Viologen-Based Electrochromic Materials: From Small Molecules, Polymers and Composites to Their Applications
Abstract
:1. Introduction
2. Viologen-Based Electrochromes
2.1. Small Molecular Viologens
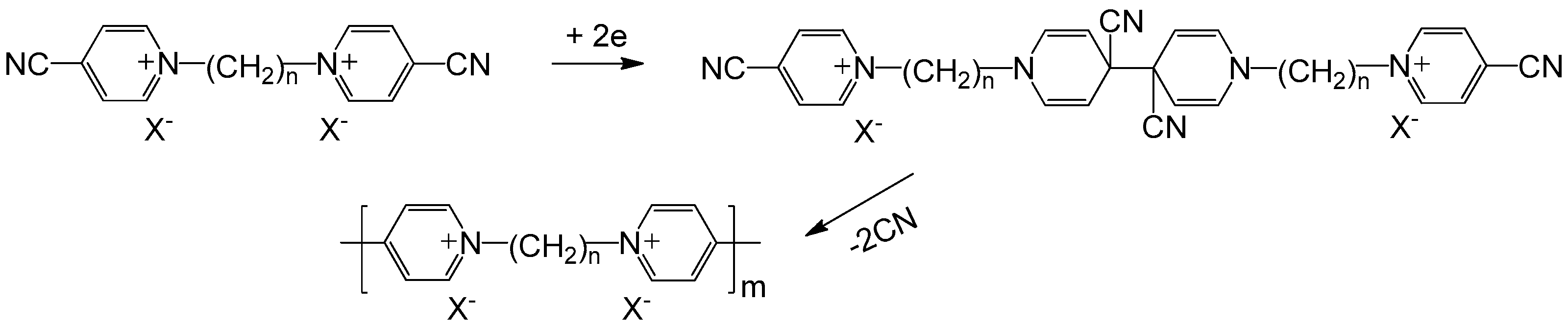
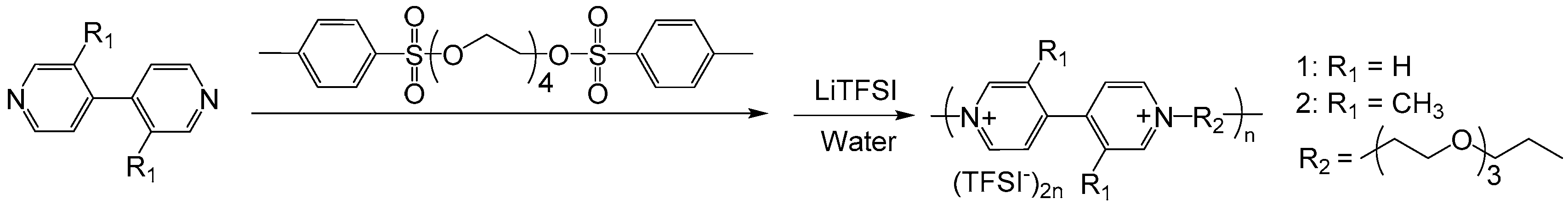
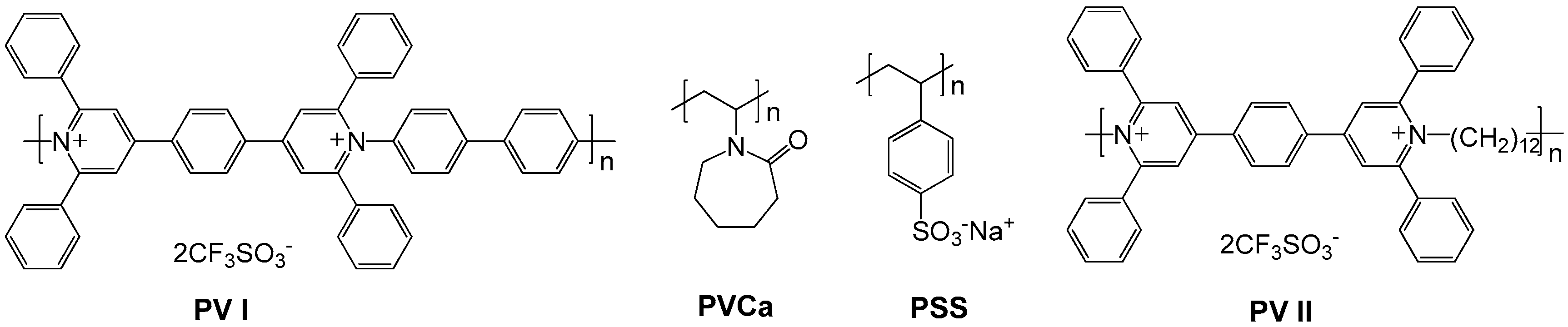
2.2. Polyviologens
2.3. Viologen-Functionalized Conjugated Polymers
2.3.1. Viologen in the Main Chain
2.3.2. Viologen as Pendant
2.4. Viologen Based Composite Materials
2.4.1. Composites with Metal Oxides
2.4.2. Composites with Carbon Nanomaterials
2.4.3. Dual EC Composites via Layer-By-Layer (LBL) Assembly
3. Commercial Applications of Viologen-Based ECDs
4. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Niklasson, G.A.; Granqvist, C.G. Electrochromics for smart windows: Thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 2007, 17, 127–156. [Google Scholar] [CrossRef]
- Svensson, J.; Granqvist, C. Electrochromic coatings for “smart windows”. Sol. Energy Mater. 1985, 12, 391–402. [Google Scholar] [CrossRef]
- Granqvist, C.; Azens, A.; Hjelm, A.; Kullman, L.; Niklasson, G.A.; Rönnow, D.; Mattsson, M.S.; Veszelei, M.; Vaivars, G. Recent advances in electrochromics for smart windows applications. Sol. Energy 1998, 63, 199–216. [Google Scholar] [CrossRef]
- Azens, A.; Granqvist, C. Electrochromic smart windows: Energy efficiency and device aspects. J. Solid State Electrochem. 2003, 7, 64–68. [Google Scholar] [CrossRef]
- Runnerstrom, E.L.; Llordés, A.; Lounis, S.D.; Milliron, D.J. Nanostructured electrochromic smart windows: Traditional materials and nir-selective plasmonic nanocrystals. Chem. Commun. 2014, 50, 10555–10572. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Varaprasad, D.V.; Habibi, H.; McCabe, I.A.; Lynam, N.R. Electrochromic Mirrors and Devices. Google Patents EP0758929A4, 6 August 1997. [Google Scholar]
- Mortimer, R.J. Switching colors with electricity: Electrochromic materials can be used in glare reduction, energy conservation and chameleonic fabrics. Am. Sci. 2013, 101, 38–46. [Google Scholar] [CrossRef]
- Itaya, K.; Shibayama, K.; Akahoshi, H.; Toshima, S. Prussian-blue-modified electrodes: An application for a stable electrochromic display device. J. Appl. Phys. 1982, 53, 804–805. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, R.J. Electrochromic materials. Chem. Soc. Rev. 1997, 26, 147–156. [Google Scholar] [CrossRef]
- Mortimer, R.J. Electrochromic materials. Annu. Rev. Mater. Res. 2011, 41, 241–268. [Google Scholar] [CrossRef]
- Granqvist, C. Electrochromic devices. J. Eur. Ceram. Soc. 2005, 25, 2907–2912. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Wu, W.; Wang, M.; Ma, J.; Cao, Y.; Deng, Y. Electrochromic metal oxides: Recent progress and prospect. Adv. Electron. Mater. 2018, 4, 1800185. [Google Scholar] [CrossRef]
- Mortimer, R.J. Organic electrochromic materials. Electrochim. Acta 1999, 44, 2971–2981. [Google Scholar] [CrossRef]
- Neo, W.T.; Ye, Q.; Chua, S.J.; Xu, J.W. Conjugated polymer-based electrochromics: Materials, device fabrication and application prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Lv, X.J.; Li, W.J.; Ouyang, M.; Zhang, Y.J.; Wright, D.S.; Zhang, C. Polymeric electrochromic materials with donor-acceptor structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- Michaelis, L.; Hill, E.S. The viologen indicators. J. Gen. Physiol. 1933, 16, 859. [Google Scholar] [CrossRef]
- Dmitrieva, E.; Rosenkranz, M.; Alesanco, Y.; Viñuales, A. The reduction mechanism of p-cyanophenylviologen in pva-borax gel polyelectrolyte-based bicolor electrochromic devices. Electrochim. Acta 2018, 292, 81–87. [Google Scholar] [CrossRef]
- Kao, S.-Y.; Lu, H.-C.; Kung, C.-W.; Chen, H.-W.; Chang, T.-H.; Ho, K.-C. Thermally cured dual functional viologen-based all-in-one electrochromic devices with panchromatic modulation. ACS Appl. Mater. Interfaces 2016, 8, 4175–4184. [Google Scholar] [CrossRef]
- Sydam, R.; Ghosh, A.; Deepa, M. Enhanced electrochromic write–erase efficiency of a device with a novel viologen: 1,1′-bis (2-(1h-indol-3-yl) ethyl)-4,4′-bipyridinium diperchlorate. Org. Electron. 2015, 17, 33–43. [Google Scholar] [CrossRef]
