1. Introduction
With the international calls for energy conservation, environmental protection, and emission reduction, a growing voice to make motor vehicles more fuel efficient has become widespread all over the world, thus calling for a high-performance tire (i.e., a green tire) [
1]. A green tire could achieve a balance of low rolling resistance, good wet skid resistance, and low external rolling noise. As one of the main components of a tire, elastomeric material is the only part that directly touches the ground. Therefore, the performance of elastomeric material plays a decisive role for low rolling resistance and good wet grip resistance of a tire [
2]. Emulsion polymerized styrene-butadiene rubber (ESBR) is one of the most widely used synthetic rubbers. Due to its molecular structure, polymerization methods, and so on, conventional ESBR has a wide molecular weight distribution, poor filler dispersion, large heat generation, high rolling resistance, and does not meet the requirements for a green tire [
3,
4,
5]. Solution polymerized styrene-butadiene rubber (SSBR) with excellent overall performance is used to manufacture green tires in the industry. However, due to a conditioned synthetic process, low production efficiency, and high production cost, SSBR still cannot completely replace ESBR in tire manufacturing on a large scale in a short time [
6,
7]. Driven by requirements of the EU Tyre Labelling Regulation and the competition threat of SSBR, it is imperative to optimize the overall performance of ESBR via physical and chemical modifications such as the functionalization of ESBR macromolecules.
Recently, the preparation of silica/polymer nanocomposites has received attention all over the world. For example, silica has been incorporated into polymers such as silica gels [
8] and poly(ethylene terephthalate) [
9,
10] to prepare silica/polymer nanocomposites, aiming to improve their overall performance. With the proposal of green tires, nonpetroleum-based silica has become one of the most widely used fillers in tire tread rubber [
11] as silica can endow the tire tread rubber with excellent overall performance (excellent mechanical properties, low rolling resistance, and good wet skid resistance). However, the surface of silica contains a large number of hydroxyl groups [
12], resulting in poor dispersion of silica in hydrophobic rubbers and poor compatibility with the rubber matrix [
13]. There are two main ways to solve this problem: (i) hydrophobation of the silica surface using a coupling agent [
14,
15] and the subsequent chemical bonding between the rubber molecules and the coupling agent [
16,
17], and (ii) improvement of interfacial interactions between rubber and silica by introducing functional groups to the ESBR molecular chains [
18].
At present, the functional modification of traditional synthetic rubber has developed rapidly through (1) epoxidation [
19,
20,
21,
22,
23], (2) grafting [
24,
25], and (3) copolymerization [
18,
26,
27,
28]. Among them, copolymerization modification is an important means for imparting special functions to rubber in emulsion polymerization. The copolymerization modification of ESBR refers to copolymerizing a third functional monomer, such as glycidyl methacrylate (GMA) [
28], hydroxypropyl methacrylate (HPMA) [
29], acrylonitrile (ACN) [
29], or methyl diethylaminoethyl acrylate [
30], during the process of ESBR synthesis. The effects of various functional monomers in silica/ESBR compounds have been investigated, and results show that hydroxylated methacrylates and acrylates are suitable third co-monomers in ESBR for improving interfacial interaction in the compounds. Qiao et al. [
27] showed demonstrated an excellent performance of silica/GMA-ESBR compounds without coupling agents. Hyunsung Mun et al. [
31] manufactured silica/GMA-ESBR compounds using wet masterbatch (WMB) technology to reduce viscosity and improve processability. However, the functional monomers used in previous studies were all petroleum-based monomers. In order to reduce the dependence on non-renewable petrochemical resources in the rubber industry, we chose bio-based functional monomers derived from biomass resources [
32,
33,
34] instead of petroleum-based functional monomers. Wang et al. [
35,
36,
37] prepared a bio-based elastomer by emulsion copolymerization of itaconate with diene. Lei et al. [
38] used various itaconates to copolymerize with isoprene to prepare bio-based elastomers with various side lengths. The results showed that dibutyl itaconate is the optimal bio-based monomer to prepare the bio-based elastomer, and that it has excellent overall performance. Based on the results shown by Lei et al., this work used dibutyl itaconate as a functional monomer to modify ESBR. Different to previously introduced petroleum-based functional monomers, dibutyl itaconate has two ester groups located on different sides, which means dibutyl itaconate can introduce higher polar group density than those petroleum-based monomers under the same conditions. Thus, we believe that dibutyl itaconate can be used to modify the molecular structure of ESBR and to improve the overall performance of ESBR.
