Dual-Layer Approach toward Self-Healing and Self-Cleaning Polyurethane Thermosets
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
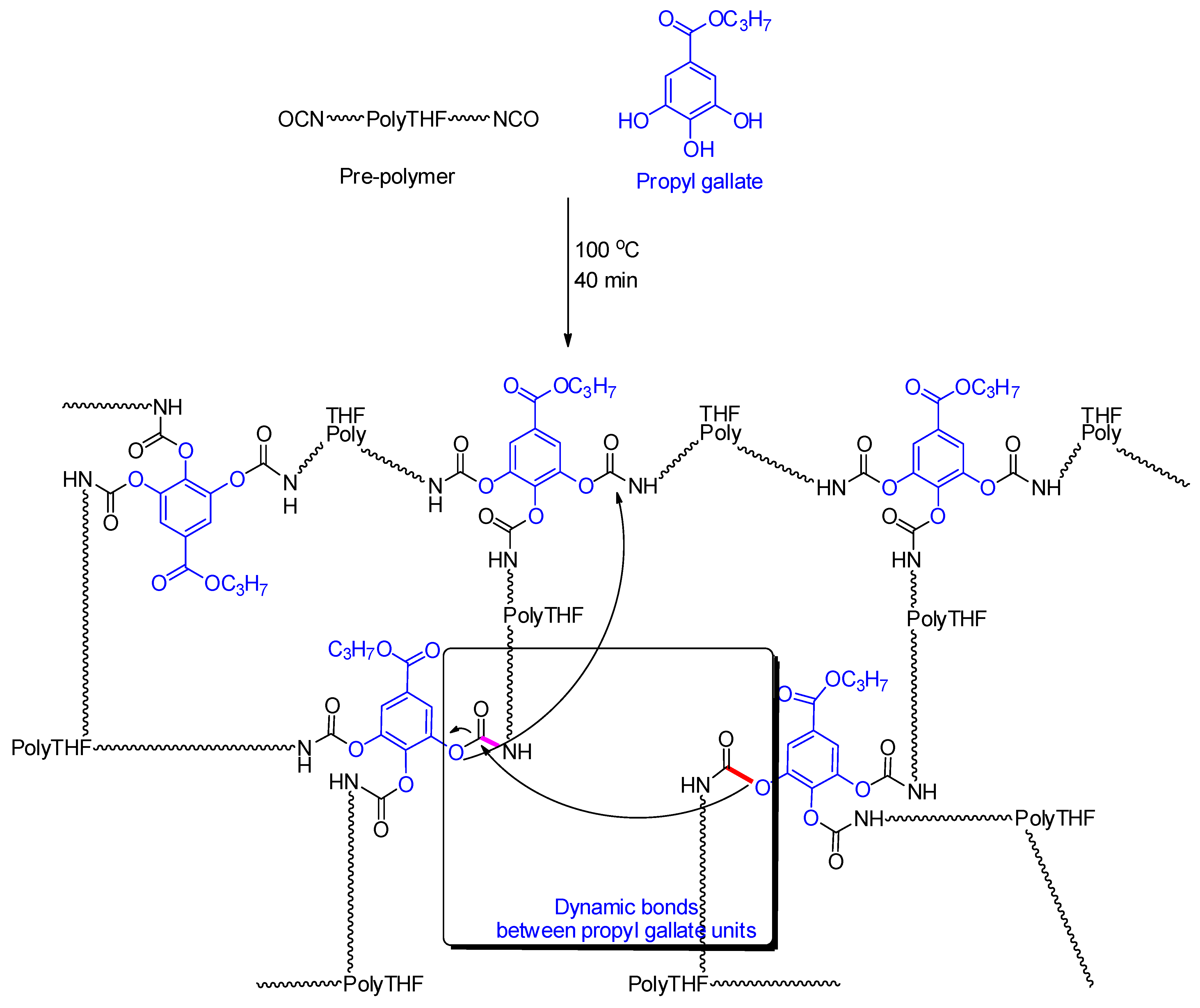
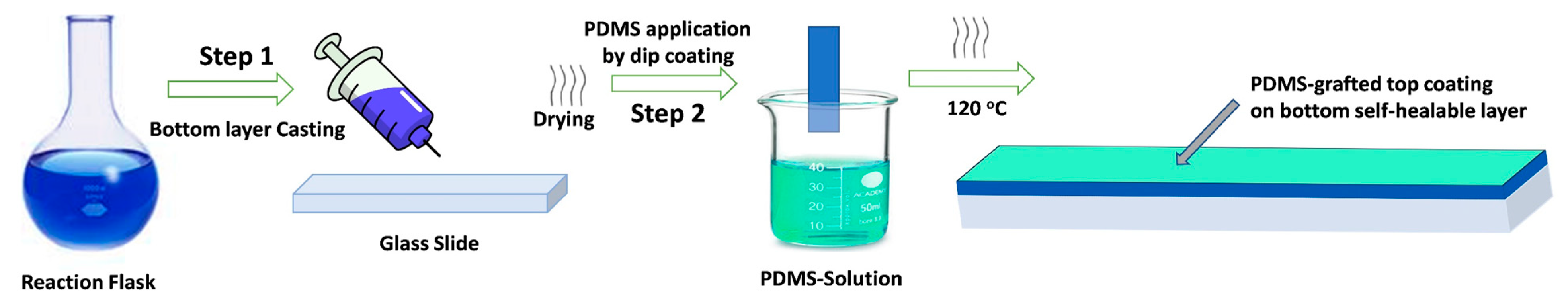
2.2. Preparation of the Self-Healing Self-Cleaning Polyurethane Coatings
2.3. Characterization
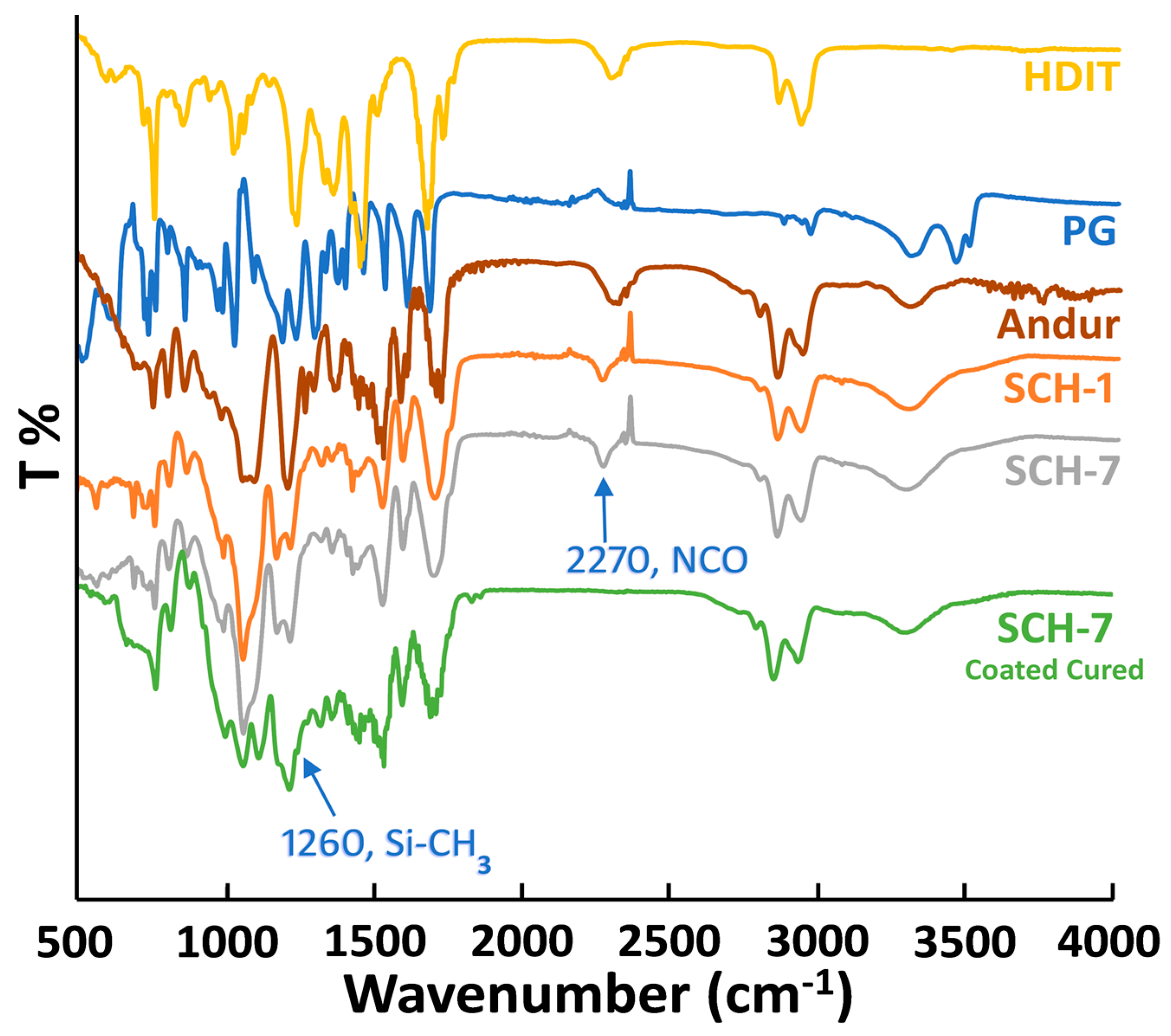
2.3.1. Attenuated Total Reflection-Fourier-Transform Infrared Analysis
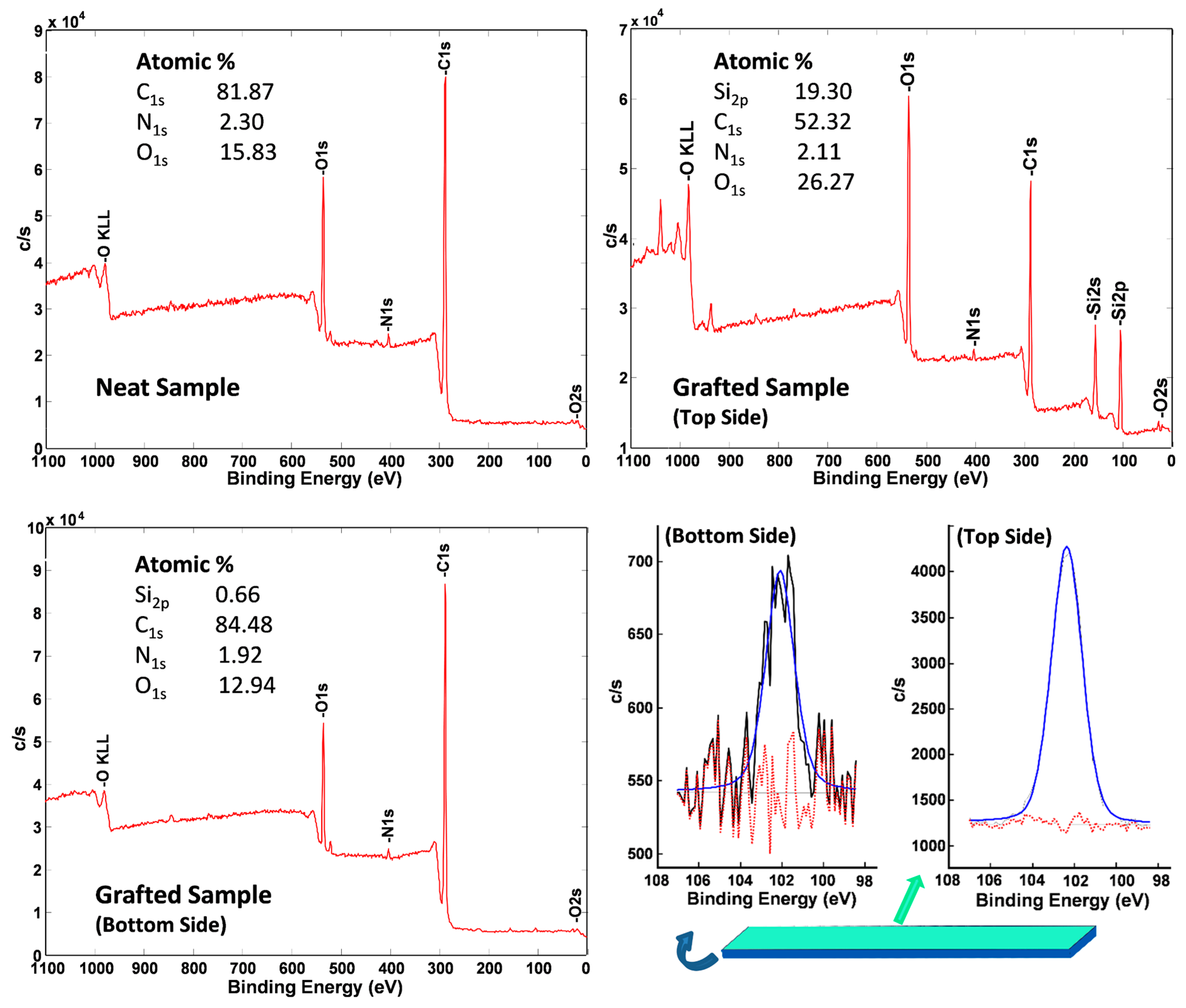
2.3.2. X-ray Photoelectron Spectroscopy (XPS)
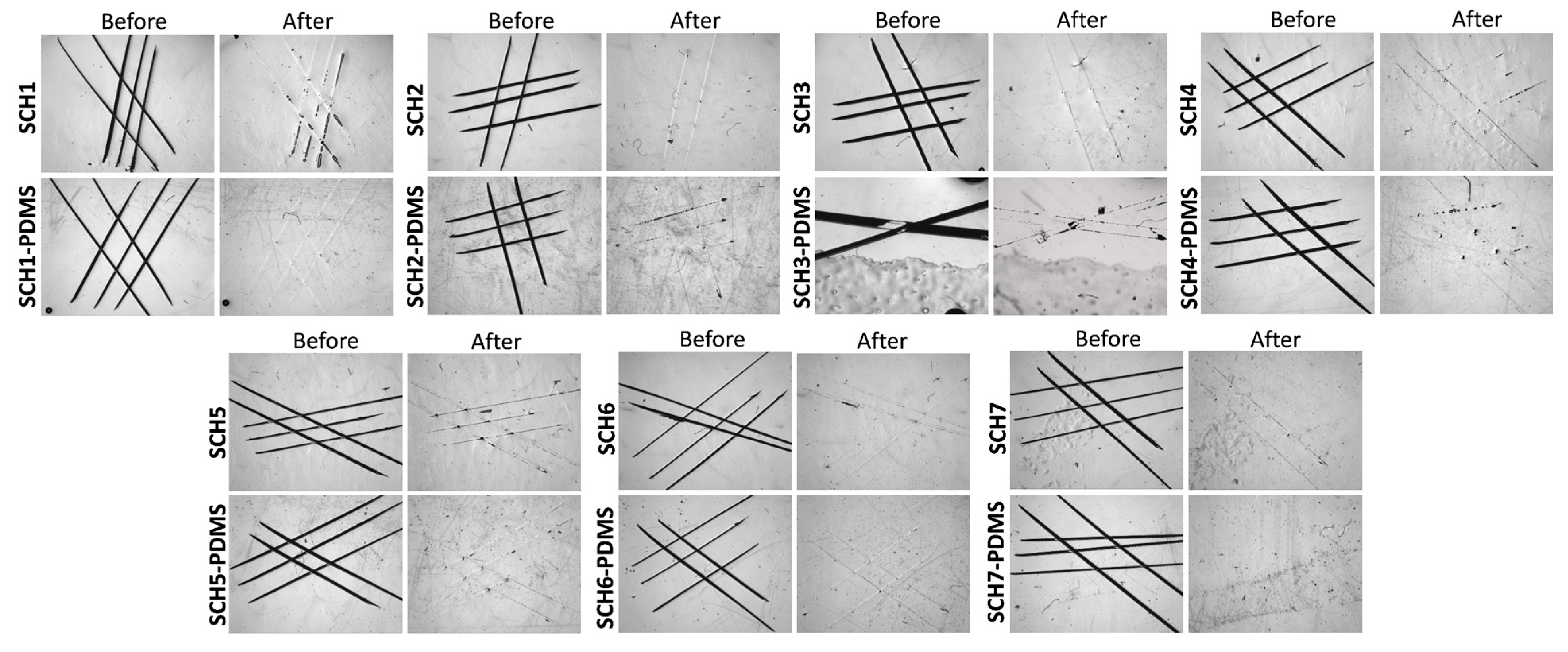
2.3.3. Validation of the Self-Healing Behavior
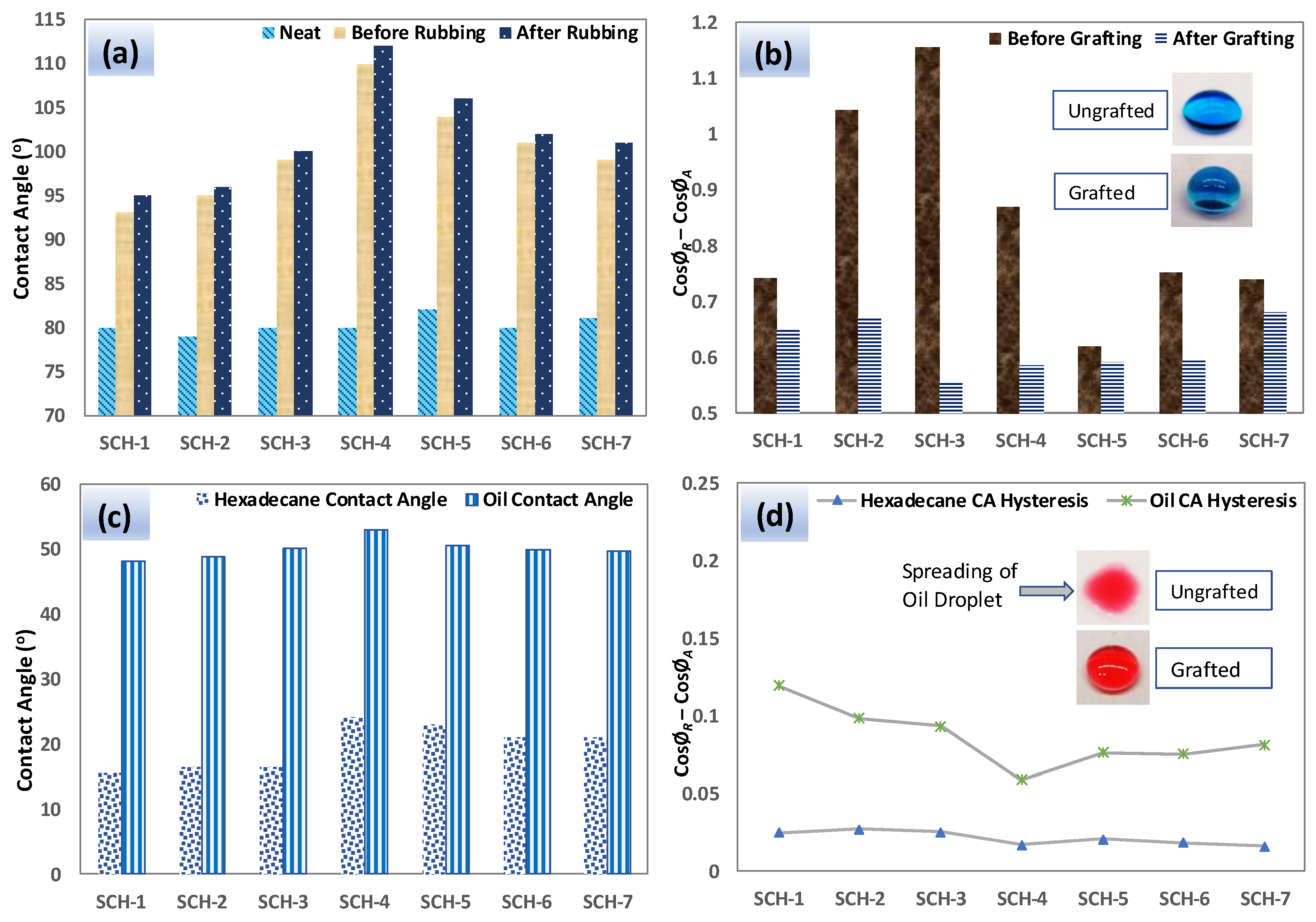
2.3.4. Water Contact Angle and Hysteresis
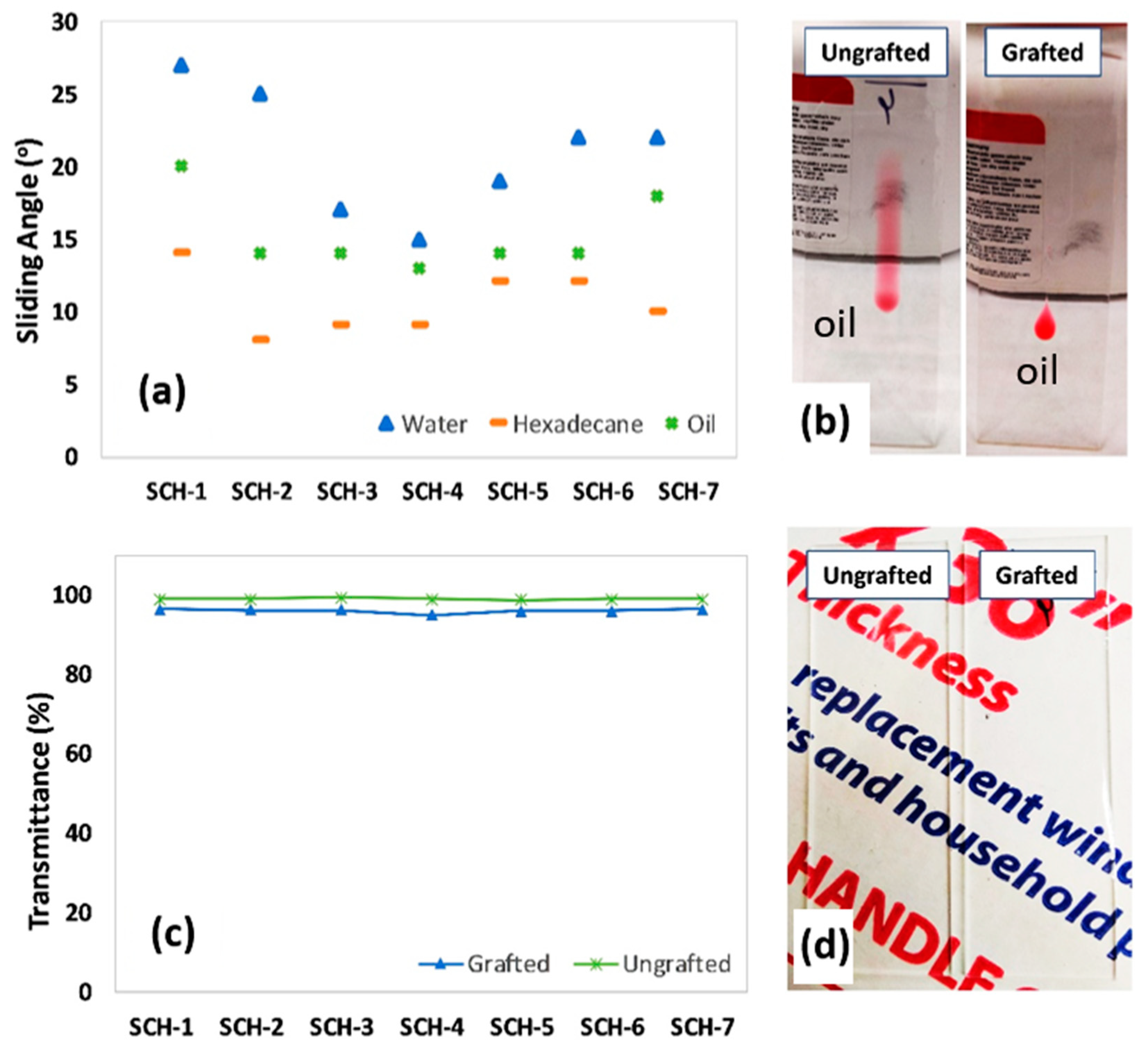
2.3.5. Sliding Contact Angles
2.3.6. Optical Transmittance
2.3.7. Abrasion Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49, 175–220. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S.; Kessler, M. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Bond, I.P.; Trask, R.S.; Williams, H.R. Self-Healing Fiber-Reinforced Polymer Composites. MRS Bull. 2008, 33, 770–774. [Google Scholar] [CrossRef]
- Polenz, I.; Datta, S.S.; Weitz, D.A. Controlling the Morphology of Polyurea Microcapsules Using Microfluidics. Langmuir 2014, 30, 13405–13410. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Darlington, T.; Starr, A.F.; Takahashi, K.; Riendeau, J.; Hahn, H.T. Multiple healing effect of thermally activated self-healing composites based on Diels–Alder reaction. Compos. Sci. Technol. 2010, 70, 2154–2159. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194. [Google Scholar] [CrossRef]
- Deng, G.; Tang, C.; Li, F.; Jiang, H.; Chen, Y. Covalent Cross-Linked Polymer Gels with Reversible Sol−Gel Transition and Self-Healing Properties. Macromolecules 2010, 43, 1191–1194. [Google Scholar] [CrossRef]
- Kuhl, N.; Bode, S.; Bose, R.K.; Vitz, J.; Seifert, A.; Hoeppener, S.; Garcia, S.J.; Spange, S.; Van Der Zwaag, S.; Hager, M.D.; et al. Acylhydrazones as Reversible Covalent Crosslinkers for Self-Healing Polymers. Adv. Funct. Mater. 2015, 25, 3295–3301. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional Hydrogel with Good Structure Integrity, Self-Healing, and Tissue-Adhesive Property Formed by Combining Diels–Alder Click Reaction and Acylhydrazone Bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef]
- Fu, F.; Huang, M.; Zhang, W.; Zhao, Y.; Liu, X. Thermally assisted self-healing behavior of anhydride modified polybenzoxazines based on transesterification. Sci. Rep. 2018, 8, 10325. [Google Scholar] [CrossRef] [PubMed]