- Pan, M.; Ke, Y.; Ma, L.; Zhao, S.; Wu, N.; Xiao, D. Single-layer electrochromic device based on hydroxyalkyl viologens with large contrast and high coloration efficiency. Electrochim. Acta 2018, 266, 395–403. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, W.; Guan, Z.; Jin, B.; Xiao, D. A novel bis (dihydroxypropyl) viologen-based all-in-one electrochromic device with high cycling stability and coloration efficiency. Electrochim. Acta 2019, 298, 533–540. [Google Scholar] [CrossRef]
- Deng, J.; Fu, X.; Wang, G.; Wu, L.; Huang, J. The synthesis and electrochemical study of new electrochromic materials vinylbipyridinium derivatives. Electrochim. Acta 2012, 85, 195–202. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Li, M.; Zheng, J.; Xu, C. Novel electrochromic-fluorescent bi-functional devices based on aromatic viologen derivates. Electrochim. Acta 2018, 285, 415–423. [Google Scholar] [CrossRef]
- Kim, S.-h.; Shim, N.; Lee, H.; Moon, B. Synthesis of a perylenediimide-viologen dyad (pdi-2v) and its electrochromism in a layer-by-layer self-assembled multilayer film with pedot: Pss. J. Mater. Chem. 2012, 22, 13558–13563. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Zhu, C.-R.; Long, J.-F.; Gong, C.-B.; Fu, X.-K. Novel dual-colored 1,1′,1″,1‴-tetrasubstituted (4,4′,4″,4‴-tetrapyridyl) cyclobutane with rapid electrochromic switching. Electrochim. Acta 2018, 259, 986–993. [Google Scholar] [CrossRef]
- Geélinas, B.; Das, D.; Rochefort, D. Air-stable, self-bleaching electrochromic device based on viologen-and ferrocene-containing triflimide redox ionic liquids. ACS Appl. Mater. Interfaces 2017, 9, 28726–28736. [Google Scholar] [CrossRef]
- Watanabe, T.; Honda, K. Measurement of the extinction coefficient of the methyl viologen cation radical and the efficiency of its formation by semiconductor photocatalysis. J. Phys. Chem. 1982, 86, 2617–2619. [Google Scholar] [CrossRef]
- Monk, P.M.S. The effect of ferrocyanide on the performance of heptyl viologen-based electrochromic display devices. J. Electroanal. Chem. 1997, 432, 175–179. [Google Scholar] [CrossRef]
- Wardman, P. The reduction potential of benzyl viologen: An important reference compound for oxidant/radical redox couples. Free Radic. Res. Commun. 1991, 14, 57–67. [Google Scholar] [CrossRef]
- Lu, H.-C.; Kao, S.-Y.; Chang, T.-H.; Kung, C.-W.; Ho, K.-C. An electrochromic device based on prussian blue, self-immobilized vinyl benzyl viologen, and ferrocene. Sol. Energy Mater. Sol. Cells 2016, 147, 75–84. [Google Scholar] [CrossRef]
- Chang, T.-H.; Lu, H.-C.; Lee, M.-H.; Kao, S.-Y.; Ho, K.-C. Multi-color electrochromic devices based on phenyl and heptyl viologens immobilized with UV–cured polymer electrolyte. Sol. Energy Mater. Sol. Cells 2018, 177, 75–81. [Google Scholar] [CrossRef]
- Ho, K.-C.; Lu, H.-C.; Yu, H.-F. Viologens-based electrochromic materials and devices. In Electrochromic Smart Materials—Fabrication and Applications; Xu, J., Chua, M.H., Shah, K.W., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 372–405. [Google Scholar]
- Monk, P.M. Comment on: “Dimer formation of viologen derivatives and their electrochromic properties”. Dyes Pigments 1998, 39, 125–128. [Google Scholar] [CrossRef]
- Moon, H.C.; Kim, C.-H.; Lodge, T.P.; Frisbie, C.D. Multicolored, low-power, flexible electrochromic devices based on ion gels. ACS Appl. Mater. Interfaces 2016, 8, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.N.; Kolesar, J.M.; Hall, S.R.; Saleh, N.-A.; Jones, D.S.; Walter, M.G. Thiazolothiazole fluorophores exhibiting strong fluorescence and viologen-like reversible electrochromism. J. Am. Chem. Soc. 2017, 139, 8467–8473. [Google Scholar] [CrossRef] [PubMed]
- Cospito, S.; Beneduci, A.; Veltri, L.; Salamonczyk, M.; Chidichimo, G. Mesomorphism and electrochemistry of thienoviologen liquid crystals. Phys. Chem. Chem. Phys. 2015, 17, 17670–17678. [Google Scholar] [CrossRef]
