Fabrication of Poly(butylene succinate)/Carbon Black Nanocomposite Foams with Good Electrical Conductivity and High Strength by a Supercritical CO2 Foaming Process
Abstract
:1. Introduction
2. Experimental Part
2.1. Raw Materials
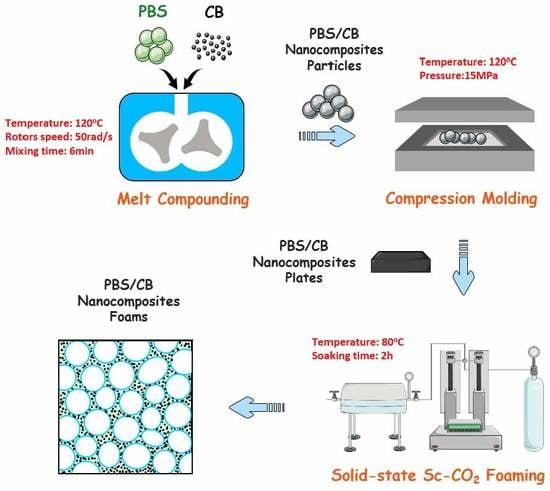
2.2. Preparation of PBS/CB Nanocomposites
2.3. Preparation of PBS/CB Nanocomposite Foams
2.4. Characterization
2.4.1. Thermal Characterization
2.4.2. Mechanical Property Test
2.4.3. Rheological Test
2.4.4. Electrical Conductivity Test
2.4.5. Microstructure Analysis
3. Results and Discussion
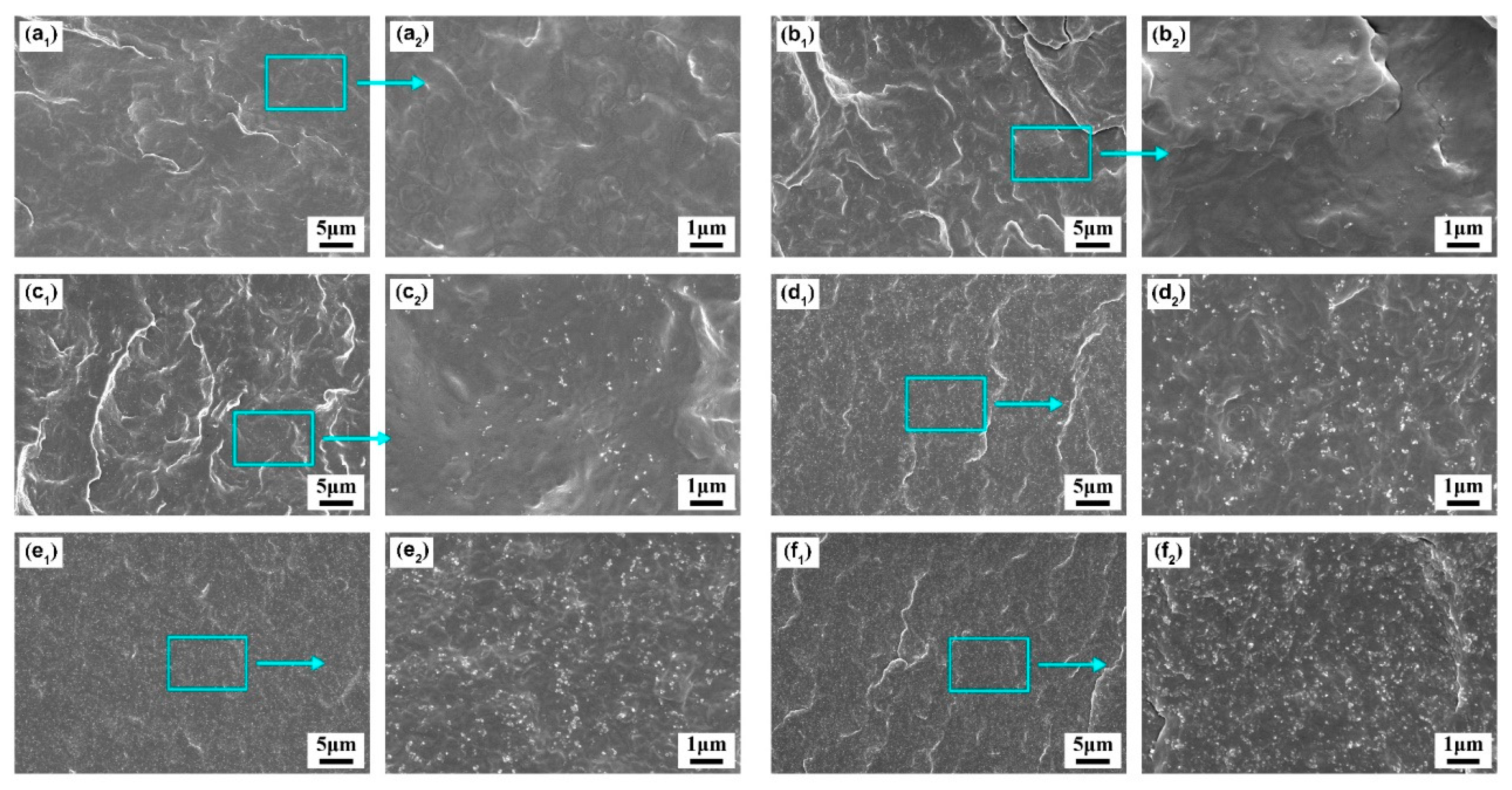
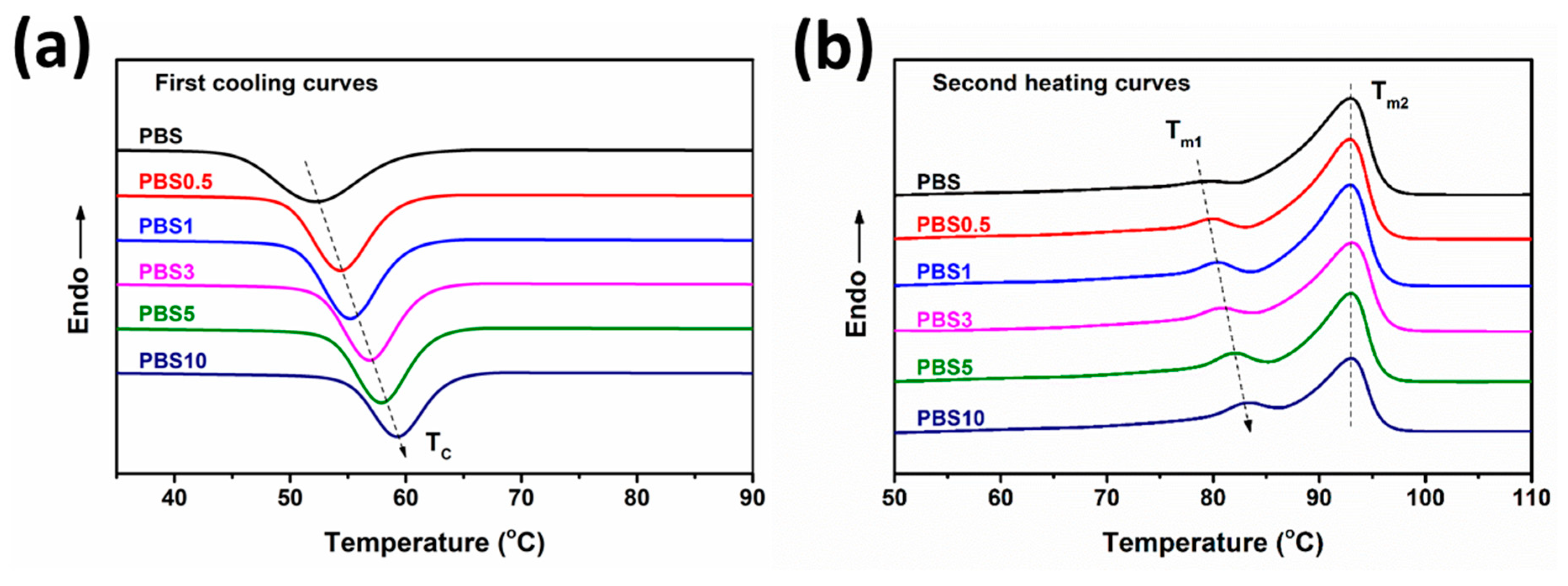
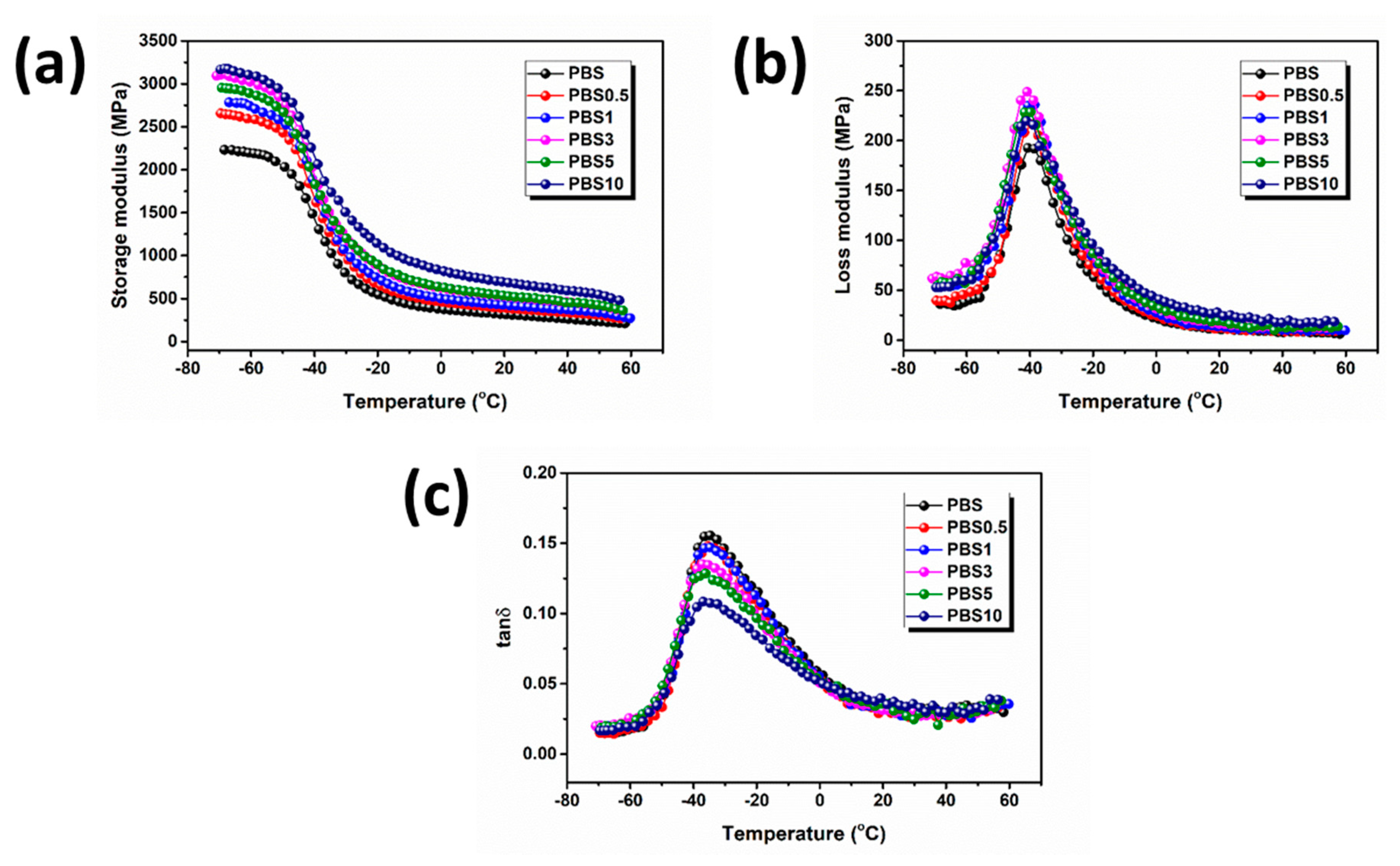
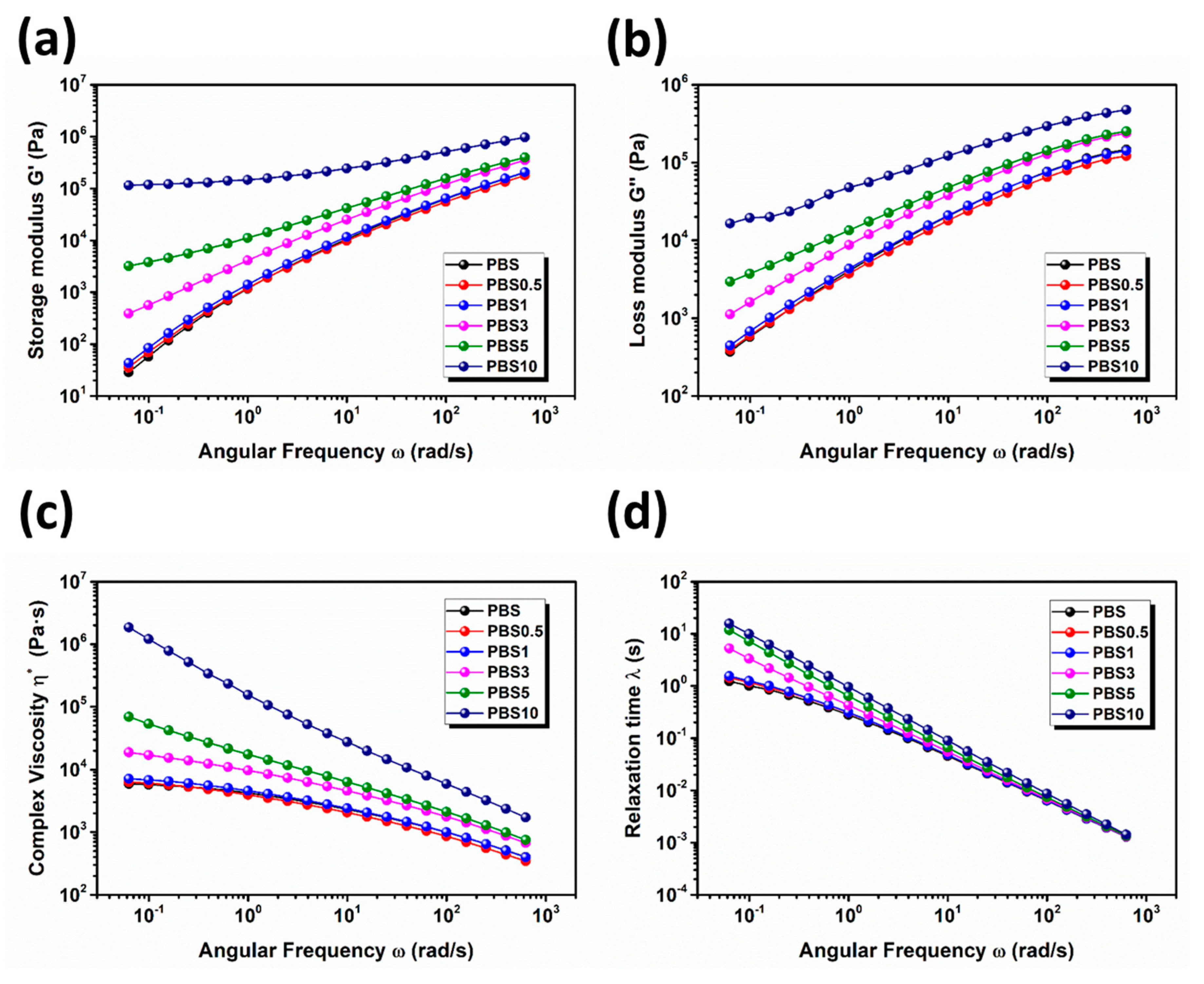
3.1. Effect of CB Content on the Physical Properties of PBS/CB Nanocomposites
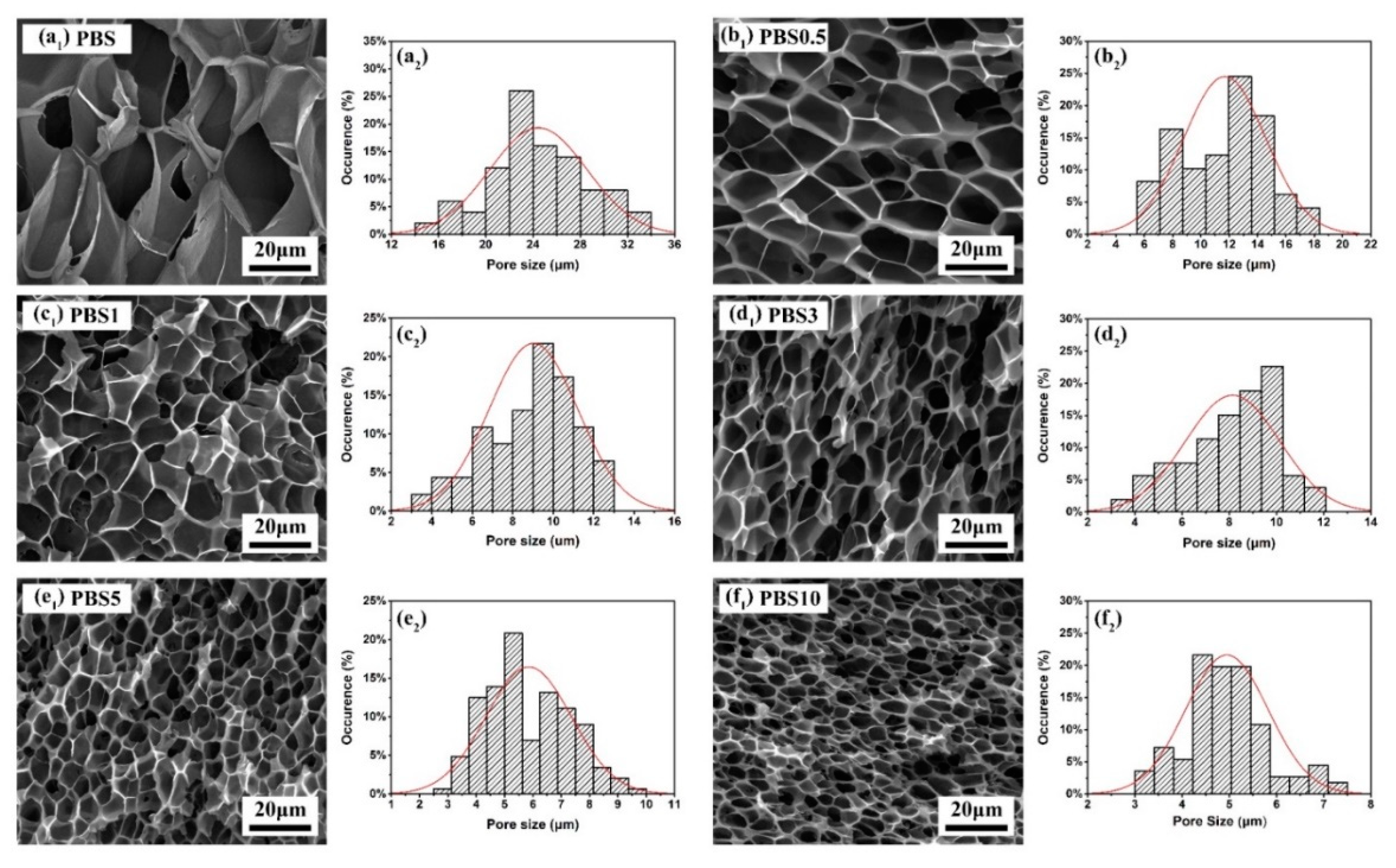
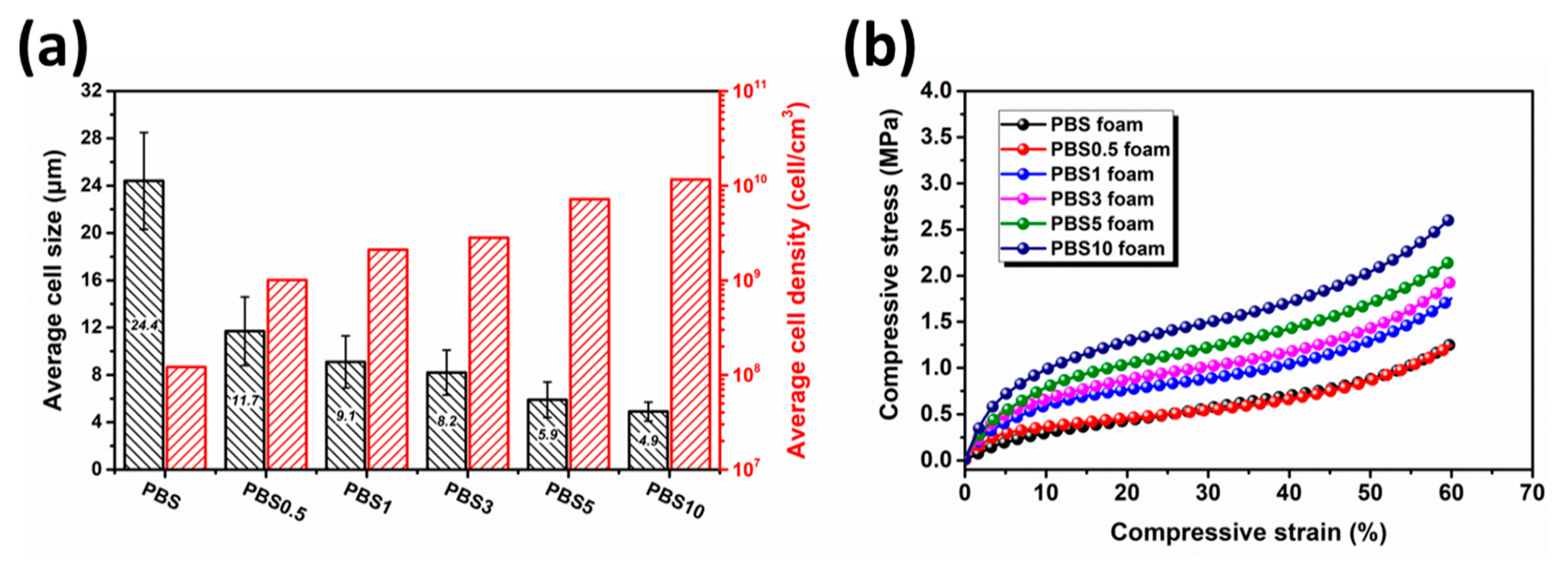
3.2. Effect of CB Content on the Cell Morphology and Cell Size Distribution of Nanocomposite Foams
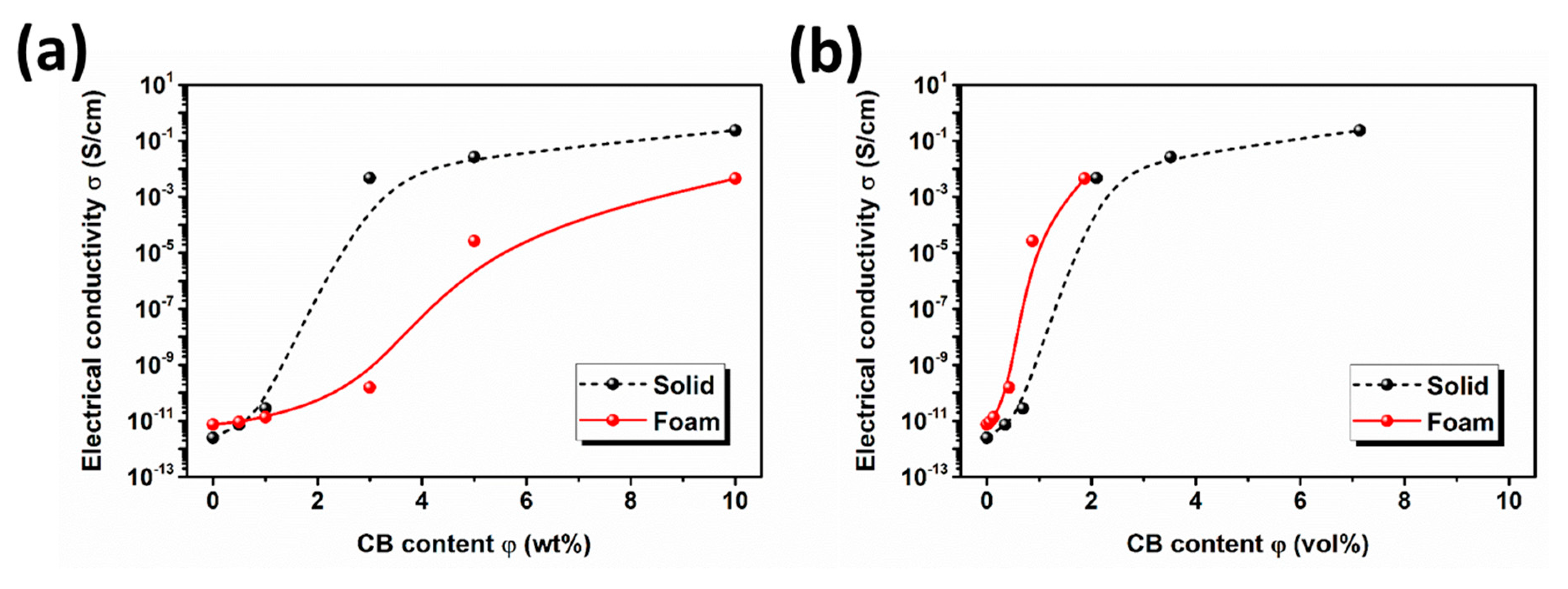
3.3. Electrical Conductivity of PBS/CB Nanocomposites and Their Nanocomposite Foams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dlouha, J.; Suryanegara, L.; Yano, H. Cellulose nanofibre-poly(lactic acid) microcellular foams exhibiting high tensile toughness. React. Funct. Polym. 2014, 85, 201–207. [Google Scholar] [CrossRef]