- Rekondo, A.; Martin, R.; De Luzuriaga, A.R.; Cabanero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horizons 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Pepels, M.; Filot, I.; Klumperman, B.; Goossens, H.; Goossens, J. Self-healing systems based on disulfide–thiol exchange reactions. Polym. Chem. 2013, 4, 4955. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kamada, J.; Koynov, K.; Mohin, J.; Nicolaÿ, R.; Zhang, Y.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. Self-Healing Polymer Films Based on Thiol–Disulfide Exchange Reactions and Self-Healing Kinetics Measured Using Atomic Force Microscopy. Macromolecules 2011, 45, 142–149. [Google Scholar] [CrossRef]
- Canadell, J.; Goossens, H.; Klumperman, B.; Goossens, J. Self-Healing Materials Based on Disulfide Links. Macromolecules 2011, 44, 2536–2541. [Google Scholar] [CrossRef]
- Yuan, C.; Rong, M.Z.; Zhang, M.Q.; Zhang, Z.P.; Yuan, Y.C. Self-Healing of Polymers via Synchronous Covalent Bond Fission/Radical Recombination. Chem. Mater. 2011, 23, 5076–5081. [Google Scholar] [CrossRef]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z. Self-Healing Multiphase Polymers via Dynamic Metal–Ligand Interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef]
- Rao, Y.-L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.-C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable Self-Healing Polymeric Dielectrics Cross-Linked Through Metal–Ligand Coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A Healable Supramolecular Polymer Blend Based on Aromatic π−π Stacking and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef]