- Stolar, M.; Borau-Garcia, J.; Toonen, M.; Baumgartner, T. Synthesis and tunability of highly electron-accepting, n-benzylated “phosphaviologens”. J. Am. Chem. Soc. 2015, 137, 3366–3371. [Google Scholar] [CrossRef]
- Durben, S.; Baumgartner, T. 3,7-diazadibenzophosphole oxide: A phosphorus-bridged viologen analogue with significantly lowered reduction threshold. Angew. Chem. Int. Ed. 2011, 50, 7948–7952. [Google Scholar] [CrossRef]
- Li, G.; Xu, L.; Zhang, W.; Zhou, K.; Ding, Y.; Liu, F.; He, X.; He, G. Narrow-bandgap chalcogenoviologens for electrochromism and visible-light-driven hydrogen evolution. Angew. Chem. Int. Ed. 2018, 57, 4897–4901. [Google Scholar] [CrossRef]
- Zhidkova, M.N.; Aysina, K.E.; Kotov, V.Y.; Ivanov, V.K.; Nelyubina, Y.V.; Ananyev, I.V.; Laurinavichyute, V.K. Synthesis and electropolymerization of bis (4-cyano-1-pyridino) alkanes: Effect of co-and counter-ions. Electrochim. Acta 2016, 219, 673–681. [Google Scholar] [CrossRef]
- Saika, T.; Iyoda, T.; Shimidzu, T. Electropolymerization of bis (4-cyano-1-pyridinio) derivatives for the preparation of polyviologen films on electrodes. Bull. Chem. Soc. Jpn. 1993, 66, 2054–2060. [Google Scholar] [CrossRef]
- Kamata, K.; Suzuki, T.; Kawai, T.; Iyoda, T. Voltammetric anion recognition by a highly cross-linked polyviologen film. J. Electroanal. Chem. 1999, 473, 145–155. [Google Scholar] [CrossRef]
- Kamata, K.; Kawai, T.; Iyoda, T. Anion-controlled redox process in a cross-linked polyviologen film toward electrochemical anion recognition. Langmuir 2001, 17, 155–163. [Google Scholar] [CrossRef]
- Hsu, Y.; Ho, K. The anionic effect on the intercalation and spectral properties of poly (butyl viologen) films. J. New Mater. Electrochem. Syst. 2005, 8, 49–57. [Google Scholar]
- DeLongchamp, D.M.; Kastantin, M.; Hammond, P.T. High-contrast electrochromism from layer-by-layer polymer films. Chem. Mater. 2003, 15, 1575–1586. [Google Scholar] [CrossRef]
- Kuo, T.-H.; Hsu, C.-Y.; Lee, K.-M.; Ho, K.-C. All-solid-state electrochromic device based on poly (butyl viologen), prussian blue, and succinonitrile. Sol. Energy Mater. Sol. Cells 2009, 93, 1755–1760. [Google Scholar] [CrossRef]
- Creager, S.E.; Fox, M.A. Solute permeation in thin adsorbed layers of poly-(p-xylyl-viologen): Solvation, counterion, and electron transfer kinetic effects. J. Electrochem. Soc. 1990, 137, 2151–2157. [Google Scholar] [CrossRef]
- Janda, P.; Weber, J.; Kavan, L. Modification of glassy carbon electrodes by a new type of polymeric viologen. J. Electroanal. Chem. Interfacial Electrochem. 1984, 180, 109–120. [Google Scholar] [CrossRef]
- Adeogun, M.J.; Hay, J.N. Synthesis of mesoporous amorphous silica via silica-polyviologen hybrids prepared by the sol−gel route. Chem. Mater. 2000, 12, 767–775. [Google Scholar] [CrossRef]
- Das, G.; Skorjanc, T.; Prakasam, T.; Nuryyeva, S.; Olsen, J.-C.; Trabolsi, A. Microwave-assisted synthesis of a viologen-based covalent organic polymer with redox-tunable polarity for dye adsorption. RSC Adv. 2017, 7, 3594–3598. [Google Scholar] [CrossRef] [Green Version]
- Adeogun, M.; Fairclough, J.; Hay, J.; Ryan, A. Structure control in sol-gel silica synthesis using ionene polymers—Evidence from x-ray scattering. J. Sol-Gel Sci. Technol. 1998, 13, 27–30. [Google Scholar]
- Adeogun, M.; Hay, J. Structure control in sol-gel silica synthesis using ionene polymers. 2: Evidence from spectroscopic analysis. J. Sol-Gel Sci. Technol. 2001, 20, 119–128. [Google Scholar]
- Sato, K.; Mizukami, R.; Mizuma, T.; Nishide, H.; Oyaizu, K. Synthesis of dimethyl-substituted polyviologen and control of charge transport in electrodes for high-resolution electrochromic displays. Polymers 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-P.; Ding, G.-J.; Wang, Y.-C.; Yang, Y.-I. Preparation of uv curing crosslinked polyviologen film and its photochromic and electrochromic performances. Appl. Surf. Sci. 2011, 258, 1184–1191. [Google Scholar] [CrossRef]
- Harris, F.W.; Chuang, K.C.; Huang, S.A.X.; Janimak, J.J.; Cheng, S.Z. Aromatic poly (pyridinium salt) s: Synthesis and structure of organo-soluble, rigid-rod poly (pyridinium tetrafluoroborate) s. Polymer 1994, 35, 4940–4948. [Google Scholar] [CrossRef]
