR (kW/m2) | THR (MJ/m2) | av-EHC (MJ/kg) | Char (wt %) | TSR (m2/m2) |
|---|---|---|---|---|---|
| PA66 | 830 ± 42 | 122.4 ± 3.7 | 29.5 ± 1.7 | 0.1 ± 1.0 | 3904 ± 158 |
| 17.0%MPP /PA66 | 201 ± 35 | 60.3 ± 0.2 | 24.4 ± 1.3 | 18.6 ± 0.7 | 1365 ± 101 |
| 5.1%PI/PA66 | 559 ± 33 | 92.8 ± 0.6 | 30.3 ± 0.2 | 0.1 ± 0.5 | 1420 ± 20 |
| 11.9%MPP/5.1%PI/PA66 | 190 ± 10 | 54.1 ± 1.0 | 24.9 ± 0.3 | 21.0 ± 0.9 | 1129 ± 96 |
| Samples | C (%) | O (%) | N (%) | P (%) | Si (%) | Ca (%) | Al (%) |
|---|---|---|---|---|---|---|---|
| 17.0%MPP/PA66 | 46.58 | 26.19 | 11.70 | 6.58 | 4.45 | 3.31 | 1.20 |
| 11.9%MPP/5.1%PI/PA66 | 69.82 | 9.19 | 18.83 | 1.59 | 0.57 | 0 | 0 |
| Samples | P in Composites (g) | P in Residue (g) | P Released (g) |
|---|---|---|---|
| 17.0%MPP/PA66 | 1.38 | 0.48 | 0.90 |
| 11.9%MPP/5.1%PI/PA66 | 0.97 | 0.13 | 0.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Cao, Y.; Qian, L.; Chen, Y.; Qiu, Y.; Xu, B.; Xin, F. Synergistic Charring Flame-Retardant Behavior of Polyimide and Melamine Polyphosphate in Glass Fiber-Reinforced Polyamide 66. Polymers 2019, 11, 1851. https://doi.org/10.3390/polym11111851
Tang W, Cao Y, Qian L, Chen Y, Qiu Y, Xu B, Xin F. Synergistic Charring Flame-Retardant Behavior of Polyimide and Melamine Polyphosphate in Glass Fiber-Reinforced Polyamide 66. Polymers. 2019; 11(11):1851. https://doi.org/10.3390/polym11111851
Chicago/Turabian StyleTang, Wei, Yanfang Cao, Lijun Qian, Yajun Chen, Yong Qiu, Bo Xu, and Fei Xin. 2019. "Synergistic Charring Flame-Retardant Behavior of Polyimide and Melamine Polyphosphate in Glass Fiber-Reinforced Polyamide 66" Polymers 11, no. 11: 1851. https://doi.org/10.3390/polym11111851
APA StyleTang, W., Cao, Y., Qian, L., Chen, Y., Qiu, Y., Xu, B., & Xin, F. (2019). Synergistic Charring Flame-Retardant Behavior of Polyimide and Melamine Polyphosphate in Glass Fiber-Reinforced Polyamide 66. Polymers, 11(11), 1851. https://doi.org/10.3390/polym11111851