- Burattini, S.; Colquhoun, H.M.; Fox, J.D.; Friedmann, D.; Greenland, B.W.; Harris, P.J.F.; Hayes, W.; Mackay, M.E.; Rowan, S.J. A self-repairing, supramolecular polymer system: healability as a consequence of donor–acceptor π–π stacking interactions. Chem. Commun. 2009, 44, 6717–6719. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.-F.; Jia, X.-Y.; Lai, J.-C.; Sun, Y.; Li, C.-H.; Wu, J.-H.; Cao, Y.; You, X.-Z.; Bao, Z. A Highly Stretchable and Autonomous Self-Healing Polymer Based on Combination of Pt···Pt and π-π Interactions. Macromol. Rapid Commun. 2016, 37, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Del Campo, A. Multivalent H-bonds for self-healing hydrogels. Chem. Commun. 2012, 48, 9302. [Google Scholar] [CrossRef] [PubMed]
- Quéré, D. Wetting and Roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef]
- Bocquet, L.; Lauga, E. A Smooth Future? Nat. Mater. 2011, 10, 334. [Google Scholar] [CrossRef]
- Poetes, R.; Holtzmann, K.; Franze, K.; Steiner, U. Metastable Underwater Superhydrophobicity. Phys. Rev. Lett. 2010, 105, 166104. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically Durable Superhydrophobic Surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef]