- Kuang, T.R.; Mi, H.Y.; Fu, D.J.; Jing, X.; Chen, B.Y.; Mou, W.J.; Peng, X.F. Fabrication of Poly(lactic acid)/Graphene Oxide Foams with Highly Oriented and Elongated Cell Structure via Unidirectional Foaming Using Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2015, 54, 758–768. [Google Scholar] [CrossRef]
- Kuang, T.R.; Chang, L.Q.; Chen, F.; Sheng, Y.; Fu, D.J.; Peng, X.F. Facile preparation of lightweight high-strength biodegradable polymer/multi-walled carbon nanotubes nanocomposite foams for electromagnetic interference shielding. Carbon 2016, 105, 305–313. [Google Scholar] [CrossRef]
- Kuang, T.R.; Chen, F.; Chang, L.Q.; Zhao, Y.N.; Fu, D.J.; Gong, X.; Peng, X. Facile preparation of open-cellular porous poly (L-lactic acid) scaffold by supercritical carbon dioxide foaming. for potential tissue engineering applications. Chem. Eng. J. 2017, 307, 1017–1025. [Google Scholar] [CrossRef]
- Kuang, T.R.; Li, K.C.; Chen, B.Y.; Peng, X.F. Poly (propylene carbonate)-based in situ nanofibrillar biocomposites with enhanced miscibility, dynamic mechanical properties, rheological behavior and extrusion foaming ability. Compos. Part B Eng. 2017, 123, 112–123. [Google Scholar] [CrossRef]
- Kuang, T.R.; Ju, J.J.; Yang, Z.Y.; Geng, L.; Peng, X.F. A facile approach towards fabrication of lightweight biodegradable poly (butylene succinate)/carbon fiber composite foams with high electrical conductivity and strength. Compos. Sci. Technol. 2018, 159, 171–179. [Google Scholar] [CrossRef]