- Huang, S.A.X.; Chuang, K.C.; Cheng, S.Z.; Harris, F.W. Aromatic poly (pyridinium salt) s part 2. Synthesis and properties of organo-soluble, rigid-rod poly (pyridinium triflate) s. Polymer 2000, 41, 5001–5009. [Google Scholar] [CrossRef]
- Keshtov, M.; Udum, Y.A.; Toppare, L.; Kochurov, V.; Khokhlov, A. Synthesis of aromatic poly (pyridinium salt) s and their electrochromic properties. Mater. Chem. Phys. 2013, 139, 936–943. [Google Scholar] [CrossRef]
- Petrov, M.M.; Makhaeva, E.E.; Keshtov, M.L.; Khokhlov, A.R. The effect of poly (n-vinylcaprolactam) on the electrochromic properties of a poly (pyridinium triflate). Electrochim. Acta 2014, 122, 159–165. [Google Scholar] [CrossRef]
- Petrov, M.M.; Pichugov, R.D.; Keshtov, M.L.; Makhaeva, E.E. Electrochromism of interpolyelectrolyte poly (pyridinium)–poly (styrene sulfonate) complexes. Org. Electron. 2016, 34, 1–11. [Google Scholar] [CrossRef]
- Frolov, D.; Petrov, M.; Makhaeva, E.; Keshtov, M.; Khokhlov, A. Electrochromic behavior of poly (pyridinium triflates) films: Electrolyte ions influence. Synth. Met. 2018, 239, 29–35. [Google Scholar] [CrossRef]
- Frolov, D.G.; Makhaeva, E.E.; Keshtov, M.L. Electrochromic behavior of films and «smart windows» prototypes based on π-conjugated and non–conjugated poly (pyridinium triflate) s. Synth. Met. 2019, 248, 14–19. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in pi-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Bendikov, M.; Chand, S. Poly(3,4-ethylenedioxyselenophene) and its derivatives: Novel organic electronic materials. Acc. Chem. Res. 2014, 47, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Barrett, M.; Duane, B.; Gu, J.; Zenhausern, F. Materials and processing of polymer-based electrochromic devices. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2018, 228, 167–174. [Google Scholar] [CrossRef]
- Beverina, L.; Pagani, G.; Sassi, M. Multichromophoric electrochromic polymers: Colour tuning of conjugated polymers through the side chain functionalization approach. Chem. Commun. 2014, 50, 5413–5430. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, B.; Damlin, P.; Ääritalo, T.; Kvarnström, C. Electrosynthesis of viologen cross-linked polythiophene in ionic liquid and its electrochromic properties. Electrochim. Acta 2014, 133, 268–274. [Google Scholar] [CrossRef]
- Gadgil, B.; Damlin, P.; Dmitrieva, E.; Ääritalo, T.; Kvarnström, C. Esr/UV–vis-nir spectroelectrochemical study and electrochromic contrast enhancement of a polythiophene derivative bearing a pendant viologen. RSC Adv. 2015, 5, 42242–42249. [Google Scholar] [CrossRef]
- Ko, H.C.; Kang, M.; Moon, B.; Lee, H. Enhancement of electrochromic contrast of poly (3,4-ethylenedioxythiophene) by incorporating a pendant viologen. Adv. Mater. 2004, 16, 1712–1716. [Google Scholar] [CrossRef]
- Yen, H.J.; Tsai, C.L.; Chen, S.H.; Liou, G.S. Electrochromism and nonvolatile memory device derived from triphenylamine-based polyimides with pendant viologen units. Macromol. Rapid Commun. 2017, 38, 1600715. [Google Scholar] [CrossRef]
- Berridge, R.; Wright, S.P.; Skabara, P.J.; Dyer, A.; Steckler, T.; Argun, A.A.; Reynolds, J.R.; Ross, W.H.; Clegg, W. Electrochromic properties of a fast switching, dual colour polythiophene bearing non-planar dithiinoquinoxaline units. J. Mater. Chem. 2007, 17, 225–231. [Google Scholar] [CrossRef]
- Nicho, M.E.; Hu, H.L.; Lopez-Mata, C.; Escalante, J. Synthesis of derivatives of polythiophene and their application in an electrochromic device. Sol. Energy Mater. Sol. Cells 2004, 82, 105–118. [Google Scholar] [CrossRef]
- Alkan, S.; Cutler, C.A.; Reynolds, J.R. High quality electrochromic polythiophenes via bf3 center dot et2o electropolymerization. Adv. Funct. Mater. 2003, 13, 331–336. [Google Scholar] [CrossRef]
- Pang, Y.; Li, X.; Ding, H.; Shi, G.; Jin, L. Electropolymerization of high quality electrochromic poly (3-alkyl-thiophene) s via a room temperature ionic liquid. Electrochim. Acta 2007, 52, 6172–6177. [Google Scholar] [CrossRef]