- Cheng, D.F.; Urata, C.; Masheder, B.; Hozumi, A. A Physical Approach To Specifically Improve the Mobility of Alkane Liquid Drops. J. Am. Chem. Soc. 2012, 134, 10191–10199. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.F.; Urata, C.; Yagihashi, M.; Hozumi, A. A Statically Oleophilic but Dynamically Oleophobic Smooth Nonperfluorinated Surface. Angew. Chem. Int. Ed. 2012, 51, 2956–2959. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.Y.; McCarthy, T.J. Trialkylsilane Monolayers Covalently Attached to Silicon Surfaces: Wettability Studies Indicating that Molecular Topography Contributes to Contact Angle Hysteresis. Langmuir 1999, 15, 3759–3766. [Google Scholar] [CrossRef]
- Cheng, D.F.; Masheder, B.; Urata, C.; Hozumi, A. Smooth Perfluorinated Surfaces with Different Chemical and Physical Natures: Their Unusual Dynamic Dewetting Behavior toward Polar and Nonpolar Liquids. Langmuir 2013, 29, 11322–11329. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, Y.; Zhang, H.; Zacharia, N.S. Surface Functionalization for a Nontextured Liquid-Infused Surface with Enhanced Lifetime. ACS Appl. Mater. Interfaces 2018, 10, 5892–5901. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Liu, G. Graft-Copolymer-Based Approach to Clear, Durable, and Anti-Smudge Polyurethane Coatings. Angew. Chem. Int. Ed. 2015, 54, 6516–6520. [Google Scholar] [CrossRef]