- Ju, J.; Peng, X.; Huang, K.; Li, L.; Liu, X.; Chitrakar, C.; Chang, L.; Gu, Z.; Kuang, T. High-performance porous PLLA-based scaffolds for bone tissue engineering: Preparation, characterization, and in vitro and in vivo evaluation. Polymer 2019, 180, 121707. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Peng, J.; Salick, M.R.; Peng, X.-F.; Turng, L.-S. Poly(ε-caprolactone) (PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose 2014, 21, 2727–2741. [Google Scholar] [CrossRef]
- Tsimpliaraki, A.; Tsivintzelis, I.; Marras, S.I.; Zuburtikudis, I.; Panayiotou, C. Foaming of PCL/clay nanocomposites with supercritical CO2 mixtures: The effect of nanocomposite fabrication route on the clay dispersion and the final porous structure. J. Supercrit. Fluids 2013, 81, 86–91. [Google Scholar] [CrossRef]
- Javadi, A.; Srithep, Y.; Lee, J.; Pilla, S.; Clemons, C.; Gong, S.Q.; Turng, L.S. Processing and characterization of solid and microcellular PHBV/PBAT blend and its RWF/nanoclay composites. Compos. Part A Appl. Sic. Manuf. 2010, 41, 982–990. [Google Scholar] [CrossRef]
- Javadi, A.; Srithep, Y.; Clemons, C.C.; Turng, L.S.; Gong, S.Q. Processing of poly(hydroxybutyrate-co-hydroxyvalerate)-based bionanocomposite foams using supercritical fluids. J. Mater. Res. 2012, 27, 1506–1517. [Google Scholar] [CrossRef]
- Gigli, M.; Lotti, N.; Gazzano, M.; Finelli, L.; Munari, A. Novel eco-friendly random copolyesters of poly(butylene succinate) containing ether-linkages. React. Funct. Polym. 2012, 72, 303–310. [Google Scholar] [CrossRef]