- Varis, S.; Ak, M.; Tanyeli, C.; Akhmedov, I.M.; Toppare, L. A soluble and multichromic conducting polythiophene derivative. Eur. Polym. J. 2006, 42, 2352–2360. [Google Scholar] [CrossRef]
- Gadgil, B.; Damlin, P.; Ääritalo, T.; Kankare, J.; Kvarnström, C. Electrosynthesis and characterization of viologen cross linked thiophene copolymer. Electrochim. Acta 2013, 97, 378–385. [Google Scholar] [CrossRef]
- Krompiec, M.; Grudzka, I.; Filapek, M.; Krompiec, S.; Łapkowski, M.; Kania, M.; Danikiewicz, W. An electrochromic diquat-quaterthiophene alternating copolymer: A polythiophene with a viologen-like moiety in the main chain. Electrochim. Acta 2011, 56, 8108–8114. [Google Scholar] [CrossRef]
- Ko, H.C.; Park, S.-a.; Paik, W.-k.; Lee, H. Electrochemistry and electrochromism of the polythiophene derivative with viologen pendant. Synth. Met. 2002, 132, 15–20. [Google Scholar] [CrossRef]
- Lim, J.Y.; Ko, H.C.; Lee, H. Single-and dual-type electrochromic devices based on polycarbazole derivative bearing pendent viologen. Synth. Met. 2006, 156, 695–698. [Google Scholar] [CrossRef]
- Ko, H.C.; Yom, J.; Moon, B.; Lee, H. Electrochemistry and electrochromism of a poly (cyclopentadithiophene) derivative with a viologen pendant. Electrochim. Acta 2003, 48, 4127–4135. [Google Scholar]
- Ko, H.C.; Kim, S.; Lee, H.; Moon, B. Multicolored electrochromism of a poly {1,4-bis [2-(3,4-ethylenedioxy) thienyl] benzene} derivative bearing viologen functional groups. Adv. Funct. Mater. 2005, 15, 905–909. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Makishi, S. Synthesis and chemical properties of electrochromic π-conjugated polyphenylenes with pendant viologen-tcnq salts. J. Appl. Polym. Sci. 2013, 129, 397–403. [Google Scholar] [CrossRef]
- Alesanco, Y.; Palenzuela, J.; Tena-Zaera, R.; Cabanero, G.; Grande, H.; Herbig, B.; Schmitt, A.; Schott, M.; Posset, U.; Guerfi, A. Plastic electrochromic devices based on viologen-modified tio2 films prepared at low temperature. Sol. Energy Mater. Sol. Cells 2016, 157, 624–635. [Google Scholar] [CrossRef]
- Alesanco, Y.; Viñuales, A.; Ugalde, J.; Azaceta, E.; Cabañero, G.; Rodriguez, J.; Tena-Zaera, R. Consecutive anchoring of symmetric viologens: Electrochromic devices providing colorless to neutral-color switching. Sol. Energy Mater. Sol. Cells 2018, 177, 110–119. [Google Scholar] [CrossRef]
- Bhandari, S.; Deepa, M.; Srivastava, A.; Lakshmikumar, S. Electrochromic response, structure optimization and ion transfer behavior in viologen adsorbed titanium oxide films. Solid State Ion. 2009, 180, 41–49. [Google Scholar] [CrossRef]
- Choi, S.Y.; Mamak, M.; Coombs, N.; Chopra, N.; Ozin, G.A. Electrochromic performance of viologen-modified periodic mesoporous nanocrystalline anatase electrodes. Nano Lett. 2004, 4, 1231–1235. [Google Scholar] [CrossRef]
- Cinnsealach, R.; Boschloo, G.; Rao, S.N.; Fitzmaurice, D. Electrochromic windows based on viologen-modified nanostructured tio2 films. Sol. Energy Mater. Sol. Cells 1998, 55, 215–223. [Google Scholar] [CrossRef]
- Edwards, M. Passive-matrix addressing of viologen–ti o 2 displays. Appl. Phys. Lett. 2005, 86, 073507. [Google Scholar] [CrossRef]
- Kim, H.J.; Seo, J.K.; Kim, Y.J.; Jeong, H.K.; Lim, G.I.; Choi, Y.S.; Lee, W.I. Formation of ultrafast-switching viologen-anchored tio2 electrochromic device by introducing sb-doped sno2 nanoparticles. Sol. Energy Mater. Sol. Cells 2009, 93, 2108–2112. [Google Scholar] [CrossRef]
- Kim, H.N.; Cho, S.M.; Ah, C.S.; Song, J.; Ryu, H.; Kim, Y.H.; Kim, T.-Y. Electrochromic mirror using viologen-anchored nanoparticles. Mater. Res. Bull. 2016, 82, 16–21. [Google Scholar] [CrossRef]
- Periyat, P.; Leyland, N.; McCormack, D.E.; Colreavy, J.; Corr, D.; Pillai, S.C. Rapid microwave synthesis of mesoporous tio 2 for electrochromic displays. J. Mater. Chem. 2010, 20, 3650–3655. [Google Scholar] [CrossRef]
- Rong, Y.; Kim, S.; Su, F.; Myers, D.; Taya, M. New effective process to fabricate fast switching and high contrast electrochromic device based on viologen and prussian blue/antimony tin oxide nano-composites with dark colored state. Electrochim. Acta 2011, 56, 6230–6236. [Google Scholar] [CrossRef]