- Grozea, C.M.; Rabnawaz, M.; Liu, G.; Zhang, G. Coating of silica particles by fluorinated diblock copolymers and use of the resultant silica for superamphiphobic surfaces. Polymer 2015, 64, 153–162. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Liu, G.; Hu, H. Fluorine-Free Anti-Smudge Polyurethane Coatings. Angew. Chemie-Int. Ed. 2015, 54, 12722–12727. [Google Scholar] [CrossRef]
- Li, Z.; Rabnawaz, M. Oil- and Water-Resistant Coatings for Porous Cellulosic Substrates. ACS Appl. Polym. Mater. 2018, 1, 103–111. [Google Scholar] [CrossRef]
- Li, Z.; Rabnawaz, M. Fabrication of Food-Safe Water-Resistant Paper Coatings Using a Melamine Primer and Polysiloxane Outer Layer. ACS Omega 2018, 3, 11909–11916. [Google Scholar] [CrossRef]
- Khan, F.; Khan, A.; Tuhin, M.O.; Rabnawaz, M.; Li, Z.; Naveed, M. A novel dual-layer approach towards omniphobic polyurethane coatings. RSC Adv. 2019, 9, 26703–26711. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Rabnawaz, M.; Li, Z.; Khan, A.; Naveed, M.; Tuhin, M.O. A Simple Design for Durable and Clear Self-Cleaning Coatings. ACS Appl. Polym. Mater. 2019, 1, 2659–2667. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Hu, H.; Liu, G.; Rabnawaz, M. Hydrophilically patterned superhydrophobic cotton fabrics and their use in ink printing. J. Mater. Chem. A 2014, 2, 8094–8102. [Google Scholar] [CrossRef]