- Li, G.; Qi, R.R.; Lu, J.Q.; Hu, X.L.; Luo, Y.; Jiang, P.K. Rheological properties and foam preparation of biodegradable poly(butylene succinate). J. Appl. Polym. Sci. 2013, 127, 3586–3594. [Google Scholar] [CrossRef]
- Yu, P.; Mi, H.Y.; Huang, A.; Geng, L.H.; Chen, B.Y.; Kuang, T.R.; Mou, W.J.; Peng, X.F. Effect of Poly(butylenes succinate) on Poly(lactic acid) Foaming Behavior: Formation of Open Cell Structure. Ind. Eng. Chem. Res. 2015, 54, 6199–6207. [Google Scholar] [CrossRef]
- Zhou, H.F.; Wang, X.D.; Du, Z.J.; Li, H.Q.; Yu, K.J. Preparation and Characterization of Chain Extended Poly(butylene succinate) Foams. Polym. Eng. Sci. 2015, 55, 988–994. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.D.; Peng, X.F.; Wang, J.S. Fabrication and mechanism of poly(butylene succinate) urethane ionomer microcellular foams with high thermal insulation and compressive feature. Eur. Polym. J. 2018, 99, 250–258. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.C.; Li, C.C.; Zhu, W.X.; Zhang, D.; Guan, G.H.; Xiao, Y. Synthesis and Properties of Biodegradable Poly(ester-co-carbonate) Multiblock Copolymers Comprising of Poly(butylene Succinate) and Poly(butylene Carbonate) by Chain Extension. Ind. Eng. Chem. Res. 2012, 51, 10785–10792. [Google Scholar] [CrossRef]
- Boonprasertpoh, A.; Pentrakoon, D.; Junkasem, J. Effect of Crosslinking Agent and Branching Agent on Morphological and Physical Properties of Poly(Butylene succinate) Foams. Cell. Polym. 2017, 36, 333–354. [Google Scholar] [CrossRef]