- Viñuales, A.; Herbig, B.; Alesanco, Y.; Palenzuela, J.; Rodriguez, J.; Schmitt, A.; Posset, U. One-step preparation of viologen-tio2 nanoparticles via a hydrothermally assisted sol–gel process for use in electrochromic films and devices. Part. Part. Syst. Charact. 2018, 35, 1800142. [Google Scholar] [CrossRef]
- Vlachopoulos, N.; Nissfolk, J.; Möller, M.; Briançon, A.; Corr, D.; Grave, C.; Leyland, N.; Mesmer, R.; Pichot, F.; Ryan, M. Electrochemical aspects of display technology based on nanostructured titanium dioxide with attached viologen chromophores. Electrochim. Acta 2008, 53, 4065–4071. [Google Scholar] [CrossRef]
- Weng, D.; Shi, Y.; Zheng, J.; Xu, C. High performance black-to-transmissive electrochromic device with panchromatic absorption based on tio2-supported viologen and triphenylamine derivatives. Org. Electron. 2016, 34, 139–145. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J. Fast switching electrochromic display using a viologen-modified zno nanowire array electrode. Nano Lett. 2008, 8, 1884–1889. [Google Scholar] [CrossRef]
- Hu, A.Z.; Wu, F.; Liu, J.P.; Jiang, J.A.; Ding, R.M.; Li, X.; Cheng, C.X.; Zhu, Z.H.; Huang, X.T. Density- and adhesion-controlled zno nanorod arrays on the ito flexible substrates and their electrochromic performance. J. Alloy. Compd. 2010, 507, 261–266. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, Y.; Wu, J.-G.; Guo, L.-F.; Ye, M.; Shao, Y.-H.; Wang, R.; Zhao, C.-E.; Wei, A. Methyl-viologen modified zno nanotubes for use in electrochromic devices. RSC Adv. 2016, 6, 72037–72043. [Google Scholar] [CrossRef]
- Nakajima, R.; Yamada, Y.; Komatsu, T.; Murashiro, K.; Saji, T.; Hoshino, K. Electrochromic properties of ito nanoparticles/viologen composite film electrodes. RSC Adv. 2012, 2, 4377–4381. [Google Scholar] [CrossRef]
- Hoshino, K.; Nakajima, R.; Okuma, M. Improved electrochromic performance of viologen at an ito-nanoparticle film electrode. Appl. Surf. Sci. 2014, 313, 569–576. [Google Scholar] [CrossRef]
- Hoshino, K.; Okuma, M.; Terashima, K. Electrochromic properties of metal oxide nanoparticles/viologen composite film electrodes. J. Phys. Chem. C 2018, 122, 22577–22587. [Google Scholar] [CrossRef]
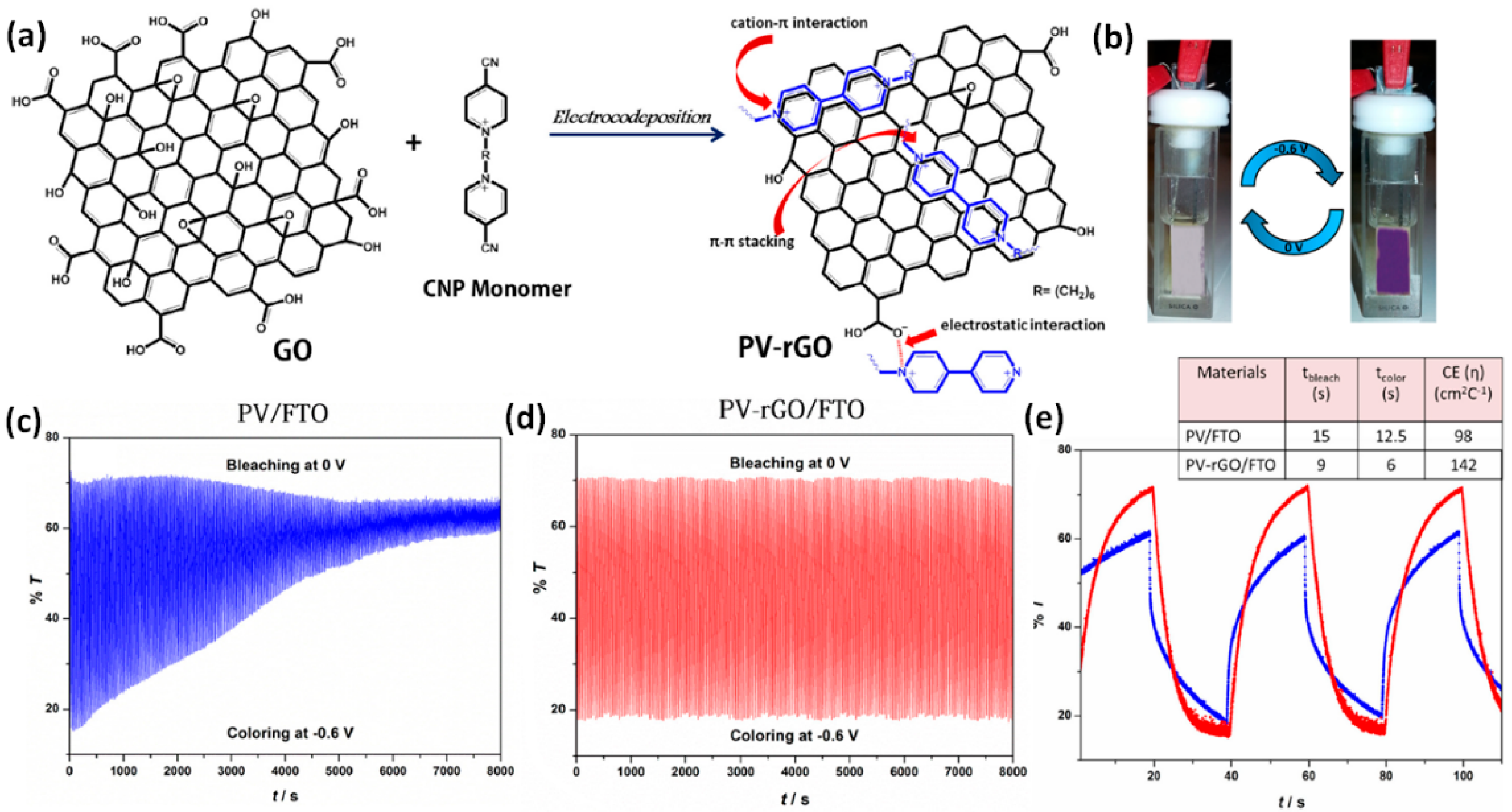
- Gadgil, B.; Damlin, P.; Heinonen, M.; Kvarnström, C. A facile one step electrostatically driven electrocodeposition of polyviologen–reduced graphene oxide nanocomposite films for enhanced electrochromic performance. Carbon 2015, 89, 53–62. [Google Scholar] [CrossRef]
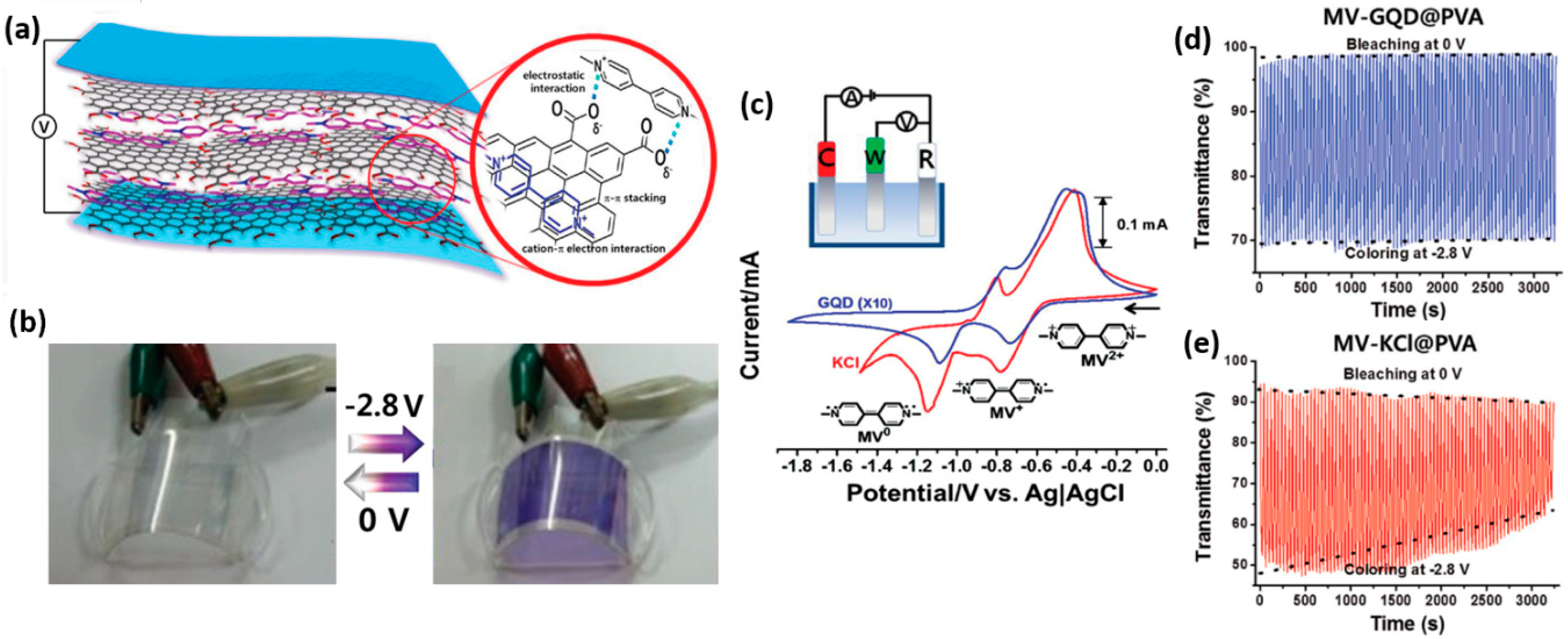
- Hwang, E.; Seo, S.; Bak, S.; Lee, H.; Min, M.; Lee, H. An electrolyte-free flexible electrochromic device using electrostatically strong graphene quantum dot–viologen nanocomposites. Adv. Mater. 2014, 26, 5129–5136. [Google Scholar] [CrossRef] [PubMed]
- Sydam, R.; Deepa, M.; Shivaprasad, S.; Srivastava, A. A wo3–poly (butyl viologen) layer-by-layer film/ruthenium purple film based electrochromic device switching by 1 volt application. Sol. Energy Mater. Sol. Cells 2015, 132, 148–161. [Google Scholar] [CrossRef]
- Pichugov, R.D.; Malyshkina, I.A.; Makhaeva, E.E. Electrochromic behavior and electrical percolation threshold of carbon nanotube/poly (pyridinium triflate) composites. J. Electroanal. Chem. 2018, 823, 601–609. [Google Scholar] [CrossRef]
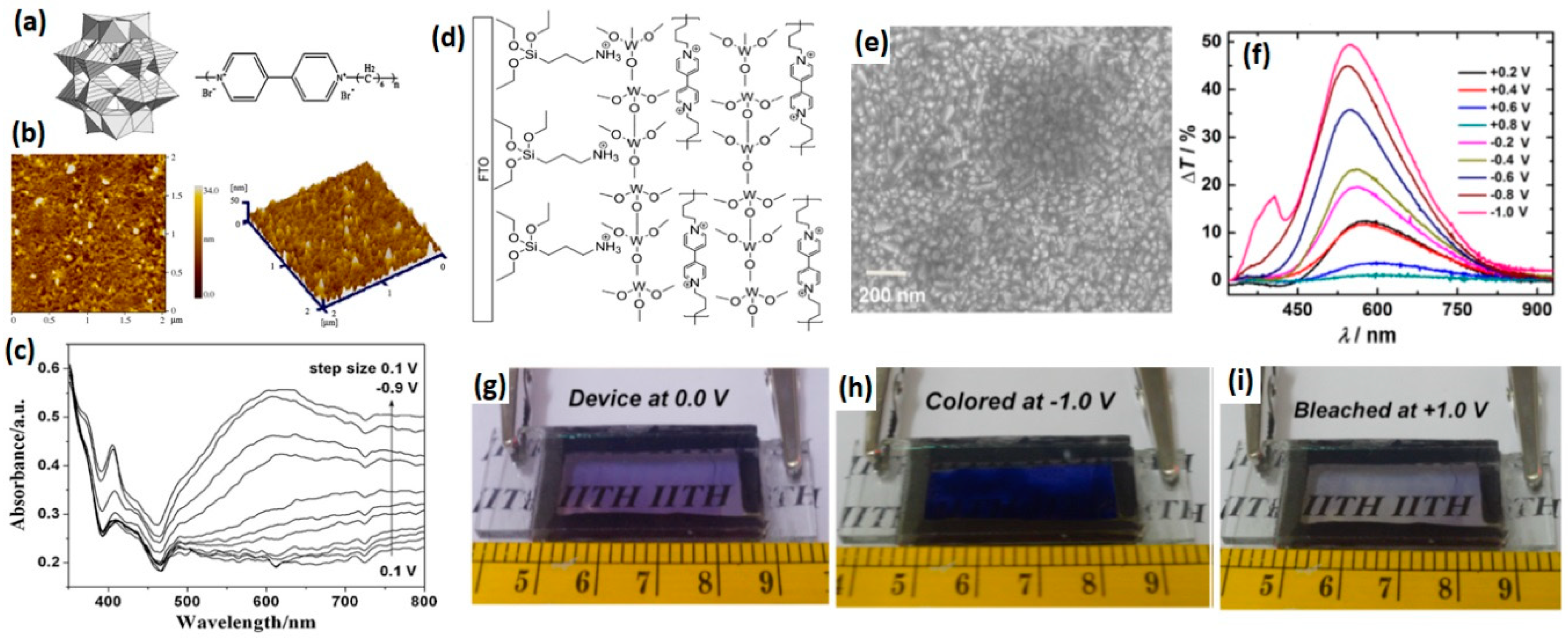
- Xu, B.; Xu, L.; Gao, G.; Yang, Y.; Guo, W.; Liu, S.; Sun, Z. Multicolor electrochromic and ph-sensitive nanocomposite thin film based on polyoxometalates and polyviologen. Electrochim. Acta 2009, 54, 2246–2252. [Google Scholar] [CrossRef]
- Jain, V.; Khiterer, M.; Montazami, R.; Yochum, H.M.; Shea, K.J.; Heflin, J.R. High-contrast solid-state electrochromic devices of viologen-bridged polysilsesquioxane nanoparticles fabricated by layer-by-layer assembly. ACS Appl. Mater. Interfaces 2009, 1, 83–89. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Das Sarma, S.; Adam, S.; Hwang, E.H.; Rossi, E. Electronic transport in two-dimensional graphene. Rev. Mod. Phys. 2011, 83, 407–470. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Lukáacs, Z.; Gadgil, B.; Damlin, P.; Janáky, C.; Kvarnström, C. Electrochemical deposition of polyviologen-reduced graphene oxide nanocomposite thin films. Electrochim. Acta 2017, 231, 279–286. [Google Scholar] [CrossRef]