- Wyman, I.; Rabnawaz, M.; Wang, Z.; Hu, H.; Liu, G. Synthesis of poly(dimethylsiloxane)-block-poly[3-(triisopropyloxysilyl) propyl methacrylate] and its use in the facile coating of hydrophilically patterned superhydrophobic fabrics. RSC Adv. 2015, 5, 39505–39511. [Google Scholar]
- Hu, H.; Liu, G.; Rabnawaz, M. Anti-Smudge and Anti-Graffiti Compositions. U.S. Patent 10/023,751, 17 July 2018. [Google Scholar]
- Dong, X.; Gao, S.; Huang, J.; Li, S.; Zhu, T.; Cheng, Y.; Zhao, Y.; Chen, Z.; Lai, Y. A self-roughened and biodegradable superhydrophobic coating with UV shielding, solar-induced self-healing and versatile oil–water separation ability. J. Mater. Chem. A 2019, 7, 2122–2128. [Google Scholar] [CrossRef]
- Li, S.; Page, K.; Sathasivam, S.; Heale, F.L.; He, G.; Lu, Y.; Lai, Y.; Chen, G.; Carmalt, C.J.; Parkin, I.P. Efficiently texturing hierarchical superhydrophobic fluoride-free translucent films by AACVD with excellent durability and self-cleaning ability. J. Mater. Chem. A 2018, 6, 17633–17641. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Li, S.; Li, M.; Xu, L.; Ding, H.; Xia, J.; Zhang, M.; Huang, K. A thermal self-healing polyurethane thermoset based on phenolic urethane. Polym. J. 2017, 49, 775–781. [Google Scholar] [CrossRef]