- Wu, W.; Cao, X.W.; Lin, H.; He, G.J.; Wang, M.M. Preparation of biodegradable poly(butylene succinate)/halloysite nanotube nanocomposite foams using supercritical CO2 as blowing agent. J. Polym. Res. 2015, 22, 177. [Google Scholar] [CrossRef]
- Wu, W.; Cao, X.W.; Zhang, Y.J.; He, G.J. Polylactide/halloysite nanotube nanocomposites: Thermal, mechanical properties, and foam processing. J. Appl. Polym. Sci. 2013, 130, 443–452. [Google Scholar] [CrossRef]
- Lim, S.K.; Lee, S.I.; Jang, S.G.; Lee, K.H.; Choi, H.J.; Chin, I.J. Synthetic Aliphatic Biodegradable Poly(Butylene Succinate)/MWNT Nanocomposite Foams and Their Physical Characteristics. J. Macromol. Sci. Part B 2011, 50, 1171–1184. [Google Scholar] [CrossRef]
- Lim, S.K.; Lee, S.I.; Jang, S.G.; Lee, K.H.; Choi, H.J.; Chin, I.J. Fabrication and Physical Characterization of Biodegradable Poly(butylene succinate)/Carbon Nanofiber Nanocomposite Foams. J. Macromol. Sci. Part B 2011, 50, 100–110. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Hosoya, M.; Kurumada, J. Grafting reaction of surface carboxyl groups on carbon black with polymers having terminal hydroxyl or amino groups using N,N′-dicyclohexylcarbodiimide as a condensing agent. React. Funct. Polym. 1995, 27, 75–81. [Google Scholar] [CrossRef]