- Morita, M. Effects of solvent and electrolyte on the electrochromic behavior and degradation of chemically prepared polyaniline-poly (vinyl alcohol) composite films. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 231–242. [Google Scholar] [CrossRef]
- Kavanagh, A.; Fraser, K.J.; Byrne, R.; Diamond, D. An electrochromic ionic liquid: Design, characterization, and performance in a solid-state platform. ACS Appl. Mater. Interfaces 2012, 5, 55–62. [Google Scholar] [CrossRef] [PubMed]
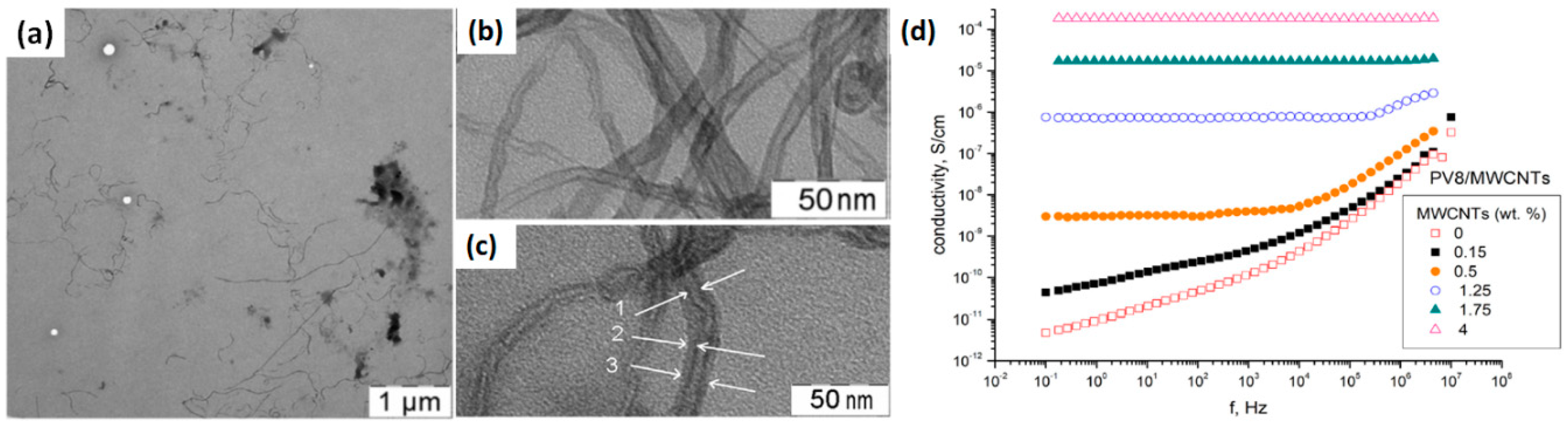
- Pichugov, R.D.; Makhaeva, E.E.; Keshtov, M.L. Fast switching electrochromic nanocomposite based on poly (pyridinium salt) and multiwalled carbon nanotubes. Electrochim. Acta 2018, 260, 139–149. [Google Scholar] [CrossRef]
- Huguenin, F.; Ferreira, M.; Zucolotto, V.; Nart, F.C.; Torresi, R.M.; Oliveira, O.N. Molecular-level manipulation of v2o5/polyaniline layer-by-layer films to control electrochromogenic and electrochemical properties. Chem. Mater. 2004, 16, 2293–2299. [Google Scholar] [CrossRef]
- Jain, V.; Sahoo, R.; Jinschek, J.R.; Montazami, R.; Yochum, H.M.; Beyer, F.L.; Kumar, A.; Heflin, J.R. High contrast solid state electrochromic devices based on ruthenium purple nanocomposites fabricated by layer-by-layer assembly. Chem. Commun. 2008, 3663–3665. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Yoo, S.J.; Sung, Y.-E.; Zentel, R. High contrast ratio and rapid switching organic polymeric electrochromic thin films based on triarylamine derivatives from layer-by-layer assembly. Chem. Mater. 2006, 18, 5823–5825. [Google Scholar] [CrossRef]
- Liu, S.; Kurth, D.G.; Bredenkötter, B.; Volkmer, D. The structure of self-assembled multilayers with polyoxometalate nanoclusters. J. Am. Chem. Soc. 2002, 124, 12279–12287. [Google Scholar] [CrossRef]
- Schoot, C.; Ponjee, J. Image Display Apparatus. US 3806229, 23 April 1974. [Google Scholar]
- Schoot, C.; Ponjee, J.; Van Dam, H.; Van Doorn, R.; Bolwijn, P. New electrochromic memory display. Appl. Phys. Lett. 1973, 23, 64–65. [Google Scholar] [CrossRef]
- Barclay, D.; Bird, C.; Kirkman, D.; Martin, D.; Moth, E. An integrated electrochromic data display. Sid 80 Dig. 1980, 124, 124–125. [Google Scholar]
- Kenworthy, J. Variable Light Transmission Device. US3712709A, 23 January 1973. [Google Scholar]
- Byker, H.J. Single-Compartment, Self-Erasing, Solution-Phase Electrochromic Devices, Solutions for Use Therein, and Uses Thereof. Google Patents US4902108A, 20 February 1990. [Google Scholar]
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic systems and the prospects for devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Corr, D.; Bach, U.; Fay, D.; Kinsella, M.; McAtamney, C.; O’Reilly, F.; Rao, S.; Stobie, N. Coloured electrochromic “paper-quality” displays based on modified mesoporous electrodes. Solid State Ion. 2003, 165, 315–321. [Google Scholar] [CrossRef]
- Pichot, F.; McAtamney, C.; Bach, U. Electrochromic Display Device. WO2004/068231A1, 12 August 2004. [Google Scholar]
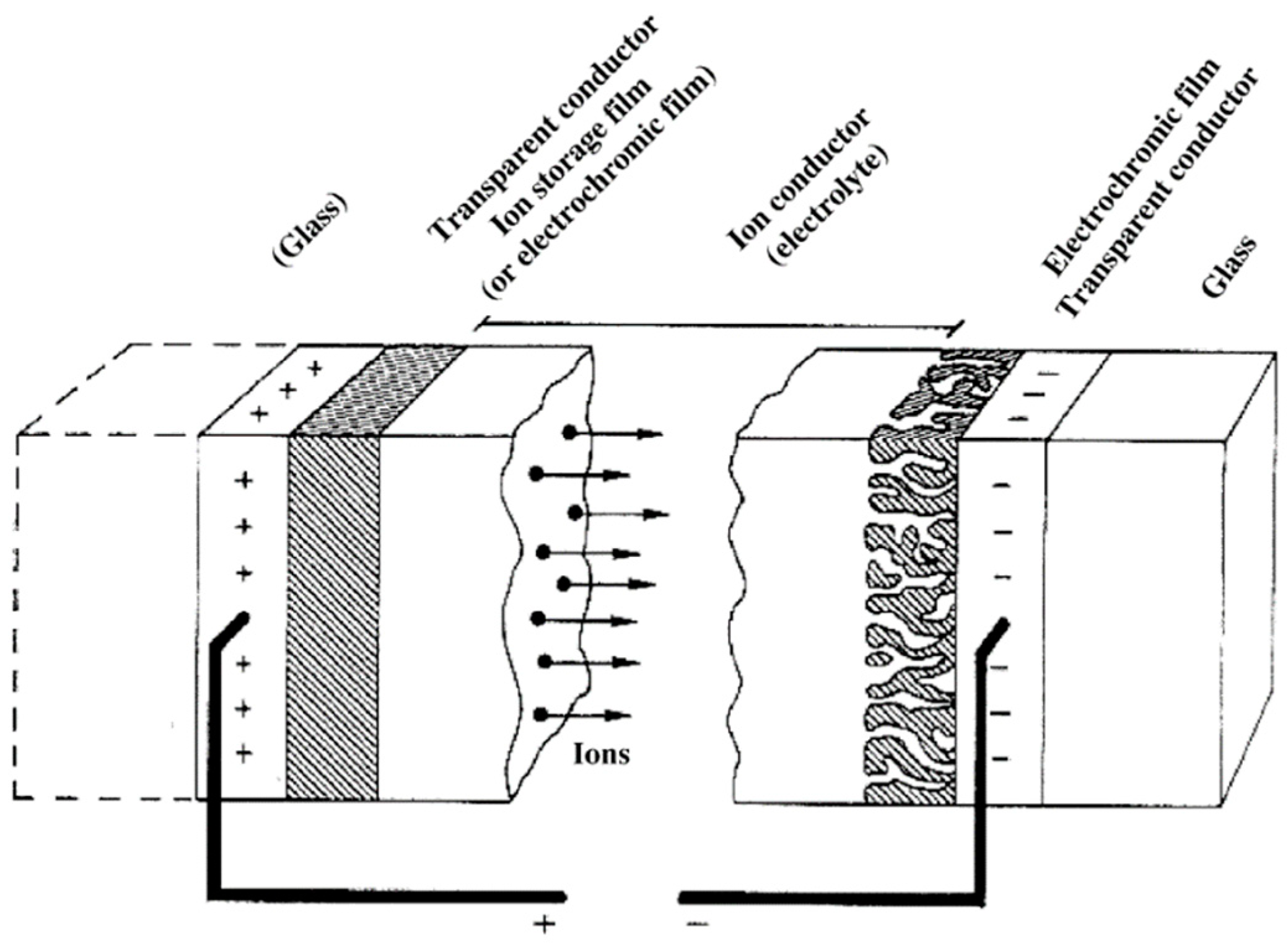

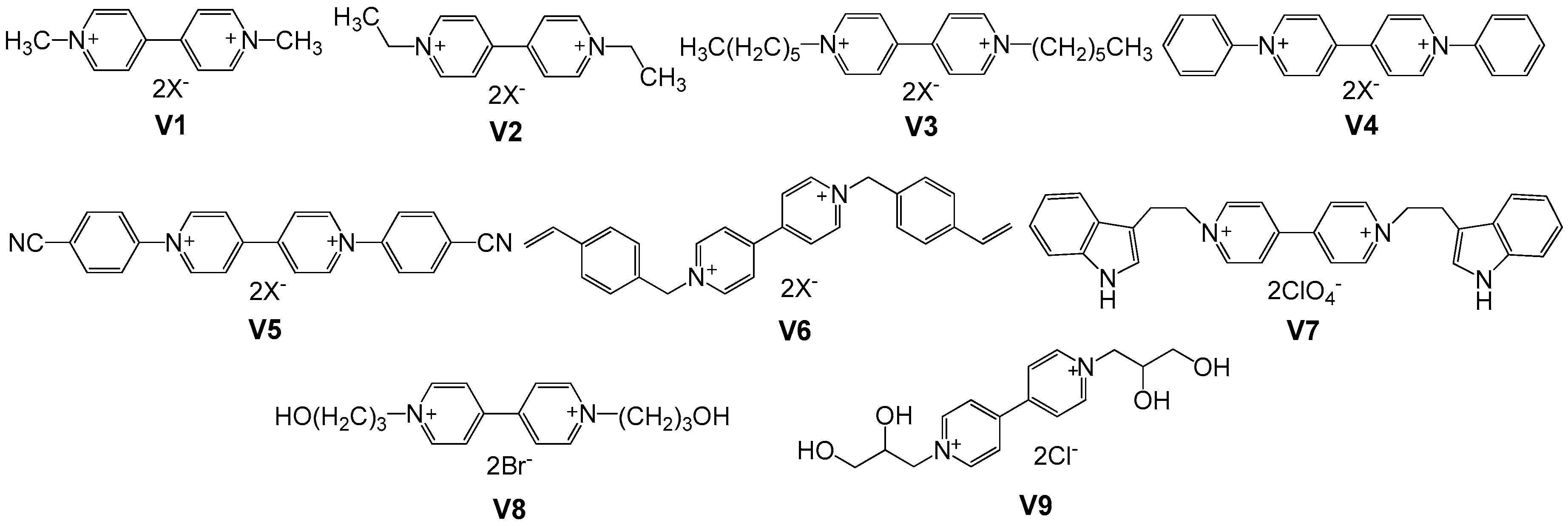
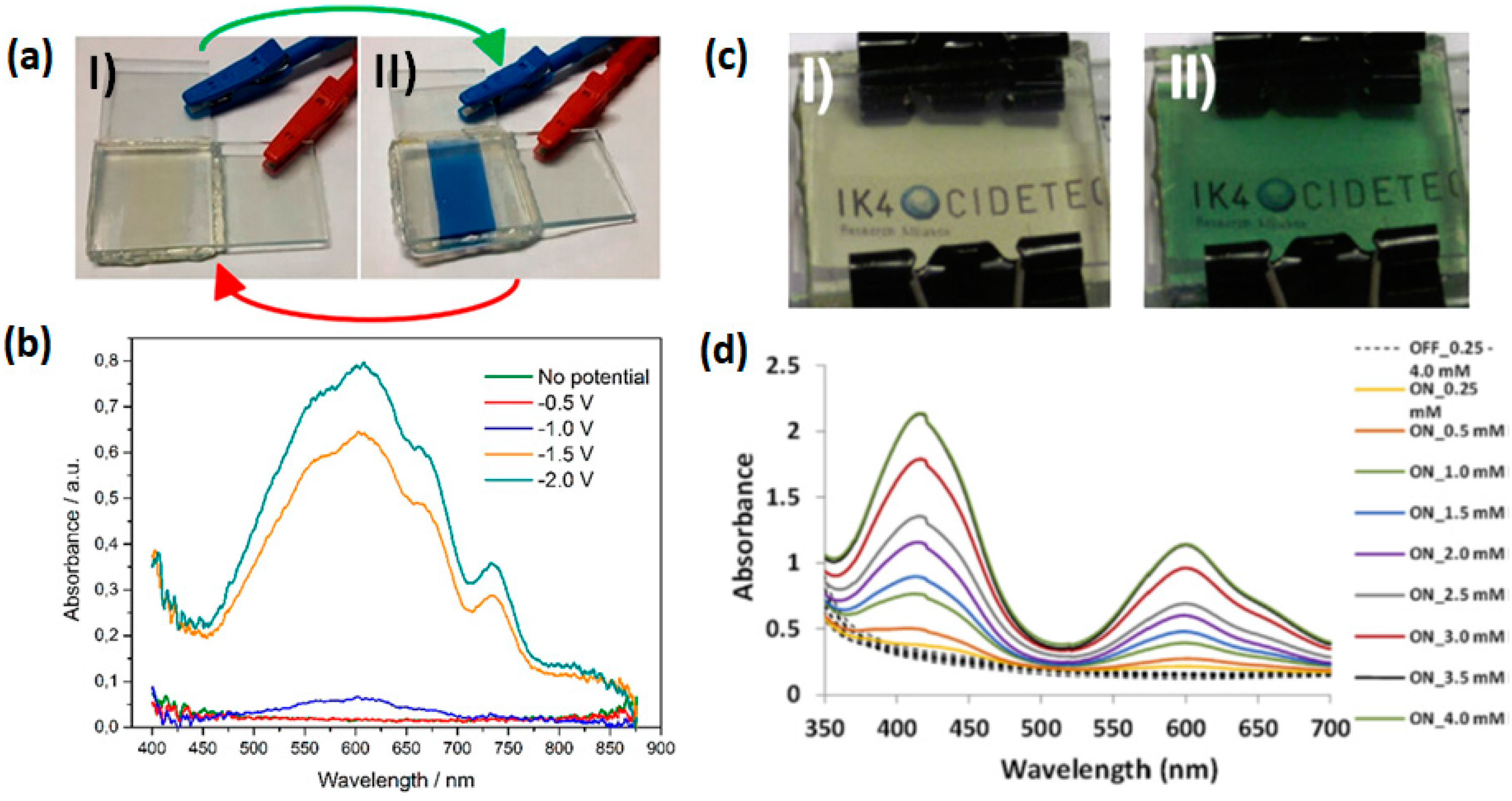
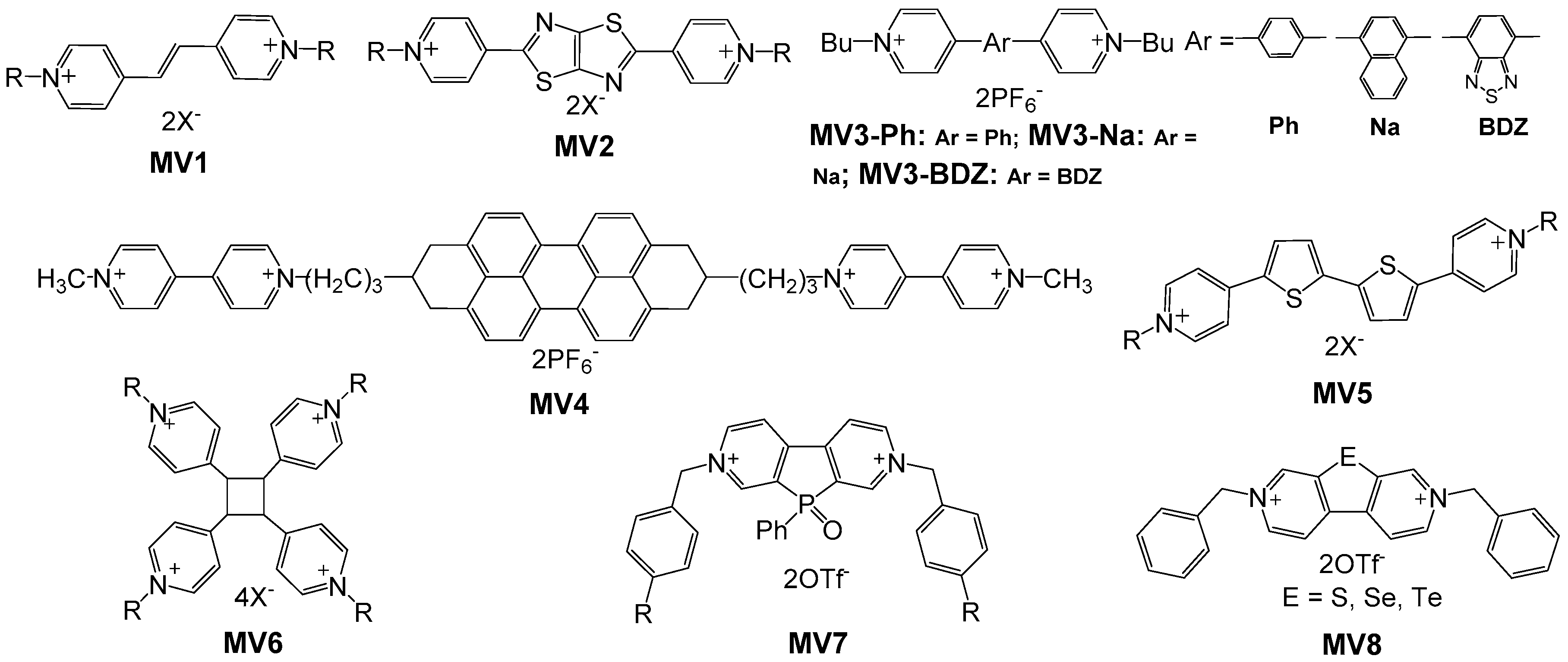
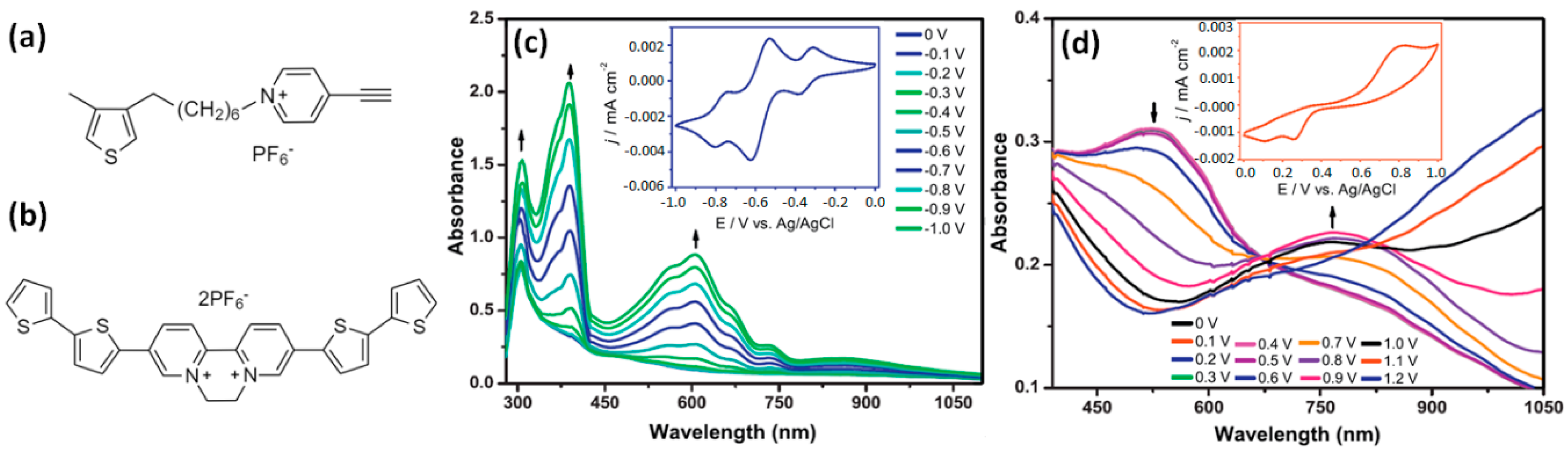
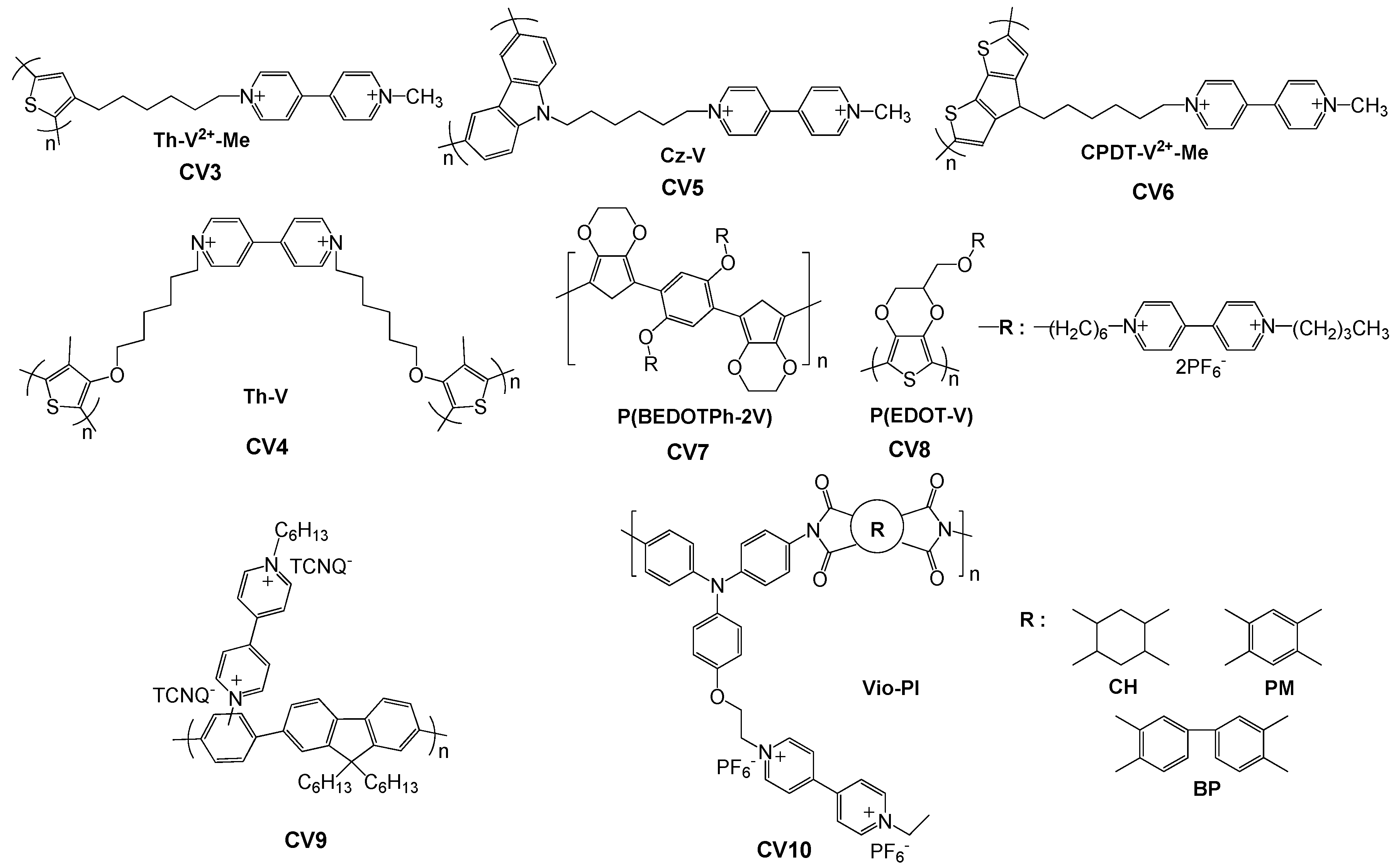
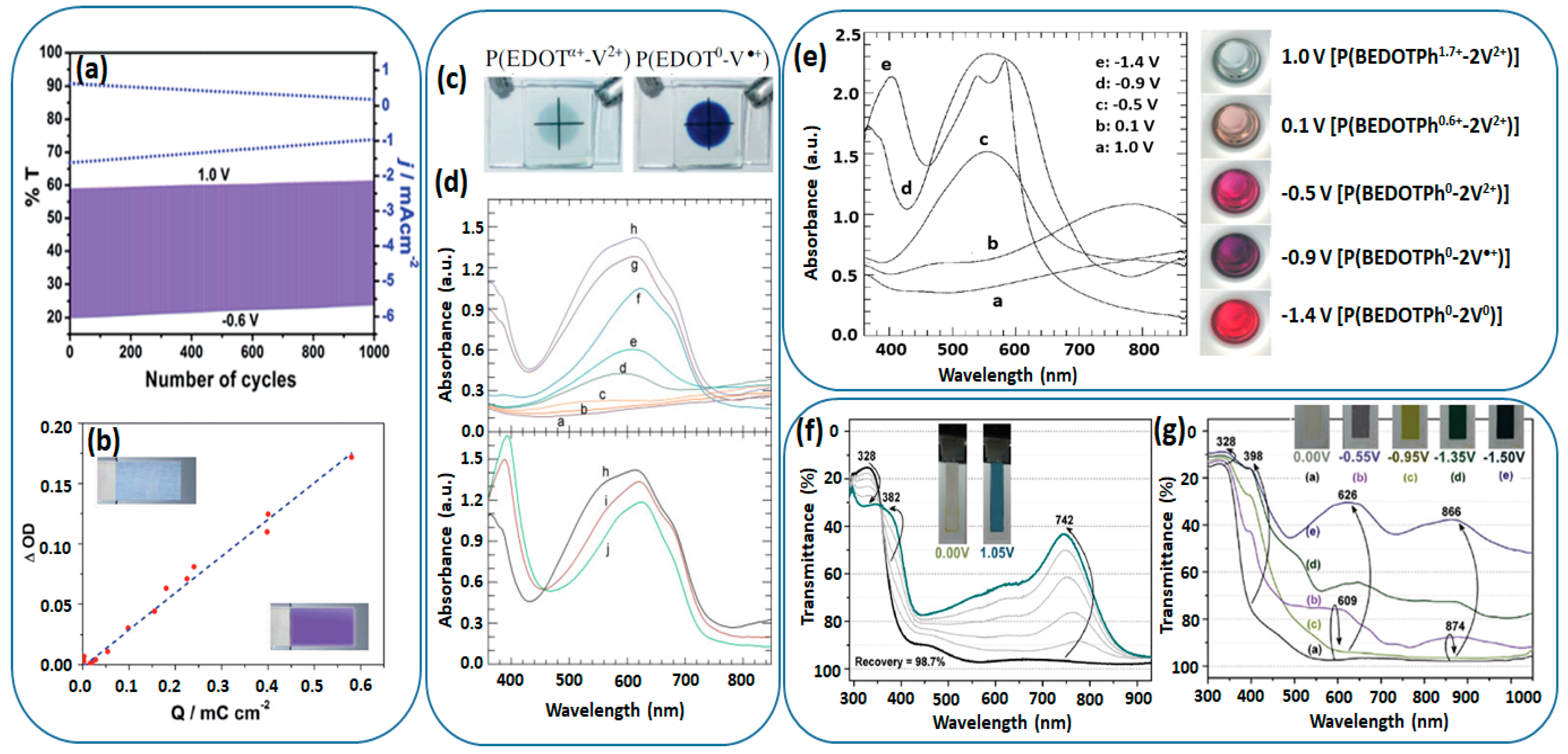
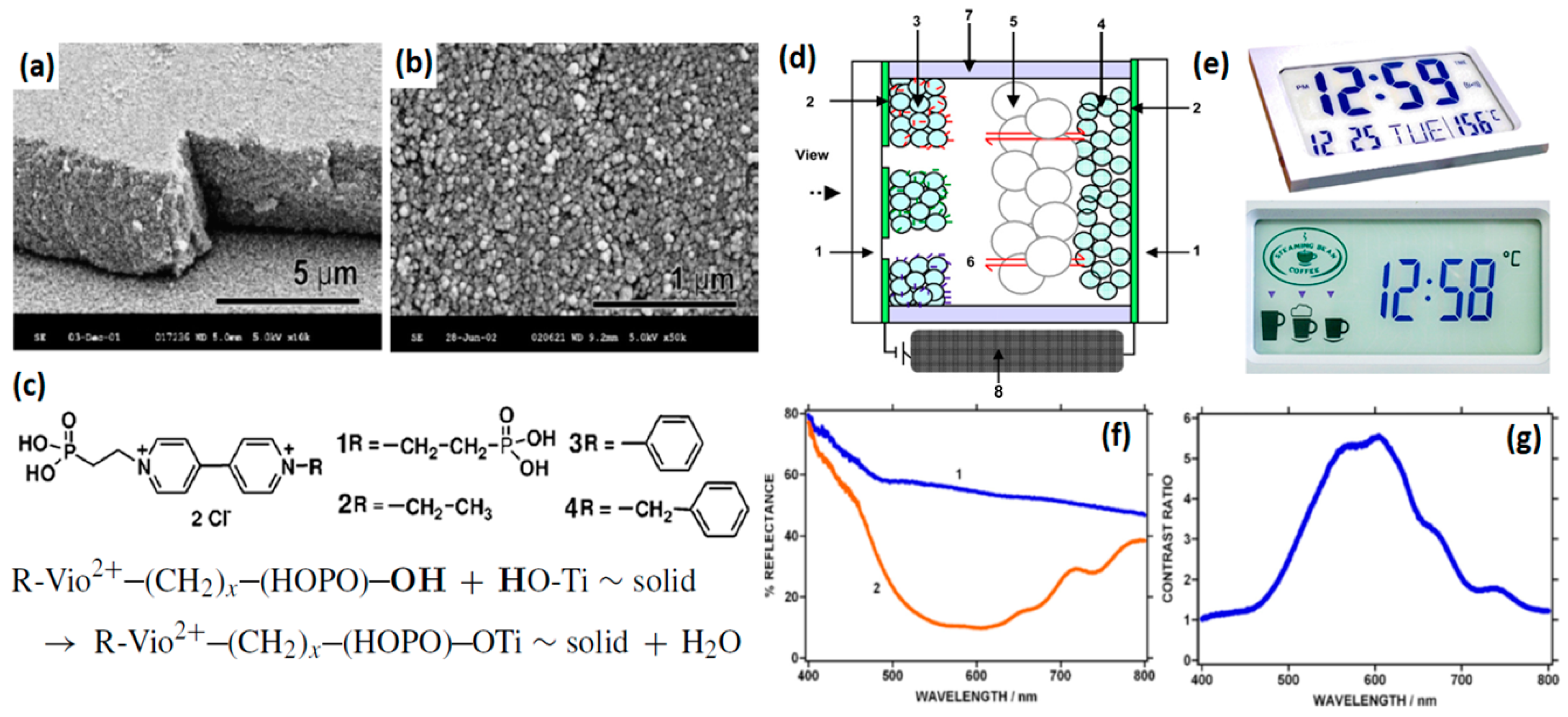
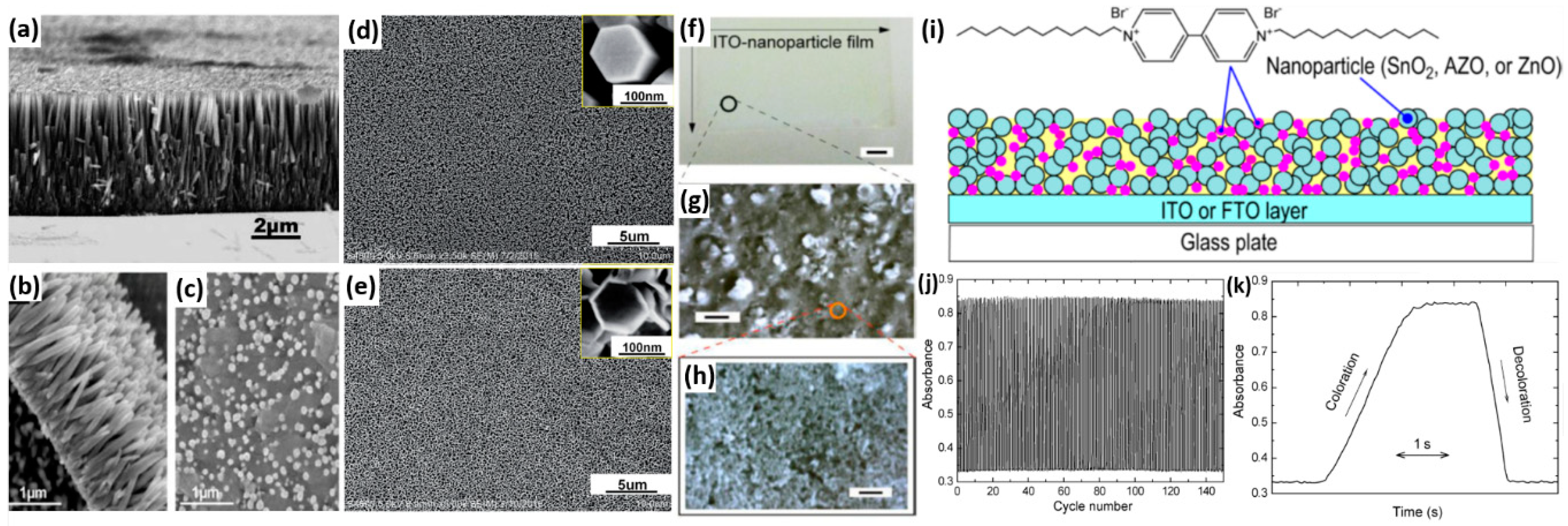
| EC Material | Color (O) a | Color (R) b | λmax c (nm) | Vb/Vc d (V) | ∆T e (%) | tb/tc f (s) | Stability (cycles) | CE g (cm2 C−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| V5 | colorless | Green or red | 600/500 | 0/−1.4 or −1.8 | 30 | - | 6000 | - | [20] |
| V6/V4 | colorless | black | 615 | 0/0.5 | 65 | 3.5/2.8 | 10,000 | - | [21] |