- Jothimuthu, P.; Carroll, A.; Bhagat, A.A.S.; Lin, G.; Mark, J.E.; Papautsky, I. Photodefinable PDMS thin films for microfabrication applications. J. Micromech. Microeng. 2009, 19, 45024. [Google Scholar] [CrossRef]
| Sample Code | Molar Equivalent ratio (PU-prepolymer:Propyl gallate) | PU-prepolymer 75 (mg) | PG (mg) | HDIT (mg) |
|---|---|---|---|---|
| SCH-1 | 1.0: 1.10 | 2000 | 380 | 140 |
| SCH-2 | 1.0: 1.05 | 2000 | 364 | 140 |
| SCH-3 | 1.0: 1.00 | 2000 | 350 | 140 |
| SCH-4 | 1.0: 0.95 | 2000 | 334 | 140 |
| SCH-5 | 1.0: 0.90 | 2000 | 320 | 140 |
| SCH-6 | 1.0: 0.85 | 2000 | 304 | 140 |
| SCH-7 | 1.0: 0.80 | 2000 | 290 | 140 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naveed, M.; Rabnawaz, M.; Khan, A.; Tuhin, M.O. Dual-Layer Approach toward Self-Healing and Self-Cleaning Polyurethane Thermosets. Polymers 2019, 11, 1849. https://doi.org/10.3390/polym11111849
Naveed M, Rabnawaz M, Khan A, Tuhin MO. Dual-Layer Approach toward Self-Healing and Self-Cleaning Polyurethane Thermosets. Polymers. 2019; 11(11):1849. https://doi.org/10.3390/polym11111849
Chicago/Turabian StyleNaveed, Muhammad, Muhammad Rabnawaz, Ajmir Khan, and Mohammad O. Tuhin. 2019. "Dual-Layer Approach toward Self-Healing and Self-Cleaning Polyurethane Thermosets" Polymers 11, no. 11: 1849. https://doi.org/10.3390/polym11111849