- Takamura, M.; Yamauchi, T.; Tsubokawa, N. Grafting and crosslinking reaction of carboxyl-terminated liquid rubber with silica nanoparticles and carbon black in the presence of Sc(OTf)3. React. Funct. Polym. 2008, 68, 1113–1118. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Chen, Y.; Yuan, M.; Yang, M.; Yuan, W. Composites of carbon black functionalized with polymers as candidates for the detection of methanol vapor. React. Funct. Polym. 2007, 67, 977–985. [Google Scholar] [CrossRef]
- Thomassin, J.; Pagnoulle, C.; Bednarz, L.; Huynen, I.; Jerome, R.; Detrembleur, C. Foams of polycaprolactone/MWNT nanocomposites for efficient EMI reduction. J. Mater. Chem. 2008, 18, 792–796. [Google Scholar] [CrossRef]
- Ling, J.; Zhai, W.; Feng, W.; Shen, B.; Zhang, J.; Zheng, W. Facile Preparation of Lightweight Microcellular Polyetherimide/Graphene Composite Foams for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2013, 5, 2677–2684. [Google Scholar] [CrossRef]
- Ameli, A.; Jung, P.; Park, C. Electrical properties and electromagnetic interference shielding effectiveness of polypropylene/carbon fiber composite foams. Carbon 2013, 60, 379–391. [Google Scholar] [CrossRef]
- Cafiero, L.; Oliviero, M.; Landi, G.; Sorrentino, L.; Sorrentino, A.; Neitzert, H.; Iannace, S. Preparation and characterization of conductive foams based on PBS, carbon nanofibers and expanded graphite nanocomposites. In Proceedings of the PPS-32: The 32nd International Conference of the Polymer Processing Society, Lyon, France, 25–29 July 2016. [Google Scholar]