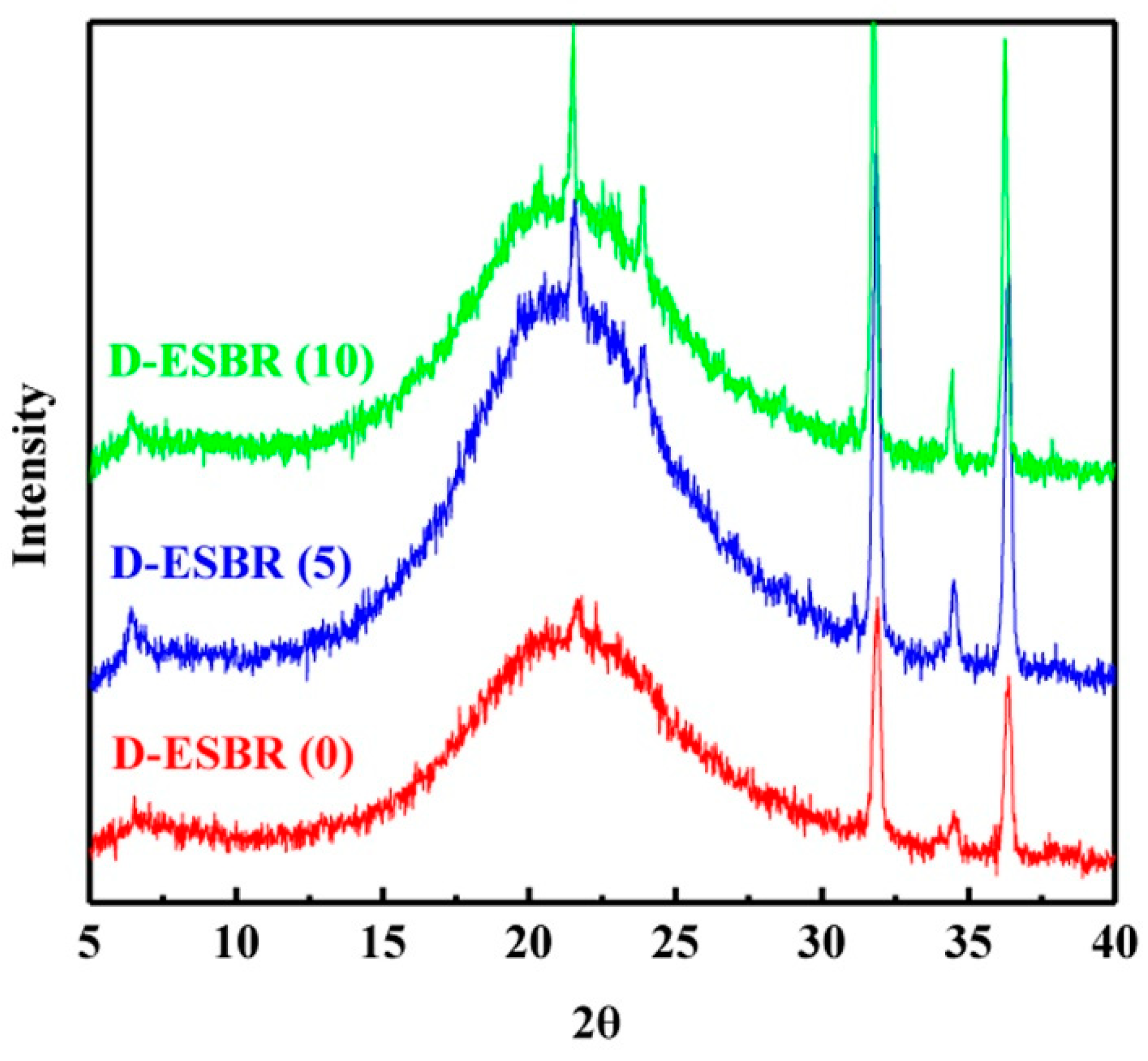
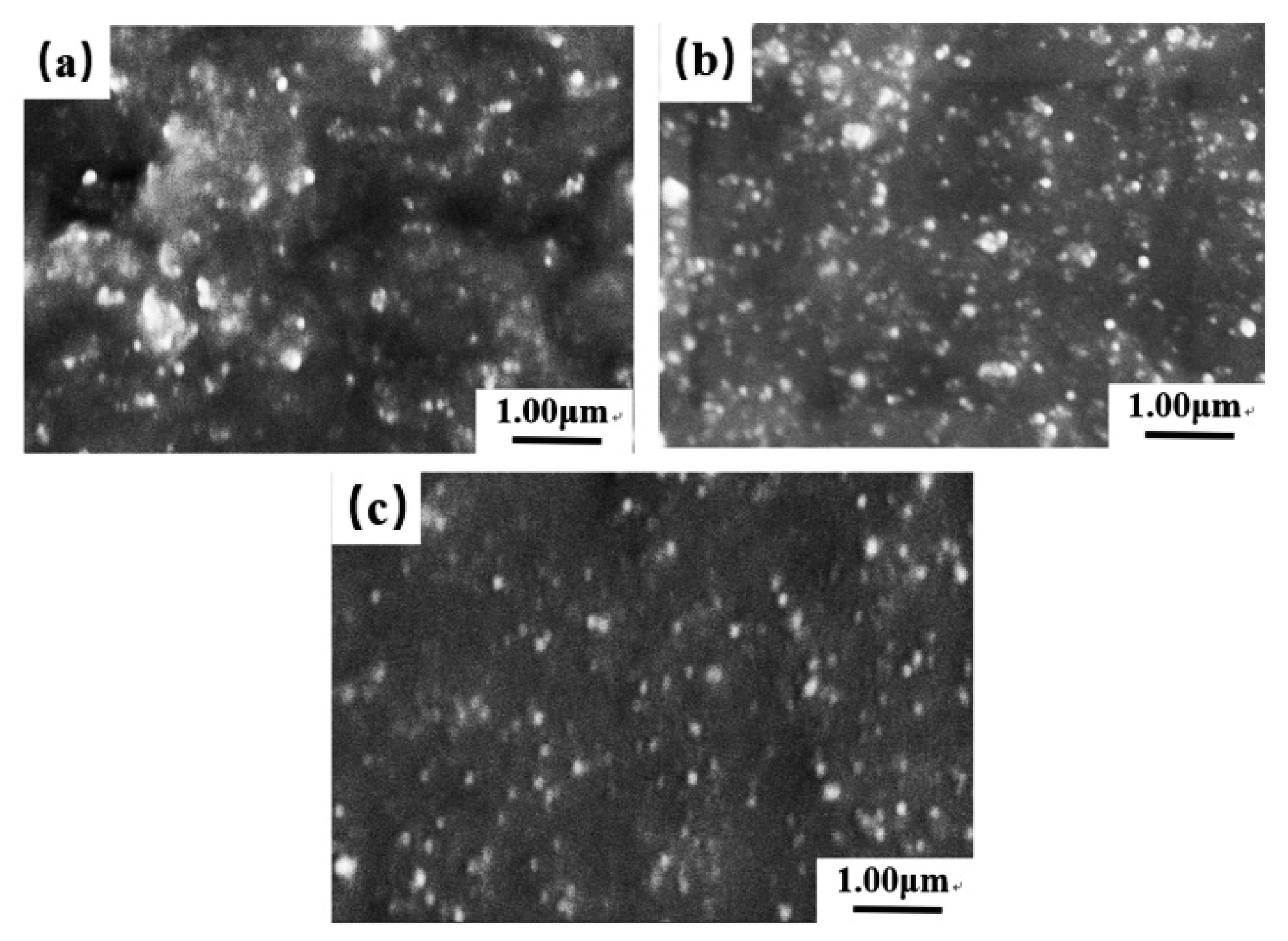
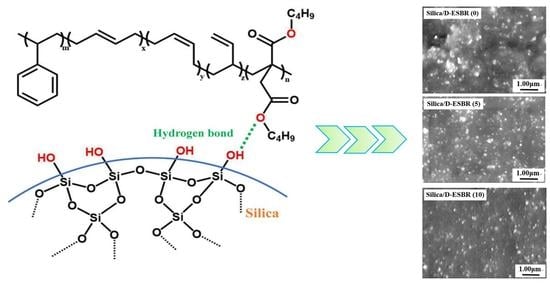
In this study, an ester-functionalized styrene-butadiene rubber (dibutyl itaconate-styrene-butadiene rubber) (D-ESBR) was synthesized through low-temperature emulsion polymerization, and silica was used as a filler to prepare silica/D-ESBR nanocomposites. We studied the effects of ester content on properties of silica/D-ESBR nanocomposites, including static and dynamic mechanical properties, dispersion of silica, and wear resistance. The hydrogen bonding formed between the hydroxyl groups on the surface of silica and the ester groups of D-ESBR can improve the dispersion of silica and enhance the interfacial interaction, resulting in a significant reduction of rolling resistance and heat generation of the silica/D-ESBR nanocomposites. This study also provides an effective strategy for preparing high-performance elastomeric nanocomposites by forming hydrogen bonding between the filler and elastomer matrixes. The proposal schematic for hydrogen bonding formed in silica/D-ESBR nanocomposites is shown in
Figure 1.
4. Conclusions
Based on bio-based dibutyl itaconate-containing ester groups, poly(dibutyl itaconate-co-styrene-co-butadiene) (dibutyl itaconate-styrene-butadiene rubber) (D-ESBR) was successfully synthesized by redox emulsion polymerization, and the silica/D-ESBR nanocomposites were prepared. After the ester group was introduced into the D-ESBR, the dispersion of silica in the rubber matrix and the interfacial interaction between silica and rubber chains were improved. The improvements were attributed to hydrogen bonds between the hydroxyl groups of silica and the ester groups of D-ESBR, which play a positive role in the reduction of rolling resistance. In addition, the physical and mechanical properties of silica/D-ESBR nanocomposites with different DBI contents were studied. The results showed that D-ESBR with 5 wt % DBI had excellent overall mechanical properties, low heat generation, the lowest rolling resistance, comparable wear resistance and the best anti-aging performance among the tested nanocomposites. The deteriorated performance of D-ESBR with 10 wt% DBI was due to a reduction in cross-linking density. All in all, small quantities of DBI were used to introduce the ester groups into D-ESBR macromolecular chains, which can improve the overall performance of D-ESBR and provide a new strategy for “high performance” (green) automotive tire materials.