| V7 | colorless | Dark violet-blue | 605 | 1.5/−1.5 | 52 | 2.1/2.2 | 2000 | 533 | [22] |
| V8 | Slight yellow | Deep blue | 607 | 0/0.9 | 83 | - | - | 315 | [23] |
| V9 | Greenish yellow | amaranth | 520 | 0/−1.0 | 51 | 2.1/4.5 | 10,000 | 301 | [24] |
| MV1 | colorless | magenta | 520 | 0/−1.5 | 68 | 1/1.5 | 1000 | 875 | [25] |
| MV3 (Ar=PV) | colorless | pink | 512 | 0/−1.5 | 76.9 | 9.5/56.7 | 1000 | - | [26] |
| MV3 (Ar=NV) | colorless | purple red | 544 | 0/−2.5 | 74.1 | 18.4/41.4 | 1000 | - | [26] |
| MV3 (Ar=BV) | colorless | deep violet-blue | 592 | 0/−2.4 | 69 | 24/38.6 | 1000 | - | [26] |
| MV4 | bright red | blue/violet | 635 | 0.5/−0.8 | 33 | 1.9/1.8 | - | - | [27] |
| MV6 (R=vinyl) | colorless | Red brown | 522 | 1.6/−1.6 | 75 | 0.79/0.48 | 1000 | 645 | [28] |
| EC Material | Color (O) a | Color (R) b | λmax c (nm) | Vb/Vc d (V) | ∆T e (%) | tb/tc f (s) | Stability (cycles) | CE g (cm2 C−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PV I | Colorless | blue | 700 | 0/−1.0 | 34 | 0.26/0.39 | 1000 | 204 | [64] |
| PV II | Colorless | blue | 650 | 0/−1.0 | 28 | 0.54/2.18 | 1000 | 117 | [64] |
| PV I/PSS | Colorless | blue | 700 | 0/−1.0 | 34.7 | 0.31/0.26 | 50 | - | [62] |
| PV I/PVCa | Slight yellow | blue | 700 | −0.5/−1.2 | 40 | 13.5/1.2 | 50 | - | [61] |
| V3/PEDOT:PSS | Colorless | blue | 525 | 0.5/−0.9 | 82.1 | 2.2/3.1 | - | - | [48] |
| PBV h/PB i | colorless | purplish-blue | 650 | −1.0/0.7 | 65 | 9.4/2.0 | 4000 | 163 | [49] |
| CV1 | colorless | Dark blue | 625 | 0/−1.0 | - | - | - | 97 | [69] |
| CV4 | colorless | Dark violet | 610 | −0.6/1.0 | 39 | 7/5 | 1000 | 305 | [70] |
| CV8 | colorless | blue | 610 | 1.0/−1.0 | 65 | 3.7/8.4 | - | - | [71] |
| CV10 (R=PM) | Pale yellow | Dark cyan | 740 | 0/1.05 | 64 | - | - | - | [72] |
| CV10 (R=BP) | Pale yellow | Dark cyan | 742 | 0/1.05 | 50 | - | - | - | [72] |
| EC Material | Color (O) a | Color (R) b | λmax c (nm) | Vb/Vc d (V) | ∆T e (%) | tb/tc f (s) | Stability (cycles) | CE g (cm2 C−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| BPV h/meso-nc-TiO2 i | colorless | deep blue | 608 | 2.5/−2.5 | 53 | 0.9/2.1 | 100 | - | [88] |
| BPV/nc-TiO2 j | colorless | deep blue | 608 | 0/−1.5 | 55 | 1/1 | 10,000 | 170 | [89] |
| PBT k/nc-TiO2 | brown yellowish | dark blue | 600 | 2.0/−2.0 | 64.8 | 0.72/0.6 | 8000 | 912 | [94] |
| PBT/nc-TiO2 | yellow green | deep black | 570 | 0.5/−1.5 | 60 | 4.8/3.0 | 120,000 | - | [97] |
| V1/ZnO NTs l | colorless | blue | 608 | 3.0/−3.0 | 70 | 3/6 | 500 | 94 | [100] |
| V1/ZnO NWs m | colorless | blue | 608 | 2.0/−2.0 | 46 | 0.14/0.17 | 200 | 196 | [98] |
| DDV o/ITO NPs n | white | violet | 512 | 0/−0.6 | 15 | 0.38/0.5 | 500 | 140 | [101] |
| DDV/SnO2 NPs | white | purple | 510 | 0/−0.5 | 32 | 0.53/1.5 | 150 | - | [103] |
| PV I/rGO p | colorless | purple | 525 | 0/−0.6 | 52 | 9/6 | 400 | 142 | [104] |
| V1/GQD q | colorless | purple | 550 | 0/−2.8 | 29 | 39/26 | 3000 | 65 | [105] |
| WO3/PBV/RP r | colorless | deep blue | 580 | 1.0/−1.0 | 49 | 0.7/0.8 | 500 | 476 | [106] |
| PPDP/MWCNTs | colorless | deep blue | 700 | 0.3/−1.0 | 52 | 0.35/0.32 | - | - | [107] |
| V3/P2W18 | colorless | violet | 600 | 0.1/−0.9 | 42.7 | 2.0/5.8 | 200 | - | [108] |
| PXV s/SQ NPs t | orange | dark purple-blue | 550 | −0.7/−1.3 | 50 | 3.5/3 | - | 205 | [109] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, K.W.; Wang, S.-X.; Soo, D.X.Y.; Xu, J. Viologen-Based Electrochromic Materials: From Small Molecules, Polymers and Composites to Their Applications. Polymers 2019, 11, 1839. https://doi.org/10.3390/polym11111839
Shah KW, Wang S-X, Soo DXY, Xu J. Viologen-Based Electrochromic Materials: From Small Molecules, Polymers and Composites to Their Applications. Polymers. 2019; 11(11):1839. https://doi.org/10.3390/polym11111839
Chicago/Turabian StyleShah, Kwok Wei, Su-Xi Wang, Debbie Xiang Yun Soo, and Jianwei Xu. 2019. "Viologen-Based Electrochromic Materials: From Small Molecules, Polymers and Composites to Their Applications" Polymers 11, no. 11: 1839. https://doi.org/10.3390/polym11111839