- Wang, X.; Yang, H.Y.; Song, L.; Hu, Y.; Xing, W.Y.; Lu, H.D. Morphology, mechanical and thermal properties of graphene-reinforced poly(butylene succinate) nanocomposites. Compos. Sci. Technol. 2011, 72, 1–6. [Google Scholar] [CrossRef]
- Li, Y.D.; Fu, Q.Q.; Wang, M.; Zeng, J.B. Morphology, crystallization and rheological behavior in poly(butylene succinate)/cellulose nanocrystal nanocomposites fabricated by solution coagulation. Carbohydr. Polym. 2017, 164, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.Y.; Zou, W.; Zhang, H.C.; Zhang, G.Z.; Yang, Z.T.; Jin, G.; Qu, J.P. Thermal behavior, dynamic mechanical properties and rheological properties of poly(butylene succinate) composites filled with nanometer calcium carbonate. Polym. Test. 2015, 42, 160–167. [Google Scholar] [CrossRef]
- Keshtkar, M.; Nofar, M.; Park, C.B.; Carreau, P.J. Extruded PLA/clay nanocomposite foams blown with supercritical CO2. Polymer 2014, 55, 4077–4090. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam with additives: Part I: Theoretical considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam with additives: Part II: Experimental results and discussion. Polym. Eng. Sci. 1987, 27, 493–499. [Google Scholar] [CrossRef]

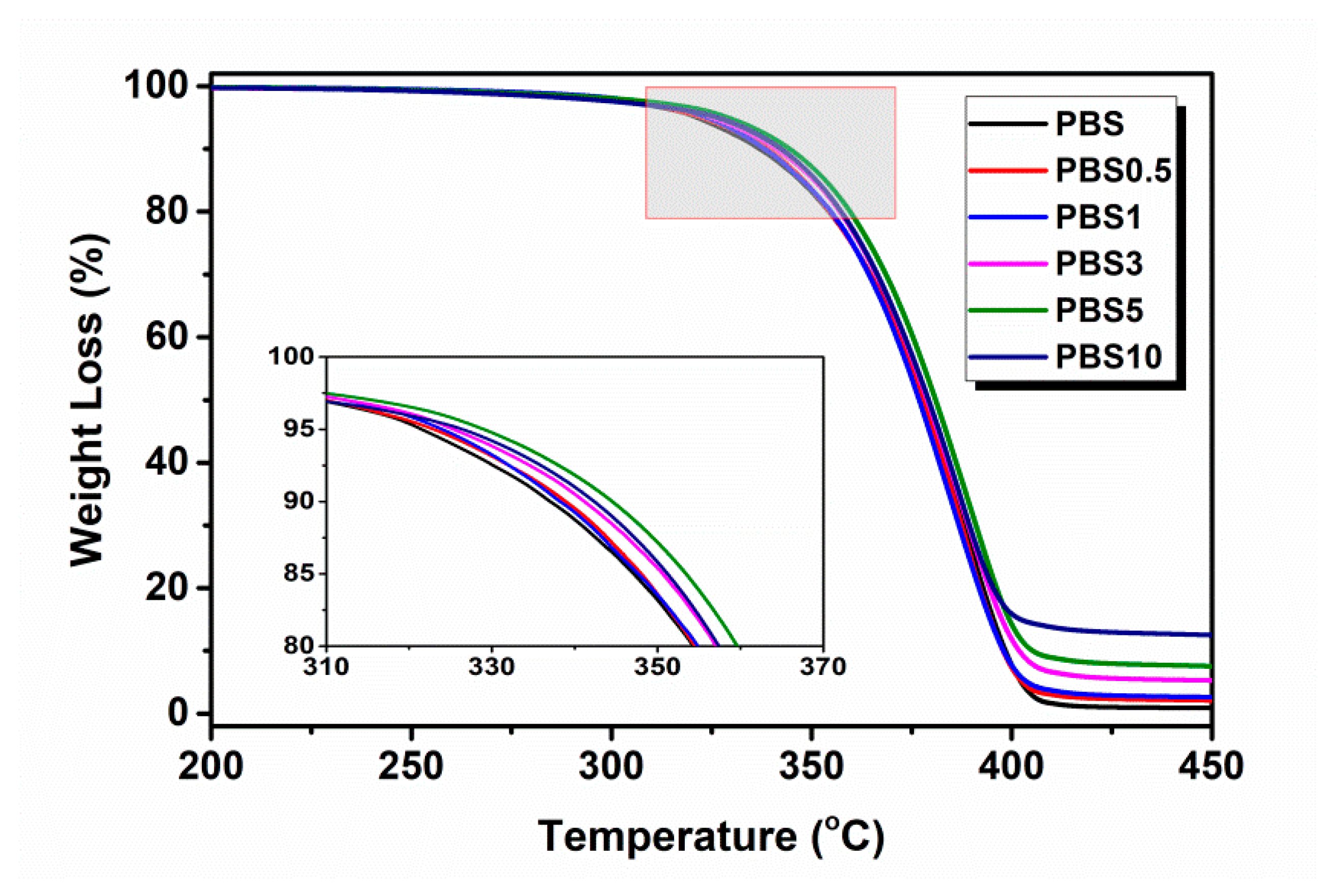
| Sample | T5% (°C) | Tmax (°C) |
|---|---|---|
| PBS | 321.5 | 388.1 |
| PBS0.5 | 323.0 | 386.1 |
| PBS1 | 324.0 | 384.4 |
| PBS3 | 325.3 | 387.8 |
| PBS5 | 329.0 | 390.3 |
| PBS10 | 326.4 | 387.2 |
| CB Content in Bulk (wt %) | CB Content in Bulk (vol %) | Density of Bulk (g·cm−3) | Density of Foam (g·cm−3) | CB Content in Foam (vol %) |
|---|---|---|---|---|
| 0 | 0 | 1.26 | 0.107 | 0 |
| 0.5 | 0.35 | 1.26 | 0.205 | 0.06 |
| 1 | 0.69 | 1.27 | 0.237 | 0.13 |
| 3 | 2.10 | 1.28 | 0.258 | 0.42 |
| 5 | 3.52 | 1.29 | 0.319 | 0.87 |
| 10 | 7.14 | 1.32 | 0.344 | 1.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Hu, J.; Ju, J.; Kuang, T. Fabrication of Poly(butylene succinate)/Carbon Black Nanocomposite Foams with Good Electrical Conductivity and High Strength by a Supercritical CO2 Foaming Process. Polymers 2019, 11, 1852. https://doi.org/10.3390/polym11111852
Chen Z, Hu J, Ju J, Kuang T. Fabrication of Poly(butylene succinate)/Carbon Black Nanocomposite Foams with Good Electrical Conductivity and High Strength by a Supercritical CO2 Foaming Process. Polymers. 2019; 11(11):1852. https://doi.org/10.3390/polym11111852
Chicago/Turabian StyleChen, Zhou, Junfeng Hu, Jiajun Ju, and Tairong Kuang. 2019. "Fabrication of Poly(butylene succinate)/Carbon Black Nanocomposite Foams with Good Electrical Conductivity and High Strength by a Supercritical CO2 Foaming Process" Polymers 11, no. 11: 1852. https://doi.org/10.3390/polym11111852
APA StyleChen, Z., Hu, J., Ju, J., & Kuang, T. (2019). Fabrication of Poly(butylene succinate)/Carbon Black Nanocomposite Foams with Good Electrical Conductivity and High Strength by a Supercritical CO2 Foaming Process. Polymers, 11(11), 1852. https://doi.org/10.3390/polym11111852